Abstract
BACKGROUND
Tumoral calcinosis, mass-like calcium deposition into the soft tissues, is an uncommon manifestation of the systemic sclerosis subtype of scleroderma. When this process affects the spinal epidural space, it can cause canal narrowing and place the spinal cord at significant risk of injury.
OBSERVATIONS
Here a 62-year-old female with systemic sclerosis and no previous evidence of spinal cord compromise who developed acute spinal cord injury and quadriparesis after a mechanical fall is described. She was found to have a large dorsal epidural calcified mass compressing her cervical spinal cord. She underwent medical management for acute spinal cord compression as well as surgical management for acute spinal cord injury and degenerative spine disease. Her case illustrates a rare etiology of simultaneous degenerative spine instability and lesional spinal cord compression with acute spinal cord injury.
LESSONS
Tumor calcinosis leading to acute spinal cord injury in the setting of systemic sclerosis is an uncommon but critical entity to recognize in patients with scleroderma and may require the physician to use a combination of medical and surgical management strategies from each of these categories of spine pathology.
Keywords: scleroderma, spinal cord injury, systemic sclerosis, trauma, calcinosis
ABBREVIATIONS : AIS = American Spinal Cord Injury Association Impairment Scale, ASIA = American Spinal Cord Injury Association, CT = computed tomography, MRI = magnetic resonance imaging, SCI = spinal cord injury
Approximately 17,000 people suffer acute spinal cord injury (SCI) in the United States each year, many of whom must live with lifelong neurological impairments.1,2 Whether attributable to chronic or degenerative spine conditions, compressive malignancies, or acute traumatic etiologies, SCI can be a debilitating diagnosis. Depending on the etiology, recommended supportive and surgical treatment interventions for SCI differ. Compression secondary to some malignancies often require steroid treatment for spinal cord edema whereas traumatic SCI necessitates blood pressure control to support and augment spinal cord perfusion in order to mitigate the effects of spinal cord ischemia resulting from trauma.3,4
Here, the authors discuss a clinical presentation of acute SCI, namely central cord syndrome, due to compressive cervical tumoral calcinosis in a patient with the systemic sclerosis subtype of scleroderma. Scleroderma is an autoimmune rheumatic disease that affects connective tissue in the body, most prominently the skin.5,6 In this disease, the body’s fibroblasts become activated causing an overproduction and accumulation of collagen resulting in the classic appearance of rigid skin and other tissues.7 However, the more diffuse form of this disease, called systemic sclerosis, can also involve the muscles, blood vessels, bones, and other organ systems.6,7 A somewhat uncommon presentation of systemic sclerosis is calcinosis of the spine whereby insoluble calcium salts are deposited into the paraspinous tissues, joints, and more rarely, the epidural tissues of the spine.7–13 When this abnormal calcified tissue forms as a solid mass, it can structurally behave as a tumor and therefore is termed tumoral calcinosis.14 This abnormal calcified tissue acts as a mass adjacent to the spinal cord and destabilizes the bony spine, thereby setting up the perfect scenario for spinal cord injury. This presentation represents a multifactorial cause of SCI with the compressive features of malignancy, spinal instability of degenerative spondylosis, and acute management concerns of acute traumatic spinal cord injury.
Tumoral calcinosis was first described in the literature in 1898 to describe a familial, hereditary condition of mass lesions that affect the periarticular space.15 However, it was not until 1943 that this entity was given the name tumoral calcinosis.15 Since then, the term has been used to describe not only the familial pattern of presentation, but also the similar deposition of calcified material with associated inflammation and fibrosis in the tissues in other diseases as well. Here, we describe a rare case of tumoral calcinosis in the cervical spine resulting in an acute spinal cord injury. Although the literature has described other cases of calcinosis in patients with systemic sclerosis, none have presented such an aggressive case of calcinosis deposition in the epidural space, with near obliteration of the spinal canal coupled with degenerative instability and associated paraspinous calcinosis changes. Features of both malignant tumoral spinal cord compression and acute traumatic spinal cord injury must be addressed and balanced against each other in management.
Illustrative Case
A 62-year-old female with limited systemic sclerosis diagnosed 9 years prior to presentation had symptoms that were controlled on oral prednisone. She had no previous symptoms of myelopathy, although she did have baseline restricted movement in bilateral wrists, hands, and fingers from her scleroderma. She suffered a mechanical ground level fall with head strike, neck hyperextension, and loss of consciousness. After the fall, she experienced immediate quadriplegia and she was transferred by ambulance to the nearest hospital, not our facility. Upon presentation, it was documented that she had 0/5 movement in her upper and lower extremities as well as absent sensation below the neck.
Computed tomography (CT) scanning of the cervical spine revealed spondylolisthesis of C3 on C4. In addition, there was evidence of disc degeneration C4–5 and C5–6 and loss of cervical lordosis. Calcified mass-like material was seen in the dorsal epidural space from C3 to C5 causing significant canal stenosis. Calcified mass-like material was observed in the bilateral parafacet joint and paraspinous regions C2–3 through C7–T1 levels as well (Fig. 1). Magnetic resonance imaging (MRI) of the cervical spine revealed severe spinal canal stenosis C3–6 (Fig. 2).
FIG. 1.
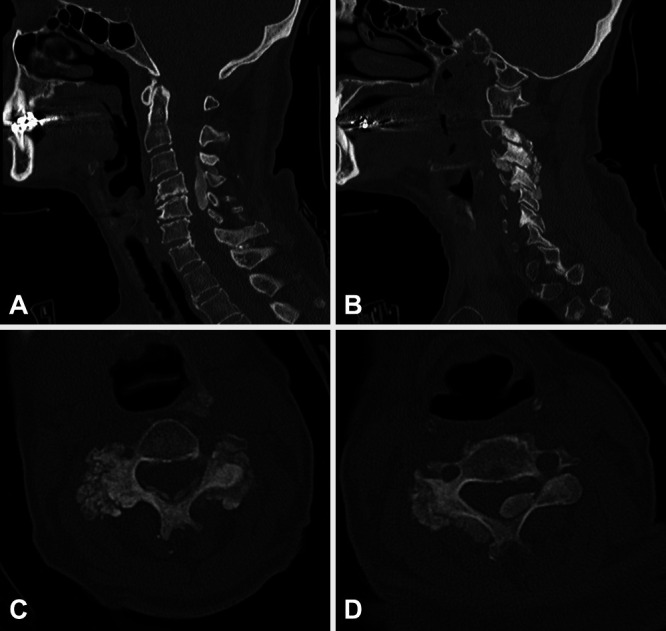
Preoperative sagittal (A and B) and axial (C and D) CT scans. The dorsal epidural tumoral calcinosis mass extends across the C3–5 levels causing central canal stenosis (A and D). There are also multilevel calcified lesions involving the facet joints and parafacet regions bilaterally (B and C). In addition, diffuse degenerative changes and spondylolisthesis C3 on C4 (A) can be appreciated.
FIG. 2.
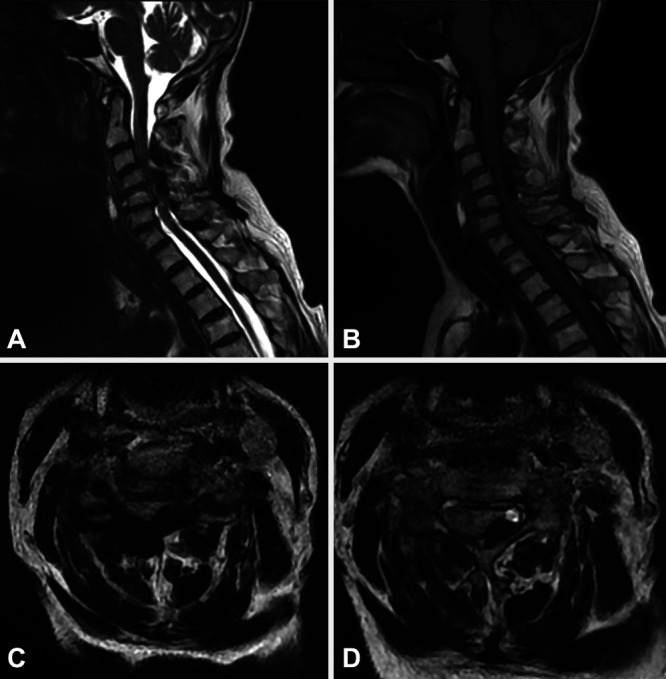
Preoperative sagittal (A and B) and axial (C and D) magnetic resonance (MR) images showing severe spinal canal stenosis and spinal cord compression due to dorsal epidural calcified mass lesion. T2-weighted (A, C, and D) and T1-weighted (B) MR images are featured.
The patient was treated with intravenous steroids and monitored. She began to show some improvement in motor and sensory function and 4 days later was transferred to our facility for acute inpatient rehabilitation. At that time, clinically, she had an incomplete SCI at the C3 level, and an American Spinal Cord Injury Association (ASIA) Impairment Scale (AIS) score of D. On physical examination, her strength in bilateral biceps and triceps was 4/5. Finger flexors, wrist extensors, and finger abductors were unable to be tested because of underlying contractures from scleroderma. Iliopsoas strength bilaterally was 4/5. The rest of her lower extremity muscles on the right were 5/5 strength. On the left, quadriceps, extensor hallucis longus, and gastrocnemius muscles were 4/5 strength while the left tibialis anterior was 3/5 strength.
Her physical examination and imaging were consistent with acute central cord syndrome secondary to severe spinal canal stenosis from likely tumoral calcinosis. In addition, she had severe degenerative spinal instability as a result of the calcinosis. She was taken to the operating room for cervical decompression with C3–5 laminectomies and removal of dorsal epidural tumoral calcinosis, as well as spinal stabilization with C3–7 posterior spine fusion and lateral mass instrumentation (Fig. 3). The calcinosis deposits encountered intraoperatively in the C3–4 through C6–7 parafacet and paraspinous regions were soft and had the semisolid consistency of toothpaste. These were debulked by suction and careful dissection of the tissue away from the surrounding normal structures. Next, the lateral mass screws were placed from the C3–7 levels. Then, laminectomies were performed using Leksell rongeurs and a high-speed matchstick drill. The dorsal epidural calcium mass encountered upon removal of the laminae was solid and flaky. It appeared to have replaced the ligamentum flavum and was severely indenting the thecal sac. It was mildly adherent to the dural surface. Once this was removed, the spinal cord was visually inspected and had reexpanded dorsally.
FIG. 3.
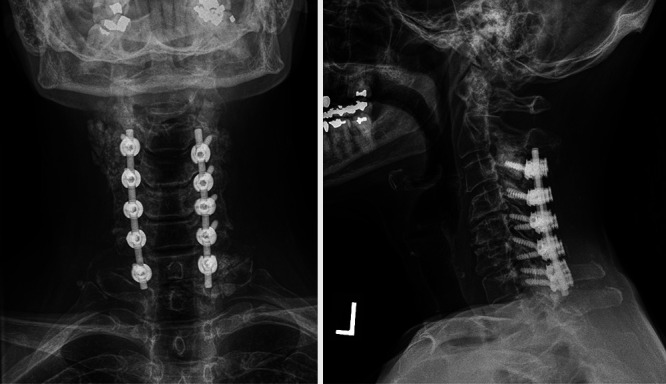
Postoperative anteroposterior (left) and lateral (right) cervical spine radiographs following C3–5 laminectomies, debulking of calcified mass, and C3–7 posterior spine fusion and instrumentation.
Postoperatively, the patient returned to the acute rehabilitation service where her strength and sensation continued to improve. Six months after the operation, she had regained full strength in all muscle groups in upper and lower extremities—sparing her baseline scleroderma-related contractures and stiffness in bilateral wrists, hands, and fingers—and had return of normal sensation.
Discussion
Observations
SCI from tumoral calcinosis of systemic sclerosis presents a unique disease state in which one must consider aspects of compressive spine malignancy, spinal degenerative disease, and acute traumatic spinal cord injury to determine the most clinically efficacious intervention(s). For the patient presented here the calcinosis acted as a mass lesion, similar to malignancy. Spinal cord compression from tumor is often treated with steroid administration for spinal cord edema.3 However, consensus for treatment of traumatic central cord syndrome patients, often due to underlying degenerative spinal canal narrowing, favors blood pressure elevation to support perfusion of the spinal cord.4 In addition, for those patients with spinal cord injury and ongoing compression, similar to patients with acute traumatic SCI, rapid surgical decompression is indicated.4,16 And finally, because of the spondylolisthesis, spine instrumentation and fusion is indicated.17 Our patient completed a course of steroids after the dorsal epidural mass lesion was identified at the presenting hospital and later, after transfer to our facility, underwent decompression and spine fusion.
Lessons
Tumoral calcinosis of the spine is a nonspecific but uncommon finding in systemic sclerosis or scleroderma, CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia), primary and secondary hyperphosphatemia syndromes, renal failure leading to uremia, rheumatoid arthritis, and other idiopathic etiologies.9,14,18,19 The calcinosis of systemic sclerosis is due to calcium hydroxyapatite deposits in connective tissues.20 This effectively converts connective tissues of the spine (including ligamentum flavum, facet capsule, muscle) into calcified structures. When these calcifications form mass lesions, they are termed tumoral calcinosis.15 Unlike other causes of spinal degenerative disease and SCI including disc herniations and ossified posterior longitudinal ligament, systemic sclerosis affects predominantly the dorsal structures of the spine. This leads to accelerated degenerative instability and spondylolisthesis of the spine at the levels affected by calcium deposition.
Depending on the morphology and location of the lesions, patients may present with a variety of symptoms ranging from pain to spinal cord dysfunction.10,21 Spinal cord compression in scleroderma-associated calcinosis is rare. Motegi et al.8 report that of 159 patients with systemic scleroderma, 27 (17%) had spinal calcinosis. Of those with spinal calcinosis, 7 of 27 (25.9%) had spinal cord compression from calcinosis, which represents 4.4% of patients with systemic scleroderma who have spinal cord compression.8
Spinal calcinosis in patients with scleroderma remains an uncommon, although potentially neurologically devastating entity. Therefore, we conducted a review of the literature of cervical spine calcinosis in patients with scleroderma/systemic sclerosis over the last 20 years (since 2000). We performed a PubMed search of the published literature and also reviewed the reference lists of articles returned from this search. We limited our search to patients with a confirmed diagnosis of scleroderma or systemic sclerosis; cases in which individual patients, their symptoms, imaging, and treatment courses, could be identified and reviewed; and English language articles. This returned 19 cases10–15,21–31 (Table 1). Fifteen of 19 (78.9%) affected patients were women, suggesting a female predominance. Three patients were treated nonoperatively and none of these patients had spinal canal stenosis due to the lesions. Their lesions were confined to the paraspinous regions and/or the facet joints. Only nine cases in the literature (47.4%) reported spinal canal compromise due to the calcinosis, and one case resulted in neural foraminal narrowing.
TABLE 1.
Literature review of reported cases of cervical calcinosis associated with scleroderma/systemic sclerosis
| Authors & Year | Sex | Age (yrs) | Preoperative Symptoms | Imaging Findings | Surgical Intervention | Outcome | ||
|---|---|---|---|---|---|---|---|---|
| Durant et al., 200114 |
M |
48 |
Unknown |
C3 on C4 subluxation, C3–4 pst element calcinosis, spinal cord compression |
Cervical laminectomies (unknown levels), pst spine fusion (unknown levels) |
Unknown |
||
| Olsen & Chew, 200415 |
F |
75 |
Torticollis |
Calcinosis of rt C2–4 facet joint, paraspinous region, & transverse process; calcinosis mass extending to anterior cervical muscle space at C2–4 levels compromising the esophagus |
“Excision of the mass” |
Unknown |
||
| Khan et al., 200522 |
F |
64 |
Neck pain, acute quadriparesis |
Calcification of cervical ligamentum flavum (unknown levels) |
C3–7 laminectomies, C2–7 pst fusion |
Resolution of neck pain, improved strength |
||
|
*Lima et al., 200623 |
F |
51 |
Weakness |
Calcification of C4–T1 PLL, “medullar compression” |
Cervical & thoracic laminectomies (unknown levels) |
Unknown |
||
|
*Smucker et al., 200624 |
M |
60 |
Neck pain, hand dysfunction, gait difficulty |
C2–3 subluxation |
Halo, C3 corpectomy & anterior fusion, C1–4 pst onlay fusion |
Unknown |
||
| F |
59 |
Neck pain |
C4–5 destructive lesion involving pst elements |
C3–7 pst fusion |
Unknown |
|||
| F |
73 |
Neck pain, hand dysfunction |
Multiple cervical levels of listhesis |
C2–T2 pst fusion, anterior C3–T1 fusion |
Unknown |
|||
| Carlson et al., 200725 |
F |
39 |
Bilat upper extremity weakness, quadriparesis, monoplegia |
Rt C4–5 facet joint calcinosis involving spinal canal causing canal narrowing |
C4–5 corpectomies |
Improved bilat upper extremity strength |
||
|
*Tuy et al., 200826 |
F |
50s |
Neck pain |
Rt C2–3 pst element calcinosis mass w/ encroachment into spinal canal |
Removal of lesion |
Symptom-free |
||
| Shoji et al., 201127 |
F |
34 |
Occipital headache |
Calcinosis around odontoid process, dislocation odontoid process due to basilar invagination w/ compression of medulla |
Halo, occipitocervical pst fusion & instrumentation |
Resolution of headaches, symptom-free |
||
| Lebl et al., 201328 |
F |
63 |
Neck pain, limited neck range of motion |
Bilat multiple cervical facet joint calcinosis (unknown levels) |
None |
NA |
||
| Al-Khudairi et al., 201530 |
F |
62 |
Bilat arm & leg weakness & numbness, myelopathy |
C3 on C4 anterolisthesis, lt C3 lateral mass destruction w/ calcinosis, lt C4 lateral mass destruction, narrowing of C2 foramen transversarium, perched rt C3 on C4 facet |
C3–4 laminectomies, reduction of C3–4 listhesis, C2–6 pst spine fusion & instrumentation |
Residual paresthesias of rt hand & elbow |
||
| Sambataro et al., 201510 |
M |
62 |
Neck pain |
Lt C4–5, C5–6 facet & lateral mass calcinosis, paraspinous region calcinosis |
“Surgical excision of the mass” |
Unknown |
||
| Nakamura et al., 201629 |
F |
74 |
Neck pain, bilat upper extremity numbness |
Calcinosis of bilat C1–2 facet joints |
“Debulking of tissue,” reconstruction of C1–2 area |
Resolution of neck pain & arm numbness, symptom-free |
||
| Nguyen et al., 201621 |
F |
57 |
Bilat hands, arms & legs numbness & weakness, gait imbalance, falls, myelopathy, incontinence |
Calcinosis of pst elements C4/5 Subluxation C4 on C5, spinal cord compression due to dorsal epidural calcified mass C4–5, spinal cord edema C3–6, rt C2–3 facet joint calcinosis, bilat C4–5 facet joint calcinosis |
C4–7 laminectomies, C3–T1 pst spine fusion & instrumentation |
Improved strength, improved bowel/bladder function |
||
| Tan et al., 201613 |
F |
79 |
Shortness of breath |
C3–5 paraspinous calcinosis masses, no spinal cord compression, L3–5 paraspinous masses |
None |
NA |
||
| Motegi et al., 201731 |
F |
54 |
Neck pain, bilat arm weakness & numbness |
C4–5 dorsal epidural tumoral calcinosis, bilat C3–4 facet calcinosis |
Resection of calcification |
Symptom-free |
||
| Logothetis et al., 201812 |
M |
67 |
Neck pain, palpable neck mass |
Rt C3–4 facet joint calcinosis mass, no spinal cord compression |
None |
NA |
||
| Karschnia et al., 201811 |
F |
58 |
Bilat hand weakness & dysesthesias, gait dysfunction, upper abdomen band-like pain |
Bilat C4–5, C5–6 facet joint calcinosis; C4–6 lamina replacement w/ calcinosis; paraspinous calcinosis; central stenosis C4–6 w/ T2 cord signal change C4–5; anterolisthesis C4 on C5 |
C4–5 laminectomies |
Residual weakness of intrinsic hand muscles, dysesthesias of hands, improvement of pain |
||
| Current case, 2021 | F | 62 | Central cord syndrome, initial quadriplegia then improved to bilat upper & lower extremity weakness | Spondylolisthesis C3 on C4, C5 on C6, C6 on C7; C3–5 dorsal epidural calcinosis mass causing severe canal stenosis & spinal cord compression; bilat C2–3 & C7–T1 facet joint calcinosis | C3–5 laminectomies, C2–7 pst fusion & instrumentation | Resolution of symptoms |
NA = not available; PLL = posterior longitudinal ligament; pst = posterior.
Data from secondary source.
Sixteen patients were treated surgically. The majority (n = 13) of patients were treated using a posterior surgical approach, either fusion alone or decompression and fusion depending on whether or not the neural elements were compromised. One patient was treated with an isolated anterior approach and two were treated with a combined anterior and posterior approach. Of those who did undergo surgical intervention, 10 patients (84.2%) required instrumentation for spine stability whereas six had decompressions alone or isolated resection of the calcified lesions. Cases reviewed did not discuss whether patients underwent medical interventions of either blood pressure support or of steroid administration during hospitalization.
Similar to the majority of reported cases associated with systemic sclerosis in the literature, our patient was female and required surgical intervention. She was treated in accordance with malignancy guidelines at the presenting hospital where she received steroids and in compliance with traumatic spinal cord injury guidelines of surgical decompression and fusion. She required posterior cervical decompression and fusion to address the compressive and destabilizing pathology of the calcinosis. This is in agreement with the most common reported practices in the literature (Table 1). Our case is unique, however, in that this patient presented with an extensive and massive lesion invading the spinal canal and causing severe cord compression. This was one of the most severe cases of such an entity presented in the literature to date. Thereby illustrating that calcinosis of scleroderma can present complex clinical management questions and may ultimately require surgical intervention in which the surgeon employs practices and principles from all 3 categories of spine pathology.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Cage, Pham, Lee. Acquisition of data: Cage, Pham, Lee. Analysis and interpretation of data: Cage, Pham, Lee. Drafting the article: Cage, Pham. Critically revising the article: Cage, Pham, Singh, Lee. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Cage. Statistical analysis: Cage, Pham. Administrative/technical/material support: Pham, Miao. Study supervision: Cage, Pham.
References
- 1. Jain NB, Ayers GD, Peterson EN, et al. Traumatic spinal cord injury in the United States, 1993-2012. JAMA. 2015;313(22):2236–2243. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merritt CH, Taylor MA, Yelton CJ, Ray SK. Economic impact of traumatic spinal cord injuries in the United States. Neuroimmunol Neuroinflamm. 2019;6:9. doi: 10.20517/2347-8659.2019.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15–24. doi: 10.1016/S1470-2045(04)01709-7. [DOI] [PubMed] [Google Scholar]
- 4. Walters B, Hadley M, Hurlbery R, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(CN_suppl_1):82–91. doi: 10.1227/01.neu.0000430319.32247.7f. [DOI] [PubMed] [Google Scholar]
- 5. Ferreli C, Gasparini G, Parodi A, Cozzani E, Rongioletti F, Atzori L. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53(3):306–336. doi: 10.1007/s12016-017-8625-4. [DOI] [PubMed] [Google Scholar]
- 6. Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis) Postgrad Med J. 1988;64(748):121–125. doi: 10.1136/pgmj.64.748.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of systemic sclerosis. Front Immunol. 2015;6:272. doi: 10.3389/fimmu.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Motegi SI, Sekiguchi A, Yonemoto Y, Mieda T, Chikuda H, Ishikawa O. Demographic and clinical characteristics of spinal calcinosis in systemic sclerosis: possible association with peripheral angiopathy. J Dermatol. 2019;46(1):33–36. doi: 10.1111/1346-8138.14704. [DOI] [PubMed] [Google Scholar]
- 9. Al-Sukaini A, Paulino Pereira NR, Yu EW, Chebib I, Bredella MA, Schwab J. Idiopathic tumoral calcinosis-like lesion in the lower cervical spine causing acute central cord syndrome: case report. J Neurosurg Spine. 2017;26(1):97–102. doi: 10.3171/2016.6.SPINE151565. [DOI] [PubMed] [Google Scholar]
- 10. Sambataro D, Sambataro G, Zaccara E, Maglione W, Vitali C, Del Papa N. Tumoral calcinosis of the spine in the course of systemic sclerosis: report of a new case and review of the literature. Clin Exp Rheumatol. 2015;33(4) suppl 91:S175–S178. [PubMed] [Google Scholar]
- 11. Karschnia P, Fulbright RK, Laurans MS, Huttner AJ, Baehring JM. Clinical reasoning: a 58-year-old woman with systemic scleroderma and progressive cervical cord compression. Neurology. 2018;91(13):e1262–e1264. doi: 10.1212/WNL.0000000000006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Logothetis CN, Emil NS, Tzamaloukas AH, Konstantinov KN. Tumoral calcinosis of the neck in a patient with systemic sclerosis. Cureus. 2018;10(11):e3585. doi: 10.7759/cureus.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan CK, Suresh E. Cervical and lumbar paraspinal calcinosis in systemic sclerosis. Reumatismo. 2016;68(4):203–205. doi: 10.4081/reumatismo.2016.900. [DOI] [PubMed] [Google Scholar]
- 14. Durant DM, Riley LH, 3rd, Burger PC, McCarthy EF. Tumoral calcinosis of the spine: a study of 21 cases. Spine (Phila Pa 1976) 2001;26(15):1673–1679. doi: 10.1097/00007632-200108010-00009. [DOI] [PubMed] [Google Scholar]
- 15. Olsen KM, Chew FS. Tumoral calcinosis: pearls, polemics, and alternative possibilities. Radiographics. 2006;26(3):871–885. doi: 10.1148/rg.263055099. [DOI] [PubMed] [Google Scholar]
- 16. Lak AM, Rahimi A, Abunimer AM, et al. Quantifying the impact of surgical decompression on quality of life and identification of factors associated with outcomes in patients with symptomatic metastatic spinal cord compression. J Neurosurg Spine. 2020;33(2):1–8. doi: 10.3171/2020.1.SPINE191326. [DOI] [PubMed] [Google Scholar]
- 17. Bambakidis NC, Feiz-Erfan I, Klopfenstein JD, Sonntag VK. Indications for surgical fusion of the cervical and lumbar motion segment. Spine (Phila Pa 1976) 2005;30(16 Suppl):S2–S6. doi: 10.1097/01.brs.0000174509.31291.26. [DOI] [PubMed] [Google Scholar]
- 18. Faraj K, Perez-Cruet K, Perez-Cruet M. CREST calcinosis affecting the lumbar and cervical spine and the use of minimally-invasive surgery. Cureus. 2017;9(4):e1145. doi: 10.7759/cureus.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westermann L, Isbell LK, Breitenfeldt MK, et al. Recuperation of severe tumoral calcinosis in a dialysis patient: A case report. World J Clin Cases. 2019;7(23):4004–4010. doi: 10.12998/wjcc.v7.i23.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenberg JN. Calcinosis in scleroderma. Proc R Soc Med. 1976;69(4):264. doi: 10.1177/003591577606900408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen HS, Sharma A, Doan N, Gelsomino M, Shabani S, Maiman D. Central cord syndrome in a patient with systemic sclerosis and cervical calcinosis: case report and review of literature. Spinal Cord Ser Cases. 2016;2:15029. doi: 10.1038/scsandc.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khan MH, Smith PN, Donaldson WF., 3rd Acute quadriparesis caused by calcification of the entire cervical ligamentum flavum in a white female--report of an unusual case and a brief review of the literature: case report. Spine (Phila Pa 1976) 2005;30(22):E687–E691. doi: 10.1097/01.brs.0000186472.88141.38. [DOI] [PubMed] [Google Scholar]
- 23. Lima IV, Galrão LA, Maia TS, Santiago MB. Spinal cord compression by ectopic calcinosis in scleroderma. Clin Exp Rheumatol. 2005;23(5):704–706. [PubMed] [Google Scholar]
- 24. Smucker JD, Heller JG, Bohlman HH, Whitesides TE., Jr Surgical treatment of destructive calcific lesions of the cervical spine in scleroderma: case series and review of the literature. Spine (Phila Pa 1976) 2006;31(17):2002–2008. doi: 10.1097/01.brs.0000229260.67357.53. [DOI] [PubMed] [Google Scholar]
- 25. Carlson AP, Yonas HM, Turner PT. Disorders of tumoral calcification of the spine: illustrative case study and review of the literature. J Spinal Disord Tech. 2007;20(1):97–103. doi: 10.1097/01.bsd.0000211278.83647.9e. [DOI] [PubMed] [Google Scholar]
- 26. Tuy BE, John TK, Uglialoro AD, Beebe KS, Vives MJ, Patterson FR. Tumoral calcinosis presenting as neck pain and mass lesion of the cervical spine. Am J Orthop. 2008;37(11):E191–E195. [PubMed] [Google Scholar]
- 27. Shoji A, Tahara K, Hayashi H, et al. Severe headache complicated by vertical atlantoaxial subluxation in diffuse systemic sclerosis with crowned dens pattern calcification. Rheumatol Int. 2011;31(9):1247–1250. doi: 10.1007/s00296-010-1703-z. [DOI] [PubMed] [Google Scholar]
- 28. Lebl DR, Girardi FP. Isolated cervical spine facet joint tumoral calcinosis. Spine J. 2013;13(2):208–209. doi: 10.1016/j.spinee.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 29. Nakamura T, Hirakawa K, Takaoka H, Iyama K. Dystrophic calcinosis with both a huge calcified mass in the cervical spine and calcification in the chest wall in a patient with rheumatoid overlap syndrome. Clin Rheumatol. 2016;35(5):1403–1409. doi: 10.1007/s10067-014-2696-x. [DOI] [PubMed] [Google Scholar]
- 30. Al-Khudairi N, Kobayashi S, Meir A. A case of symptomatic cervical spine calcinosis in systemic sclerosis. Spine J. 2015;15(9):2099–2100. doi: 10.1016/j.spinee.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 31. Motegi SI, Sekiguchi A, Toki S, Amano H, Ishikawa O. Progressive myelopathy in systemic sclerosis patient with cervical intraspinal calcinosis. J Dermatol. 2017;44(2):209–210. doi: 10.1111/1346-8138.13452. [DOI] [PubMed] [Google Scholar]


