Abstract
Cardiovascular disease (CVD) has overtaken infectious illnesses as the leading cause of mortality and disability worldwide. The pathology that underpins CVD is atherosclerosis, characterized by chronic inflammation caused by the accumulation of plaques in the arteries. As our knowledge about the microenvironment of blood vessel walls deepens, there is an opportunity to fine-tune treatments to target the mechanisms driving atherosclerosis more directly. The application of non-coding RNAs (ncRNAs) as biomarkers or intervention targets is increasing. Although these ncRNAs play an important role in driving atherosclerosis and vascular dysfunction, the cellular and extracellular environments pose a challenge for targeted transmission and therapeutic regulation of ncRNAs. Specificity, delivery, and tolerance have hampered the clinical translation of ncRNA-based therapeutics. Nanomedicine is an emerging field that uses nanotechnology for targeted drug delivery and advanced imaging. Recently, nanoscale carriers have shown promising results and have introduced new possibilities for nucleic acid targeted drug delivery, particularly for atherosclerosis. In this review, we discuss the latest developments in nanoparticles to aid ncRNA-based drug development, particularly miRNA, and we analyze the current challenges in ncRNA targeted delivery. In particular, we highlight the emergence of various kinds of nanotherapeutic approaches based on ncRNAs, which can improve treatment options for atherosclerosis.
Keywords: nanotherapy, gene delivery, non-coding RNA, atherosclerosis, nanomedicine
Graphical abstract
Li et al. reviewed the progress, challenges, indispensable delivery systems, emerging clinical trials, and future directions of ncRNAs-based targeted drug delivery related to the clinical transformation of atherosclerosis. These reviews provide important insights into the application of nanomedicine in atherosclerosis and provide potential therapeutic strategies of this disease clinically.
Introduction
Cardiovascular disease (CVD) is one of the world’s most significant causes of mortality and morbidity. The American Heart Association has reported that approximately 19 million people worldwide have died of CVD, a growth rate of 18.7% in the past 10 years.1 Despite contemporary interventions and recently available secondary prophylaxis, recurrent cardiovascular events continue to affect 10% of patients discharged from hospitals following a myocardial infarction (MI).2 Atherosclerosis, a chronic systemic inflammatory disease that affects the large and middle arteries, is considered to be the main cause of CVD. Atherosclerosis is characterized by oxidized low-density lipoprotein (ox-LDL) accumulation and increased inflammatory cytokines in the vascular wall, the dysfunction of endothelial cells and vascular smooth muscle cells, and the formation of foam cells initiated by monocytes/macrophages.3, 4, 5 At present, the treatment of atherosclerosis mainly focuses on preventing plaque growth and instability and reducing the risk of plaque rupture. Statins and new lipid-lowering drugs can reduce LDL levels and have significant advantages in preventing non-fatal cardiovascular events6 but increase the risk associated with myopathy and a large number of residual problems.7,8 Antiplatelet adhesion drugs, such as aspirin, can effectively prevent the occurrence of vascular obstruction but also have side effects on the gastrointestinal tract. In addition, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce circulating LDL-C, effectively control low-density lipoprotein cholesterol, and reduce the risk of CVD by blocking PCSK9-mediated LDL receptor degradation. The high cost of PCSK9 inhibitors may hinder patients' long-term compliance with treatment.9 However, with the in-depth study of vascular diseases, there is an opportunity to fine-tune the treatment to more directly target the mechanism that drives atherosclerosis.
Non-coding RNAs (ncRNAs) are widely involved in key physiological processes, such as metabolism and immunity,10 and they have been used for treating and preventing various diseases. For example, nucleic acid-based therapy improved clinical outcomes in patients with inherited transthyretin-mediated amyloidosis and homozygous familial hypercholesterolemia.11 In addition, experimental nucleic acid vaccines against cancer and Zika virus have recently been developed, and nucleic acid vaccines against COVID-19 have been approved for clinical use.12 In addition, the onset and progression of cardiovascular illnesses are linked to ncRNAs.13 NcRNAs are involved in the progression of atherosclerosis (Figure 1). NcRNAs can be divided into the long and short type based on their length. Short RNAs (fewer than 40 base pairs [bp]) include microRNAs (miRNAs), small interfering RNAs (siRNAs), p element-induced weak testis (PIWI) interacting RNAs (piRNAs), and tRNA-derived small RNAs (tsRNAs).14 NcRNAs with a length of more than 200 bp are long RNAs. With the further study of non-coding nucleic acids, another special class of circular RNAs (circRNAs) was identified; these circRNAs are resistant to ectoenzyme-mediated degradation and may be more stable than most linear RNAs in cells. Large-scale studies have corroborated that ncRNAs are widely involved in metabolism, immunity, and other important physiological processes and are linked to the development and incidence of malignancies, cardiovascular disorders, neurological system diseases, nephropathy, and other illnesses.15 NcRNAs can regulate gene expression by directly combining with DNA or transcription factors at the transcriptional level and play a role by targeting mRNA, miRNA, or protein and regulating their activity and stability at the post-transcriptional level.16 Furthermore, ncRNAs can have an epigenetic effect by interacting with chromatin complexes and suppressing or activating gene expression regulation.17,18 However, ncRNAs also have characteristics that are disadvantageous for the treatment of CVD and clinical applications, such as low delivery efficiency, poor targeting specificity, and off-target activity.19
Figure 1.
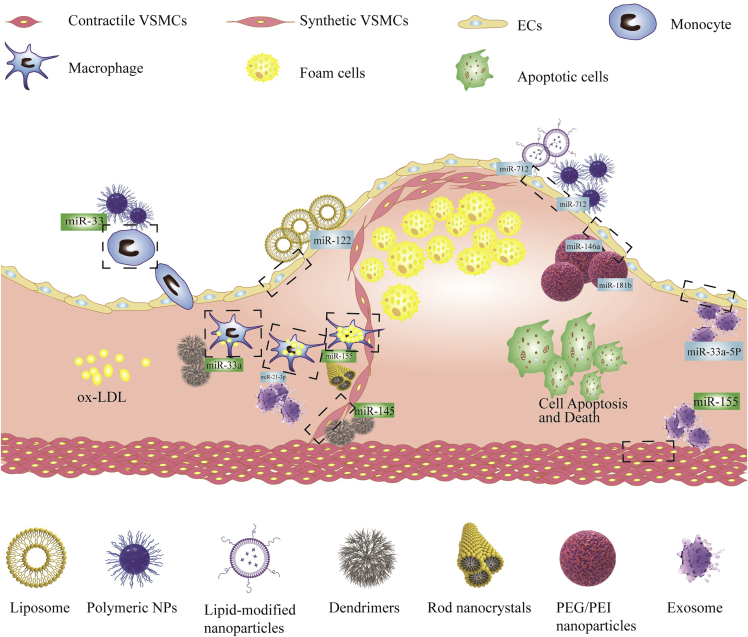
NcRNAs in the progression of atherosclerosis will benefit from nanoparticle (NP) delivery for gene therapy
With improvement in targeted and efficient drug delivery, the wide application of nanomedicines is expected to provide options for the treatment of atherosclerosis. Through the reasonable design of nanocarriers, nano-based targeted delivery systems enable improvement in the targeting selectivity of a drug and limit its distribution in the whole body and deliver the drug more efficiently to the diseased site, overcome biological obstacles, and improve the therapeutic drug index.20,21 Nanomedicine has not only made breakthroughs in the treatment of cancer but has also become the focus for the treatment of atherosclerosis.22, 23, 24 Several recent studies have shown that anti-inflammatory nanomedicines can significantly reduce inflammation and enhance the stability of atherosclerotic plaques.25,26 Reconstituted high-density lipoprotein (rHDL) nanoparticles (NPs) can target statins to deliver them to atherosclerotic plaques and reduce late atherosclerotic plaque inflammation by inhibiting the mevalonate pathway.27 The nuclear factor kappa-B (NF-κB) inhibitor celastrol loaded poly(ethylene glycol)-b-poly(propylene sulfide) (PEG-b-PPS) micelles can significantly reduce cytotoxicity, the secretion of tumor necrosis factor-α (TNF-α), and the number of inflammatory cells in atherosclerotic plaques.28 A novel ultrasound-targeted microbubble disruption (UTMD)-aided delivery system, which enables regional or tissue-specific gene delivery, can assist exosomal distribution of miR-21 against chemotherapy-associated cardiotoxicity.29 The drug-eluting stent (DES) and drug-eluting balloon models, which are sustainable drug release systems allowing the precise delivery of active substances to the lesion at the right dose, provided insight into non-coding nucleic acid delivery.30 An obvious disadvantage of the implant, however, is the potential for polymer buildup in the body, which requires surgical removal.31 At present, many nanomedicines are available for human use following successful clinical trials, such as Abraxane, Caelyx, Mepact, Myocet, Emend, and Rapamune. In addition to drugs/compounds, DESs and balloons, nucleic acids (DNA and RNA), and gene drugs have also shown potential for nanomedicines therapy. Furthermore, gene therapy based on nanomaterials has attracted extensive attention. According to a survey by Alhakamy et al.,32 5.0% of gene therapy clinical trials in the past 30 years focused on CVD, demonstrating that gene therapy is particularly attractive for CVD. In this article, we review the progress, challenges, indispensable delivery systems, emerging clinical trials, and future directions of ncRNAs-based targeted drug delivery related to the clinical transformation of atherosclerosis.
Results and discussion
RNA-based modification schemes
Several nucleic acid-based therapies have been developed, including (1) siRNAs, which have an interfering effect through mRNA degradation, (2) antisense oligonucleotides (ASO), which bind to complementary target mRNA sequences, thereby blocking protein translation, and (3) RNase H-dependent mRNA degradation directed by short chimeric antisense oligonucleotides (gapmers). Recently, these well-defined mechanisms of action have resulted in the development of several Food and Drug Administration (FDA)-approved drugs that have brought RNA-based therapeutics to the clinical stage.33,34
RNase H-mediated RNA degradation (gapmers) can potentially be a therapeutic modality for gene suppression.35 In the early gapmer studies, unmodified oligonucleotides were shown to be susceptible to degradation by nucleases.36 De Clercq et al. discovered phosphorothioate modification inhibited endogenous nuclease degradation and increased the pharmacokinetics via binding to serum proteins.37 Mipomersen, a gapmer developed by Ionis Pharmaceuticals (San Diego, CA, USA), targets apo-B-100 mRNA to treat familial hypercholesterolemia by lowering plasma LDL cholesterol levels.38 Most therapeutic oligonucleotides have phosphorothioate modifications, in which a sulfur atom replaces an oxygen atom in the phosphodiester group and the 2′-OH group in the ribose is replaced by 2′-O-methyl (2′-OMe), ensuring stability and increasing affinity for the target sequence.39 Various chemical modifications confer different biological properties to oligonucleotides. For example, locked nucleic acid oligos, phosphorodiamidate morpholino oligomers (PMOs), peptide-nucleic acid oligos (PNA), 2′-fluor (2′-F), 5-methylcytosine (m5C), and 2′-methoxyethyl (2′-MOE) are used to increase affinity to the target RNA. PNAs, 2′-MOE, and m5C reduce the chance of an oligonucleotide-induced innate immune response. SiRNAs complement target genes, effectively shutting them down and lowering the RNA and protein expression levels.40 SiRNAs with various chemical modifications, such as 2′-OMe, 2′-MOE, and 2′-F reduce immunogenicity while increasing affinity for target binding.41,42 The optimization of these chemical modifications opens up new avenues for the future development of siRNAs.
One of the biggest challenges in RNA drug delivery is that once in the body, the RNA is trapped in endosomes through cellular uptake and cannot reach the subcellular target location.43,44 Although the FDA has approved several drugs, such as mipomersen for hypercholesterolemia, they have also denied marketing due to significant liver damage.45 In summary, various RNA-based therapies are moving toward phase III trials. Novel nanomaterials are constantly updated and offer new options for developing RNA drugs in the future.
The superiority of ncRNA-based therapy
MiRNAs account for approximately 1%–5% of the human genome and are short, single-stranded ncRNAs. MiRNAs suppress protein synthesis by cleaving and degrading their target mRNAs. The degree of complementarity between the miRNA complex and its target mRNA determines the mechanism of action of silenced genes. This is because miRNA plays a vital role in silencing its target genes, affecting protein synthesis, and interfering with cell function. Recently, studies have shown that miRNA plays a role in the pathophysiology of atherosclerosis by regulating atherosclerotic genes and their role in regulating post-transcriptional gene expression.46,47 In vascular smooth muscle cells (VSMCs), miR-22 acts as a regulator of phenotype switching. Yang et al. found that overexpression of miR-22 can reverse the phenotypic transition of VSMC and inhibit the therapeutic potential of neointimal hyperplasia after arterial injury by targeting MECP2 (methyl-CpG binding protein 2), HDAC4 (histone deacetylase 4), and EVI1 (ecotropic virus integration site 1 protein homolog).46 Using Framingham risk scores, Wang et al. estimated that patients with lower levels of circulating miR-204 had a higher risk of developing CVD.48 The expression of macrophage calcineurin-nuclear factor of activated T cell (NFATc3), a negative regulator of atherosclerosis, was decreased in macrophages in human and mouse atherosclerotic lesions. The NFATc3 axis in macrophages upregulates miR-204 to lower SR-An and CD36 levels, reducing foam cell production and atherosclerosis. This suggests that the NFATc3/miR-204 axis might be a possible therapeutic target for anti-atherosclerosis.49 Interestingly, MRG-110 is an antisense oligonucleotide targeting miR-92a-3p.50 A single dose of MRG-110 was effective in reducing miR-92a levels in human peripheral blood. Anti-miR-92a has also been reported to confer atheroprotective effects.51 This is the first clinical trial conducted in patients with myocardial ischemia. Thus miR-92a plays an important role in atherosclerosis.
LncRNA is a biologically active RNA with a length of more than 200 bp. LncRNAs can act as a sponge of other transcripts and miRNA molecules and can interact with RNA, DNA, proteins, or RNA binding proteins, thus becoming a new molecule in vascular inflammation and CVD. The discovery of lncRNAs that are expressed in the intima of atherosclerotic lesions may contribute to an in-depth understanding of their role in the formation of atherosclerosis and may reveal the mechanism of apoptosis in advanced lesions. Moreover, studies in non-human primates showed that the liver-expressed liver X receptor (LXR)-mediated deletion of lncRNA CHROME increased post-transcriptional regulation of miR-27b, miR-33a, miR-33b, and miR-128, which exhibited atheroprotective effects through the upregulation of cholesterol efflux and HDL biogenesis.52 Recent studies suggest that lncRNA-H19 plays a key role in atherosclerosis regulation.53 The pro-angiogenic, pro-adipogenic, pro-inflammatory, pro-proliferative, and anti-apoptotic activities of lncRNA-H19 are involved in the initiation, development, and progression of atherosclerosis.54, 55, 56, 57, 58 In the vascular system, the lncRNA MALAT1, which is highly expressed in endothelial cells, can regulate vessel growth and function. Sebastian et al. found that ApoE−/− Malat1−/− mice on a high-fat diet had increased atherosclerotic plaque volume and infiltration of inflammatory CD45+ cells. The deletion of the MALAT1 gene leads to the aggravation of atherosclerotic lesions. Surprisingly, the anti-inflammatory effect of MALAT1 is achieved in part by reducing the expression of miR-503. Furthermore, reduced MALAT1 expression in human plaque is linked to advanced plaque stage and a poor prognosis.59 It has also been reported that even on a regular diet, MALAT1 deficiency produces severe immune-mediated atherosclerosis in ApoE−/− mice.60
CircRNAs are a newly identified type of endogenous transcripts. Because they do not have 3′- and 5′- ends, they can form covalently closed continuous loops.61 Compared with linear transcripts, they have higher stability, which also makes them excellent candidates for diagnostic biomarkers and molecular therapeutics. There is increasing evidence that the abnormal expression of circRNAs is related to neurodegenerative diseases and cancer.62 By serving as an miRNA sponge, holding RNA binding protein, and influencing alternative splicing and parental gene expression, circRNA is a significant regulatory factor at the transcriptional and post-transcriptional stages.63,64 Chen et al. discovered that the circRNA hsa circ 0003575 was significantly upregulated in ox-LDL-induced human umbilical vein endothelial cells and that silencing this circRNA increased proliferation and angiogenesis.65
PiRNA is a novel kind of short RNA with a length of around 24–32 nucleotides that can create a piRNA silencing complex by interacting with members of the PIWI protein family that are preferentially produced in germ cells (piRISC). The research on piRNA is mainly focused on cancer therapy. With more in-depth research, it has been found that piRNA exists widely in a variety of mammalian tissues and participates in a variety of diseases. Li et al. reviewed piRNA as a potential biomarker of CVD from the point of view of biogenesis, characteristics, biological function, and regulatory mechanism, which provided new insights.66
TsRNAs are short RNA fragments formed by specialized ribonucleases, such as dicer and angiogenin (ANG), clipping on the rings of transfer RNAs (tRNAs) in certain cells and tissues under specific circumstances. tsRNAs can be divided into two groups based on where the splicing site is located: tRNA-derived stress-induced RNAs (tiRNAs) and tRNA-derived fragments (tRFs). Recent studies have analyzed the expression patterns of tiRNA in atherosclerotic patients and identified 315 differentially expressed tiRNAs. tRF-Gly-GCC regulates cell adhesion, proliferation, migration, and phenotypic change in human umbilical vein endothelial cells and VSMCs, suggesting that it may have a role in the pathophysiology of atherosclerosis.67 They were lauded for their potential as indicators and therapeutic targets in clinical applications. Although our understanding of tsRNAs is still in its early stages, the unique properties in this sector are worth investigating.
As a potential new molecule for disease diagnosis and treatment, when compared with standard medications, small nucleic acid pharmaceuticals have technical advantages for targeting proteins. (1) Small nucleic acid drugs artificially designed according to target RNA have strong target specificity. (2) Preclinical research and the development of small nucleic acid drugs first determine the gene sequence and make a reasonable design for the disease gene to silence the gene targeting to avoid blind development and save on research and development time. (3) Candidate targets are abundant as small nucleic acid drugs can break through some special targets that are difficult to have a curative effect. (4) Small nucleic acids are easy to transform from the laboratory to the clinic, and the development of RNA interference technology is well researched.68 Furthermore, using a mouse MI model, a biomimetic exocrine nanocomplex delivered microRNA accurately and with a therapeutic effect. This development paves the way for nucleic acid therapy based on biomimicry. In summary, small nucleic acid drugs have become a research hotspot as a new target molecule for CVD.
Challenges of ncRNA delivery
The safety of gene therapy is a top priority. Although small nucleic acid drugs offer a wide range of therapeutic promises, each of these RNA regulators has its own set of problems that must be overcome to develop appropriate RNA treatment regimens. The clinical transformation of RNA-based therapies is hampered by problems related to specificity, delivery, and tolerability (shown in Table 1). At present, targeted delivery of gene drugs is an important method to solve these problems.
Table 1.
Challenges of ncRNA delivery
| Problem | Reason | Solution | References |
|---|---|---|---|
| Chemical instability characteristics of ncRNA | Physical and chemical properties Easily degraded by endogenous nuclease |
Chemical or structural modification | Lee et al.; Sarett et al.; Whitehead et al.; Alexis et al.; Broderick et al.; Wilson et al.; Kooi et al.; Hacein-Bey-Abina69, 70, 71, 72, 73, 74, 75, 76 |
| Extracellular and intracellular barriers | Irregular and heterogeneously high permeability of vascular tissue ncRNAs are susceptible to phagocytosis by late endosomes |
Specifically targeting the vascular system and increasing the solubility of drugs Neutrally charged ionizable lipids can be used to induce lysosomal rupture, releasing ncRNAs |
Wang et al.; Danquah et al.; Akhtar et al.; Semble et al.77, 78, 79, 80 |
| The hurdle of immunogenicity | RNA interference, NF-κB activation, and pro-inflammatory cytokine production Type I interferon response |
Third-generation modifications, such as PMOs | Winkle et al.; Barton et al.; Fabbri et al.; Cirak et al.; Sledz et al.17,81, 82, 83, 84) |
| Off-target effects | Induction of interferon response Partial complementarity of nucleic acid and unexpected targets |
Specific targeted receptor ligand | Hanagata et al.; Jackson et al.; Juliano and Carver85, 86, 87) |
Chemical instability characteristics of ncRNA
RNA delivery has broad prospects, but RNA-based therapy is still challenging due to problems related to the stability and delivery of ncRNAs. Subcutaneous injection of ncRNA can enter the circulation through lymphatic drainage of capillaries or interstitial space, bypassing the first-pass effect.69 ncRNAs are unstable in body fluid in their natural state, and when they enter the bloodstream, they are quickly destroyed by endogenous nucleases. The kidneys filter the degraded ncRNAs, which are subsequently taken up by phagocytes or the reticuloendothelial system. The half-life of unedited siRNA in serum has been observed to be around 20 min.70 To avoid phagocytosis, the lipophilicity and size of the carrier must be closely reviewed.
Because of their unfavorable physical and chemical properties, such as a considerable molecular weight, poor stability, negative charge, and significant structural rigidity, ncRNAs are challenging to transport to the cytoplasm.71 Unlike locally targeted drug delivery, many tissues can only be accessed through a blood-based medication delivery method. ncRNA preparations used in systemic systems face several obstacles in vivo before reaching the target cells. After entering the blood, ncRNAs must escape renal filtration, phagocyte absorption, serum protein buildup, and endogenous nucleic acid enzymatic destruction.72 The half-life of naked ncRNA in serum is short, and chemical or structural modifications can improve the stability of ncRNA in blood.88 Modification of siRNAs with hydrophilic and neutral chemicals like polyethylene glycol or non-anionic surfactants, for instance, significantly extends their circulation period.89 Compared with the naked version, the half-life of the modified siRNAs can be up to 20 times longer.90,91 Despite the benefits of chemically modified miRNA modulation techniques, cellular uptake, biodistribution, off-target effects, and toxicity are still barriers to miRNA therapeutics. miRNA mimics and anti-miRs are attracted to negativity due to the phosphate groups on the backbone, making it challenging to leave the bloodstream and easily penetrate membranes.73 To overcome these obstacles, viral and non-viral nucleotide delivery systems have been investigated as delivery vehicles for ncRNA. However, these methods are not without flaws. Many virus particles, such as the adenovirus, cause a severe inflammatory reaction with elevated cytokine levels, resulting in shock and liver problems.74 As opposed to viral vectors that carry ncRNA in the form of viral genomes, non-viral delivery options have been used to deliver native ncRNAs, including bacteria, exosomes, virus-like particles, and biological liposomes.92,93 The natural properties of these biological agents for specific gene delivery have accelerated the development of delivery technologies. Non-viral vectors are non-infectious, have no vector capacity limitations, offer a wide source of materials, have a controlled chemical structure, and are easy to prepare in large quantities. Therefore, viral vectors have an irreplaceable role in the targeted transfer of expression plasmids, antisense oligonucleotides, and antisense expression plasmids to eukaryotic cells.94,95 However, the transduction efficiency of non-viral vectors is low, the target gene is only expressed instantaneously, and the particles of their delivery systems are large, which can easily trigger immune reactions and be cleared by the organism.96 The most critical aspect impacting ncRNA treatments is selecting the optimal delivery platform to enhance circulation durability, advance the spatial distribution of ncRNAs, and achieve effective endosomal escape.
Extracellular and intracellular barriers
siRNAs and miRNAs must reach the cytoplasm of target cells to fully exploit the capabilities of RNA therapy. These RNA molecules must travel through the tight junction of the vascular endothelium before being transported to the target site. The cross-membrane trafficking of siRNAs in tumor tissue is more straightforward than in healthy tissue due to the leaky, irregular capillaries at tumor locations.77 However, there are also physiological obstacles in tumor microvascular systems, such as irregular tissue in blood vessels and heterogeneously high permeability.78 Methods have been developed to circumvent these obstacles, such as targeting tumor vasculature and enhancing drug solubility in water.78 Upon entering the cell, ncRNAs are transported, beginning with the early endosome, which merges with the sorting endosome to transfer the contents to the late endosome.79 ncRNAs must exit the endosome in the cytoplasm through endosomolysis or by rupturing the lysosome membrane to reach the RNA-induced silencing complex. Neutrally charged ionizable lipids can be used for lysosome rupture, thereby releasing the siRNA.80
The hurdle of immunogenicity
As a virus defense mechanism, our immune system recognizes foreign RNA in different ways. Toll-like receptors (TLRs) 3, 7, and 8 in endocytosis mediate external recognition, while receptors in the cytoplasm such as EIF2AK2, RIG1, IFIH1, and NOD1/2, promote intracellular recognition.17 The first reported immune activation originated from RNA interference (RNAi). TLR signaling is the most common pathway that recognizes RNA therapeutics and is facilitated through the activation of various pathways by MyD88, including NF-κB, the production of pro-inflammatory cytokines (IL-6, IL-8, IL-12, and TNF), or the activation of type I interferons, which trigger diverse downstream immune responses.81 Multiple tumor-secreted miRNAs have been found to serve as TLR agonists, suggesting that endogenous miRNAs may also do so. Exosome-secreted miR-21 and miR-29a from lung tumor cells may enter the endosomal compartment of surrounding macrophages and activate TLR8 (and TLR7 in animals), inducing NF-κB activity and inflammatory cytokine production.82 Third-generation modifications are currently used to solve the immunogenicity of RNA therapy by neutralizing the charge of small RNA therapies.97 Compounds like PMOs can further reduce immunogenicity, thus preventing them from interacting with proteins, including TLRs.83 Although some progress has been made in the immunogenicity of RNA therapy, all obstacles have not been overcome, and there are still many areas to be explored.
Off-target effects
Owing to their complex characteristics, large, polar, and highly charged duplex RNA contained in nucleic acid-based drugs can cause off-target effects in various ways. Proteins on the cell surface and within cells can bind nucleic acids causing immune regulation, including the induction of the interferon response. However, antisense oligonucleotides that stimulate the immune response can be developed as vaccine adjuvants.85 The partial complementarity of nucleic acids to unintended targets is a fundamental reason for the off-target effects of nucleic acid drugs. SiRNA demonstrates off-target silencing of a substantial number of unwanted transcripts with partial sequence similarity to miRNA.86 This process has unforeseeable cellular repercussions, including significant phenotypic toxicity.
Additionally, siRNA is more easily destroyed in the cytoplasm, contributing to off-target silencing.98 It has been demonstrated that the 5′-phosphorylation state of the siRNA strand is a determinant of its involvement in silencing and that adding other chemical components to one or both strands can also reduce specific off-target effects. For example, replacing the 5′-phosphorylation group on the siRNA strand with methyl diminished its involvement in silencing.99 Undesirable targeting can also occur when RNA therapeutics are administered systemically. For example, miR-34a exerts anti-atherosclerotic effects by regulating NF-κB signaling in T cells and ATP-binding cassette transporter A1 (ABCA1) in macrophages.100,101 However, David et al. found MRX34, a liposomal miR-34a mimic, in leukocytes.102 Although in vitro research has shown that the miR-34a mimic affects macrophage and T cell chemokine patterns, the in vivo absorption rate remains unknown.103
Another critical factor to consider is the dose of the therapeutic RNA. Compared with targeted gene silencing, which requires a near-perfect sequence match between the siRNA guide strand and the target mRNA, the off-target effect was more responsive to the siRNA dose.104 It is apparent that as targeted delivery technologies progress, challenges like target selection, effector efficacy, delivery vehicle design, and off-target consequences need to be addressed. Nanoproducts are an evolving component in the shift to effective and safe atherosclerosis therapies. Unfortunately, dosing represents a significant limitation in ncRNA therapy, and clinical studies have rarely shown the precise dose of RNA therapy successfully delivered to the target cells.105 Moreover, a high concentration of synthetic nucleic acid was transfected into cells by a cationic lipid delivery vector, which may affect cell proliferation in vitro.87 This is another possible cause of off-target effects. In conclusion, the relationship between dose and targeting in the clinical setting remains to be explored.
Targeted delivery system
NPs containing ncRNA are utilized to deliver naked or chemically modified ncRNA to tissues and cells that are not receptive to naked or chemically modified ncRNA delivery. In principle, delivery vehicles are designed to enhance absorption into the target tissue of interest while also protecting ncRNA payloads, inhibiting nonspecific distribution, and improving the therapeutic efficacy and safety of therapeutic molecules.106 In the following sections, we introduce the most commonly used nucleic acid drug delivery carriers, including NPs (for example, polymers, ceramics, and metals), liposomes, micelles, and dendrimers (Figures 1 and 2).
Figure 2.
Schematics of ncRNA-based nanomaterials for atherosclerosis therapy
Liposomes
Liposomes, both unilamellar and multilamellar, are often utilized for pharmacologically targeted delivery.107 Certain materials can create liposomes in an aquatic environment, in which a lipid bilayer forms a sphere with an aqueous center. Liposomes are lipophilic substances that can contain both hydrophilic and ionic molecules. Liposomes, like biofilms, can be absorbed by fusing with the plasma membrane; once within the cells, endocytosis treats the liposomes, and the genetic material is released into the cytoplasm. Cationic liposomes can generate "lipoplexes" by forming complexes with negatively charged anions, such as siRNAs and polycations, which improve biocompatibility and make clinical applications easier.108,109 Stable nucleic acid-lipid particle (SNALP) formulations have recently been shown to be effective in vivo in a number of animal models. Morrissey et al. found that HBV replication could be stopped by delivering an siRNA-SNALP combination that targeted HBV RNA.110 Liu et al. proposed that using a polyethylene glycol liposome delivery system, the expression of ICAM-1 could be reduced by delivering miR-122 mimic, which could maintain vascular integrity after temporary middle cerebral artery occlusion.111 In addition, after middle cerebral artery occlusion, the miR-122 mimic also reduced the expression of direct and indirect target genes in blood, which are thought to alter cell adhesion, diapedesis, leukocyte extravasation, eicosanoid, and atherosclerosis signaling. Therefore, the polyethylene glycol liposome delivery system has become a promising candidate for the treatment of atherosclerosis.111
Even though liposomes are one of the most widely used nucleic acid delivery methods, there are concerns over their safety in therapeutic applications. Certain cationic lipid particles have been shown to be cytotoxic in vitro and in vivo.112,113 On the other hand, naked chitosan liposomes are incompatible with biological body fluids, which will lead to particle degradation and reduce efficiency. Therefore, how to further reduce the side effects of liposomes in vivo and improve bioavailability has become the focus of current research.
Polymeric NPs
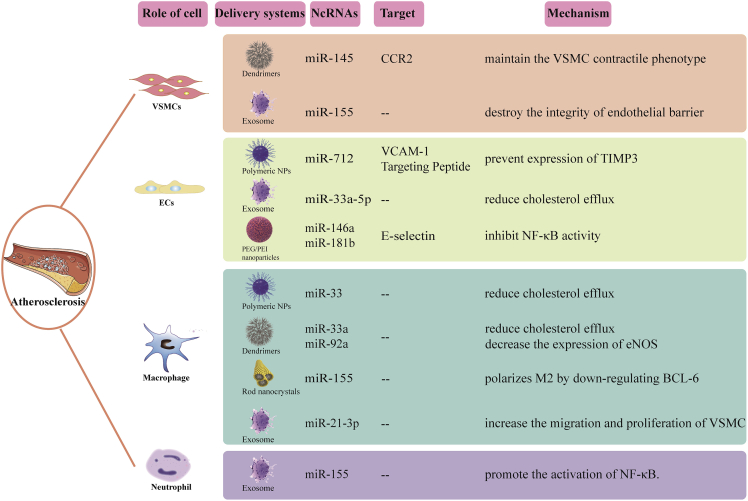
Polymeric NPs and liposomes are commonly employed in nanomedicine research because they are biodegradable and biocompatible polymeric materials that are either hydrophobic, hydrophilic, or amphiphilic in nature. Most polymer NPs are widely known to be based on poly(lactic acid), poly(glycolide), poly(caprolactone), and polylactic acid-co-glycolide. Polymer NPs have varying size, shape, and drug release qualities and are thus utilized as drug delivery carriers in a wide range of therapies. A novel polymer that can target vascular cell adhesion molecule-1 protein (β-amino ester) NP specifically delivers anti-microRNA-712 (anti-miR-712) to inflammatory endothelial cells and reduces the high expression of pro-atherosclerotic miR-712, thus protecting from atherosclerosis.114 Nguyen et al. developed chitosan NPs by ionic gel method using tripolyphosphate as a cross-linking agent, which can transfer exogenous miR-33 to initial macrophages and reduce the expression of ABCA1, showing reduced cholesterol efflux.115
Dendrimers
Dendrimers are chemically synthesized macromolecules with clear dendrimer structures. In general, dendrimers consist of a central core and two or more reactive functional groups that are covalently linked to repeating chemical components (generations). They can encapsulate or covalently bind various therapeutic and imaging agents in their dendrites or surfaces for various biomedical applications.116 Deborah et al. reduced the development of atherosclerosis by synthesizing miR-145 micelles targeting CC chemokine receptor 2 and inhibiting plaque proliferative cell types derived from VSMC.117 Moreover, the use of targeted polyelectrolyte composite micelles to target maladjusted miRNA in atherosclerosis can protect atherosclerosis. For example, REKA-bound polyelectrolyte micelles are used to deliver miR-33a inhibitors to regulate cholesterol efflux from macrophages. VHPKQHR/REKA coupled micelles were used to deliver miR-92a inhibitors, upregulate KLF2, and play an anti-inflammatory role and reduce atherosclerosis.118
Rod nanocrystals
Rod-shaped NPs have been shown to have better cellular absorption, intracellular processing, and transit ability through tissues and organs than spherical NPs.119 The electrical and thermodynamic properties of nanocrystals show a strong size dependence, so these properties can be controlled through a detailed manufacturing process.120 Anti-inflammatory baicalein nanorods are used as anti-miR-155 intracellular delivery carriers for combined therapy for atherosclerosis. Anti-miR-155 intracellular delivery polarizes M2 by downregulating BCL-6, while baicalein quickly inhibits inflammation mainly by inhibiting the secretion of NF-κB.121 As a promising preparation method, nanocrystals can improve the efficacy of anti-inflammatory drugs because they are released continuously over time after internalization.
Lipoprotein-modified NPs
Lipoprotein-modified NPs are organic NPs derived from HDL. While HDL is well recognized for its involvement in cholesterol transport, it can also serve as a carrier for systemic medication and imaging agent delivery.122 Azadeh et al. made coated cationic lipid particles (CCL) with anti-miR-712 in the core, decorated with 5 mol % peptide (VHPK) to target vascular cell adhesion molecule-1. The results showed that the delivery of VHPK-CCL-anti-miR-712 effectively downregulated the expression of miR-712 and saved the expression of the tissue inhibitor of metalloproteinase-3 and inhibited atherosclerosis in ApoE−/−mice.123
Polyethylenimine-based delivery system
Polyethylenimine (PEI) is a commonly used and researched polymer for gene delivery. PEI is positively charged in the physiological environment due to protonation of the amine groups and may thus be employed to condense nucleic acids. PEI and nucleic acid cationic polyplexes often retain a net positive charge (ζ-potential), facilitating interactions with negatively charged polysaccharides on the cell surface.124 Ma et al. packaged miR-146a and miR-181b in polyethylene glycol-polyethyleneimine (PEG/PEI) NPs and loaded them into E-selectin targeted multistage carrier (ESTA-MSV) particles. After intravenous injection into ApoE−/− mice, it was found that ESTA-MSV/miRs could reduce plaque size and stabilize plaques in ApoE−/−mice.125 Recently, studies also found ATP-responsive nanocarriers demonstrated high selectivity and stability in circulation.126 Jiang et al. synthesized an ATP-responsive low-molecular-weight polyethylenimine-based supramolecular assembly, which prevented oxidized LDL absorption by delivering SR-A siRNA via energy-dependent endocytosis and protected the siRNA from RNase destruction.127 The same group then created a multifunctional core-shell nanoplatform with SR-A siRNA/catalase/ATP-responsive cationic carrier ternary polyplexes as the core, thereby suppressing cholesterol deposition and decreasing atherosclerosis via improved macrophage CD36 targeting in plaque.128 PEG/PEI nanoparticles are a safe and more effective in vivo delivery system compared with viral vectors and liposomes, and they are prospective stars in the battle against atherosclerosis.129,130
The physicochemical features of NP platforms (size, shape, surface charge, stiffness) can impact their absorption by various organs, tissues, and cells. As a result, there have been significant efforts to optimize these features through the effective design of NPs.131,132 Several preparation techniques for distinct NP platforms have been established based on the application and physiochemical characteristics of the payload, which vary in the manner of emulsification, solvent evaporation, and film hydration.133 Although delivery to the atherosclerotic plaque has yet to be refined, the flexibility of NP design will almost certainly allow for fine-tuning NP features to obtain more acceptable and on-target effects.
Others
Exosomes are natural biological carriers with the same topological structure as cells.134 Exosomes, endogenous nano-vesicles with a diameter of 30–200 nm, are the top priority for the delivery of protein and nucleic acid drugs. They have the advantages of high ability to overcome biological obstacles, few off-target effects, and low immunogenicity, among others.135
Exosomes were first proposed as drug delivery carriers by injecting exosomes secreted by dendritic cells to deliver siRNA to the brain of mice.136 Nowadays, exosome drugs in the treatment of CVDs have become research hot spots.137,138 EC-derived exosomes loaded with anti-miR-33a-5p can upregulate cholesterol efflux from macrophages and VSMCs by increasing the expression of ABCA1, a molecule that controls cholesterol efflux and attenuates atherosclerosis.139 Moreover, miR-155 can also be transmitted through VSMC-derived exosomes, destroy the integrity of the endothelial barrier, and accelerate atherosclerosis.140 Therefore, miR-155 can be used as a regulator of atherosclerosis. It has been reported that exocrine-derived miRNA can regulate macrophage heterogeneity in atherosclerotic plaques.141 For example, secreted microRNA produced by bone marrow-derived macrophages can promote M2 polarization of recipient macrophages.142 After nicotine stimulation, the expression of exosome miR-21-3p in macrophages was upregulated, which increased the migration and proliferation of VSMC through the phosphatase and tension homolog (PTEN) pathway and accelerated atherosclerosis.143,144
Microvesicles (MVs), also known as microparticles, are irregularly shaped submicron extracellular vesicles (100–1000 nm).145 MVs are not only released in many types of cells, such as platelets, ECs, neutrophils, and erythrocytes, but also in the extracellular spaces of solid organs, atherosclerotic plaques, and tumors.146 It has been shown that MVs convey genetic information between cells in cardiovascular disease, and miRNA-containing MVs play a specific role in the chronic inflammation associated with atherosclerotic disease.138,147,148 For example, through the delivery of miR-155, neutrophil MVs enhance inflammatory gene expression and NF-κB activation, ultimately increasing vascular inflammation and atherosclerosis.149 Endothelial MVs-mediated transfer of miR-19b dramatically increases lipids, decreases collagen content in plaques, and exaggerates atherosclerosis progression.150 CircNPHP4 in monocyte-derived extracellular vesicles (EVs) reduces ICAM-1 and VCAM-1 expression by controlling heterogeneous adhesion in coronary artery atherosclerotic disease.151 EVs carry membrane receptors, nucleic acids, lipids, and proteins that trigger and extend the inflammatory response in CVD. They may also be exploited prognostically, diagnostically, and therapeutically and play a significant role in targeted transport.
EVs represented by exocrine bodies are promising carriers that can be modified to restore or inhibit the expression of pathogenic lncRNA in lncRNA-dominated diseases. Compared with other nanocarriers, exosomes have lower immunogenicity and higher in vivo stability. The effective integration of exosomes and liposomes is a key development of precision medicine, such as CRISPR-Cas9 system delivery.152,153 Moreover, the surface modification of exosomes is also an optimization strategy154,155 as various ligands can be fixed on the exocrine surface without damaging the vesicle structure to enhance targeted delivery.156
Clinical application of gene therapy
CVD is a complex multifactorial disease, and even with current advances in medical methods, the increasing prevalence of CVD requires early and accurate detection and more effective treatments. The latest advances in materials science and the advent of nanotechnology provide new methods for the use of nanoscale drugs with the potential to target the desired location for simultaneous imaging, diagnosis, and treatment, known as therapeutic diagnostics, or “diapeutics,” NPs containing active substances are effective in the treatment of atherosclerosis, and the rapid development of this field demonstrates that nanomedicines can provide enhanced drug delivery.22,24 Improved pharmacokinetics and pharmacodynamics are the main advantages of nanoscale drug delivery systems. Through passive and active targeting mechanisms, there is greater accumulation in the diseased site to achieve the purpose of diagnosis and treatment. At present, the delivery vector of ncRNA has not entered the clinic in the diagnosis of atherosclerosis, but many materials have shown potential.
Promising diagnosis
At present, nanotechnology has had a significant impact on the management of atherosclerosis. For the management of early subclinical atherosclerosis, noninvasive imaging of adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1) or E-selectin, can identify activated ECs and inflammatory cells in atherosclerosis.157 For the management of formed atherosclerotic plaque, Karen et al. completed in vivo magnetic resonance detection of atherosclerotic lesions by using lipid-coated ultra-small superparamagnetic iron particles (<20 nm), mainly because ox-LDL is an important component of plaque and is thus an easy to identify target.158 For the specific recognition of vulnerable plaques, prior studies have shown that gadolinium (Gd) micelles labeled with oxidation-specific antibodies allow the detection of vulnerable plaques in vivo using magnetic resonance imaging (MRI). Targeting Gd micelles accumulate in macrophages after extracellular binding to ox-LDL and are thus another promising imaging technology for identifying macrophages in plaque in vivo.159 However, problems associated with the biotransformation/retention of Gd may limit clinical transformation.160 Due to its high expression in atherosclerotic plaques, fibrin can also be used to identify vulnerable plaques.161 MRI and computerized spectral tomography (CT) imaging of targeted fibrin can provide sensitive detection and localization of fibrin and can directly identify vulnerable plaques in the early stage.162,163 More than that, David et al. developed a clot-binding peptide cysteine-arginine-glutamate-lysine-alanine micelle -containing anticoagulants, which can be targeted and delivered to the site of thrombus and effectively reduce the risk of thrombus formation and clot expansion.164 In addition to fibrin, macrophages are also a key component of plaque vulnerability, so atherosclerotic plaques can be detected by different imaging methods (such as PET, MRI, and CT).165, 166, 167 Jason et al. used distinct wavelength spectra for optical localization and light-activated therapy targeting macrophage ablation as a local anti-inflammatory therapy for atherosclerosis.168
Remarkable treatment
The unique and varying properties of nano materials (such as shape, size, and charge) make them promising tools for diagnosis and treatment. Nanotechnology was initially applied to the targeted delivery of anticancer drugs, including liposomal DaunoXome (GileadSciences), used in patients with Posey’s sarcoma, and albumin-bound NPs (Abraxane; AbraxisBioScience), used to treat breast cancer.169 The FDA has authorized a range of nano-drugs for oral (Gastromark; silicon-coated superparamagnetic iron oxide), local (DepoCyt; cytarabine liposomes; Estrasorb; micellar estradiol), and systemic (LipoDox) delivery. Inorganic nanomaterials (iron oxide, silica, gold, etc.) have been employed for several therapeutic applications, including as contrast agents for in vivo imaging, photothermal treatment of malignancies, and cancer-related anemia.170 However, some restrictions hinder the effective conversion of nano-drugs from the laboratory to the clinic. For example, the expression of adhesion molecules occurs throughout the development of atherosclerosis lesions, and ox-LDL also exists in blood and organs, as well as plaque. Therefore, the detection of cell adhesion molecules and ox-LDL specifically targeted by MRI is not precise to some plaque phenotypes. It is well known that macrophages actively engulf foreign bodies; therefore, any NPs accumulated in plaques will eventually enter the macrophages, thus limiting the specificity of various imaging methods for the detection of atherosclerotic plaques.75 For light-activated macrophage ablation, which may lead to potential vascular wall damage and related inflammation, there is also a challenge in the treatment of deeper plaques. With our in-depth understanding of the pathophysiology of atherosclerosis and continuous improvement of the current delivery system, we can reduce nano-drug toxicity, immunostimulatory and immunosuppressive characteristics, as well as address its limited diffusivity and other shortcomings. The continuous discovery of new molecular targets, as well as the continuous development of NP synthesis methods and imaging technology, will make improve the therapeutic prospects for atherosclerosis.
Conclusions and future perspective
Nucleic acid therapy has huge market potential, and the potential disease spectrum is broad; however, at present, most research is focused on genetic diseases, tumors, and other fields. In addition, the drug research and development cycle is long, and the current pace is sluggish. In the field of nucleic acid drugs, although there are plenty of potential therapeutic targets, choosing a suitable drug carrier so that it can reach the lesion site accurately and efficiently has restricted drug research and development. Different drug loading systems can be selected according to different therapeutic purposes and drug characteristics.
Atherosclerosis originates from endothelial dysfunction and a cascade of events. Various cells and molecules are involved in the formation and progression of atherosclerotic plaque and arterial stenosis. Therefore, it is reasonable to use ncRNA nanomedicines in targeted delivery to effectively prevent disease progression.
Gene drug therapy is an important therapeutic treatment that can regulate diseases at the gene level and overcome the limitations of incompatible proteins. RNAi has been widely used as a potential treatment strategy for a variety of pathologic illnesses during the last 15 years. Progress has been made in the study of target genes and the creation of RNAi delivery mechanisms. Furthermore, the effectiveness of delivery and gene silencing has considerably increased in recent years. However, obstacles to the effective clinical implementation of RNAi-based therapies persist. The primary cause for the withdrawal of clinical studies of certain RNAi treatments has been safety concerns. Another problem is innate immune activation caused by nanocarriers and RNAi itself. Significant progress has been made in the clinical application of miRNA-based therapies. Furthermore, the FDA and the European Medicines Agency have authorized 11 RNA-based therapies aimed at gene modifications in the muscle, tumor, liver, and central nervous system.171 Anti-miRNAs and miRNA mimics are being tested in clinical trials with a large number of RNA therapies in phase II or III, but therapies including ncRNA other than miRNA have not yet entered the clinic. Along with the increasing understanding of ncRNAs, emerging molecules such as tsRNA, piRNA, and other ncRNAs are actively being explored as biomarkers that are closely associated with diseases such as osteosarcoma,172 pancreatic cancer, and aortic dissection.173, 174, 175 In the future, specific ncRNA types such as circRNA, tsRNA, or piRNA represent novel therapeutic approaches. Targeting ncRNA might be useful in gene therapy and give a new option for precision medicine. However, there are still challenges in the successful clinical application of ncRNA-based therapy. Safety issues and targeted delivery systems, as well as innate immune activation caused by ncRNA itself, are the main reasons for the withdrawal of clinical trials of gene therapy. An ideal delivery system is vital for achieving effective, targeted, and non-toxic drug delivery in vivo. First, identifying the most suitable ncRNA is the key factor in the development of gene drugs. To clarify their involvement in physiological activity in vitro and in vivo, all conceivable connections between druggable and intracellular miRNAs or proteins they control should be identified. This poses a challenge to the in-depth basic research on the function and mechanism of ncRNA. Second, tissue-specific ligands with higher stability and affinity are also expected to improve delivery systems. Based on our present understanding of ncRNA delivery systems, we anticipate that these initiatives will usher in a new age of molecular medicine, allowing patients to benefit from safe, efficient, and individualized treatment.
Acknowledgments
This work was supported by The National Natural Science Foundation of China (No. 81870331, China), the Natural Science Foundation of Shandong Province (ZR2021MH280), and the Qingdao Municipal Science and Technology Bureau project (No.21-1-4-rkjk-12-nsh, China).
Author contributions
X.L. collected materials and wrote the manuscript. T.Y. and Y.Y. provided the idea. X.L., H.M., and T.L. are responsible for the schematic diagram within this article. T.Y., Y.Y., X.L., Z.W., W.C., and X.F. helped with the final revision of the article. All authors reviewed the manuscript and approved the final manuscript.
Declaration of interests
The authors have no conflicts of interest to declare.
Contributor Information
Yanyan Yang, Email: yangyy1201@qdu.edu.cn.
Tao Yu, Email: yutao0112@qdu.edu.cn.
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Jernberg T., Hasvold P., Henriksson M., Hjelm H., Thuresson M., Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur. Heart J. 2015;36:1163–1170. doi: 10.1093/eurheartj/ehu505. [DOI] [PubMed] [Google Scholar]
- 3.Li X., Yang Y., Wang Z., Jiang S., Meng Y., Song X., Zhao L., Zou L., Li M., Yu T. Targeting non-coding RNAs in unstable atherosclerotic plaques: mechanism, regulation, possibilities, and limitations. Int. J. Biol. Sci. 2021;17:3413–3427. doi: 10.7150/ijbs.62506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allahverdian S., Chehroudi A.C., McManus B.M., Abraham T., Francis G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 5.Allahverdian S., Chaabane C., Boukais K., Francis G.A., Bochaton-Piallat M.L. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc. Res. 2018;114:540–550. doi: 10.1093/cvr/cvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon C.P., Steinberg B.A., Murphy S.A., Mega J.L., Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J. Am. Coll. Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 7.Sampson U.K., Fazio S., Linton M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr. Atheroscler. Rep. 2012;14:1–10. doi: 10.1007/s11883-011-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine SEARCH Collaborative Group. Homocysteine Collaborative G., Armitage J., Bowman L., Wallendszus K., Bulbulia R., Rahimi K., Haynes R., Parish S., Peto R., et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12, 064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–1669. doi: 10.1016/S0140-6736(10)60310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hlatky M.A., Kazi D.S. PCSK9 inhibitors: economics and policy. J. Am. Coll. Cardiol. 2017;70:2677–2687. doi: 10.1016/j.jacc.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Bai B., Yang Y., Ji S., Wang S., Peng X., Tian C., Sun R.C., Yu T., Chu X.M. MicroRNA-302c-3p inhibits endothelial cell pyroptosis via directly targeting NOD-LRR- and pyrin domain-containing protein 3 in atherosclerosis. J. Cell. Mol. Med. 2021;25:4373–4386. doi: 10.1111/jcmm.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crooke S.T., Wang S., Vickers T.A., Shen W., Liang X.H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017;35:230–237. doi: 10.1038/nbt.3779. [DOI] [PubMed] [Google Scholar]
- 12.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X., Lian Z., Yang Y., Wang Z., Fu X., Liu Y., Li M., Tian J., Yu T., Xin H. Long non-coding RNA PEBP1P2 suppresses proliferative VSMCs phenotypic switching and proliferation in atherosclerosis. Mol. Ther. Nucleic Acids. 2020;22:84–98. doi: 10.1016/j.omtn.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czech B., Munafò M., Ciabrelli F., Eastwood E.L., Fabry M.H., Kneuss E., Hannon G.J. piRNA-guided genome defense: from biogenesis to silencing. Annu. Rev. Genet. 2018;52:131–157. doi: 10.1146/annurev-genet-120417-031441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M., et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 16.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 2021;20:629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchese F.P., Raimondi I., Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fordyce C.B., Roe M.T., Ahmad T., Libby P., Borer J.S., Hiatt W.R., Bristow M.R., Packer M., Wasserman S.M., Braunstein N., et al. Cardiovascular drug development: is it dead or just hibernating? J. Am. Coll. Cardiol. 2015;65:1567–1582. doi: 10.1016/j.jacc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Yang Y., Wang Z., Ju H., Fu X., Zou L., Li M., Xue Q., Ma H., Meng Y., et al. Multistage-responsive nanocomplexes attenuate ulcerative colitis by improving the accumulation and distribution of oral nucleic acid drugs in the colon. ACS Appl. Mater. Inter. 2022;14:2058–2070. doi: 10.1021/acsami.1c21595. [DOI] [PubMed] [Google Scholar]
- 21.Qi H., Yang J., Yu J., Yang L., Shan P., Zhu S., Wang Y., Li P., Wang K., Zhou Q. Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes. Nanotechnol. Rev. 2022;11:1511–1524. doi: 10.1515/ntrev-2022-0095. [DOI] [Google Scholar]
- 22.Lobatto M.E., Fuster V., Fayad Z.A., Mulder W.J.M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011;10:835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang H., Stiles W.R., Baek Y., Nomura S., Bao K., Hu S., Park G.K., Jo M.J., I H., Coll J., et al. Renal clearable theranostic nanoplatforms for gastrointestinal stromal tumors. Adv. Mater. 2020;32:e1905899. doi: 10.1002/adma.201905899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duivenvoorden R., Senders M.L., van Leent M.M.T., Pérez-Medina C., Nahrendorf M., Fayad Z.A., Mulder W.J.M. Nanoimmunotherapy to treat ischaemic heart disease. Nat. Rev. Cardiol. 2019;16:21–32. doi: 10.1038/s41569-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard M.D., Hood E.D., Zern B., Shuvaev V.V., Grosser T., Muzykantov V.R. Nanocarriers for vascular delivery of anti-inflammatory agents. Annu. Rev. Pharmacol. Toxicol. 2014;54:205–226. doi: 10.1146/annurev-pharmtox-011613-140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hood E.D., Chorny M., Greineder C.F., S Alferiev I., Levy R.J., Muzykantov V.R. Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials. 2014;35:3708–3715. doi: 10.1016/j.biomaterials.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duivenvoorden R., Tang J., Cormode D.P., Mieszawska A.J., Izquierdo-Garcia D., Ozcan C., Otten M.J., Zaidi N., Lobatto M.E., van Rijs S.M., et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen S.D., Liu Y.G., Kim T., Bobbala S., Yi S., Zhang X., Choi J., Scott E.A. Celastrol-loaded PEG-b-PPS nanocarriers as an anti-inflammatory treatment for atherosclerosis. Biomater. Sci. 2019;7:657–668. doi: 10.1039/c8bm01224e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun W., Zhao P., Zhou Y., Xing C., Zhao L., Li Z., Yuan L. Ultrasound targeted microbubble destruction assisted exosomal delivery of miR-21 protects the heart from chemotherapy associated cardiotoxicity. Biochem. Biophys. Res. Commun. 2020;532:60–67. doi: 10.1016/j.bbrc.2020.05.044. [DOI] [PubMed] [Google Scholar]
- 30.Chen D., Jepson N. Coronary stent technology: a narrative review. Med. J. Aust. 2016;205:277–281. doi: 10.5694/mja16.00444. [DOI] [PubMed] [Google Scholar]
- 31.Turner E., Erwin M., Atigh M., Christians U., Saul J.M., Yazdani S.K. In vitro and in vivo assessment of Keratose as a novel excipient of paclitaxel coated balloons. Front. Pharmacol. 2018;9:808. doi: 10.3389/fphar.2018.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhakamy N.A., Curiel D.T., Berkland C.J. The era of gene therapy: from preclinical development to clinical application. Drug Discov. Today. 2021;26:1602–1619. doi: 10.1016/j.drudis.2021.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Stein C.A., Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol. Ther. 2017;25:1069–1075. doi: 10.1016/j.ymthe.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rüger J., Ioannou S., Castanotto D., Stein C.A. Oligonucleotides to the (gene) rescue: FDA approvals 2017-2019. Trends Pharmacol. Sci. 2020;41:27–41. doi: 10.1016/j.tips.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979;7:179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crooke S.T., Vickers T.A., Liang X.H. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020;48:5235–5253. doi: 10.1093/nar/gkaa299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Clercq E., Eckstein E., Merigan T.C. [Interferon induction increased through chemical modification of a synthetic polyribonucleotide] Science. 1969;165:1137–1139. doi: 10.1126/science.165.3898.1137. [DOI] [PubMed] [Google Scholar]
- 38.Parham J.S., Goldberg A.C. Mipomersen and its use in familial hypercholesterolemia. Expert Opin. Pharmacother. 2019;20:127–131. doi: 10.1080/14656566.2018.1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckstein F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 2014;24:374–387. doi: 10.1089/nat.2014.0506. [DOI] [PubMed] [Google Scholar]
- 40.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 41.Robbins M., Judge A., Liang L., McClintock K., Yaworski E., MacLachlan I. 2'-O-methyl-modified RNAs act as TLR7 antagonists. Mol. Ther. 2007;15:1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- 42.Khvorova A., Watts J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017;35:238–248. doi: 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paunovska K., Loughrey D., Dahlman J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022;23:265–280. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He F., Wen N., Xiao D., Yan J., Xiong H., Cai S., Liu Z., Liu Y. Aptamer-based targeted drug delivery systems: current potential and challenges. Curr. Med. Chem. 2020;27:2189–2219. doi: 10.2174/0929867325666181008142831. [DOI] [PubMed] [Google Scholar]
- 45.France M. Homozygous familial hypercholesterolaemia: update on management. Paediatr. Int. Child Health. 2016;36:243–247. doi: 10.1080/20469047.2016.1246640. [DOI] [PubMed] [Google Scholar]
- 46.Yang F., Chen Q., He S., Yang M., Maguire E.M., An W., Afzal T.A., Luong L.A., Zhang L., Xiao Q. miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation. 2018;137:1824–1841. doi: 10.1161/CIRCULATIONAHA.117.027799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang W.Q., Wei P., Lin R.Q., Huang F. Protective effects of microrna-22 against endothelial cell injury by targeting NLRP3 through suppression of the inflammasome signaling pathway in a rat model of coronary heart disease. Cell. Physiol. Biochem. 2017;43:1346–1358. doi: 10.1159/000481846. [DOI] [PubMed] [Google Scholar]
- 48.Wang R., Ding Y.D., Gao W., Pei Y.Q., Yang J.X., Zhao Y.X., Liu X.L., Shen H., Zhang S., Yu L., Ge H.L. Serum microRNA-204 levels are associated with long-term cardiovascular disease risk based on the Framingham risk score in patients with type 2 diabetes: results from an observational study. J. Geriatr. Cardiol. 2020;17:330–337. doi: 10.11909/j.issn.1671-5411.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Guo J.W., Lin X.C., Tuo Y.H., Peng W.L., He S.Y., Li Z.Q., Ye Y.C., Yu J., Zhang F.R., et al. Macrophage NFATc3 prevents foam cell formation and atherosclerosis: evidence and mechanisms. Eur. Heart J. 2021;42:4847–4861. doi: 10.1093/eurheartj/ehab660. [DOI] [PubMed] [Google Scholar]
- 50.Abplanalp W.T., Fischer A., John D., Zeiher A.M., Gosgnach W., Darville H., Montgomery R., Pestano L., Allée G., Paty I., et al. Efficiency and target derepression of anti-miR-92a: results of a first in human study. Nucleic Acid Ther. 2020;30:335–345. doi: 10.1089/nat.2020.0871. [DOI] [PubMed] [Google Scholar]
- 51.Loyer X., Potteaux S., Vion A.C., Guérin C.L., Boulkroun S., Rautou P.E., Ramkhelawon B., Esposito B., Dalloz M., Paul J.L., et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ. Res. 2014;114:434–443. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- 52.Hennessy E.J., van Solingen C., Scacalossi K.R., Ouimet M., Afonso M.S., Prins J., Koelwyn G.J., Sharma M., Ramkhelawon B., Carpenter S., et al. The long noncoding RNA CHROME regulates cholesterol homeostasis in primate. Nat. Metab. 2019;1:98–110. doi: 10.1038/s42255-018-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi X., Wei Y.T., Li H., Jiang T., Zheng X.L., Yin K., Zhao G.J. Long non-coding RNA H19 in atherosclerosis: what role? Mol. Med. 2020;26:72. doi: 10.1186/s10020-020-00196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L., Cheng H., Yue Y., Li S., Zhang D., He R. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/beta-catenin in ox-LDL -stimulated vascular smooth muscle cells. J. Biomed. Sci. 2018;25:11. doi: 10.1186/s12929-018-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y., Tang F., Wei F., Yang L., Kuang C., Zhang H., Deng J., Wu Q. Silencing of long non-coding RNA H19 downregulates CTCF to protect against atherosclerosis by upregulating PKD1 expression in ApoE knockout mice. Aging. 2019;11:10016–10030. doi: 10.18632/aging.102388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Y., Zhong L., He X., Wang S., Lai Y., Wu W., Song H., Chen Y., Yang Y., Liao W., et al. LncRNA H19 promotes vascular inflammation and abdominal aortic aneurysm formation by functioning as a competing endogenous RNA. J. Mol. Cell. Cardiol. 2019;131:66–81. doi: 10.1016/j.yjmcc.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Li D.Y., Busch A., Jin H., Chernogubova E., Pelisek J., Karlsson J., Sennblad B., Liu S., Lao S., Hofmann P., et al. H19 induces abdominal aortic aneurysm development and progression. Circulation. 2018;138:1551–1568. doi: 10.1161/CIRCULATIONAHA.117.032184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Y., Ma J., Wang J., Wang L. Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Mol. Immunol. 2018;93:107–114. doi: 10.1016/j.molimm.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 59.Cremer S., Michalik K.M., Fischer A., Pfisterer L., Jaé N., Winter C., Boon R.A., Muhly-Reinholz M., John D., Uchida S., et al. Hematopoietic deficiency of the long noncoding RNA MALAT1 promotes atherosclerosis and plaque inflammation. Circulation. 2019;139:1320–1334. doi: 10.1161/CIRCULATIONAHA.117.029015. [DOI] [PubMed] [Google Scholar]
- 60.Gast M., Rauch B.H., Nakagawa S., Haghikia A., Jasina A., Haas J., Nath N., Jensen L., Stroux A., Böhm A., et al. Immune system-mediated atherosclerosis caused by deficiency of long non-coding RNA MALAT1 in ApoE-/-mice. Cardiovasc. Res. 2019;115:302–314. doi: 10.1093/cvr/cvy202. [DOI] [PubMed] [Google Scholar]
- 61.Zeng X., Lin W., Guo M., Zou Q. A comprehensive overview and evaluation of circular RNA detection tools. PLoS Comput. Biol. 2017;13:e1005420. doi: 10.1371/journal.pcbi.1005420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holdt L.M., Stahringer A., Sass K., Pichler G., Kulak N.A., Wilfert W., Kohlmaier A., Herbst A., Northoff B.H., Nicolaou A., et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsui M., Corey D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017;16:167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y., Yang Y., Ju H., He X., Sun P., Tian Y., Yang P., Song X.X., Yu T., Jiang Z. Comprehensive profile of circRNAs in formaldehyde induced heart development. Food Chem. Toxicol. 2022;162:112899. doi: 10.1016/j.fct.2022.112899. [DOI] [PubMed] [Google Scholar]
- 65.Li C.Y., Ma L., Yu B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed. Pharmacother. 2017;95:1514–1519. doi: 10.1016/j.biopha.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 66.Li M., Yang Y., Wang Z., Zong T., Fu X., Aung L.H.H., Wang K., Wang J.X., Yu T. Piwi-interacting RNAs (piRNAs) as potential biomarkers and therapeutic targets for cardiovascular diseases. Angiogenesis. 2021;24:19–34. doi: 10.1007/s10456-020-09750-w. [DOI] [PubMed] [Google Scholar]
- 67.He X., Yang Y., Wang Q., Wang J., Li S., Li C., Zong T., Li X., Zhang Y., Zou Y., Yu T. Expression profiles and potential roles of transfer RNA-derived small RNAs in atherosclerosis. J. Cell. Mol. Med. 2021;25:7052–7065. doi: 10.1111/jcmm.16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han H. RNA interference to Knock down gene expression. Methods Mol. Biol. 2018;1706:293–302. doi: 10.1007/978-1-4939-7471-9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S.J., Son S., Yhee J.Y., Choi K., Kwon I.C., Kim S.H., Kim K. Structural modification of siRNA for efficient gene silencing. Biotechnol. Adv. 2013;31:491–503. doi: 10.1016/j.biotechadv.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Sarett S.M., Kilchrist K.V., Miteva M., Duvall C.L. Conjugation of palmitic acid improves potency and longevity of siRNA delivered via endosomolytic polymer nanoparticles. J. Biomed. Mater. Res. A. 2015;103:3107–3116. doi: 10.1002/jbm.a.35413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexis F., Pridgen E., Molnar L.K., Farokhzad O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broderick J.A., Zamore P.D. MicroRNA therapeutics. Gene Ther. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson J.M. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2009;96:151–157. doi: 10.1016/j.ymgme.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 75.Kooi M.E., Cappendijk V.C., Cleutjens K.B., Kessels A.G., Kitslaar P.J., Borgers M., Frederik P.M., Daemen M.J., van Engelshoven J.M. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 76.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 77.Wang J., Lu Z., Wientjes M.G., Au J.L.S. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Danquah M.K., Zhang X.A., Mahato R.I. Extravasation of polymeric nanomedicines across tumor vasculature. Adv. Drug Deliv. Rev. 2011;63:623–639. doi: 10.1016/j.addr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Akhtar S., Benter I.F. Nonviral delivery of synthetic siRNAs in vivo. J. Clin. Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., Sah D.W.Y., Stebbing D., Crosley E.J., Yaworski E., et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 81.Barton G.M., Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 82.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cirak S., Arechavala-Gomeza V., Guglieri M., Feng L., Torelli S., Anthony K., Abbs S., Garralda M.E., Bourke J., Wells D.J., et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sledz C.A., Holko M., de Veer M.J., Silverman R.H., Williams B.R.G. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 85.Hanagata N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int. J. Nanomed. 2017;12:515–531. doi: 10.2147/IJN.S114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson A.L., Bartz S.R., Schelter J., Kobayashi S.V., Burchard J., Mao M., Li B., Cavet G., Linsley P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 87.Juliano R.L., Carver K. Cellular uptake and intracellular trafficking of oligonucleotides. Adv. Drug Deliv. Rev. 2015;87:35–45. doi: 10.1016/j.addr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hickerson R.P., Vlassov A.V., Wang Q., Leake D., Ilves H., Gonzalez-Gonzalez E., Contag C.H., Johnston B.H., Kaspar R.L. Stability study of unmodified siRNA and relevance to clinical use. Oligonucleotides. 2008;18:345–354. doi: 10.1089/oli.2008.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tokatlian T., Segura T. siRNA applications in nanomedicine. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol. 2010;2:305–315. doi: 10.1002/wnan.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bramsen J.B., Kjems J. Engineering small interfering RNAs by strategic chemical modification. Methods Mol. Biol. 2013;942:87–109. doi: 10.1007/978-1-62703-119-6_5. [DOI] [PubMed] [Google Scholar]
- 91.Behlke M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18:305–319. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- 92.Keller M. Nanomedicinal delivery approaches for therapeutic siRNA. Int. J. Pharm. 2009;379:210–211. doi: 10.1016/j.ijpharm.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 93.Giacca M., Zacchigna S. Virus-mediated gene delivery for human gene therapy. J. Control Release. 2012;161:377–388. doi: 10.1016/j.jconrel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 94.Zhang S., Zhao Y., Zhi D., Zhang S. Non-viral vectors for the mediation of RNAi. Bioorg. Chem. 2012;40:10–18. doi: 10.1016/j.bioorg.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J.H., Wang W.J., Zhang J., Xiao Y.P., Liu Y.H., Yu X.Q. ROS-responsive fluorinated polycations as non-viral gene vectors. Eur. J. Med. Chem. 2019;182:111666. doi: 10.1016/j.ejmech.2019.111666. [DOI] [PubMed] [Google Scholar]
- 96.Seow Y., Wood M.J. Biological gene delivery vehicles: beyond viral vectors. Mol. Ther. 2009;17:767–777. doi: 10.1038/mt.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Furlan M., Delgado-Tejedor A., Mulroney L., Pelizzola M., Novoa E.M., Leonardi T. Computational methods for RNA modification detection from nanopore direct RNA sequencing data. RNA Biol. 2021;18:31–40. doi: 10.1080/15476286.2021.1978215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rao D.D., Senzer N., Cleary M.A., Nemunaitis J. Comparative assessment of siRNA and shRNA off target effects: what is slowing clinical development. Cancer Gene Ther. 2009;16:807–809. doi: 10.1038/cgt.2009.53. [DOI] [PubMed] [Google Scholar]
- 99.Chen P.Y., Weinmann L., Gaidatzis D., Pei Y., Zavolan M., Tuschl T., Meister G. Strand-specific 5'-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity. RNA. 2008;14:263–274. doi: 10.1261/rna.789808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu Y., Xu Y., Zhu Y., Sun H., Juguilon C., Li F., Fan D., Yin L., Zhang Y. Macrophage miR-34a is a key regulator of cholesterol efflux and atherosclerosis. Mol. Ther. 2020;28:202–216. doi: 10.1016/j.ymthe.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hart M., Walch-Rückheim B., Friedmann K.S., Rheinheimer S., Tänzer T., Glombitza B., Sester M., Lenhof H.P., Hoth M., Schwarz E.C., et al. miR-34a: a new player in the regulation of T cell function by modulation of NF-kappaB signaling. Cell Death Dis. 2019;10:46. doi: 10.1038/s41419-018-1295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong D.S., Kang Y.K., Borad M., Sachdev J., Ejadi S., Lim H.Y., Brenner A.J., Park K., Lee J.L., Kim T.Y., et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer. 2020;122:1630–1637. doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hart M., Nickl L., Walch-Rueckheim B., Krammes L., Rheinheimer S., Diener C., Taenzer T., Kehl T., Sester M., Lenhof H.P., et al. Wrinkle in the plan: miR-34a-5p impacts chemokine signaling by modulating CXCL10/CXCL11/CXCR3-axis in CD4(+), CD8(+) T cells, and M1 macrophages. J. Immunother. Cancer. 2020;8:e001617. doi: 10.1136/jitc-2020-001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ui-Tei K., Naito Y., Nishi K., Juni A., Saigo K. Thermodynamic stability and Watson-Crick base pairing in the seed duplex are major determinants of the efficiency of the siRNA-based off-target effect. Nucleic Acids Res. 2008;36:7100–7109. doi: 10.1093/nar/gkn902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh M.S., Peer D. RNA nanomedicines: the next generation drugs? Curr. Opin. Biotechnol. 2016;39:28–34. doi: 10.1016/j.copbio.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 106.Patel D.K., Senapati S., Mourya P., Singh M.M., Aswal V.K., Ray B., Maiti P. Functionalized graphene tagged polyurethanes for corrosion inhibitor and sustained drug delivery. ACS Biomater. Sci. Eng. 2017;3:3351–3363. doi: 10.1021/acsbiomaterials.7b00342. [DOI] [PubMed] [Google Scholar]
- 107.Torchilin V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 108.Rezaee M., Oskuee R.K., Nassirli H., Malaekeh-Nikouei B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control Release. 2016;236:1–14. doi: 10.1016/j.jconrel.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 109.Chen X., Mangala L.S., Rodriguez-Aguayo C., Kong X., Lopez-Berestein G., Sood A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018;37:107–124. doi: 10.1007/s10555-017-9717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morrissey D.V., Lockridge J.A., Shaw L., Blanchard K., Jensen K., Breen W., Hartsough K., Machemer L., Radka S., Jadhav V., et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 111.Liu D.Z., Jickling G.C., Ander B.P., Hull H., Zhan X., Cox C., Shroff N., Dykstra-Aiello C., Stamova B., Sharp F.R. Elevating microRNA-122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 2016;36:1374–1383. doi: 10.1177/0271678X15610786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lv H., Zhang S., Wang B., Cui S., Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 113.Akhtar S., Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv. Drug Deliv. Rev. 2007;59:164–182. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 114.Dosta P., Tamargo I., Ramos V., Kumar S., Kang D.W., Borrós S., Jo H. Delivery of anti-microRNA-712 to inflamed endothelial cells using poly(beta-amino ester) nanoparticles conjugated with VCAM-1 targeting peptide. Adv. Healthc. Mater. 2021;10:e2001894. doi: 10.1002/adhm.202001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen M.A., Wyatt H., Susser L., Geoffrion M., Rasheed A., Duchez A.C., Cottee M.L., Afolayan E., Farah E., Kahiel Z., et al. Delivery of MicroRNAs by chitosan nanoparticles to functionally alter macrophage cholesterol efflux in vitro and in vivo. ACS Nano. 2019;13:6491–6505. doi: 10.1021/acsnano.8b09679. [DOI] [PubMed] [Google Scholar]
- 116.Lyu Z., Ding L., Tintaru A., Peng L. Self-assembling supramolecular dendrimers for biomedical applications: lessons learned from poly(amidoamine) dendrimers. Acc. Chem. Res. 2020;53:2936–2949. doi: 10.1021/acs.accounts.0c00589. [DOI] [PubMed] [Google Scholar]
- 117.Chin D.D., Poon C., Wang J., Joo J., Ong V., Jiang Z., Cheng K., Plotkin A., Magee G.A., Chung E.J. miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype. Biomaterials. 2021;273:120810. doi: 10.1016/j.biomaterials.2021.120810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuo C.H., Leon L., Chung E.J., Huang R.T., Sontag T.J., Reardon C.A., Getz G.S., Tirrell M., Fang Y. Inhibition of atherosclerosis-promoting microRNAs via targeted polyelectrolyte complex micelles. J. Mater. Chem. B. 2014;2:8142–8153. doi: 10.1039/C4TB00977K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li D., Li X., Bai J., Liu Y., de Vries R., Li Y. Rod-shaped polypeptide nanoparticles for siRNA delivery. Int. J. Biol. Macromol. 2021;166:401–408. doi: 10.1016/j.ijbiomac.2020.10.198. [DOI] [PubMed] [Google Scholar]
- 120.Vasiliu S., Racovita S., Gugoasa I.A., Lungan M.A., Popa M., Desbrieres J. The benefits of smart nanoparticles in dental applications. Int. J. Mol. Sci. 2021;22:2585. doi: 10.3390/ijms22052585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teng C., Lin C., Huang F., Xing X., Chen S., Ye L., Azevedo H.S., Xu C., Wu Z., Chen Z., He W. Intracellular codelivery of anti-inflammatory drug and anti-miR 155 to treat inflammatory disease. Acta Pharm. Sin. B. 2020;10:1521–1533. doi: 10.1016/j.apsb.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kornmueller K., Vidakovic I., Prassl R. Artificial high density lipoprotein nanoparticles in cardiovascular research. Molecules. 2019;24:E2829. doi: 10.3390/molecules24152829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kheirolomoom A., Kim C.W., Seo J.W., Kumar S., Son D.J., Gagnon M.K.J., Ingham E.S., Ferrara K.W., Jo H. Multifunctional nanoparticles facilitate molecular targeting and miRNA delivery to inhibit atherosclerosis in ApoE(-/-) mice. ACS Nano. 2015;9:8885–8897. doi: 10.1021/acsnano.5b02611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boussif O., Lezoualc'h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ma S., Tian X.Y., Zhang Y., Mu C., Shen H., Bismuth J., Pownall H.J., Huang Y., Wong W.T. E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci. Rep. 2016;6:22910. doi: 10.1038/srep22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoshinaga N., Uchida S., Dirisala A., Naito M., Osada K., Cabral H., Kataoka K. mRNA loading into ATP-responsive polyplex micelles with optimal density of phenylboronate ester crosslinking to balance robustness in the biological milieu and intracellular translational efficiency. J. Control Release. 2021;330:317–328. doi: 10.1016/j.jconrel.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 127.Jiang C., Qi Z., Jia H., Huang Y., Wang Y., Zhang W., Wu Z., Yang H., Liu J. ATP-responsive low-molecular-weight polyethylenimine-based supramolecular assembly via host-guest interaction for gene delivery. Biomacromolecules. 2019;20:478–489. doi: 10.1021/acs.biomac.8b01395. [DOI] [PubMed] [Google Scholar]
- 128.Jiang C., Qi Z., He W., Li Z., Tang Y., Wang Y., Huang Y., Zang H., Yang H., Liu J. Dynamically enhancing plaque targeting via a positive feedback loop using multifunctional biomimetic nanoparticles for plaque regression. J. Control Release. 2019;308:71–85. doi: 10.1016/j.jconrel.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 129.Malam Y., Loizidou M., Seifalian A.M. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 130.Jokerst J.V., Lobovkina T., Zare R.N., Gambhir S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kamaly N., Xiao Z., Valencia P.M., Radovic-Moreno A.F., Farokhzad O.C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gadde S., Rayner K.J. Nanomedicine meets microRNA: current advances in RNA-based nanotherapies for atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016;36 doi: 10.1161/ATVBAHA.116.307481. e73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kamaly N., Fredman G., Fojas J.J.R., Subramanian M., Choi W.I., Zepeda K., Vilos C., Yu M., Gadde S., Wu J., et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 2016;10:5280–5292. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang L., Han D., Zhan Q., Li X., Shan P., Hu Y., Ding H., Wang Y., Zhang L., Zhang Y., et al. Blood TfR+ exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy. Theranostics. 2019;9:7680–7696. doi: 10.7150/thno.37220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 137.Wang C., Li Z., Liu Y., Yuan L. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11:3996–4010. doi: 10.7150/thno.56035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loyer X., Vion A.C., Tedgui A., Boulanger C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014;114:345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 139.Stamatikos A., Knight E., Vojtech L., Bi L., Wacker B.K., Tang C., Dichek D.A. Exosome-mediated transfer of anti-miR-33a-5p from transduced endothelial cells enhances macrophage and vascular smooth muscle cell cholesterol efflux. Hum. Gene Ther. 2020;31:219–232. doi: 10.1089/hum.2019.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng B., Yin W.N., Suzuki T., Zhang X.H., Zhang Y., Song L.L., Jin L.S., Zhan H., Zhang H., Li J.S., Wen J.K. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol. Ther. 2017;25:1279–1294. doi: 10.1016/j.ymthe.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Li X., He X., Wang J., Wang D., Cong P., Zhu A., Chen W. The regulation of exosome-derived miRNA on heterogeneity of macrophages in atherosclerotic plaques. Front. Immunol. 2020;11:2175. doi: 10.3389/fimmu.2020.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bouchareychas L., Duong P., Covarrubias S., Alsop E., Phu T.A., Chung A., Gomes M., Wong D., Meechoovet B., Capili A., et al. Macrophage exosomes resolve atherosclerosis by regulating hematopoiesis and inflammation via MicroRNA cargo. Cell Rep. 2020;32:107881. doi: 10.1016/j.celrep.2020.107881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu J., Liu B., Wang Z., Wang D., Ni H., Zhang L., Wang Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901–6919. doi: 10.7150/thno.37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang Y., Li M., Liu Y., Wang Z., Fu X., He X., Wang Q., Li X.X., Ma H., Wang K., et al. The lncRNA punisher regulates apoptosis and mitochondrial homeostasis of vascular smooth muscle cells via targeting miR-664a-5p and OPA1. Oxid. Med. Cell. Longev. 2022;2022:5477024. doi: 10.1155/2022/5477024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Extracellular vesicles and atherosclerotic disease. Cell. Mol. Life Sci. 2015;72:2697–2708. doi: 10.1007/s00018-015-1906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.György B., Módos K., Pállinger E., Pálóczi K., Pásztói M., Misják P., Deli M.A., Sipos A., Szalai A., Voszka I., et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–e48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 147.Hulsmans M., Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc. Res. 2013;100:7–18. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 148.Blaser M.C., Aikawa E. Differential miRNA loading underpins dual harmful and protective roles for extracellular vesicles in atherogenesis. Circ. Res. 2019;124:467–469. doi: 10.1161/CIRCRESAHA.119.314596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gomez I., Ward B., Souilhol C., Recarti C., Ariaans M., Johnston J., Burnett A., Mahmoud M., Luong L.A., West L., et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat. Commun. 2020;11:214. doi: 10.1038/s41467-019-14043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li C., Li S., Zhang F., Wu M., Liang H., Song J., Lee C., Chen H. Endothelial microparticles-mediated transfer of microRNA-19b promotes atherosclerosis via activating perivascular adipose tissue inflammation in apoE(-/-) mice. Biochem. Biophys. Res. Commun. 2018;495:1922–1929. doi: 10.1016/j.bbrc.2017.11.195. [DOI] [PubMed] [Google Scholar]
- 151.Xiong F., Mao R., Zhang L., Zhao R., Tan K., Liu C., Xu J., Du G., Zhang T. CircNPHP4 in monocyte-derived small extracellular vesicles controls heterogeneous adhesion in coronary heart atherosclerotic disease. Cell Death Dis. 2021;12:948. doi: 10.1038/s41419-021-04253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tao S.C., Guo S.C., Zhang C.Q. Modularized extracellular vesicles: the dawn of prospective personalized and precision medicine. Adv. Sci. 2018;5:1700449. doi: 10.1002/advs.201700449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. 2018;5:1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu H., Liao C., Liang S., Ye B.C. A novel peptide-equipped exosomes platform for delivery of antisense oligonucleotides. ACS Appl. Mater. Inter. 2021;13:10760–10767. doi: 10.1021/acsami.1c00016. [DOI] [PubMed] [Google Scholar]
- 155.Kwon S., Shin S., Do M., Oh B.H., Song Y., Bui V.D., Lee E.S., Jo D.G., Cho Y.W., Kim D.H., Park J.H. Engineering approaches for effective therapeutic applications based on extracellular vesicles. J. Control Release. 2021;330:15–30. doi: 10.1016/j.jconrel.2020.11.062. [DOI] [PubMed] [Google Scholar]
- 156.Chen C., Sun M., Liu X., Wu W., Su L., Li Y., Liu G., Yan X. General and mild modification of food-derived extracellular vesicles for enhanced cell targeting. Nanoscale. 2021;13:3061–3069. doi: 10.1039/d0nr06309f. [DOI] [PubMed] [Google Scholar]
- 157.Nahrendorf M., Jaffer F.A., Kelly K.A., Sosnovik D.E., Aikawa E., Libby P., Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 158.Briley-Saebo K.C., Cho Y.S., Shaw P.X., Ryu S.K., Mani V., Dickson S., Izadmehr E., Green S., Fayad Z.A., Tsimikas S. Targeted iron oxide particles for in vivo magnetic resonance detection of atherosclerotic lesions with antibodies directed to oxidation-specific epitopes. J. Am. Coll. Cardiol. 2011;57:337–347. doi: 10.1016/j.jacc.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Briley-Saebo K.C., Shaw P.X., Mulder W.J.M., Choi S.H., Vucic E., Aguinaldo J.G.S., Witztum J.L., Fuster V., Tsimikas S., Fayad Z.A. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation. 2008;117:3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pedersen M. Safety update on the possible causal relationship between gadolinium-containing MRI agents and nephrogenic systemic fibrosis. J. Magn. Reson. Imaging. 2007;25:881–883. doi: 10.1002/jmri.20983. [DOI] [PubMed] [Google Scholar]
- 161.Mitchell J.L., Lionikiene A.S., Georgiev G., Klemmer A., Brain C., Kim P.Y., Mutch N.J. Polyphosphate colocalizes with factor XII on platelet-bound fibrin and augments its plasminogen activator activity. Blood. 2016;128:2834–2845. doi: 10.1182/blood-2015-10-673285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pan D., Roessl E., Schlomka J.P., Caruthers S.D., Senpan A., Scott M.J., Allen J.S., Zhang H., Hu G., Gaffney P.J., et al. Computed tomography in color: NanoK-enhanced spectral CT molecular imaging. Angew. Chem. Int. Ed. Engl. 2010;49:9635–9639. doi: 10.1002/anie.201005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Flacke S., Fischer S., Scott M.J., Fuhrhop R.J., Allen J.S., McLean M., Winter P., Sicard G.A., Gaffney P.J., Wickline S.A., Lanza G.M. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104:1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 164.Peters D., Kastantin M., Kotamraju V.R., Karmali P.P., Gujraty K., Tirrell M., Ruoslahti E. Targeting atherosclerosis by using modular, multifunctional micelles. Proc. Natl. Acad. Sci. USA. 2009;106:9815–9819. doi: 10.1073/pnas.0903369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hyafil F., Cornily J.C., Feig J.E., Gordon R., Vucic E., Amirbekian V., Fisher E.A., Fuster V., Feldman L.J., Fayad Z.A. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 2007;13:636–641. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 166.Cormode D.P., Roessl E., Thran A., Skajaa T., Gordon R.E., Schlomka J.P., Fuster V., Fisher E.A., Mulder W.J.M., Proksa R., Fayad Z.A. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256:774–782. doi: 10.1148/radiol.10092473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amirbekian V., Lipinski M.J., Briley-Saebo K.C., Amirbekian S., Aguinaldo J.G.S., Weinreb D.B., Vucic E., Frias J.C., Hyafil F., Mani V., et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc. Natl. Acad. Sci. USA. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McCarthy J.R., Korngold E., Weissleder R., Jaffer F.A. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6:2041–2049. doi: 10.1002/smll.201000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Davis M.E., Chen Z.G., Shin D.M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 170.Huang H.C., Barua S., Sharma G., Dey S.K., Rege K. Inorganic nanoparticles for cancer imaging and therapy. J. Control Release. 2011;155:344–357. doi: 10.1016/j.jconrel.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 171.Rinoldi C., Zargarian S.S., Nakielski P., Li X., Liguori A., Petronella F., Presutti D., Wang Q., Costantini M., De Sio L., et al. Nanotechnology-Assisted RNA delivery: from nucleic acid therapeutics to COVID-19 vaccines. Small Methods. 2021;5:2100402. doi: 10.1002/smtd.202100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cui P., Xin D., Li F., Deng L., Gao Y. Butorphanol suppresses the proliferation and migration of osteosarcoma by promoting the expression of piRNA hsa_piR_006613. Front. Oncol. 2022;12:775132. doi: 10.3389/fonc.2022.775132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jin F., Yang L., Wang W., Yuan N., Zhan S., Yang P., Chen X., Ma T., Wang Y. A novel class of tsRNA signatures as biomarkers for diagnosis and prognosis of pancreatic cancer. Mol. Cancer. 2021;20:95. doi: 10.1186/s12943-021-01389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zong T., Yang Y., Lin X., Jiang S., Zhao H., Liu M., Meng Y., Li Y., Zhao L., Tang G., et al. 5'-tiRNA-Cys-GCA regulates VSMC proliferation and phenotypic transition by targeting STAT4 in aortic dissection. Mol. Ther. Nucleic Acids. 2021;26:295–306. doi: 10.1016/j.omtn.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li M., Yang Y., Zong J., Wang Z., Jiang S., Fu X., He X., Li X., Xue Q., Wang J.X., Yu T. miR-564: a potential regulator of vascular smooth muscle cells and therapeutic target for aortic dissection. J. Mol. Cell. Cardiol. 2022;170:100–114. doi: 10.1016/j.yjmcc.2022.06.003. [DOI] [PubMed] [Google Scholar]