Abstract
The gene encoding the noncoupled NADH:ubiquinone oxidoreductase (NDH II) from Azotobacter vinelandii was cloned, sequenced, and used to construct an NDH II-deficient mutant strain. Compared to the wild type, this strain showed a marked decrease in respiratory activity. It was unable to grow diazotrophically at high aeration, while it was fully capable of growth at low aeration or in the presence of NH4+. This result suggests that the role of NDH II is as a vital component of the respiratory protection mechanism of the nitrogenase complex in A. vinelandii. It was also found that the oxidation of NADPH in the A. vinelandii respiratory chain is catalyzed solely by NDH II.
Molecular nitrogen reduction is mediated by the nitrogenase complex, an enzyme known to be highly vulnerable to oxygen damage. Thus, the majority of bacteria are capable of reducing N2 only in anaerobic or microaerobic conditions. Contrary to this, Azotobacter vinelandii is an obligate aerobe capable of fixing molecular nitrogen even at very high ambient O2 concentrations. The purified nitrogenase complex of this bacterium was shown to be as susceptible to oxygen damage as those in other microorganisms (26). Dalton and Postgate hypothesized that the concentration of O2 in the cytoplasm of Azotobacter is effectively reduced below nitrogenase-damaging levels by the extremely active respiration characteristic of this bacterium (5, 6). The existence of such a mechanism, termed respiratory protection, was later substantiated in a large number of studies. In particular, it was found that the A. vinelandii bd-type quinol oxidase is essential for respiratory protection (15, 21). It was also shown that respiration mediated by the bd-type oxidase is dominant in cells growing diazotrophically or at high oxygen concentrations (18, 24). Mutant strains with disruptions in the gene encoding the bd-type oxidase failed to fix N2 at high ambient oxygen concentrations (15).
It would be logical to assume that in order to match the above-mentioned highly rapid oxygen consumption at the bd terminus of the A. vinelandii respiratory chain, there must exist a mechanism at the initial segment that feeds electrons into the chain just as effectively. Moreover, this putative enzyme should utilize a major respiratory substrate as well as have no coupling with proton translocation in order to avoid limitations imposed by the generated transmembrane proton potential.
In our previous study (3), it was shown that NADH oxidation in A. vinelandii is mediated by two distinct NADH:quinone oxidoreductases; one is coupled to the generation of the transmembrane proton potential (NDH I), and the other is not (NDH II). We also showed that the expression of NDH II, in contrast to that of NDH I, is induced by high O2 concentration and by switching of the bacterial culture to diazotrophic growth. Induction of NDH II activity was observed under the same conditions in which induction of the bd-type oxidase was shown to take place (3). It is known that the latter effect is mediated by a regulatory A. vinelandii protein, CydR (homologous to FNR in Escherichia coli) (24, 25). It was shown that the A. vinelandii strains lacking this protein overproduce the bd-type oxidase even during growth at lowered O2 concentrations. Later it was found that the cydR mutation also leads to induction of NDH II and repression of NDH I (3). Thus, at all growth conditions NADH is oxidized in the respiratory chain of this mutant almost entirely via NDH II.
The properties of the noncoupled NADH dehydrogenase (NDH II) and the conditions of its induction in A. vinelandii suggest the possible role of this enzyme in respiratory protection. To prove this assumption, in the current work we constructed an NDH II-deficient strain of A. vinelandii and tested its nitrogen-fixing capabilities at high ambient oxygen concentrations.
MATERIALS AND METHODS
Bacterial strains, plasmids, growth, and medium compositions.
The A. vinelandii and E. coli strains used in this study are listed in Table 1. A. vinelandii cells were grown in modified Burke's media BS and BSN (BS with ammonia salts added) (7). The cells were grown in a rotary shaker at 250 rpm and 30°C. Antibiotics (where used) were added to the following final concentrations: rifampicin, 10 μg/ml; kanamycin, 1 μg/ml; ampicillin, 50 μg/ml; tetracycline, 10 μg/ml. E. coli cells were routinely grown in Luria-Bertani (LB) medium.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strains and plasmids | Characteristic(s) | Reference or source |
|---|---|---|
| A. vinelandii UW136 | Rifr | 15 |
| A. vinelandii MK8 | UW136 cydR::Tn5 Rifr Kmr | 15 |
| A. vinelandii DN165 | UW136 ndh::ΩTc Rifr Tcr | This work |
| A. vinelandii N24 | UW136 ndh::pNDT6 Rifr Tcr Ampr | This work |
| E. coli TG1 | F′ traD36 lacIqZΔM15 proAB+/supE Δ(hsdM-mcrB)5 (rK− mK+msrB) thi Δ(lac-proAB) | Promega |
| pGEM-T | PCR product cloning vector; Ampr | Promega |
| pHP45ΩTc | Tetracycline resistance | 9 |
| pND7 | pGEM-T with 400-bp fragment of A. vinelandii ndh; Ampr | This work |
| pNDT6 | pND7 with ΩTc inserted in SmaI site of ndh fragment; Ampr Tcr | This work |
KLα.
The oxygen transfer coefficient (KLα) was determined by the sulfite method as described previously (10) with some modifications: 100 g of KI/liter and 63.5 g of I2/liter were used to prepare a 0.25 M iodine solution, and Na2S2O3 was used instead of Na2S2O4 for titration. To establish high aeration (measured KLα = 14 mmol of O2 liter−1 h−1), 100 ml of growth medium was shaken in 1-liter flasks. Decreased aeration (KLα = 1.4 mmol of O2 liter−1 h−1) was established by shaking 200 ml of medium in 350-ml flasks.
SBPs.
Subbacterial particles (SBPs) from A. vinelandii cells were prepared as described previously (2).
(i) Respiration of A. vinelandii cells and SBPs.
Respiration of A. vinelandii cells and SBPs was monitored using a standard Clark-type electrode at 30°C. The following buffer was used as the respiration medium for SBPs: 60 mM KCl–2 mM MgSO4–20 mM HEPES-KOH (pH 7.5). Respiration of whole A. vinelandii cells was measured in BSN medium. Logarithmic cells containing to 30 to 60 μg of overall protein were sampled from the growth medium and directly injected into a polarograph chamber.
(ii) Oxidation rates of NADH, NADPH, and reduced nicotinamide hypoxanthine dinucleotide (dNADH) by A. vinelandii SBPs.
Oxidation rates were monitored by means of a Hitachi-557 spectrophotometer as described previously (3). All reduced pyridine dinucleotides were used at a final concentration of 150 μM.
Estimation of Km values of A. vinelandii NDH I and NDH II for reduced pyridine nucleotide oxidation.
Estimation of Km values for NDH I and NDH II were carried out using SBPs from A. vinelandii strains DN165 and MK8, respectively. NADH and dNADH oxidation was monitored by registering the decrease in optical density at 340 nm (OD340) (for both NADH and dNADH, ɛ340 = 6.22 mM−1 cm−1 was used). The rates of NADH and dNADH oxidation at different concentrations of these reduced pyridine dinucleotides were determined by analyzing the first derivative of the (d)NADH oxidation progress curves. Briefly, the (d)NADH concentration can be calculated from a direct OD340-versus-time curve, while the values of the first derivative of the direct curve are proportional to the rates of the (d)NADH oxidation. The data obtained were fitted to the Michaelis-Menten equation using the nonlinear regression analysis method.
The Km value for NADPH was determined by recording initial rates of oxygen consumption by NADPH-oxidizing SBPs at different NADPH concentrations.
Construction of an NDH II-deficient A. vinelandii strain by site-directed mutagenesis.
Amplification of the A. vinelandii ndh fragment was carried out using the PCR with degenerative primers ndhb1a [5′-CACCT(G/C)TTCCAGCCGCTGCT] and ndhb2a [5′-CCGGT(G/C)GGGCC(A/C)GC(G/C)CCGAC] (17). The amplified ndh fragment was cloned into pGEM-T vector, resulting in plasmid pND7.
Construction of the ndh::ΩTc strain of A. vinelandii was carried out as follows. A tetracycline resistance cassette from pHP45ΩTc was ligated into the SmaI site of pND7, and a ΩTc-containing plasmid (pNDT6) bearing the ndh gene together with the unidirectionally transcribing tetracycline resistance cassette was selected. Competent A. vinelandii cells were transformed by the pNDT6 plasmid. This transformation resulted in a series of tetracycline-resistant clones. Screening for both tetracycline and ampicillin resistance led to selection of an Amps Tcr clone (DN165) characteristic of a double-crossover introduced mutation. A single recombinant Ampr Tcr clone (N24) was also selected and later used for ndh cloning by the plasmid rescue technique.
Segregation of the ndh mutation in A. vinelandii.
In order to obtain an A. vinelandii strain with a mutated ndh in all copies of the chromosome, A. vinelandii DN165 obtained on selective medium with 10 μg of tetracycline per ml was plated on solid medium supplied with 30 μg of tetracycline per ml. An individual clone was selected and plated on solid medium supplied with 60 μg of tetracycline per ml.
Based on the determined primary sequence of the cloned ndh fragment, a p_ndh (5′-CGGCGACGAACTGAACTA) and r_ndh (5′-GTGGGCACGCAAGTAGTG) primer pair was selected. With these primers, a unique 300-bp PCR product from wild-type A. vinelandii DNA was formed. It was employed as a specific marker to monitor the segregation process. PCR analysis of DNA from the segregated mutant clone showed the presence of the major 2.4-kbp fragment only (comprising the 2.1- and 0.3-kbp components corresponding to the tetracycline resistance cassette and the ndh fragment, respectively). The 0.3-kbp wild-type fragment was not present.
Sequencing of the whole ndh gene from A. vinelandii.
The 3′ part of ndh was cloned by the plasmid rescue technique (8). Four micrograms of chromosomal DNA of the single recombinant clone, N24, was incubated with the AatII restrictase. The restriction mixture was treated with T4 DNA ligase in a 300-μl volume and used for E. coli transformation. As a result, an ampicillin-resistant clone bearing the pGEM plasmid with an ∼4-kbp insert of A. vinelandii genomic DNA was obtained. This plasmid was used for sequencing of the 3′ part of the ndh gene.
The 5′ part of ndh was cloned by an inverse PCR technique (19). Two micrograms of A. vinelandii UW136 DNA was incubated with the PstI restrictase. The restriction mixture was treated with T4 DNA ligase in a 150-μl volume. Subsequently, 1 μl of the ligase mixture was used as the template for PCR with the rev_ph (5′-GGTCGAGTTCAGCGAGC) and rev_mdh (5′-CACTACTTGCGTGCCCAC) primers. A unique ∼2.8-kbp PCR product was obtained and later used for sequencing of the 5′ part of ndh.
Nucleotide sequence was analyzed with double-stranded templates (both strands were sequenced) using synthesized oligonucleotide primers. This analysis was carried out by the sequencing group of the Engelhard Institute of Molecular Biology using an Applied Biosystems ABI 373A DNA sequencer.
Competent A. vinelandii cells were obtained as described by Page and von Tigerstrom (20). Transformation of A. vinelandii cells was carried out as described by Glick et al. (11).
Protein concentration was measured by means of the bicinchoninic acid method with bovine serum albumin (Serva, type V) as the standard.
Nucleotide sequence accession number.
The novel DNA sequence reported here has been deposited into the GenBank database and is available under accession number AF346487.
RESULTS
Construction of an NDH II-deficient A. vinelandii strain. (i) Cloning of a fragment of the ndh gene from A. vinelandii.
An ndh gene fragment from the A. vinelandii DNA was amplified by means of a PCR technique using degenerative primers (17). PCR with these primers and the A. vinelandii DNA as a template resulted in amplification of a variety of fragments of different-lengths. The PCR products were separated using gel electrophoresis. In accordance with expected size of the ndh gene, the amplified product was searched for in the 400-bp band. This band was isolated, cloned into a pGEM-T vector (resulting in plasmid pND7), and sequenced. A BLASTX analysis of the acquired fragment (with the exclusion of primer sequences) showed that the putative peptide corresponding to this nucleotide fragment is homologous to NDH II from a wide variety of bacteria. The highest homologies were was found with the sequence of NDH II from Pseudomonas aeruginosa (79%) and Pseudomonas fluorescence (77%). Thus, the ndh fragment of A. vinelandii DNA was cloned.
(ii) Construction of an ndh::ΩTc strain of A. vinelandii.
The sequence data for the cloned ndh fragment revealed the presence of a unique SmaI site located 263 bp away from the 5′ end of the PCR product. Subsequently, a tetracycline resistance cassette from pHP45ΩTc was ligated into this SmaI site of plasmid pND7, resulting in plasmid pNDT6. In order to construct a strain of A. vinelandii with the insertion-inactivated fragment replacing the wild-type sequence, competent A. vinelandii cells were transformed using plasmid pNDT6 (this plasmid is unstable in A. vinelandii because of its ColE1 replicon). An Amps Tcr clone (DN165) characteristic of a double-crossover introduced mutation was selected. Localization of this mutation in the ndh gene of the acquired strain was verified by PCR analysis.
In order to clone the entire A. vinelandii ndh gene, the plasmid rescue technique was applied. This method is based on obtaining a strain with studied gene disruption by a single-crossover plasmid insert. Briefly, DNA of such a mutant is cut by a variety of different endonucleases, and the fragments are ligated and used to transform E. coli cells. Such a procedure may lead to obtaining a clone bearing the plasmid with the insert of the DNA which flanked the crossover site in the chromosome (8). This procedure may be successful provided there are two restriction sites in the flanking DNA regions that are near enough to the start and stop codons of the studied gene while there are no such sites inside the gene. In our case, the N24 single recombinant A. vinelandii strain was used. A desired clone was obtained only by using the AatII restrictase (see Materials and Methods for details). As this restrictase is located in the pNDT6 polylinker, we succeeded in cloning and sequencing only the DNA fragment flanking the pNDT6 plasmid insert from 3′ side of the ndh gene.
In order to clone the 5′ side of ndh, the inverse PCR technique was used. This involved cutting the wild-type DNA with an appropriate endonuclease, ligating the fragments, amplifying the fragment carrying the part of the known sequence of ndh with inverse primers, and, finally, isolating and sequencing the amplified DNA. Again, this technique can be successful provided that one restriction site is in the known region of ndh while the second site is in the 5′-flanking DNA close enough so that the resulting fragment can be ligated and amplified. Based upon the determined sequence of ndh, a set of restrictases was chosen. The choice of the PstI restrictase proved to be successful and lad to cloning and sequencing of the 5′ part of ndh (see Materials and Methods for details).
Thus, having applied the inverse PCR and the plasmid rescue techniques, we succeeded in sequencing the whole A. vinelandii ndh gene. The resulting data were deposited into GenBank (accession number AF346487).
Properties of the NDH II-deficient A. vinelandii strain. (i) Respiration rates of whole A. vinelandii cells and subbacterial particles.
As experiments showed, the DN165 mutant cells have a much lower respiratory activity than the wild type. Generally, the wild-type cells grown in highly aerated BSN medium consumed 2.1 ± 0.6 μg-atom of oxygen per min per mg of protein, whereas the figure for DN165 was as low as 0.4 ± 0.1 (average from three independent experiments).
NADH- and dNADH-oxidizing activities of the A. vinelandii DN165 SBPs were also tested (Table 2). It is well known that NDH I can oxidize either NADH or its analog dNADH, while NDH II utilizes only NADH and not dNADH (3). SBPs from wild-type A. vinelandii oxidized NADH at much higher rates than dNADH due to operation of both NADH dehydrogenases (Table 2). The difference between NADH and dNADH oxidase rates is much more profound in the MK8 (ΔcydR) strain, which contains very low levels of NDH I (3). On the other hand, in the constructed NDH II-deficient mutant strain DN165, NADH and dNADH oxidation rates were equal and low (Table 2). This confirms that the DN165 mutant does not possess significant NDH II activity.
TABLE 2.
NADH-, dNADH-, and NADPH-oxidase activities of SBPs from different A. vinelandii strains
| Strain | Activitya
|
||
|---|---|---|---|
| NADH | dNADH | NADPH | |
| UW136 (wild type) | 8.2 | 2.0 | 1.1 |
| MK8 (ΔcydR) | 10.4 | 0.5 | 1.7 |
| DN165 (Δndh) | 1.6 | 1.75 | 0.005 |
The concentration of all dinucleotides was 150 μM. Activities are given in micromoles of reduced pyridine dinucleotides oxidized per minute per milligram of protein.
(ii) Participation of the A. vinelandii NDH II in respiratory protection.
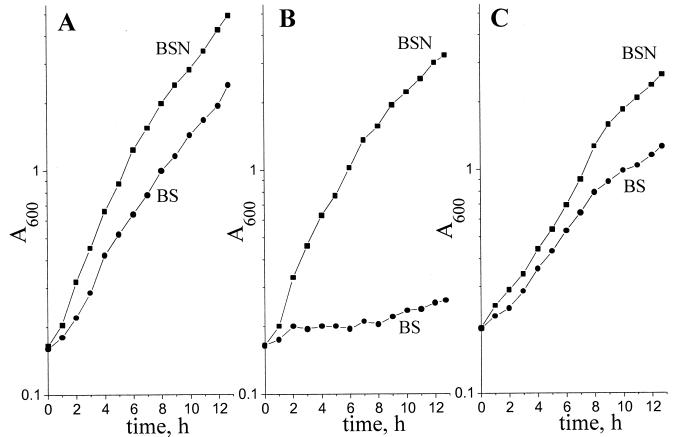
Growth of three A. vinelandii strains was tested at different aeration levels in media with (BSN) and without (BS) ammonium acetate. As seen from Fig. 1, at high aeration (KLα = 14 mmol of O2 liter−1 h−1) the DN165 strain lacking NDH II is capable of growing in the presence of NH4+ at a rate comparable to that of the wild-type strain (doubling time, 150 and 140 min, respectively), but contrary to the wild type, it failed to grow at a high oxygen concentration if N2 fixation was necessary. For comparison, Fig. 1 also includes growth curves for A. vinelandii MK8, which was shown to most preferably oxidize NADH via NDH II (3). As seen from Fig. 1C, the absence of NDH I activity (in contrast to NDH II) does not lead to any increase in O2 sensitivity of the N2-fixing bacterial culture.
FIG. 1.
Growth curves for various A. vinelandii strains grown at high aeration (KLα = 14 mmol of O2 liter−1 h−1) in BSN medium containing NH4+ (■) and BS minimal medium (●) (see Materials and Methods for details). (A) A. vinelandii UW 136 (wild type); (B) A. vinelandii DN165 (Δndh); (C) A. vinelandii MK8 (ΔcydR).
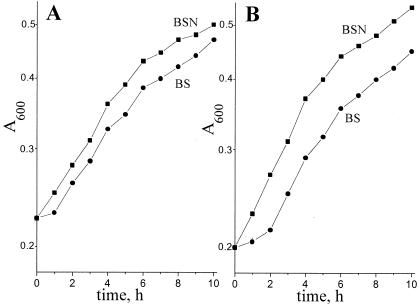
The data presented in Fig. 2 shows that growth rates of the wild type and the mutant strain DN165 at low aeration (KLα = 1.4 mmol of O2 liter−1 h−1) were similar both in the presence and in the absence of NH4+. An assumption can be drawn that NDH II is vital for the protection of the nitrogenase complex against oxygen insult, i.e., during diazotrophic growth at a high ambient oxygen concentration.
FIG. 2.
Growth curves for various A. vinelandii strains grown at low aeration (KLα = 1.4 mmol of O2 liter−1 h−1) in BSN medium containing NH4+ (■) and BS minimal medium (●) (see Materials and Methods for details). (A) A. vinelandii UW 136 (wild type); (B) A. vinelandii DN165 (Δndh).
(iii) Role of NDH II in the NADPH oxidation activity of A. vinelandii.
Earlier studies (13) have shown that A. vinelandii membranes are capable of oxidizing NADPH. Ackrell and coworkers (1) have postulated the presence of a specific enzyme mediating this reaction. In the present study, we tested the effect of ndh mutation on the NADPH-oxidizing activity of A. vinelandii. Our data show (Table 2) that SBPs from A. vinelandii strain DN165 completely lack the ability to oxidize NADPH. This provides proof that NDH II is solely responsible for the NADPH dehydrogenase activity in the respiratory chain of A. vinelandii. It is noteworthy that a similar conclusion has been recently drawn for NDH II of Corynebacterium glutamicum (17).
(iv) Estimation of Km values of A. vinelandii NDH I and NDH II for reduced pyridine nucleotide oxidation.
Using A. vinelandii strains that respire almost solely via NDH I (MK8 [3]) or entirely via NDH II (DN165), we measured the Km values of these enzymes for various respiratory substrates (Table 3). Tables 2 and 3 show that NDH I is capable of oxidizing both NADH and dNADH, but not NADPH, while NDH II oxidizes NADH and NADPH but cannot utilize dNADH. The affinity of NDH II for NADPH is very poor, which indicates that it is not a major substrate under physiological conditions. When comparing Km values for NADH, we expected to see higher values for NDH II then for NDH I, as in theory this would render NDH II efficient only when NDH I is either substrate saturated or limited by respiratory control. Test results showed that the Km value of NDH II for NADH turned out to be twice that of NDH I (Table 3). It is possible, however, that the electron flow through the two different NADH dehydrogenases is downregulated at the level of quinone, i.e., via their different affinities for ubiquinone-8. Investigation of this speculation, however, is left for future research.
TABLE 3.
Km values of A. vinelandii NADH:ubiquinone oxidoreductases for different reduced pyridine dinucleotides
| Enzyme |
Km (μM)a
|
||
|---|---|---|---|
| NADH | dNADH | NADPH | |
| NDH I | 24.2 ± 1.2 | 43.8 ± 2.7 | |
| NDH II | 13.0 ± 1.0 | 1,720 ± 270 | |
The Km values for NDH I and NDH II were determined using SBPs from A. vinelandii strains DN165 and MK8, respectively (see Materials and Methods for details).
DISCUSSION
The current work clearly indicates that NDH II in A. vinelandii is vital for diazotrophic growth at high ambient oxygen concentrations. Thus, the result may suggest that the role of NDH II is as a key component of the oxygen protection mechanism in this bacterium. In earlier work (15, 21), a similar function has been assigned to the bd-type terminal oxidase from the same organism. Studies by our group (3) found NDH II induction by increased ambient oxygen concentration or by the absence of NH4+ in the medium, i.e., under the same conditions that induce cytochrome bd (18, 24). Such coordinated expression of both of these enzymes is achieved through their joint induction by the CydR regulator protein (3, 24, 25).
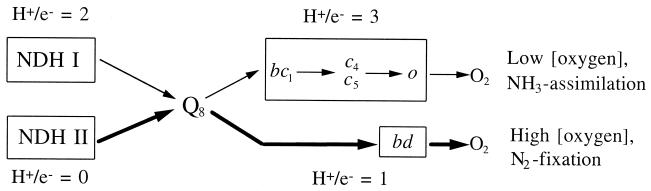
Taking into account the results of previous work (2, 3, 15, 16, 22), the A. vinelandii respiratory chain may be described by the scheme shown in Fig. 3. It is assumed that A. vinelandii cells possess at least two respiratory chains differing in enzyme composition and physiological function. One of them is completely coupled (thin arrows); it includes NDH I (3), the bc1-complex (2), cytochromes c4 and c5 (22), and the o-type oxidase (16). The other chain (thick arrows) is much simpler and includes the noncoupled NDH II (3) and the bd-type quinol oxidase (15). The energy-conserving efficiency of this chain (H+/e− = 1 [H+/e− is the number of H+ ions pumped across the membrane for every one electron passed down the respiratory chain]) is much lower than that of the coupled one (H+/e− = 5) (2, 3). This allows avoidance of limitations of oxygen consumption rates by systems utilizing transmembrane electrochemical proton potential (ΔμH+).
FIG. 3.
Putative scheme of the A. vinelandii respiratory chain. The electron transport pathway dominating in cells grown at high [O2] or low [NH3] is shown by thick arrows.
It is also worth noting that both NDH II and the bd oxidase possess unusually high specific activities (more so for NDH II), i.e., 560 μg-atom of oxygen min−1 mg of protein−1 (14) and 106 mmol of NADH min−1 mg of protein−1 (4) for the bd-type quinol oxidase and NDH II, respectively. Such high rates may be due to the absence of the proton-translocating function for these enzymes (the function of the bd-type oxidase does in fact generate ΔμH+ but solely due to scalar proton effects [2, 12]). Exceptionally high turnover of NDH II should be favorable for optimally efficient utilization of the membrane-occupied space which can limit overall rates of oxygen consumption in a cell. Such a high turnover rate of the respiratory protection chain is what makes it possible to decrease the intracellular oxygen level required for the operation of the nitrogenase.
While the respiratory chain in the inner membrane of animal mitochondria possesses a single NADH-oxidizing enzyme (complex I, analogous to bacterial NDH I) (23), most bacteria also harbor a second enzyme (i.e., NDH II) the physiological role of which has not yet been revealed. The analysis of the A. vinelandii NDH II sequence indicates that this protein is very similar to the NDH II of microorganisms from the gamma subdivision of proteobacteria. This suggests that NDH II from A. vinelandii is a typical representative of this class of enzymes and that in other organisms harboring NDH II the enzyme may also be employed similarly, i.e., for very fast quinone reduction (or very fast NADH oxidation). The possible physiological role(s) of NDH II in bacteria other than Azotobacter is left for future research.
ACKNOWLEDGMENTS
This work was supported in part by RFBR grant 99-04-49161. Y.V.B. and A.V.B. are indebted to the RFBR for fellowships (no. 01-04-06480 and 01-04-06481).
We thank D. Molenaar for generously providing the degenerative primers, R. K. Poole for kindly providing A. vinelandii strains, I. V. Elanskaya for help in the cloning experiments and for fruitful discussion, and A. I. Shestopalov for help with preparation of the manuscript.
REFERENCES
- 1.Ackrell B A, Erickson S K, Jones C W. The respiratory-chain NADPH dehydrogenase of Azotobacter vinelandii. Eur J Biochem. 1972;26:387–392. doi: 10.1111/j.1432-1033.1972.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 2.Bertsova Y V, Bogachev A V, Skulachev V P. Generation of protonic potential by the bd-type quinol oxidase of Azotobacter vinelandii. FEBS Lett. 1997;414:369–372. doi: 10.1016/s0014-5793(97)01047-8. [DOI] [PubMed] [Google Scholar]
- 3.Bertsova Y V, Bogachev A V, Skulachev V P. Two NADH:ubiquinone oxidoreductases of Azotobacter vinelandii and their role in the respiratory protection. Biochim Biophys Acta. 1998;1363:125–133. doi: 10.1016/s0005-2728(97)00094-7. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklof K, Zickermann V, Finel M. Purification of the 45 kDa, membrane bound NADH dehydrogenase of Escherichia coli (NDH-2) and analysis of its interaction with ubiquinone analogues. FEBS Lett. 2000;467:105–110. doi: 10.1016/s0014-5793(00)01130-3. [DOI] [PubMed] [Google Scholar]
- 5.Dalton H, Postgate J R. Effect of oxygen on growth of Azotobacter chroococcum in continuous culture. J Gen Microbiol. 1968;54:463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- 6.Dalton H, Postgate J R. Growth and physiology of Azotobacter chroococcum in continuous culture. J Gen Microbiol. 1969;56:307–319. doi: 10.1099/00221287-56-3-307. [DOI] [PubMed] [Google Scholar]
- 7.D'Mello R, Hill S, Poole R K. Determination of the oxygen affinities of terminal oxidases in Azotobacter vinelandii using the deoxygenation of oxyleghaemoglobin and oxymyoglobin: cytochrome bd is a low-affinity oxidase. Microbiology. 1994;140:1395–1402. [Google Scholar]
- 8.Dolganov N, Grossman A R. Insertional inactivation of genes to isolate mutants of Synechococcus sp. strain PCC 7942: isolation of filamentous strains. J Bacteriol. 1993;175:7644–7651. doi: 10.1128/jb.175.23.7644-7651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 10.Gil A, Kroll R G, Poole R K. The cytochrome composition of the meat spoilage bacterium Brochothrix thermosphacta: identification of cytochrome a3-and d-type terminal oxidases under various conditions. Arch Microbiol. 1992;158:226–233. doi: 10.1007/BF00290819. [DOI] [PubMed] [Google Scholar]
- 11.Glick B R, Brooks H E, Pasternak J J. Transformation of Azotobacter vinelandii with plasmid DNA. J Bacteriol. 1985;162:276–279. doi: 10.1128/jb.162.1.276-279.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasaitis A, Borisov V B, Belevich N P, Morgan J E, Konstantinov A A, Verkhovsky M I. Electrogenic reactions of cytochrome bd. Biochemistry. 2000;39:13800–13809. doi: 10.1021/bi001165n. [DOI] [PubMed] [Google Scholar]
- 13.Jones C W, Ackrell B A, Erickson S K. Respiratory control in Azotobacter vinelandii membranes. Biochim Biophys Acta. 1971;245:54–62. doi: 10.1016/0005-2728(71)90007-7. [DOI] [PubMed] [Google Scholar]
- 14.Junemann S, Butterworth P J, Wrigglesworth J M. A suggested mechanism for the catalytic cycle of cytochrome bd terminal oxidase based on kinetic analysis. Biochemistry. 1995;34:14861–14867. doi: 10.1021/bi00045a029. [DOI] [PubMed] [Google Scholar]
- 15.Kelly M J S, Poole R K, Yates M G, Kennedy C. Cloning and mutagenesis of genes encoding the cytochrome bd terminal oxidase complex in Azotobacter vinelandii: mutants deficient in the cytochrome d complex are unable to fix nitrogen in air. J Bacteriol. 1990;172:6010–6019. doi: 10.1128/jb.172.10.6010-6019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung D, van der Oost J, Kelly M, Saraste M, Hill S, Poole R K. Mutagenesis of a gene encoding a cytochrome o-like terminal oxidase of Azotobacter vinelandii: a cytochrome o mutant is aero-tolerant during nitrogen fixation. FEMS Microbiol Lett. 1994;119:351–358. doi: 10.1111/j.1574-6968.1994.tb06912.x. [DOI] [PubMed] [Google Scholar]
- 17.Molenaar D, van der Rest M E, Drysch A, Yucel R. Functions of the membrane-associated and cytoplasmic malate dehydrogenases in the citric acid cycle of Corynebacterium glutamicum. J Bacteriol. 2000;182:6884–6891. doi: 10.1128/jb.182.24.6884-6891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moshiri F, Smith E G, Taormino J P, Maier R J. Transcriptional regulation of cytochrome d in nitrogen-fixing Azotobacter vinelandii. Evidence that up-regulation during N2 fixation is independent of nifA but dependent on ntrA. J Biol Chem. 1991;266:23169–23174. [PubMed] [Google Scholar]
- 19.Ochman H, Gerber A S, Hartl D L. Genetic applications of an inversed polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page W J, von Tigerstrom M. Induction of transformation competence in Azotobacter vinelandii iron-limited cultures. Can J Microbiol. 1978;24:1590–1594. doi: 10.1139/m78-254. [DOI] [PubMed] [Google Scholar]
- 21.Poole R K, Hill S. Respiratory protection of nitrogenase activity in Azotobacter vinelandii—roles of the terminal oxidases. Biosci Rep. 1997;17:303–317. doi: 10.1023/a:1027336712748. [DOI] [PubMed] [Google Scholar]
- 22.Rey L, Maier R J. Cytochrome c terminal oxidase pathways of Azotobacter vinelandii: analysis of cytochrome c4 and c5 mutants and up-regulation of cytochrome c-dependent pathways with N2 fixation. J Bacteriol. 1997;179:7191–7196. doi: 10.1128/jb.179.22.7191-7196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sled' V D, Friedrich T, Leif H, Weiss H, Meinhardt S W, Fukumori Y, Calhoun M W, Gennis R B, Ohnishi T. Bacterial NADH-quinone oxidoreductases: iron-sulfur clusters and related problems. J Bioenerg Biomembr. 1993;25:347–356. doi: 10.1007/BF00762460. [DOI] [PubMed] [Google Scholar]
- 24.Wu G, Hill S, Kelly M J S, Sawers G, Poole R K. The cydR gene product, required for cytochrome bd expression in obligate aerobe Azotobacter vinelandii, is an FNR-like protein. Microbiology. 1997;143:2197–2207. doi: 10.1099/00221287-143-7-2197. [DOI] [PubMed] [Google Scholar]
- 25.Wu G, Cruz-Ramos H, Hill S, Green J, Sawers G, Poole R K. Regulation of cytochrome bd expression in the obligate aerobe Azotobacter vinelandii by CydR (Fnr). Sensitivity to oxygen, reactive oxygen species, and nitric oxide. J Biol Chem. 2000;275:4679–4686. doi: 10.1074/jbc.275.7.4679. [DOI] [PubMed] [Google Scholar]
- 26.Yates M G. The role of oxygen and hydrogen in nitrogen fixation. In: Cole I A, Ferguson S I, editors. The nitrogen and sulphur cycles. Vol. 42. Cambridge, United Kingdom: University Press; 1988. pp. 386–416. [Google Scholar]