Abstract
After vaccination with enterovirus 71 (EV-A71), the prevalence of hand-foot-and-mouth disease (HFMD) remained high, and the spatial–temporal distribution of enteroviruses changed. Therefore, it is essential to define the temporal features, spatial distributions, and epidemiological and etiological characteristics of HFMD in Kunming. Between 2017 and 2020, a total of 36,540 children were diagnosed with HFMD in Kunming, including 32,754 children with enterovirus-positive clinical samples. Demographic, geographical, epidemiological and etiological data of the cases were acquired and analyzed. Other enteroviruses replaced EV-A71, and the incidence of EV-A71 decreased dramatically, whereas coxsackievirus A6 (CV-A6) and coxsackievirus A16 (CV-A16) had substantial outbreaks in 2018 and 2019, respectively. The major and minor peaks all extended for 2–4 months compared to before vaccination with the EV-A71 vaccine. From 2019 to 2020, CV-A6, as the predominant serotype, showed only a single peak. Although a high incidence of HFMD was observed in Guandu, Chenggong and Xishan, the annual incidence of different enterovirus serotypes was different in different regions. In 2017, other enteroviruses were most prevalent in Shilin. In 2018, CV-A16 and CV-A6 were most prevalent in Luquan and Shilin, respectively. In 2019, CV-A16 was most prevalent in Jinning. In 2020, CV-A6 and coxsackievirus A10 (CV-A10) were most prevalent in Luquan and Shilin, respectively. Meanwhile, the epidemic cycle of CV-A6 and CV-A16 was only 1 year, and CV-A10 and other enteroviruses were potential risk pathogens. The spatial and temporal distribution of HFMD varies at different scales, and the incidence of HFMD associated with different pathogens has obvious regional differences and seasonal trends. Therefore, research on multivalent combined vaccines is urgently needed, and proper preventive and protective measures could effectively control the incidence of HFMD-like diseases.
Subject terms: Infectious diseases, Disease prevention, Paediatrics, Public health
Introduction
Since 1997, hand-foot-and-mouth disease (HFMD) has been primarily prevalent in the Asia–Pacific region, including China, Japan, Singapore, Malaysia, Vietnam, South Korea, Thailand, Cuba, India and Cambodia. In mainland China, with an incidence of 37.01/100,000–205.06/100,000 people1,2, a total of 23.5 million HFMD cases were reported to the Chinese Center for Disease Control and Prevention (CDC) during 2000–20183,4 and showed a continuous upward trend in recent years. Previous studies have focused on the eastern coastal, densely populated and economically developed cities (including Shandong, Anhui, Jiangxi, Jiangsu, Zhejiang, Fujian, Guangdong Province, Beijing, Shanghai and Shenzhen municipality) and indicated the epidemiological characteristics of EV-A71 and CV-A16, which were considered the most common etiological agents of HFMD in the past two decades5. However, less is known about what causes the different characteristics of the descriptive epidemiology of HFMD between different areas6–10.
To control the HFMD epidemic, the inactivated monovalent EV-A71 vaccine has been wildly administered throughout China since 2016, and is highly effective against EV-A71-associated HFMD11,12, but the prevalence of HFMD induced by other serotypes remains high13,14. Meanwhile, due to the coronavirus disease 2019 (COVID-19) pandemic, public areas were closed, and quarantine of infected patients was imposed beginning in December 2019. We implemented measures of regional surveillance of epidemics and no physical contact with infected patients, which played an important role in reducing HFMD-like disease cases in 2020.
As a medium-sized developing inland city in Southwest China with a high altitude and complex terrain, Kunming urgently needs research on the temporal features, spatial distributions, and epidemiological and etiological characteristics of HFMD, with an in-depth analysis before and after the EV-A71 vaccination campaign and COVID-19 pandemic.
Materials and methods
Study area
Kunming is the largest city in Yunnan Province in Southwest China. Within an area of approximately 21,473 square kilometers, the city has a complex terrain consisting of mountains, hills, plains, basins and plateaus and a subtropical monsoon continental climate. The city administers 7 districts, 1 county-level cities, 3 counties and 3 autonomous counties, and has a population of 8.46 million people now, including 1,267,713 people aged 0–14, accounting for 14.98%.
Kunming Children’s Hospital is a unique tertiary care pediatric hospital in Yunnan Province that integrates medical treatment, scientific research, teaching, health care and rehabilitation, with over 1200 inpatient beds.
Data collection
We collected surveillance data on the prevalence of HFMD from Kunming Children’s Hospital from 2017 to 2020, including sex, age, population classification, address, type of diagnosis and laboratory test results (confirmed by fluorescence quantitative real-time reverse transcriptase PCR (qRT‒PCR) in the clinical laboratory of the hospital) of 36,540 HFMD cases.
Clinical definition
According to the guidelines of the National Health Commission of the People’s Republic of China, a clinical case of HFMD was defined as a patient with maculopapular or vesicular rash on the hands, feet, mouth, or buttocks, with or without fever. Severe HFMD patients had at least one of the following: cardiopulmonary collapse, pulmonary hemorrhage, pulmonary edema, encephalitis, aseptic meningitis, acute flaccid paralysis, myocarditis, or death.
Pathogenic detection
After the preliminary clinical diagnosis of HFMD, the enterovirus genotypes were determined using qRT‒PCR for clinical specimens. Feces were collected from sampling cases and kept at − 80 °C until examination for enterovirus by qRT‒PCR. Viral RNA was extracted from the clinical specimens using the Viral RNA Mini Kit (QIAamp Viral RNA Mini Kit (50) 52,904 Qiagen, Germany) in accordance with the manufacturer’s instructions and was subjected to qRT‒PCR. Laboratory test results were divided into 6 categories: EV-A71 positive, CV-A16 positive, CV-A6 positive, CV-A10 positive and other enteroviruses positive and enterovirus negative.
Vaccination criteria
The inactivated EV-A71 vaccine can be used for preventing HFMD caused by EV-A71 infection in children aged 6 months to 5 years. The basic immunization procedure is 2 doses with an interval of 1 month. It is recommended that vaccination be completed before the age of 12 months.
Statistical analysis
Descriptive epidemiological methods were used to analyze the demographic (including age, sex and population classification), etiological (EV-A71, CV-A16, CV-A6, CV-A10 and other enteroviruses), temporal and spatial characteristics of HFMD by month.
SPSS 22.0 statistical software was used for statistical analysis. Basic data were analyzed by descriptive analysis, and the counting data were analyzed by the chi-square test. The alpha value α = 0.05 was used. The geographic distribution map of HFMD cases was drawn by MapInfo 10.0 spatial distribution software.
Ethics statement
All the study procedures were reviewed and approved by the Ethics Committee of Kunming Children’s Hospital. Written consent was obtained from each study participant’s parents or guardians.
Results
Demographic characteristics
A total of 32,754 positive cases of HFMD were monitored from 2017 to 2020 in Kunming. The annual incidence rates were 71.58/100,000 at minimum and 163.24/100,000 at maximum, and the average incidence rate was 118.97/100,000 (Table 1). HFMD patients under 5 years of age predominated and accounted for 93.30%, 52.17% of HFMD patients were aged 0–3 years, and 41.13% were aged 3–5 years. The male-to-female ratio of HFMD patients was 1.46:1 (ranging from 1.43:1 to 1.49:1) and showed a steady trend. The highest proportion of classified HFMD cases was observed among children of different ages, accounting for 55.77%; the second highest proportion was observed among nursery children, accounting for 39.97%; and the proportion of school-age-children was small, accounting for 4.26% (Table 2).
Table 1.
The spatial distribution of HFMD annual incidence of districts and counties in Kunming, 2017–2020.
| Years | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|
| Number | 4855 | 11,182 | 10,311 | 6406 |
| Incidence | 71.58/100,000 | 163.24/100,000 | 148.37/100,000 | 92.17/100,000 |
| Location | ||||
| Anning | 39.72 | 53.54 | 65.55 | 41.39 |
| Chenggong | 82.48 | 245.82 | 302.61 | 154.94 |
| Dongchuan | 31.10 | 65.49 | 51.93 | 37.54 |
| Fumin | 28.75 | 74.00 | 38.36 | 33.96 |
| Guandu | 176.27 | 384.51 | 344.32 | 204.89 |
| Jinning | 38.51 | 89.59 | 68.95 | 36.86 |
| Luquan | 14.97 | 78.17 | 45.33 | 81.02 |
| Panlong | 67.44 | 149.05 | 133.22 | 77.90 |
| Shilin | 1.52 | 11.37 | 9.02 | 5.26 |
| Songming | 76.92 | 167.99 | 156.58 | 73.39 |
| Wuhua | 75.50 | 154.86 | 125.78 | 102.87 |
| Xishan | 108.87 | 235.78 | 217.55 | 131.79 |
| Xundian | 19.27 | 70.68 | 52.14 | 32.24 |
| Yiliang | 21.37 | 60.74 | 59.76 | 17.01 |
Table 2.
The epidemiological description of HFMD in Kunming, 2017–2020.
| Years | 2017 | 2018 | 2019 | 2020 | P-value* | |
|---|---|---|---|---|---|---|
| Total | 4855 | 11,182 | 10,311 | 6406 | ||
| Age group | < 0.001** | |||||
| 0–3 | Number | 2508 | 6010 | 5255 | 3316 | |
| Percentage | 51.7% | 53.7% | 51.0% | 51.8% | ||
| 3–5 | Number | 2093 | 4458 | 4287 | 2632 | |
| Percentage | 43.1% | 39.9% | 41.6% | 41.1% | ||
| ≥ 6 | Number | 254 | 714 | 769 | 458 | |
| Percentage | 5.2% | 6.4% | 7.5% | 7.1% | ||
| Gender | 0.364*** | |||||
| Male | Number | 2864 | 6694 | 6061 | 3825 | |
| Percentage | 59.0% | 59.9% | 58.8% | 59.7% | ||
| Female | Number | 1991 | 4488 | 4250 | 2581 | |
| Percentage | 41.0% | 40.1% | 41.2% | 40.3% | ||
| Hosting classification | < 0.001** | |||||
| Scatter children | Number | 2559 | 6396 | 5783 | 3529 | |
| Percentage | 52.7% | 57.2% | 56.1% | 55.1% | ||
| Childcare | Number | 2141 | 4362 | 4085 | 2502 | |
| Percentage | 44.1% | 39.0% | 39.6% | 39.1% | ||
| School | Number | 155 | 424 | 443 | 375 | |
| Percentage | 3.2% | 3.8% | 4.3% | 5.9% | ||
| Location | ||||||
| Anning | Number | 150 | 204 | 255 | 161 | |
| Percentage | 3.1% | 1.8% | 2.5% | 2.5% | ||
| Chenggong | Number | 281 | 868 | 1123 | 575 | |
| Percentage | 5.8% | 7.8% | 10.9% | 9.0% | ||
| Dongchuan | Number | 88 | 186 | 148 | 107 | |
| Percentage | 1.8% | 1.7% | 1.4% | 1.7% | ||
| Fumin | Number | 45 | 117 | 61 | 54 | |
| Percentage | 0.9% | 1.0% | 0.6% | 0.8% | ||
| Guandu | Number | 1584 | 3514 | 3250 | 1934 | |
| Percentage | 32.6% | 31.4% | 31.5% | 30.2% | ||
| Jinning | Number | 118 | 277 | 217 | 116 | |
| Percentage | 2.4% | 2.5% | 2.1% | 1.8% | ||
| Luquan | Number | 62 | 324 | 188 | 336 | |
| Percentage | 1.3% | 2.9% | 1.8% | 5.2% | ||
| Panlong | Number | 565 | 1255 | 1127 | 659 | |
| Percentage | 11.6% | 11.2% | 10.9% | 10.3% | ||
| Shilin | Number | 4 | 30 | 24 | 14 | |
| Percentage | 0.1% | 0.3% | 0.2% | 0.2% | ||
| Songming | Number | 253 | 573 | 559 | 262 | |
| Percentage | 5.2% | 5.1% | 5.4% | 4.1% | ||
| Wuhua | Number | 661 | 1360 | 1109 | 907 | |
| Percentage | 13.6% | 12.2% | 10.8% | 14.2% | ||
| Xishan | Number | 859 | 1869 | 1735 | 1051 | |
| Percentage | 17.7% | 16.7% | 16.8% | 16.4% | ||
| Xundian | Number | 91 | 336 | 249 | 154 | |
| Percentage | 1.9% | 3.0% | 2.4% | 2.4% | ||
| Yiliang | Number | 94 | 269 | 267 | 76 | |
| Percentage | 1.9% | 2.4% | 2.6% | 1.2% | ||
| Identification of enterovirus | ||||||
| EV-A71 | Number | 504 | 430 | 130 | 2 | |
| Percentage | 10.4% | 3.8% | 1.3% | 0.0% | ||
| CV-A16 | Number | 629 | 3359 | 4866 | 263 | |
| Percentage | 13.0% | 30.0% | 47.2% | 4.1% | ||
| CV-A6 | Number | 10 | 3728 | 3473 | 4537 | |
| Percentage | 0.2% | 33.3% | 33.7% | 70.8% | ||
| CV-A10 | Number | 11 | 450 | 167 | 457 | |
| Percentage | 0.2% | 4.0% | 1.6% | 7.1% | ||
| Other enteroviruses | Number | 3701 | 3215 | 1676 | 1147 | |
| Percentage | 76.2% | 28.8% | 16.3% | 17.9% | ||
*Comparison of HFMD patients with age group, gender and hosting classification in 2017–2020 by chi-square test.
**Significant statistical differences, P < 0.001.
***No statistically significant differences, P > 0.05.
Enterovirus serotype distribution
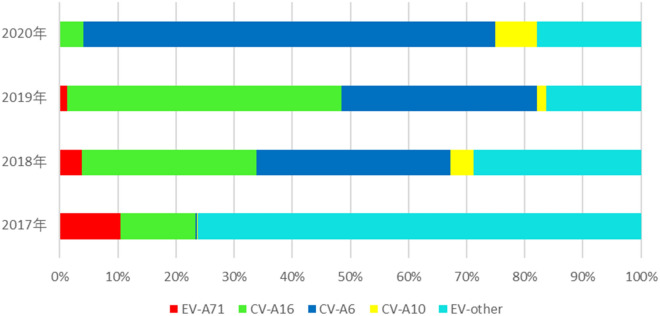
The numbers of serotypes of laboratory-confirmed cases fluctuated during 2017–2020, and we observed that there were two important time inflection points of change in enterovirus serotypes in 2017 and 2020. The annual percentages of EV-A71 were 10.4%, 3.8%, 1.3%, and only two cases from 2017 to 2020, respectively, which decreased continuously. The number of other enteroviruses and CV-A16 first trended upward and then decreased. Other enteroviruses increased substantially and accounted for 76.2% in 2017 but decreased yearly to 28.8% in 2018, 16.3% in 2019, and 17.9% in 2020. CV-A16 increased gradually and accounted for 30.0% and 47.2% in 2018 and 2019, respectively, but decreased dramatically to 4.1% in 2020. The numbers of CV-A6 and CV-A10 cases trended upward continuously; in particular, CV-A6 increased substantially and accounted for 33.3% and 33.7% in 2018 and 2019, respectively, and suddenly rose to 70.8% in 2020. The annual number of CV-A10 cases remained at a low level from 2017 to 2019, 0.2%, 4.0%, and 1.6%, respectively, but increased to 7.1% in 2020 (Table 2 and Fig. 1).
Figure 1.
The annual percentages of positive enteroviruses of HFMD in Kunming, 2017–2020.
Temporal characteristics
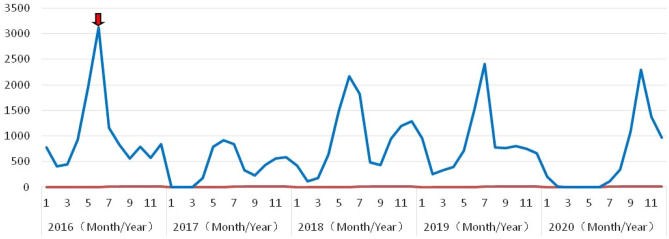
The annual incidence of HFMD in Kunming initially increased and presented a seasonal distribution with semiannual or annual peaks. One peak was observed in 2016 from April to June before vaccination with the EV-A71 vaccine. Two peaks were observed in 2017 and 2018, a major peak from April to August and another from September to the following February, and the peak value in 2018 was much higher than that in 2017. A single peak appeared in 2019 and 2020, and although the peak values were similar, they showed obvious differences. The peak in 2019 was followed by a plateau until the following February; in 2020, there was only one peak from July to the following February (Fig. 2).
Figure 2.
The temporal distribution with periodic peak of HFMD in Kunming, 2016–2020. The red arrow indicates that the local CDC began to vaccinate local children with EV-A71 vaccine in June 2016.
Spatial characteristics
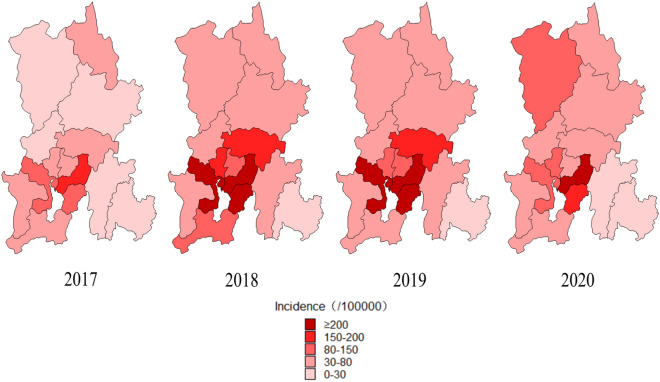
During 2017–2020, 7 districts, 1 county-level city, 3 counties and 3 autonomous counties in Kunming reported the occurrence of HFMD. The spatial distribution of the incidence of HFMD was heterogeneous at the county level, ranging from 1.52/100,000 (Shilin) to 384.51/100,000 people (Guandu). The counties with the highest incidence of HFMD were Guandu, Chenggong, Xishan, Songming, Wuhua and Panlong. In some regions, such as Luquan, the incidence increased gradually from 14.97/100,000 in 2017 to 78.17/100,000 in 2018 and 81.02/100,000 in 2020. Overall, the area with the highest incidence was the core area of Kunming, which has a concentrated population and prosperous economy (Table 1 and Fig. 3).
Figure 3.
The spatial distribution map of HFMD annual incidence of districts and counties in Kunming, 2017–2020. The number of HFMD cases mainly concentrated in Guandu, Xishan, Wuhua, Panlong and Chenggong, and the high incidence of HFMD areas were Guandu, Chenggong and Xishan.
This figure was drawn by MapInfo 10.0 spatial distribution software [url = http://www.i-mapinfo.com]MapInfo[/url].
Spatial–temporal clusters
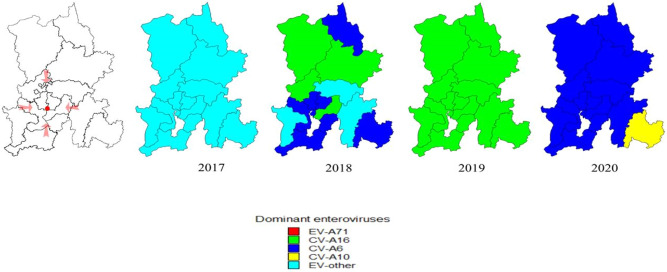
During 2017–2020, the periodic peaks of HFMD showed spatial–temporal clusters, and different pathogens showed different spatial–temporal distributions (Fig. 4). In 2017, the areas with a high incidence of other enteroviruses were Shilin (100%), Yiliang (94.7%), Anning (89.3%), Chenggong (85.1%) and Luquan (79.0%). In 2018, the areas with a high incidence of CV-A16 were Luquan (43.5%), Xundian (39.3%), Fumin (37.6%) and Guandu (33.2%); the areas with a high incidence of CV-A6 were Shilin (60.0%), Dongchuan (49.5%), Jinning (45.8%), Chenggong (37.8%) and Wuhua (36.5%). In 2019, the areas with a high incidence of CV-A16 were Jinning (59.9%), Yiliang (55.8%), Chenggong (49.5%), Panlong (48.4%) and Anning (48.2%). In 2020, the areas with a high incidence of CV-A6 were Luquan (77.4%), Dongchuan (75.7%), Anning (74.5%), Fumin (74.1%) and Xundian (73.4%); the areas with a high incidence of CV-A10 were Shilin (50.0%), Songming (19.1%) and Yiliang (17.1%).
Figure 4.
The spatial distribution map of other enteroviruses as dominant enteroviruses of districts and counties in Kunming, 2017–2020. The red dot shows the location of Kunming Children's Hospital, which is located at the junction of the main urban area. In 2017, the areas with a high proportion of other enteroviruses as dominant enteroviruses were Shilin (100%), Yiliang (94.7%), Anning (89.3%), Chenggong (85.1%) and Luquan (79.0%). In 2018, CV-A16 was mainly prevalent in Luquan (43.5%), Xundian (39.3%), Fumin (37.6%) and Guandu (33.2%); CV-A6 was mainly prevalent in Shilin (60.0%), Dongchuan (49.5%), Jinning (45.8%), Chenggong (37.8%) and Wuhua (36.5%). In 2019, the areas with a high proportion of CV-A16 as dominant enteroviruses were Jinning (59.9%), Yiliang (55.8%), Chenggong (49.5%), Panlong (48.4%) and Anning (48.2%). In 2020, CV-A6 was mainly prevalent in Luquan (77.4%), Dongchuan (75.7%), Anning (74.5%), Fumin (74.1%) and Xundian (73.4%); CV-A10 was mainly prevalent in Shilin (50.0%), Songming (19.1%) and Yiliang (17.1%).
This figure was drawn by MapInfo 10.0 spatial distribution software [url = http://www.i-mapinfo.com] MapInfo[/url].
Discussion
According to World Health Organization (WHO) reports, China is one of the countries with a high incidence of HFMD15. Since the 2008 outbreak, HFMD has been listed as a type C statutory notifiable infectious disease16 that need constant surveillance. In the eastern coastal cities of China, previous studies have been carried out and summarized some regional epidemiological characteristics of HFMD, including the prevalence among children under five years of age17, a higher incidence among local males, one peak in a year18, and EV-A71 as the most common causative pathogen1,7,19.
In recent years, research on HFMD has gradually been conducted in central and western cities in China, such as Sichuan and Xinjiang, and obvious regional epidemiological characteristics have been found, such as two peak epidemic seasons in a year1, and EV-A71 and CV-A16 as the most common etiological agents in local areas7,21. Kunming, as an inland city in Southwest China, has also continuously surveilled HFMD for over a decade. A few reports showed the research results of the local incidence of HFMD before 2017, and found that children aged 1–3 who lived in different areas were at the highest risk, the ratio of males to females was 1.5:1, a major peak occurred during April to June followed by an autumn peak during October to November, and EV-A71, CV-A16, and other enteroviruses were the most common causative pathogens22. These results reflected some regional epidemiological and etiological characteristics of HFMD in Kunming before the EV-A71 vaccination campaign, and played a certain role in prevention and control.
Since 2016, the inactivated monovalent EV-A71 vaccine has been administered nationwide in China, which substantially reduces EV-A71-associated HFMD by over 90%23. However, due to the lack of cross-protection from the vaccine and the high rate of gene mutations and recombinations in other serotypes24, the prevalence of HFMD remains high; for example, CV-A16 replaced EV-A71 in Guangzhou, Shenzhen and Xinjiang, and CV-A6 prevailed as the dominant serotype in Xiangyang and Sichuan4,14. From 2017 to 2020, approximately 600,000 doses of inactivated monovalent EV-A71 vaccines were administered by the local CDC in Kunming, and the incidence of EV-A71-associated HFMD decreased dramatically, from 10.4% in 2017 to only 1.3% in 2019. In 2017, other enteroviruses replaced EV-A71 as the predominant serotypes and accounted for 76.2%. However, CV-A6 and CV-A16 showed an upward trend from 2017 to 2019, with sizable outbreaks in 2018 (33.3%) and 2019 (47.2%), respectively. Meanwhile, the peak time was longer than that before vaccination with the EV-A71 vaccine; the major peak extended for two months from April to August, and the minor peak extended for four months from September to the following February (Fig. 2). This phenomenon is mainly due to the EV-A71 vaccine imparting selective pressure for these other serotypes to emerge at high proportions25 and the different enterovirus genotypes with dissimilar activity and transmission characteristics under the influences of complex social and climatic factors7,26, which can cause differing scales and peak patterns27.
In addition, after the COVID-19 outbreak, we found that the incidence of CV-A6 and CV-A10 increased substantially, and CV-A6 replaced CV-A16 as the predominant serotype and accounted for 70.8%. From the end of 2019, the minor peak of CV-A6 disappeared and plateaued until the following February. In 2020, CV-A6 only presented a single peak that continued from July to the following February. We speculate that the main reasons are as follows. First, EVA71, CV-A16, CV-A6 and other enteroviruses circulate in 2–3- or 3–4- year cycles. The year 2020 may have been the end of the epidemic cycle of CV-A16, whereas the epidemic cycle of CV-A6 was beginning in Kunming. Second, effective personal hygiene measures during the epidemic period, such as reducing physical contact, wearing masks and washing hands, effectively reduced the transmission routes of HFMD. Third, public places were closed and children with HFMD were completely isolated at home. Fourth, the surveillance of population flow has obviously affected the epidemic and outbreak cycle of different pathogens.
To further understand the evolutionary dynamics of HFMD enteroviruses and enhance vaccination strategies in Kunming, we presented a the spatial and temporal description of their epidemiological and etiological characteristics. Spatial–temporal scanning includes geographic information, corrects nonuniform population density in different places, makes up for the deficiencies in the simple epidemiological morbidity comparisons, and objectively and comprehensively evaluates abnormal increases and the incidence aggregation area in both time and space dimensions28.
From 2017 to 2020, by using spatial–temporal scanning, we analyzed the number of cases of HFMD mainly concentrated in Guandu, Xishan, Wuhua, Panlong and Chenggong, and the high-incidence HFMD areas were Guandu, Chenggong and Xishan. Both the number and incidence of HFMD cases showed outbreaks in 2018 and 2019 and continued to increase in Chenggong. Spatial aggregation means that the risk of HFMD is substantially higher in some areas than in others, probably because of greater residential and population density, which can lead to clustered infections. In addition, it is interesting to note that these areas are mainly concentrated near the Panlong River and Dianchi Lake, which indicates that the prevalence of HFMD may be associated with humidity, pollution or other factors related to the lake. Moreover, different pathogens have different spatial and temporal distributions, and which also occur for the same pathogen. In 2017, other enteroviruses were the predominant serotypes, and the areas with high proportions of enteroviruses were Shilin, Yiliang and Anning. In 2018, CV-A16 was mainly prevalent in Luquan, Xundian and Fumin; CV-A6 was mainly prevalent in Shilin, Dongchuan and Jinning. In 2019, CV-A16 was the predominant serotype, and the areas with high proportions were Jinning, Yiliang and Chenggong. In 2020, CV-A6 was the predominant serotype, and the areas with high proportions were Luquan, Dongchuan and Anning; CV-A10 was mainly prevalent in Shilin, Songming and Yiliang. Although the annual total incidence of HFMD is directly related to population density, the annual incidence of different enterovirus serotypes differs in different regions, which pose a great challenges to the epidemic prevention of HFMD. It was reported that epidemics of EV-A71 or CV-A16 circulate in a cyclical pattern every 2–3 years29. Throughout Kunming after EV-A71 vaccination, we noticed that other enteroviruses have decreased substantially since 2018 but have remained stable at 15–20% since 2019. CV-A16 gradually increased from 2018 and led to an outbreak in 2019. CV-A6 had a regional outbreak in 2018 and gradually increased in 2019. CV-A10 cases have increased since 2018. After the COVID-19 pandemic, cases of CV-A6 broke out throughout Kunming, cases of CV-A10 broke out regionally, and other enteroviruses remained stable at 20% and surpassed CV-A6. We found that the epidemics and outbreaks of CV-A6- and CV-A16- associated HFMD appeared alternately, and the cycle was only 1 year; CV-A10 and other enteroviruses were potentially associated risk pathogens for outbreaks of HFMD in the next cycle period, although their incidence was not high.
This study has several limitations that should be acknowledged. First, HFMD is asymptomatic and self-limiting; thus, some cases of HFMD may be ignored through the passive surveillance system, and the actual number of cases may have been underestimated. Second, we did not test for enterovirus serotypes beyond EV-A71, CV-A16, CV-A6, CV-A10 and other serotypes because they had not been dominant in the past based on other studies. Third, we analyzed the association between the spatial and temporal distribution of infectious diseases, but the spread of disease is influenced by a variety of factors, such as meteorological, socioeconomic, health resource and traffic variables. However, due to limited data access, these factors were not considered in this study.
Conclusions
This study confirms that the spatial and temporal distribution of HFMD varies at different scales, and the incidence of different pathogens associated with HFMD has obvious regional differences and seasonal trends. Therefore, the research and development of multivalent combined vaccines, including those for EV-A71, CV-A16, CV-A6 and CV-A10, are urgently needed, which could cover over 90% of pathogens associated with HFMD in the southwestern region of China. In addition, due to the COVID-19 pandemic, the number of infected patients was considerably low for 2020. Therefore, taking proper preventive and protective measures could effectively control the incidence of HFMD-like diseases in Kunming and even nationwide (Supplementary Information).
Supplementary Information
Acknowledgements
The manuscript is an original work and has not been published or is under consideration for publication in another journal. The study complies with current ethical consideration. The authors confirmed that all the listed authors have participated actively in the research, and have seen and approved the submitted manuscript.
Author contributions
J.L., M.W. and T.C. wrote the main manuscript text. M.W., J.P., Y.L., L.D., Z.L., J.H., C.L., Q.G., W.M., Z.C., Q.Z., W.C., Y.C. and M.H. collected sample. M.W., M.H., G.L. and J.L. performed the experiments. M.H., G.L. and J.L. prepared figures and tables. J.L. and M.W. wrote and revised the paper. All authors reviewed the manuscript.
Funding
This work was supported by the Kunming "Spring City Plan" high-level talent training project, Kunming municipal government "spring city famous doctor" special project [Grant No. 2020-23]; Kunming Science and technology planning project [Grant number 2019-1-S-25318000001241]; Kunming health and Family Planning Commission Project [Grant No. 2019-SW-33]; Yunnan Province’s reserve medical talents project [Grant No. H-2019002]; Kunming Medical University Applied Basic Research Joint Special Project [Grant No. 202001AY070001-170].
Data availability
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Meifen Wang and Tao Chen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21312-2.
References
- 1.Puenpa J, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg. Infect. Dis. 2013;19(4):641–643. doi: 10.3201/eid1904.121666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saki T, Liao Q, Van B, et al. Hand, Foot, and mouth disease in China: Modeling epidemic dynamics of enterovirus serotypes and implications for vaccination. PLoS Med. 2016;13(2):e1001958. doi: 10.1371/journal.pmed.1001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumi A, Toyoda S, Kanou K, et al. Association between meteorological factors and reported cases of hand, foot, and mouth disease from 2000 to 2015 in Japan. Epidemiol. Infect. 2017;145(14):2896–2911. doi: 10.1017/S0950268817001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang B, Liu F, Liao Q, et al. Epidemiology of hand, foot and mouth disease in China, 2008 to 2015 prior to the introduction of EV-A71 vaccine. Euro Surveill. 2017;22(50):38–47. doi: 10.2807/1560-7917.ES.2017.22.50.16-00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi H, Li Y, Zhang J, et al. Quantifying the risk of hand, foot, and mouth disease (HFMD) attributable to meteorological factors in East China: A time series modelling study. Sci. Total Environ. 2020;728:138548. doi: 10.1016/j.scitotenv.2020.138548. [DOI] [PubMed] [Google Scholar]
- 6.Liang YK, Li N, Yang JZ, Deng B, Xie RH, Shu S, et al. Epidemiologic characteristics of hand-foot-mouth disease in Guiyang between 2008 and 2010. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14(3):195–197. [PubMed] [Google Scholar]
- 7.Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet. Infect. Dis. 2014;14(4):308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun LM, Wu SL, Tan XH, Li H, Yang F, Zeng HR, et al. Epidemiological characteristics of Coxsackie virus A16 caused hand foot and mouth disease cases in Guangdong province, 2012–2016. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39(3):342–346. doi: 10.3760/cma.j.issn.0254-6450.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Bao H, Zhang X, Zhai M, Bao X, Wang D, et al. Epidemiological and genetic analysis concerning the non-enterovirus 71 and non-coxsackievirus A16 causative agents related to hand, foot and mouth disease in Anyang city, Henan Province, China, from 2011 to 2015. J. Med. Virol. 2017;89(10):1749–1758. doi: 10.1002/jmv.24847. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Xing XS, Wu Y, Liao QH, Liu GP, Jiang XQ, et al. Hand, foot and mouth disease in Hubei province, 2009–2015: an epidemiological and etiological study. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(4):441–445. doi: 10.3760/cma.j.issn.0254-6450.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 2014;370(9):829–837. doi: 10.1056/NEJMoa1303224. [DOI] [PubMed] [Google Scholar]
- 12.Zhu F, Xu W, Xia J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N. Engl. J. Med. 2014;370(9):818–828. doi: 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Li JX, Jin PF, et al. Enterovirus 71: a whole virion inactivated enterovirus 71 vaccine. Expert Rev. Vac. 2016;15(7):803–813. doi: 10.1080/14760584.2016.1191357. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J. Trend of epidemics and variation of pathogens of hand, foot and mouth disease in China: a dynamic series analysis, 2008–2017. Chin. J. Endemiol. 2019;40(2):147–154. doi: 10.3760/cma.j.issn.0254-6450.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Owino CO, Chu J. Recent advances on the role of host factors during non-poliovirus enteroviral infections. J. Biomed. Sci. 2019;26(1):1–17. doi: 10.1186/s12929-019-0540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jian C, Wu J, Xu Z, et al. Associations between extreme precipitation and childhood hand, foot and mouth disease in urban and rural areas in Hefei China. Sci. Total Environ. 2014;497:484–490. doi: 10.1016/j.scitotenv.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Wu J, Ding C, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect. Dis. 2017;17(7):716–725. doi: 10.1016/S1473-3099(17)30227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Teng Z, Cui X, et al. Epidemiological and serological surveillance of hand-foot-and-mouth disease in Shanghai, China, 2012–2016. Emerg. Microbes Infect. 2018;7(1):8. doi: 10.1038/s41426-017-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooi MH, et al. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9(11):1097–1105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 20.Gopalkrishna V, Patil PR, Patil GP, et al. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J. Med. Microbiol. 2012;61(3):420–425. doi: 10.1099/jmm.0.036400-0. [DOI] [PubMed] [Google Scholar]
- 21.Yuwei W, Wei C, Meng H, et al. Epidemiology and etiology of hand, foot, and mouth disease in Fujian province, 2008–2014. Arch. Virol. 2017;162(2):535–542. doi: 10.1007/s00705-016-3127-x. [DOI] [PubMed] [Google Scholar]
- 22.Hongchao J, Zhen Z, Qing R, et al. The epidemiological characteristics of enterovirus infection before and after the use of enterovirus 71 inactivated vaccine in Kunming China. Emerg. Microbes Infect. 2021;10(1):619–628. doi: 10.1080/22221751.2021.1899772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin JY, Kung YA, Shih SR. Antivirals and vaccines for enterovirus A71. J Biomed. Sci. 2019;26(1):65. doi: 10.1186/s12929-019-0560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholson E, Piedra PA. Local versus global enterovirus (EV) surveillance: A discussion for the need for active surveillance to guide EV-A71 vaccines. J. Infect. Dis. 2017;216(11):1337–1339. doi: 10.1093/infdis/jix493. [DOI] [PubMed] [Google Scholar]
- 25.Meng XD, Tong Y, Wei ZN, et al. Epidemical and etiological study on hand, foot and mouth disease following EV-A71 vaccination in Xiangyang, China. Sci. Rep. 2020;10(1):20909. doi: 10.1038/s41598-020-77768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M, He S, Yan Q, et al. Severe hand, foot and mouth disease associated with Coxsackievirus A10 infections in Xiamen, China in 2015. Clin. Virol. 2017;93:20–24. doi: 10.1016/j.jcv.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Di P, Yue Ma, Yaqiong L, et al. Epidemiological and aetiological characteristics of hand, foot, and mouth disease in Sichuan Province, China, 2011–2017. Sci. Rep. 2020;10(1):6117. doi: 10.1038/s41598-020-63274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Zhao H, Ou R, et al. Epidemiological and clinical characteristics of severe hand-foot-and-mouth disease (HFMD) among children: A 6-year population-based study. BMC Public Health. 2020;20(1):801. doi: 10.1186/s12889-020-08961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao Q, Wang Y, Shao J, et al. The compatibility of inactivated-enterovirus 71 vaccination with coxsackievirus A16 and poliovirus immunizations in humans and animals. Hum. Vaccin. Immunother. 2015;11(11):2723–2733. doi: 10.1080/21645515.2015.1011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.