Abstract
Profound natural killer (NK) cell suppression after cancer surgery is a main driver of metastases and recurrence, for which there is no clinically approved intervention available. Surgical stress is known to cause systemic postoperative changes that negatively modulate NK cell function including the expansion of surgery-induced myeloid-derived suppressor cells (Sx-MDSCs) and a marked reduction in arginine bioavailability. In this study, we determine that Sx-MDSCs regulate systemic arginine levels in the postoperative period and that restoring arginine imbalance after surgery by dietary intake alone was sufficient to significantly reduce surgery-induced metastases in our preclinical murine models. Importantly, the effects of perioperative arginine were dependent upon NK cells. Although perioperative arginine did not prevent immediate NK cell immunoparalysis after surgery, it did accelerate their return to preoperative cytotoxicity, interferon gamma secretion, and activating receptor expression. Finally, in a cohort of patients with colorectal cancer, postoperative arginine levels were shown to correlate with their Sx-MDSC levels. Therefore, this study lends further support for the use of perioperative arginine supplementation by improving NK cell recovery after surgery.
Keywords: metastasis, surgery, arginine, immunology, immunotherapy, natural killer cells, myeloid-derived suppressor cells, colorectal cancer, surgical oncology
Graphical abstract
Surgery causes a period of immune suppression, which can allow the formation of metastases. In this preclinical study, we investigated an immunonutritional intervention comprised of arginine supplementation around the time of surgery that led to enhanced recovery of natural killer cell function and prevented surgery-induced metastases in mice.
Introduction
Natural killer (NK) cells are the cytolytic cells of the innate immune system that are central for controlling metastatic disease.1 Our group and others have shown that surgery results in a profound impairment in NK cell cytotoxicity and interferon gamma (IFNγ) secretion in both patients with cancer and in murine studies.2, 3, 4, 5, 6, 7, 8 This period of diminished NK cell function after surgery is directly linked to increased postoperative lung metastases in preclinical studies and is the result of physiological changes that occur in response to surgery.8,9 Notably, there is an expansion of myeloid-derived suppressor cells (MDSCs) in response to the proinflammatory state that ensues immediately after surgical insult.10, 11, 12 These immature myeloid cells are known to express the arginine consuming enzymes arginase-1 (Arg1) or inducible nitric oxide synthase (iNOS), depending on the inflammatory environment.13 We have previously demonstrated that these MDSCs are responsible for postoperative NK cell suppression.12 Since the postoperative period is characterized by elevated interleukin-10 (IL-10), a Th2 cytokine known to induce Arg1, we hypothesize that the large accumulation of Arg1-expressing MDSCs leads to the systemic depletion of arginine after surgery.14,15
Arginine is a conditionally essential amino acid that is necessary for proper immune cell function but is rapidly depleted after surgical trauma.9,16,17 Insufficient arginine is known to impair T cells and NK cells.18, 19, 20, 21, 22, 23 In the absence of arginine, T cells are less responsive due to reduced T cell receptor expression, and NK cells have decreased proliferation, cytotoxicity, and IFNγ production.16,24 To combat the catabolic effects of surgery, perioperative arginine supplements have been investigated and shown to reduce the length of patient recovery time and the number of postoperative infections and complications.25, 26, 27, 28 Whether or not arginine supplementation has anti-metastatic properties as a result of beneficial immunomodulatory effects on NK cells has yet to be studied.
Here, we investigated the cause of postoperative arginine depletion and assessed the therapeutic potential of an arginine-enriched diet (AED) to modulate perioperative arginine levels and prevent surgery-induced NK cell suppression and metastases. We report that surgery-induced MDSCs (Sx-MDSCs) regulate postoperative arginine bioavailability and that a perioperative AED attenuates metastases by accelerating NK cell recovery after surgery, a phenomenon and mechanism that has not been previously reported. We suspect that postoperative arginine modulation is one of many suppressive mechanisms that MDSCs use to regulate the immune system after surgery.
Results
Surgery-induced MDSCs expand and persist postoperatively with increased Arg1 expression and activity
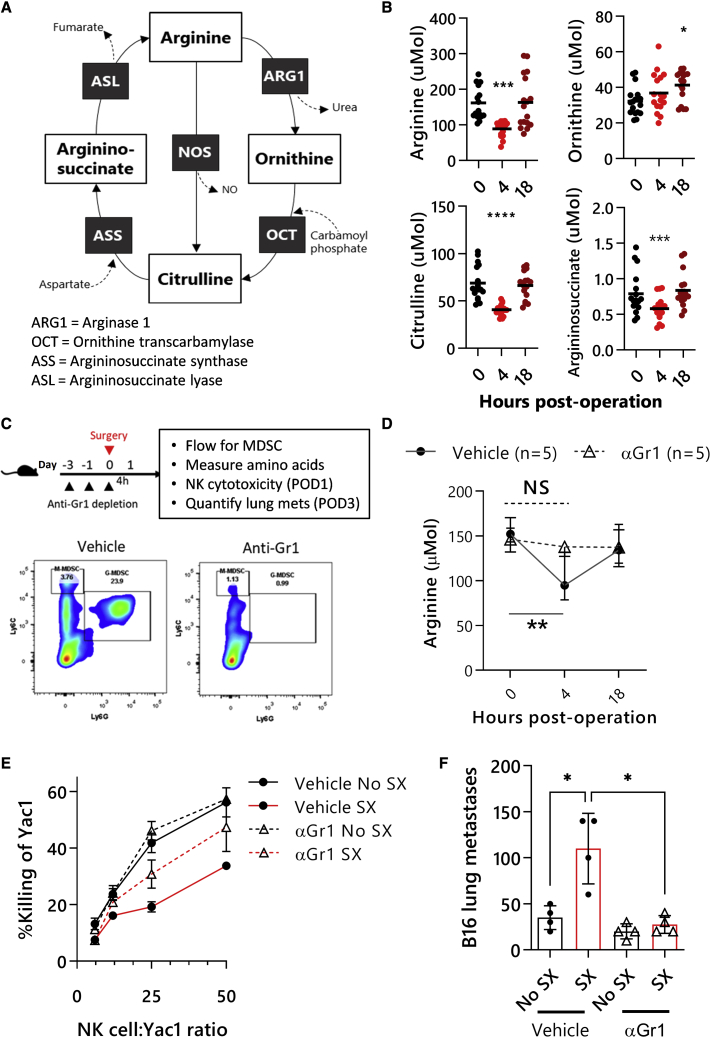
To assess the prometastatic effects of surgery, we utilized two models of surgical stress: (1) the experimental B16F10 melanoma metastasis model in C57BL6 mice (Figure 1A) and (2) the spontaneous orthotopic 4T1 breast cancer model in Balb/c mice (Figure 1B). As previously reported, lung metastases in these models are significantly increased (B16F10, n = 18/group, p < 0.0001; 4T1, n = 12/group, p = 0.0008) in response to surgical stress.6,8,12 Surgical stress resulted in a significant expansion of monocytic MDSCs (M-MDSCs; SSClow, CD3-CD11b+Ly6ChiLy6Gneg) and polymorphonuclear MDSCs (PMN-MDSCs; SSChi, CD3-CD11b+Ly6GhiLy6C+), which we will refer to collectively as Sx-MDSCs (Figures 1C, 1D, and S1). On postoperative day (POD) 1, PMN Sx-MDSCs had already significantly increased in both spleen and blood samples (p < 0.0001). In contrast, although circulating monocytic Sx-MDSCs were increased on POD1 (p = 0.0006) and POD3 (p = 0.0002), significant increases in the spleen were not observed until POD3.
Figure 1.
Surgery increases postoperative metastases and MDSCs
(A) Representative lung images of mice inoculated with B16F10lacZ cells intravenously (i.v.) prior to surgery (left). Three days after surgery, lungs were harvested, and metastases were quantified by microscopy (right; n = 18/group from 3 experiments; Mann Whitney U tests). (B) Orthotopic 4T1 tumors were inoculated in Balb/c mice and resected after 14 days ± nephrectomy. Fourteen days after resection, mice were sacrificed, and spontaneous lung metastases were quantified by microscopy (right; n = 12/group from 2 experiments; Mann Whitney U tests). (C and D) M-MDSCs and PMN-MDSCs were quantified by flow cytometry from (C) no surgery (n = 43), POD1 (n = 17), and POD3 (n = 36) splenocytes (pooled from 11 experiments) or (D) no surgery (n = 22), POD1 (n = 16), and POD3 (n = 8) blood (pooled from 4 experiments, Kruskal-Wallis test) of C57Bl/6 mice. (E) Schematic of fluorescence based MDSC:NK cell co-culture and suppression assay. (F) NK cells were co-cultured with MDSCs from surgery or no-surgery mice at a ratio of 4:1 (MDSC:NK cells) overnight in the presence of 100 U/mL of IL-2. 5 × 103 Yac1 target cells were then added to the co-cultures for 4 h at a ratio of 8:1 (NK:YAC), and cytotoxicity was measured by flow cytometry (n = 2 mice/group, average of triplicates, Kruskal-Wallis test). (G) Intracellular flow cytometry was used to determine the fold increase of arginase-1 MFI gated on PMN-MDSCs (CD11b+Gr1hi) (n = 8, Mann-Whitney U test). (H) Lysates of isolated Ly6G+ cells were used to measure arginase-1 activity via urea production from no-surgery or surgery mouse cohorts (n = 4, Mann-Whitney U test). ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001. POD, postoperation day; MFI, mean fluorescence intensity.
Since the majority of Sx-MDSCs on POD1 were the PMN subtype, we measured their ability to suppress NK cell effector functions. Consistent with previous reports, on POD1, magnetically sorted PMN Sx-MDSCs significantly suppressed NK cell cytotoxicity (p = 0.03) compared with MDSCs isolated from non-surgically stressed mice (Figures 1E and 1F).12 Arg1 has been postulated to contribute to the suppressive function of PMN Sx-MDSCs through indirect depletion of arginine; therefore, we measured the expression and enzymatic activity of Arg1.16,29,30 We observed a >2-fold increase in Arg1 mean fluorescence intensity (MFI) in PMN Sx-MDSCs after surgery (Figure 1G; n = 8, p = 0.0002). Likewise, the lysates of Sx-MDSCs converted exogenous arginine into urea at a >2-fold rate compared with naive mice (Figure 1H; n = 4, p = 0.03).31 Therefore, the large increase in Sx-MDSCs after surgery coupled with increased Arg1 activity may heavily contribute to the substantial drop in arginine levels documented after surgery.15,17,32
Sx-MDSCs regulate systemic arginine bioavailability
Arginine, ornithine, citrulline, and argininosuccinate (Figure 2A), as well as other amino acids (Figure S2A), were measured at 0, 4, and 18 h postoperatively in the B16F10 surgery model and the 4T1 model (Figure S2B). Consistent with previous reports, we observed a significant decrease in blood arginine at 4 h postsurgery that had normalized by 18 h postsurgery (Figure 2B; n = 17, p = 0.0002).32 Importantly, increased ornithine levels were evident at 18 h postsurgery, suggesting the conversion of arginine into ornithine by Arg1. To determine whether Sx-MDSCs are responsible for the postoperative reduction in arginine, we depleted MDSCs before surgery and measured blood arginine levels at 4 and 18 h after surgery (Figure 2C). MDSC depletion attenuated the decrease in arginine concentrations after surgery without affecting preoperative arginine levels (Figure 2D), supporting the hypothesis that Sx-MDSCs are responsible for the postoperative reduction in systemic arginine availability. The NK cells isolated from MDSC-depleted mice had greater cytotoxic potential compared with NK cells isolated from vehicle control mice on POD1 (Figure 2E); however, cytotoxicity was not fully restored, suggesting that other mechanisms of NK cell dysfunction likely exist. Lastly, the prometastatic effects of surgery were completely abrogated on POD3 in MDSC-depleted mice (Figure 2F). Together, these findings suggest that Sx-MDSCs mediate arginine depletion and contribute to NK cell dysfunction postoperatively, and since arginine is necessary for NK cell function, we hypothesized that restoring the arginine imbalance after surgery will improve NK cell function and reduce metastases.21
Figure 2.
Surgery results in a rapid decrease in systemic arginine levels mediated by Sx-MDSCs
(A) The arginine cycle. (B) Amino-acid levels were quantified by liquid chromatography-mass spectrometry from murine dried-blood-spot samples taken at 0, 4, and 18 h postsurgery (n = 17 per group; Friedman test). (C) Anti-Gr1 (clone RB6-8C5) depletion timeline and experimental endpoints (top panels). Representative flow plots from spleens of vehicle- or anti-Gr1-treated mice gated on live CD11b+ CD3- cells (bottom panels; n = 5 per group). (D) Blood arginine levels from vehicle- or anti-Gr1-depleted mice at 0, 4, and 18 h postoperation (two-way ANOVA, Dunnett’s post test). (E) NK cells were isolated from MDSC-depleted and control animals after surgery and plated with Yac1 target cells for 4 h at increasing ratios in the presence of 100 U/mL IL-2. Cytotoxicity was measured against Yac1 target cells (n = 5 per group). (F) Depleting MDSCs prior to surgery attenuates postoperative metastases (n = 5 per group; Mann-Whitney U test). ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001. POD, postoperation day; SX, surgery.
Perioperative arginine supplementation controls metastatic disease
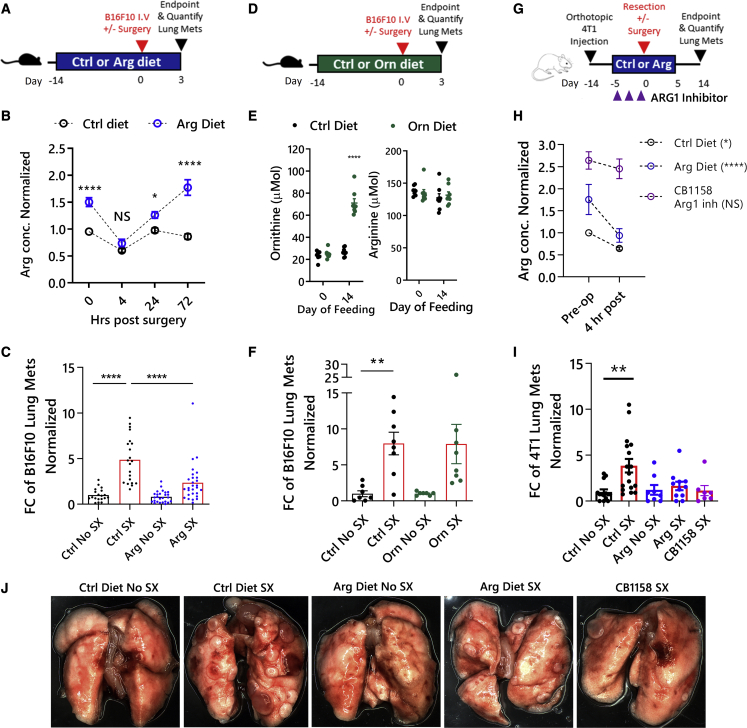
Although arginine can be produced de novo, in times of physiological stress, arginine must be obtained externally through dietary sources.33 Dietary arginine has been shown to have beneficial effects when given to patients perioperatively.25,34 Therefore, to assess the effects of a perioperative arginine diet on postoperative metastases, we fed mice either an AED or a control diet ad libitum (Figures 3A–3D). After 14 days of feeding in the B16F10 model, blood arginine levels from AED-fed mice were significantly higher than control-diet-fed mice (1.5-fold at 0 h; Figures 3B and S2B). Unexpectedly, at 4 h postsurgery, the AED-fed mice had similar levels of arginine as the control-fed mice, despite being significantly higher preoperatively. However, the AED-fed mice had a quicker return to baseline levels (pre-AED feeding) and were significantly higher (>1.5-fold) than control-diet-fed mice by 72 h postsurgery. While there were no significant differences in the number of Sx-MDSCs in the AED compared with control-diet-fed mice (Figure S3A), AED-fed mice had significantly reduced metastatic lung tumor burden following surgery (Figure 3C). Additionally, we assessed whether an ornithine-enriched diet could protect against surgery-induced metastases (Figure 3D). The ornithine-enriched diet was able to increase perioperative ornithine levels without affecting arginine, but there was no protection against surgery-induced metastases (Figures 3E and 3F). This suggests that arginine, rather than the downstream metabolite ornithine, is required postoperatively.
Figure 3.
Dietary arginine supplementation increases arginine levels and reduces postoperative metastases in two murine models of surgical stress
(A) Schematic of the arginine-diet regimen in B16F10 model of surgical stress. (B) Arginine concentrations from dried blood spots after 14 days of feeding, normalized to their baseline levels (n = 12–28 mice/group, mixed-effects model with Bonferroni’s multiple comparisons test). (C) Lung metastases were quantified on POD3 and normalized to the control no-surgery group (n = 19–28 mice from 6 experiments, Mann-Whitney test). (D) Schematic of ornithine-diet treatment in B16F10 model of surgical stress. (E) Ornithine and arginine concentrations following ornithine-enriched diet. (F) Lung metastases were quantified on POD3 and normalized to the control no-surgery group (n = 8/group, Mann-Whitney U test). (G) Schematic of 4T1 model of surgical stress. The ARG1 inhibitor CB1158 was given b.i.d. for 3 days pre-operatively with control diet (n = 6 mice/group; mixed-effects model with Bonferroni’s multiple comparisons test). (H) Arginine concentrations after pre-op treatment in Balb/c mice normalized to baseline. (I) Lung metastases were quantified on POD14 and normalized to the control no-surgery group (n = 8–17 mice from 3 experiments, Kruskal-Wallis). (J) Representative images of lungs from the orthotopic 4T1 surgery model. ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001. POD, postoperation day; ARG1, arginase-1; Mets, metastases; Arg, arginine; Orn, ornithine; Ctrl, control; ARG1 inh, CB1158; FC, fold change; SX, surgery; pre-op, pre-operation.
To corroborate this observation in a second model of surgical stress, we used a therapeutically relevant operation, feeding schedule, and mouse model that results in spontaneous pulmonary metastases in the 4T1 model in Balb/c mice (Figures 3G and S4). In this model, the AED was given perioperatively (5 days before and 5 days after surgery). This feeding regimen was sufficient to increase preoperative arginine levels of AED-fed mice 1.5-fold higher than control-diet-fed mice (Figure 3H) and had no effect on the primary 4T1 tumor growth (Figures S4A and S4B) but was able to prevent the increase in spontaneous metastases after surgery (Figure 3I). Furthermore, we assessed the ability of a non-specific, extracellular Arg1 inhibitor, CB1158, to attenuate postoperative metastases (Figure 3G). Notably, oral gavage of CB1158 twice daily (b.i.d.) was able to attain much higher levels of pre- and postoperative arginine levels compared with AED feeding, and there was no decrease in arginine at 4 h postsurgery (Figure 3H), further supporting that Sx-MDSC Arg1 regulates postoperative arginine levels. Lastly, CB1158 attenuated postoperative metastases to a similar extent as the AED (Figure 3I). Since these results show that therapies that improve arginine levels perioperatively can protect against postoperative metastases, we next sought to determine whether this was due to immunomodulatory effects on NK cell function.
Arginine accelerates NK cell recovery
To identify the cellular target mediating the anti-metastatic effects of dietary arginine supplementation, we depleted CD4 (clone: GK1.5) and CD8 (clone: 53-5.8) T cells (Figure S5) or NK cells (anti-NK1.1) in the B16F10 model of surgical stress. While T cell depletion did not alter the number of surgery-induced metastases, the anti-metastatic effect of the AED was completely abrogated in the absence of NK cells, suggesting an NK-dependent mechanism (Figure 4A). Since NK cells are critical for controlling metastases and are suppressed after surgery, we investigated whether the AED exerts its anti-metastatic effects by preserving NK cell function after surgery. To that end, we first quantified the amount of lung metastases at 4, 24, and 72 h after surgery in the AED-fed mice (Figure 4A). There was no significant difference in the number of seeding tumor cells (4 h) or early lung metastases (24 h) postsurgery between the AED and control-diet groups; however, lung metastases were reduced at 72 h postsurgery in the AED group (Figure 3, Figure 4B and 3C). Therefore, while the AED does not influence lung tumor seeding immediately after surgery (4 h), it appears to enhance tumor cell clearance in the postoperative recovery period.
Figure 4.
Arginine accelerates NK cell recovery from surgery-induced dysfunction
(A) Schematic of NK cell depletion (anti-NK1.1) in C57Bl/6 mice fed a perioperative arginine diet (left panel). Lung metastases were quantified on POD3 (right panel). (B) Experimental outline assessing NK cell function at 4, 24, and 72 h postsurgery (left panel). C57Bl/6 mice were fed an arginine-enriched diet or a control diet, and metastatic lung tumor burden was enumerated at 4, 24, and 72 h postoperation (right panel). (C) Pooled cytotoxicity data from NK cells isolated from control- or arginine-fed mice (left; n = 11–15 mice/group; normalized 8:1 ratio). Murine NK cell IFNγ from whole blood following overnight stimulation with NK Vue (right panel; n = 7–18 mice/group). (D) Proportion of splenic NK cells that are DNAM1+ (left) and NKG2D+ (right). ∗p ≤ 0.05; ∗∗∗p ≤ 0.001. POD, postoperation day; IFNγ, interferon gamma; Sac, sacrifice; Mets, Metastases; Arg, Arginine; Ctrl, control; Sx, surgery; Periop, perioperative.
To correlate this finding with postoperative NK cell dysfunction, we evaluated the impact of the AED on NK cell cytotoxicity (NKC) and on NK cell activity (NKA), which is the ability of NK cells to secrete IFN-γ after stimulation.5 Consistent with our observation that Sx-MDSCs accumulate (Figure S3) and arginine levels are reduced to a similar extent in both control- and AED-fed mice at 4 h postoperation (Figures 3B–3H), NKC and NKA were also suppressed to the same extent in both groups on POD1 (Figure 4C). However, both NKC and NKA were significantly improved in the AED-fed mice compared with the control-diet-fed mice by POD3, suggesting a beneficial effect of perioperative arginine supplementation on NK cell function (Figure 4C). In agreement with these functional assays, we also observed higher expression of the activating receptors DNAX accessory molecule 1 (DNAM1) and NKG2D on POD3, but not on POD1 (Figure 4D), further supporting that perioperative arginine supplementation does not prevent NK cell dysfunction but accelerates their recovery from surgical stress.
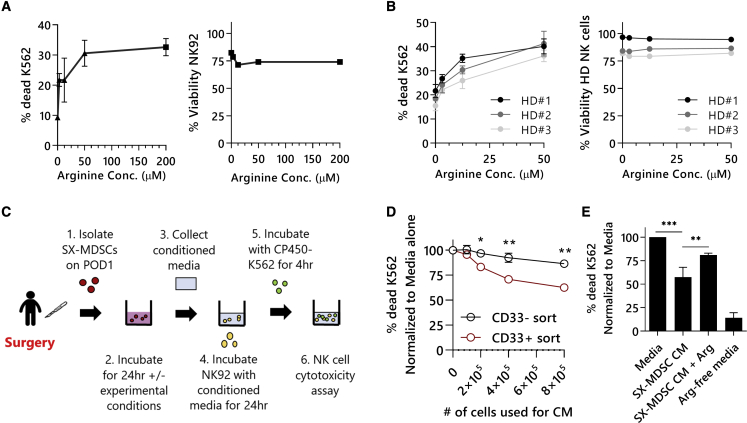
Arginine supplementation can restore the function of human NK cells cultured in Sx-MDSC conditioned media
Since arginine is required for NKC, we first sought to establish the minimal concentration of arginine required for NKC activity of the human NK cell line NK92-MI.22 By supplementing arginine-free media with increasing concentrations of arginine prior to measuring NKC against CP450-labeled K562 target cells, we observed that NKC is impacted when cultured in media containing <50 μM arginine (Figure 5A).35 Primary healthy human donor (n = 3) NK cells were similarly affected (Figure 5B). Next, we investigated whether Sx-MDSCs isolated from patients having cancer surgery could mediate NK cell suppression through the depletion of arginine from the culture media. To accomplish this, cell-free conditioned media was collected following the incubation of Sx-MDSCs in media containing the minimal concentration of arginine required for maximal NKC (50 μM) for 24 h (Figure 5C). Culturing NK92-MI cells in media conditioned by human Sx-MDSCs (CD33+ magnetic sort) isolated from peripheral blood mononuclear cells (PBMCs) on POD1 is sufficient to significantly reduce NKC. This inhibitory effect was not present when the media was conditioned with PBMCs depleted of Sx-MDSCs (CD33-) from the same patient with cancer on POD1 (Figure 5D). Together, these findings confirm that the suppressive activity of Sx-MDSCs is not strictly dependent upon direct cell contact, which is consistent with previous findings.36 To determine whether the inhibitory effects of Sx-MDSC under these conditions is due to a depletion of arginine from the culture media, we supplemented the conditioned media with exogenous 200 μM of arginine. Importantly, arginine supplementation is sufficient to significantly increase NKC under these conditions (Figure 5E; n = 3 experiments, p = 0.008).
Figure 5.
NK cells require arginine for cytotoxicity
NK cells were incubated for 24 h in arginine-free media reconstituted with increasing amounts of arginine. (A) NK92-MI cytotoxicity (left) and viability (right). Representative results from n = 2 separate experiments done in triplicates. (B) Healthy donor primary NK cells (n = 3) cytotoxicity (left) and viability (right). (C) Schematic of Sx-MDSC conditioned media (CM) transfer onto NK92-MI cells and subsequent NK cytotoxicity assay. (D) Increasing number of Sx-MDSCs (CD33+ sort) or CD33- cells from a POD1 patient with cancer were used to condition media. NK92-MIs were subsequently incubated with CM and tested for their cytotoxic potential (two-way ANOVA with Bonferroni’s multiple comparisons test, CD33- versus CD33+ at each cell concentration). (E) NK cell cytotoxicity following conditioned-media transfers. ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001. HD, healthy donor; POD, postoperation day; CM, conditioned media; Arg, arginine; Conc., concentration.
Arginine levels correlate to NK cell function in patients with colorectal cancer having surgery
The combined preclinical murine data and ex vivo co-culture experiments suggest that surgical stress results in the postoperative expansion of Arg1-expressing Sx-MDSCs, which suppress NKA in part through the depletion of systemic arginine concentrations. To explore whether Sx-MDSCs similarly impact arginine levels in patients having cancer surgery, we analyzed the amino-acid levels in a cohort of patients with colorectal cancer (n = 22) preoperatively on the morning of surgery (POD0), and on POD1, POD3, and POD35, as well as in healthy donors (n = 9; Figure S6). Consistent with our preclinical data, we observed a significant decrease in arginine on POD1 (Figure 6A). There was a significant increase in both M-MDSCs (p = 0.01) and PMN-MDSCs (p = 0.0007) on POD1, which remained elevated on POD3 (Figures 6B and 6C). M-MDSCs had reduced HLA-DR expression after surgery, which returned to preoperative levels by POD35 (Figure 6D). We observed a significant inverse correlation between the absolute number of Sx-MDSCs and the concentration of arginine on POD1 (Figure 6E; p = 0.03, R2 = 0.2). Additionally, we observed a significant positive correlation between arginine concentration and NKC on POD0 (Figure 6F; p = 0.006, R2 = 0.4).
Figure 6.
Preop and postoperative arginine levels correlate with NK cytotoxicity in patients with colorectal cancer
(A) Blood amino-acid concentrations from healthy donors (n = 9) and colorectal cancer (CRC) patients (n = 22). (B) Gating strategy used to identify Sx-MDSC subtypes from patient PBMC samples. (C) Absolute number of circulating M-MDSCs and PMN-MDSCs per μL blood. (D) HLA-DR MFI gated on M-MDSCs. (E) Correlations between the absolute number of circulating Sx-MDSCs versus arginine concentrations on POD0 (left) and POD1 (right). (F) Correlations between raw NKC and arginine concentrations on POD0 (left) and POD1 (right). ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001. POD, postoperative day; Lin, lineage; NKC, NK cytotoxicity; ARG, arginine concentration; PBMC, peripheral blood mononuclear cells.
Discussion
It is well established that surgery results in a systemic decrease in the blood concentration of arginine; however, the impact of reduced arginine after surgery on immunity and, in particular, metastases have not been investigated previously. Here, we have shown that following surgical insult, Sx-MDSCs expand and reduce blood concentrations of arginine via increased Sx-MDSC Arg1 activity. Different MDSC subsets have been reported to contribute to suppression via multiple mechanisms. M-MDSCs are known to express higher levels of iNOS and expand the greatest in the PBMC fraction in human blood compared with PMN-MDSCs.37,38 While iNOS is less effective than Arg1 in reducing arginine bioavailability, an increased expansion of M-MDSCs (Figure 6C) would still result in a reduction in arginine through iNOS as well.39,40 It is worth noting that in our murine models, postoperative arginine levels were normalized by POD1, but Sx-MDSCs remained significantly elevated until POD3. This discrepancy may be explained by the multiple ways that arginine can be restored, such as through de novo synthesis from citrulline, amino-acid recycling from the breakdown of proteins, or directly from dietary sources.38 Since Western diets can contribute up to 30% of the arginine in circulation, dietary arginine supplementation is an effective way of replenishing arginine bioavailability.41
Although the use of perioperative arginine has been recommended by product manufacturers for many years, it has not been universally adopted in surgical practice, and the mechanisms underlying its efficacy are poorly understood.42 Interestingly, despite the 1.5-fold increase in arginine after preoperative AED feeding, arginine levels were reduced to similar levels as the control-diet group immediately (4 h) after surgery. Since the number of Sx-MDSCs did not differ between groups, this suggests that either Sx-MDSCs have an excessive capacity to deplete arginine levels or the excess supplemental arginine is being metabolized by other immune cells. It has been shown that, following CD3/CD28 stimulation, T cells have enhanced arginine uptake compared with unstimulated T cells due to increased transcription and translation of the cationic amino acid transport-1 (CAT-1; gene SLC7A1).43 While the same conditions have not been replicated in NK cells, NK cells are known to metabolically adapt from glycolysis during quiescence to aerobic glycolysis and oxidative phosphorylation under stimulating conditions.44, 45, 46 Thus, it is possible that NK cells upregulate amino-acid transporters in response to surgical stress, and the supplemental arginine accelerates recovery and function by fueling their metabolic needs when they encounter micrometastases.
Interestingly, unstimulated NK92 cells have been shown to upregulate CAT-1 and CAT-2B transcription in the absence of arginine.22 Therefore, in times of immune stimulation or arginine depletion, immune effector cells respond by increasing their capacity for arginine uptake. This may explain why, in the absence of surgery, we observed no benefit of arginine supplementation on NK cell function. Since supraphysiological levels of arginine do not improve immune cell function beyond normal levels, immune cells may only need to meet a threshold of arginine, and any excess arginine would not necessarily lead to better immune cell function.16 This threshold for human NK cytotoxicity was between 12.5 and 50 μM of arginine in our ex vivo culture conditions. Given that the blood concentrations of arginine did not reach a level below this minimum threshold in control- or AED-fed mice, the reduction in arginine bioavailability alone after surgery cannot explain surgery-induced NK cell dysfunction.
Reduced arginine after traumatic stress/injury may be evolutionarily conserved to aid in wound healing via Arg1 to produce polyamines and proline, which enhance collagen synthesis and wound closure.47 Furthermore, Arg1 may be used by myeloid cells to limit the amount of available substrate for NO production, thus regulating the pro-inflammatory phase after surgery. This resembles what is observed in preterm newborns, where reduced arginine bioavailability prevents excessive pro-inflammatory responses to foreign pathogens newborns are exposed to at birth and during commensal colonization.48 Thus, in the context of cancer surgery where postoperative immune surveillance for micrometastases is so critical, it would be advantageous to quickly re-establish anti-tumor immunity.
It has been shown that post- and perioperative arginine supplementation schedules are associated with better postoperative outcomes compared with preoperative supplementation alone.25,26,42 This does not discredit the use of preoperative arginine supplementation, since patients with cancer present with lower arginine levels than healthy individuals.19 Furthermore, preoperative arginine levels were significantly correlated to preoperative NKC in our patient cohort. Since NKA is associated with cancer prognosis, and Arg1 expression increases with advanced stages of colorectal cancer, arginine status may also indicate the severity of cancer progression.49,50
The therapeutic necessity of preoperative arginine supplementation should still be confirmed because of the potential for fueling cancer outgrowth in tumors that are auxotrophic for arginine.51 Although we did not see an effect on primary tumor growth in our experimental murine models, arginine is known to have a role in tumor growth and proliferation.52 For this reason, an alternative cancer therapeutics strategy involves arginine deprivation via pegylated-Arg1.52,53 Amino-acid-deprivation therapies (mainly targeting glutamine, asparagine, or arginine) have been devised to starve growing tumors of essential amino acids.54 For example, acute lymphoblastic leukemias (ALLs) are auxotrophic for L-asparagine (they cannot synthesize adequate amounts) and therefore depend on exogenous sources for cell growth, and asparaginase therapy to limit the amount of available asparagine for cancer growth is an approved treatment option for patients with ALLs.55,56
The efficacy of perioperative dietary arginine was dependent on the presence of NK cells, as there was no effect on metastases when the AED was given in NK-cell-depleted mice. Other studies have demonstrated a role for arginine in the expression of cell surface receptors such as CD25 (IL-2 receptor) in neonatal lymphocytes57 and NKp46 and NKp30 on the human NK cell line NK92.22 Many of these receptors are already reduced in patients with cancer in the absence of surgical stress, and their reduction after surgery may be the combined result of reduced arginine levels and the increase in anti-inflammatory cytokines such as transforming growth factor beta (TGF-β), which has been shown to inversely correlate with the expression of NKG2D, DNAM1, NKp30, and NKp46 on NK cells.8,58, 59, 60, 61 In our study, an AED resulted in a quicker recovery of NKC, IFN-γ secretion, and the expression of the activating receptors DNAM1 and NKG2D.
If the efficacy of perioperative arginine supplementation is only seen postoperatively, then postoperative administration is essential. Unfortunately, dietary intake may be limited immediately following surgery, especially for procedures involving the gastrointestinal tract, where patients are often unable to tolerate a diet in the immediate postoperative period. In these situations, parenteral arginine administration (such as intravenous) or small-molecule inhibitors of Arg1 activity may be more effective in preserving arginine levels after surgery. The Arg1 inhibitor CB1158 has been shown to have antitumor and antimetastatic effects in tumor mouse models as a monotherapy or in combination with checkpoint inhibitors and is currently being evaluated in several clinical trials both in solid tumors and hematologic malignancies (ClinicalTrials.gov: NCT03837509, NCT03910530, NCT02903914, and NCT03314935).62 This remains a promising avenue for perioperative investigation; however, it should be noted that Arg1 plays an important role in wound healing via the production of ornithine, which is a substrate for polyamines and tissue repair, suggesting that supplemental ornithine might also be required.63
The immunosuppressive effects of surgery disarm the immune system’s ability to destroy invading tumor cells at metastatic sites, particularly through NK cell immunoparalysis. In our models of surgical stress, we have elucidated a role for Sx-MDSCs in facilitating the systemic reduction in arginine bioavailability, which is necessary for NK cell function. However, this is only one piece of a multifactorial response to surgery, as simultaneous immunosuppressive mechanisms in the postoperative period are likely at play. In addition to depleting arginine, MDSCs can suppress the immune system through the generation of ROS/NOS, cytokines (e.g., IL-10, PGE2), and direct inhibitory receptor engagement.13 Figure 5E suggests this because adding arginine back in the conditioned media only partially restored NKC. Although depleting Sx-MDSCs in our murine models was an effective strategy in reversing these effects, this is not a viable option for patients having surgery, as a specific marker for Sx-MDSCs has yet to be identified. Total ablation of CD33+ myeloid cells in patients with cancer would, in theory, prevent Sx-MDSC accumulation; however, this would also remove myeloid progenitor cells, which give rise to neutrophils that are vital to fighting postoperative infections.64 Therefore, as a non-invasive approach to restoring arginine balance postoperatively, we investigated the potential of perioperative arginine supplementation in a preclinical model of surgical stress. This study gives more support to the promising results on short-term surgical outcomes with arginine immunonutrition after surgery.25 Much is still unknown about the complex relationship between nutrition and immunity; however, through this study, we have outlined how perioperative arginine can positively impact innate immunity through improved NK cell recovery after surgery.
Materials and methods
Study design
The objective of this research was to determine a therapeutic effect for a perioperative diet enriched in arginine in reducing postoperative metastases. For all animal experiments, the sample sizes can be found within the figure legends. Two murine models were used that allowed us to study perioperative arginine supplementation from two unique perspectives. C57BL/6 mice were intravenously inoculated with B16F10 cells just prior to surgical stress, which allowed us to isolate the acute stress events of surgery on the immune system. The orthotopic 4T1 model in Balb/c mice was used as a therapeutically relevant surgical model whereby a primary tumor is resected after 2 weeks of growth. In both models, all samples were normalized to the average number of metastases quantified in the no-surgery control group. This allowed us to compare the increase in metastases, as a fold change, between different experimental runs. For the 4T1 model, we prospectively determined that lung metastases from mice with primary tumors ≥0.3g would be analyzed in a separate analysis from primary tumors <0.3g. For cytotoxicity and suppression assays, samples were plated in triplicates and replicated in subsequent experiments under the same conditions. To ensure we collected as much data as possible from our animal experiments, we would often perform many experimental endpoints simultaneously such as quantifying lung metastases, characterizing immune cells by flow cytometry, and collecting blood samples for amino-acid measurements.
We also used data that was collected from the phase II clinical trial (ClinicalTrials.gov: NCT02987296) to assess if arginine levels correlated with the number of Sx-MDSCs or NKC.
Mice and cell lines
Six-week-old female C57BL/6 and Balb/c mice were purchased from Charles Rivers Laboratory and housed in pathogen-free conditions. All studies were done in compliance with the guidelines of the Animal Care Veterinary Service facility (University of Ottawa). The B16F10LacZ melanoma cell line was obtained from Dr. K. Graham (London, ON, Canada) and maintained in cDMEM. 4T1, YAC-1, K562, and NK92-MI cell lines were purchased from ATCC and maintained in cRPMI.
Murine models of surgical stress and treatment regimens
The experimental metastasis model was performed in C57BL/6 mice by inoculating 3 × 105 B16F10LacZ melanoma cells via tail-vein injection 30 min prior to inflicting surgical stress by abdominal laparotomy and left nephrectomy. On POD3, lungs from euthanized mice were stained with X-gal to visualize the tumor metastases for quantification.8 The AED (4% arginine + Teklad Global 16% protein rodent diet, ENVIGO) in the B16F10 model were given for 14 days prior to surgery and on all PODs. Balb/c mice were used in the orthotopic 4T1 breast cancer model, which leads to spontaneous lung metastases. 1 × 105 4T1 cells were inoculated orthotopically and resected 14 days later, with or without major surgical stress (abdominal laparotomy and left nephrectomy). Mice were left to recover for another 14 days and then euthanized in order to quantify the macroscopically visible 4T1 lung metastases.65 The AED was given 5 days before and 5 days after surgery (perioperatively) in the 4T1 model. The Arg1 inhibitor CB1158 (Calithera Biosciences) was given b.i.d. by oral gavage starting 3 days before surgery.62
Flow-cytometry staining and analysis
Mouse spleen and saphenous blood samples were collected, and red blood cells (RBCs) were lysed with ammonia chloride potassium (ACK) lysis buffer. 1 × 106 cells were stained with Fixable Viability Stain 510 (BD Bioscience #564406) and Mouse BD Fc Block (BD Biosciences, #553142) prior to extracellular staining. Anti-mouse antibodies used in this study included CD3e (Clone 500A2, AF700 BD); CD11b (Clone M1/70, FITC, PeCy7 Biolegend); Ly6G (Clone 1A8; BD; PE-CF594, PeCy7); Ly6C (Clone AL-21 PerCP-Cy5.5 BD); Gr-1 (Clone RB6-8C5; Biolegend; FITC, Pe-Cy7); Arg1 (R&D Systems APC, cat. no. IC5868A); DX5/CD49b (PE BD); NK1.1 (PE-CF594 BD); DNAM-1/CD226 (BV421 Biolegend); and NKG2D/CD314 (FITC Biolegend). Samples were acquired with the BD Fortessa LSRII and analyzed with FlowJo v.10.
Isolation of surgery-induced MDSCs
Sx-MDSCs (CD11b+Ly6G+ cells) were isolated from mouse splenocytes on POD1 by magnetic separation using the Myeloid-Derived Suppressor Cell Isolation kit (Miltenyi #130-094-538) with a purity of >80%. Human Sx-MDSCs were isolated from human PBMC samples on POD1 by magnetic separation using anti-CD33 microbeads (Miltenyi #130-045-501), which results in a bulk population of Sx-MDSCs (Lin-CD33+CD14+CD15+). The Miltenyi autoMACS was used to obtain both sorted cell populations.
NK cell isolation
Murine NK cells were isolated with the EasySep Mouse NK cell Isolation Kit (StemCell #19855) via magnetic-bead separation from splenocytes. Human NK cells were isolated with the StraightFrom Whole Blood CD56 MicroBeads magnetic separation kit (Miltenyi #130-090-875).
NKC and MDSC:NK cell suppression assays
NK cell cytotoxic potential was determined by isolating NK cells from surgery or no surgery, treated or untreated, mice and incubating them for 4 h with CP450-labeled (eBioscience) NK-specific target tumor cells (either Yac1 or K562 for murine or human studies, respectively) in a 96-V bottom plate in triplicates. Following incubation, ethidium homodimer (Invitrogen, E1169) was added to each sample well before collecting and analyzing by flow cytometry. NKC was measured by quantifying the proportion of dead EtHD+CP450+ target cells.35 To measure the suppressive effects of MDSCs on NKC, MDSCs were isolated before surgery or on POD1 and seeded together with naive NK cells at increasing MDSC:NK cell ratios for 24 h at 37°C. The following day, CP450-labeled target cells were added to the MDSC:NK co-cultures for 4 h, and NKC was quantified as described above. In both assays, NK cells were treated with 100 U/mL IL-2 to maintain viability throughout the duration of the co-culture. In addition, 5,000 target cells were used to determine the number of effector or suppressor cells to plate to reach the desired suppressor-to-effector-to-target cell ratios.
Arg1 activity assay
Arg1 activity in MDSC lysates was determined by measuring the conversion of arginine to urea as previously described.31 Briefly, lysates, collected by lysing 1 × 105 magnetically sorted MDSCs with 0.1% Triton X-100 + 1X EDTA-free protease inhibitor, were heat activated by incubating at 50°C for 15 min. L-arginine was then added to the lysates for 2 h at 37°C, and Arg1 activity was quantified by the amount of urea produced (detected by adding α-isonitrosopropiophenone and measuring absorbance at 490 nm).
Peripheral blood amino-acid measurements
Murine and human blood samples (75 μL) were spotted onto Whatman 903 Protein Saver Cards (Sigma-Aldrich) and immediately stored at -20⁰C until analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS) at Newborn Screening Ontario, Children’s Hospital of Eastern Ontario as previously described.66 The amino acids measured included arginine, ornithine, citrulline, argininosuccinate, leucine, valine, phenylalanine, alanine, glycine, methionine, and tyrosine.
In vivo MDSC, NK cell, or T cell depletion experiments
MDSCs were depleted prior to surgery by 3 preoperative intraperitoneal (i.p.) injections of 100 μg/mouse InVivoMab anti-mouse Ly6G/Ly6C (Gr-1; RB6-8C5) (BioxCell; BE0075-5MG-A) on days -3 and -1 and the day of surgery. MDSC depletion was confirmed by flow-cytometry staining with the Ly6G clone 1A8 (PeCy7 Biolegend). NK cells were depleted by injecting 200 μg of anti-NK1.1 (PK136) i.p. on PODs -4, -1, and +1, as previously described.8 Double depletion of CD4 and CD8 T cells were performed on PODs -2, -1, and +1 via 100 μg of anti-CD4 (GK1.5) and 50 μg of anti-CD8β2 (53–5.8) injected i.p. Control mice were given 100 μg of immunoglobulin G (IgG) control i.p.
NKA assay
The murine NK-Vue kit (ATGen Canada/NKMax) was used to measure the amount of IFN-γ secreted (ELISA) by NK cells after a whole-blood stim using a proprietary NK cell-specific cytokine cocktail (Promoca).
Sx-MDSC conditioned media/arginine depleted media
RPMI-1640 without arginine, leucine, lysine, and phenol red (R1780, Sigma-Aldrich) was reconstituted with L-leucine (0.05 g/L, Sigma), L-lysine (0.04 g/L, Sigma), and increasing amounts of L-arginine (Sigma). Sx-MDSCs were purified from PBMCs using the CD33+ microbead magnetic sorting kit (Miltenyi) on POD1 and plated in reconstituted 50 μM arginine media for 24 h. Following the incubation, Sx-MDSCs were removed by pelleting and collecting the supernatants two times. NK92-MI cells were counted and plated in PBS and immediately spun down to remove the PBS. The NK92-MI cells were resuspended in the Sx-MDSC conditioned media/arginine depleted media and incubated for 24 h. CP450-labeled K562 target cells were then added to the plates, and NKC was measured by flow cytometry after 4 h of incubation.
Human patient dataset
All patients that participated in this study have given informed consent, and the research ethics board has approved our study under Ottawa Health Science Network Research Ethics Board (20160732-01H). For the human studies, we used the patient dataset from the PERIOP-02 clinical trial (ClinicalTrials.gov: NCT02987296) of patients undergoing abdominal surgeries for colorectal cancer.
Statistical analysis
Statistical tests were performed using GraphPad Prism 8 and are described within the figure legends. Generally, unpaired, non-parametric Mann-Whitney U tests were performed when comparing two groups (e.g., no surgery versus surgery). When assessing changes over time from the same animal (e.g., amino-acid concentration time course), a paired, non-parametric, Friedman test was used. For the Sx-MDSC depletion studies, we used a matched two-way ANOVA, with the Dunnett’s multiple comparisons test. Wilcoxon rank-sum tests were used to compare matched patient samples at different time points. Significance was assigned when ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
Data and materials availability
All data associated with this study are available in the main text or the supplemental information.
Acknowledgments
We would like to thank and acknowledge J.N., A.J., D.M.B., M.C., and M.S. for their assistance in quantifying lung metastases. We also thank Newborn Screening Ontario (CHEO), the University of Ottawa ACVS, and Flow Core for the use of their services. This work was supported by grants from the Canadian Institutes of Health Research, the Cancer Research Society, and the Terry Fox Research Institute. The Ottawa Hospital Academic Medical Organization and the Canadian Association of General Surgeons provided funding for the phase II clinical trial.
Author contributions
L.A. performed all the in vitro experiments with help from K.E.B., S.T.K., M.M., A.B.M., M.A.K., and L.-H.T. C.T.D.S. performed all the tumor cell inoculations, animal surgeries, and euthanizations. L.A., M.A.K., J.C.B., and R.C.A. compiled, wrote, and edited the final manuscript. L.A., L.-H.T., M.A.K., J.C.B., and R.C.A. formulated the design and execution of the study.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.05.024.
Supplemental information
References
- 1.Miller J.S., Lanier L.L. Natural killer cells in cancer immunotherapy. Annu. Rev. Cancer Biol. 2019;3:77–103. doi: 10.1146/annurev-cancerbio-030518-055653. [DOI] [Google Scholar]
- 2.Iannone F., Porzia A., Peruzzi G., Birarelli P., Milana B., Sacco L., Dinatale G., Peparini N., Prezioso G., Battella S., et al. Effect of surgery on pancreatic tumor-dependent lymphocyte asset: modulation of natural killer cell frequency and cytotoxic function. Pancreas. 2015;44:386–393. doi: 10.1097/mpa.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velásquez J.F., Ramírez M.F., Ai D.I., Lewis V., Cata J.P. Impaired immune function in patients undergoing surgery for bone cancer. Anticancer Res. 2015;35:5461–5466. [PubMed] [Google Scholar]
- 4.Reinhardt R., Pohlmann S., Kleinertz H., Hepner-Schefczyk M., Paul A., Flohé S.B. Invasive surgery impairs the regulatory function of human CD56 bright natural killer cells in response to Staphylococcus aureus. Suppression of interferon-γ synthesis. PLoS One. 2015;10:e0130155. doi: 10.1371/journal.pone.0130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angka L., Martel A.B., Kilgour M., Jeong A., Sadiq M., de Souza C.T., Baker L., Kennedy M.A., Kekre N., Auer R.C. Natural killer cell IFNγ secretion is profoundly suppressed following colorectal cancer surgery. Ann. Surg. Oncol. 2018;25:3747–3754. doi: 10.1245/s10434-018-6691-3. [DOI] [PubMed] [Google Scholar]
- 6.Seth R., Tai L.-H., Falls T., de Souza C.T., Bell J.C., Carrier M., Atkins H., Boushey R., Auer R.A. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann. Surg. 2013;258:158–168. doi: 10.1097/sla.0b013e31826fcbdb. [DOI] [PubMed] [Google Scholar]
- 7.Tai L.H., Zhang J., Scott K.J., De Souza C.T., Alkayyal A.A., Ananth A.A., Sahi S., Adair R.A., Mahmoud A.B., Sad S., et al. Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery-induced dysfunction in natural killer cells. Clin. Cancer Res. 2013;19:5104–5115. doi: 10.1158/1078-0432.ccr-13-0246. [DOI] [PubMed] [Google Scholar]
- 8.Tai L.-H., de Souza C.T., Bélanger S., Ly L., Alkayyal A.A., Zhang J., Rintoul J.L., Ananth A.A., Lam T., Breitbach C.J., et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013;73:97–107. doi: 10.1158/0008-5472.can-12-1993. [DOI] [PubMed] [Google Scholar]
- 9.Angka L., Khan S., Kilgour M., Xu R., Kennedy M., Auer R. Dysfunctional natural killer cells in the aftermath of cancer surgery. Int. J. Mol. Sci. 2017;18:1787. doi: 10.3390/ijms18081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talmadge J.E., Gabrilovich D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alazawi W., Pirmadjid N., Lahiri R., Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann. Surg. 2016;264:73–80. doi: 10.1097/sla.0000000000001691. [DOI] [PubMed] [Google Scholar]
- 12.Tai L.-H., Alkayyal A.A., Leslie A.L., Sahi S., Bennett S., Tanese de Souza C., Baxter K., Angka L., Xu R., Kennedy M.A., Auer R.C. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology. 2018;7:e1431082. doi: 10.1080/2162402x.2018.1431082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato M., Honda I., Suzuki H., Murakami M., Matsukawa S., Hashimoto Y. Interleukin-10 production during and after upper abdominal surgery. J. Clin. Anesth. 1998;10:184–188. doi: 10.1016/s0952-8180(97)00264-x. [DOI] [PubMed] [Google Scholar]
- 15.Ochoa J.B., Bernard A.C., O’Brien W.E., Griffen M.M., Maley M.E., Rockich A.K., Tsuei B.J., Boulanger B.R., Kearney P.A., Morris S.M., Jr. Arginase I expression and activity in human mononuclear cells after injury. Ann. Surg. 2001;233:393–399. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popovic P.J., Zeh H.J., 3rd, Ochoa J.B. Arginine and immunity. J. Nutr. 2007;137:1681S–1686S. doi: 10.1093/jn/137.6.1681s. [DOI] [PubMed] [Google Scholar]
- 17.Nijveldt R.J., Prins H.A., Siroen M., Rauwerda J.A., Teerlink T., van Leeuwen P. Low arginine plasma levels in patients after thoracoabdominal aortic surgery. Eur. J. Clin. Nutr. 2000;54:615–617. doi: 10.1038/sj.ejcn.1601062. [DOI] [PubMed] [Google Scholar]
- 18.Lind D.S. Arginine and cancer. J. Nutr. 2004;134:2837S–2841S. doi: 10.1093/jn/134.10.2837s. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- 19.Vissers Y.L., Dejong C.H., Luiking Y.C., Fearon K.C., von Meyenfeldt M.F., Deutz N.E. Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency? Am. J. Clin. Nutr. 2005;81:1142–1146. doi: 10.1093/ajcn/81.5.1142. [DOI] [PubMed] [Google Scholar]
- 20.Van de Velde L.-A., Subramanian C., Smith A.M., Barron L., Qualls J.E., Neale G., Alfonso-Pecchio A., Jackowski S., Rock C.O., Wynn T.A., Murray P.J. T cells encountering myeloid cells programmed for amino acid-dependent immunosuppression use rictor/mTORC2 protein for proliferative checkpoint decisions. J. Biol. Chem. 2017;292:15–30. doi: 10.1074/jbc.m116.766238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberlies J., Watzl C., Giese T., Luckner C., Kropf P., Müller I., Ho A.D., Munder M. Regulation of NK cell function by human granulocyte arginase. J. Immunol. 2009;182:5259–5267. doi: 10.4049/jimmunol.0803523. [DOI] [PubMed] [Google Scholar]
- 22.Lamas B., Vergnaud-Gauduchon J., Goncalves-Mendes N., Perche O., Rossary A., Vasson M.-P., Farges M.-C. Altered functions of natural killer cells in response to L-Arginine availability. Cell. Immunol. 2012;280:182–190. doi: 10.1016/j.cellimm.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Goh C.C., Roggerson K.M., Lee H.-C., Golden-Mason L., Rosen H.R., Hahn Y.S. Hepatitis C virus–induced myeloid-derived suppressor cells suppress NK cell IFN-γ production by altering cellular metabolism via arginase-1. J. Immunol. 2016;196:2283–2292. doi: 10.4049/jimmunol.1501881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kedia-Mehta N., Finlay D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019;10:2123. doi: 10.1038/s41467-019-10015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drover J.W., Dhaliwal R., Weitzel L., Wischmeyer P.E., Ochoa J.B., Heyland D.K. Perioperative use of arginine-supplemented diets: a systematic review of the evidence. J. Am. Coll. Surg. 2011;212:385–399.e1. doi: 10.1016/j.jamcollsurg.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Osland E., Hossain M.B., Khan S., Memon M.A. Effect of timing of pharmaconutrition (immunonutrition) administration on outcomes of elective surgery for gastrointestinal malignancies: a systematic review and meta-analysis. JPEN J. Parenter. Enteral Nutr. 2014;38:53–69. doi: 10.1177/0148607112474825. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y., Feng Y., Zhang Y., Zhu X., Jin F. L-Arginine supplementation inhibits the growth of breast cancer by enhancing innate and adaptive immune responses mediated by suppression of MDSCs in vivo. BMC Cancer. 2016;16:343. doi: 10.1186/s12885-016-2376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adiamah A., Skořepa P., Weimann A., Lobo D.N. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysis. Ann. Surg. 2019;270:247–256. doi: 10.1097/sla.0000000000003256. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez P.C., Ernstoff M.S., Hernandez C., Atkins M., Zabaleta J., Sierra R., Ochoa A.C. Arginase I–producing myeloid-derived suppressor cells in Renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronte V., Brandau S., Chen S.-H., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-Rosenberg S., et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J., Huang X., Yang Y. Myeloid-derived suppressor cells regulate natural killer cell response to adenovirus-mediated gene transfer. J. Virol. 2012;86:13689–13696. doi: 10.1128/jvi.01595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pribis J.P., Zhu X., Vodovotz Y., Ochoa J.B. Systemic arginine depletion after a murine model of surgery or trauma. J. Parenter. Enteral Nutr. 2012;36:53–59. doi: 10.1177/0148607111414579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez P.C., Ochoa A.C., Al-Khami A.A. Arginine metabolism in myeloid cells shapes innate and adaptive immunity. Front. Immunol. 2017;8:93. doi: 10.3389/fimmu.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buijs N., van Bokhorst-de van der Schueren M.A.E., Langius J.A., Leemans C.R., Kuik D.J., Vermeulen M.A., van Leeuwen P.A. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am. J. Clin. Nutr. 2010;92:1151–1156. doi: 10.3945/ajcn.2010.29532. [DOI] [PubMed] [Google Scholar]
- 35.Kandarian F., Sunga G.M., Arango-Saenz D., Rossetti M. A flow cytometry-based cytotoxicity assay for the assessment of human NK cell activity. J. Vis. Exp. 2017 doi: 10.3791/56191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiff A., Trikha P., Mundy-Bosse B., McMichael E., Mace T.A., Benner B., Kendra K., Campbell A., Gautam S., Abood D., et al. Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clin. Cancer Res. 2018;24:1891–1904. doi: 10.1158/1078-0432.ccr-17-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raber P., Ochoa A.C., Rodríguez P.C. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol. Invest. 2012;41:614–634. doi: 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rath M., Müller I., Kropf P., Closs E.I., Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front. Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez P.C., Zea A.H., DeSalvo J., Culotta K.S., Zabaleta J., Quiceno D.G., Ochoa J.B., Ochoa A.C. l-Arginine consumption by macrophages modulates the expression of CD3ζ chain in T lymphocytes. J. Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 40.Durante W., Johnson F.K., Johnson R.A. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenthal M.D., Carrott P.W., Patel J., Kiraly L., Martindale R.G. Parenteral or enteral arginine supplementation safety and efficacy. J. Nutr. 2016;146:2594S–2600S. doi: 10.3945/jn.115.228544. [DOI] [PubMed] [Google Scholar]
- 42.Weimann A., Braga M., Carli F., Higashiguchi T., Hübner M., Klek S., Laviano A., Ljungqvist O., Lobo D.N., Martindale R., et al. ESPEN guideline: clinical nutrition in surgery. Clin. Nutr. 2017;36:623–650. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Werner A., Amann E., Schnitzius V., Habermeier A., Luckner-Minden C., Leuchtner N., Rupp J., Closs E.I., Munder M. Induced arginine transport via cationic amino acid transporter-1 is necessary for human T-cell proliferation. Eur. J. Immunol. 2016;46:92–103. doi: 10.1002/eji.201546047. [DOI] [PubMed] [Google Scholar]
- 44.Gardiner C.M., Finlay D.K. What fuels natural killers? Metabolism and NK cell responses. Front. Immunol. 2017;8:367. doi: 10.3389/fimmu.2017.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loftus R.M., Assmann N., Kedia-Mehta N., O’Brien K.L., Garcia A., Gillespie C., Hukelmann J.L., Oefner P.J., Lamond A.I., Gardiner C.M., et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 2018;9:2341. doi: 10.1038/s41467-018-04719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almutairi S.M., Ali A.K., He W., Yang D.-S., Ghorbani P., Wang L., Fullerton M.D., Lee S.-H. Interleukin-18 up-regulates amino acid transporters and facilitates amino acid–induced mTORC1 activation in natural killer cells. J. Biol. Chem. 2019;294:4644–4655. doi: 10.1074/jbc.RA118.005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gould A.N., Candy G.P. In: L-arginine in Clinical Nutrition. Patel V.B., Preedy V.R., Rajendram R., editors. Springer International Publishing; 2017. pp. 577–588. [Google Scholar]
- 48.Badurdeen S., Mulongo M., Berkley J.A. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatr. Res. 2015;77:290–297. doi: 10.1038/pr.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imai K., Matsuyama S., Miyake S., Suga K., Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/s0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 50.Ma Z., Lian J., Yang M., Wuyang J., Zhao C., Chen W., Liu C., Zhao Q., Lou C., Han J., Zhang Y. Overexpression of Arginase-1 is an indicator of poor prognosis in patients with colorectal cancer. Pathol. Res. Pract. 2019;215:152383. doi: 10.1016/j.prp.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Patil M.D., Bhaumik J., Babykutty S., Banerjee U.C., Fukumura D. Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene. 2016;35:4957–4972. doi: 10.1038/onc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albaugh V.L., Pinzon-Guzman C., Barbul A. Arginine-dual roles as an onconutrient and immunonutrient. J. Surg. Oncol. 2017;115:273–280. doi: 10.1002/jso.24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riess C., Shokraie F., Classen C., Kreikemeyer B., Fiedler T., Junghanss C., Maletzki C. Arginine-depleting enzymes - an increasingly Recognized treatment strategy for therapy-refractory malignancies. Cell. Physiol. Biochem. 2018;51:854–870. doi: 10.1159/000495382. [DOI] [PubMed] [Google Scholar]
- 54.Fung M.K.L., Chan G.C.-F. Drug-induced amino acid deprivation as strategy for cancer therapy. J. Hematol. Oncol. 2017;10:144. doi: 10.1186/s13045-017-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egler R.A., Ahuja S.P., Matloub Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J. Pharmacol. Pharmacother. 2016;7:62–71. doi: 10.4103/0976-500x.184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boissel N., Sender L.S. Best practices in adolescent and young adult patients with acute lymphoblastic leukemia: a focus on asparaginase. J. Adolesc. Young Adult Oncol. 2015;4:118–128. doi: 10.1089/jayao.2015.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu H.-R., Kuo H.-C., Huang L.-T., Chen C.-C., Tain Y.-L., Sheen J.-M., Tiao M.-M., Huang H.-C., Yang K.D., Ou C.-Y., Hsu T.-Y. l -Arginine modulates neonatal lymphocyte proliferation through an interleukin-2 independent pathway. Immunology. 2014;143:184–192. doi: 10.1111/imm.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng Y.-P., Zhu Y., Zhang J.-J., Xu Z.-K., Qian Z.-Y., Dai C.-C., Jiang K.-R., Wu J.-L., Gao W.-T., Li Q., et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J. Transl. Med. 2013;11:262. doi: 10.1186/1479-5876-11-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rocca Y.S., Roberti M.P., Juliá E.P., Pampena M.B., Bruno L., Rivero S., Huertas E., Sánchez Loria F., Sánchez Loria F., Pairola A., Caignard A., Mordoh J., Levy E.M. Phenotypic and functional dysregulated blood NK cells in colorectal cancer patients can be activated by cetuximab plus IL-2 or IL-15. Front. Immunol. 2016;7:413. doi: 10.3389/fimmu.2016.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han B., Mao F.-Y., Zhao Y.-L., Lv Y.-P., Teng Y.-S., Duan M., Chen W., Cheng P., Wang T.-T., Liang Z.-Y., et al. Altered NKp30, NKp46, NKG2D, and DNAM-1 expression on circulating NK cells is associated with tumor progression in human gastric cancer. J. Immunol. Res. 2018;2018:6248590. doi: 10.1155/2018/6248590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niavarani S.R., Lawson C., Bakos O., Boudaud M., Batenchuk C., Rouleau S., Tai L.-H. Lipid accumulation impairs natural killer cell cytotoxicity and tumor control in the postoperative period. BMC Cancer. 2019;19:823. doi: 10.1186/s12885-019-6045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steggerda S.M., Bennett M.K., Chen J., Emberley E., Huang T., Janes J.R., Li W., MacKinnon A.L., Makkouk A., Marguier G., et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J. Immunother. Cancer. 2017;5:101. doi: 10.1186/s40425-017-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campbell L., Saville C.R., Murray P.J., Cruickshank S.M., Hardman M.J. Local arginase 1 activity is required for cutaneous wound healing. J. Invest. Dermatol. 2013;133:2461–2470. doi: 10.1038/jid.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hesselink L., Spijkerman R., van Wessem K.J.P., Koenderman L., Leenen L.P.H., Huber-Lang M., Hietbrink F. Neutrophil heterogeneity and its role in infectious complications after severe trauma. World J. Emerg. Surg. 2019;14:24. doi: 10.1186/s13017-019-0244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tai L.-H., Tanese de Souza C., Sahi S., Zhang J., Alkayyal A.A., Ananth A.A., Auer R.A. A mouse tumor model of surgical stress to explore the mechanisms of postoperative immunosuppression and evaluate novel perioperative immunotherapies. J. Vis. Exp. 2014:51253. doi: 10.3791/51253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Dirbashi O.Y., Fisher L., McRoberts C., Siriwardena K., Geraghty M., Chakraborty P. Identification of a neonate with hepatorenal tyrosinemia by combined routine newborn screening for succinylacetone, acylcarnitines and amino acids. Clin. Biochem. 2010;43:691–693. doi: 10.1016/j.clinbiochem.2009.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.