Abstract
Recanatesis and co-authors show the need for directed noise fluctuations within cortical spike trains then find evidence for them. The directed fluctuations can produce the observed reliable sequence of discrete activity patterns, while maintaining the observed variability in the timing of transitions between patterns as rats engage in self-initiated actions.
Life is full of sequences of self-initiated actions. Typically, the order of actions in a sequence is important, while the timing can vary. For example, to take a sip of a drink we may reach for a cup, then grip the handle, then move the cup to our lips, open our mouth slightly, then tilt the cup toward our mouth. It would matter little if we paused or took more or less time for some of the steps, but if we got the order wrong, we would make a mess! The next step in a sequence of actions is dependent on the current state of the system, here for example, whether we are holding the cup or not and its current location relative to our mouth. In a paper appearing in this issue of Neuron (Recanatesis et al., 2022), the authors identify the neural representation of such states as activity patterns in secondary motor cortex (M2), an area associated with action plans. Importantly, the patterns are relatively stable until a fluctuation kicks them into the next state in a sequence, suggesting they are discrete attractor states. A key question they address is that given the large number of potential states, “How can the random fluctuation causing the state-change ensure that change is to the correct next state in the sequence?”
State transitions are apparent to us when we stare at an ambiguous image. For example, the image in Figure 1A can give rise to two different percepts, either a leftward-facing duck or a rightward-facing rabbit. If we stare at the image, it appears to flip at somewhat random intervals between the two percepts in a phenomenon called perceptual rivalry. The wide distribution of dwelling times in each state for an individual viewer indicates that state transitions include a strong random component. Models of the process aimed to match the distribution of dwelling times suggest transitions between the meta-stable attractor states are induced by random fluctuations, i. e., noise (Moreno-Bote et al., 2007).
Figure 1. Itinerancy from one attractor state to another.
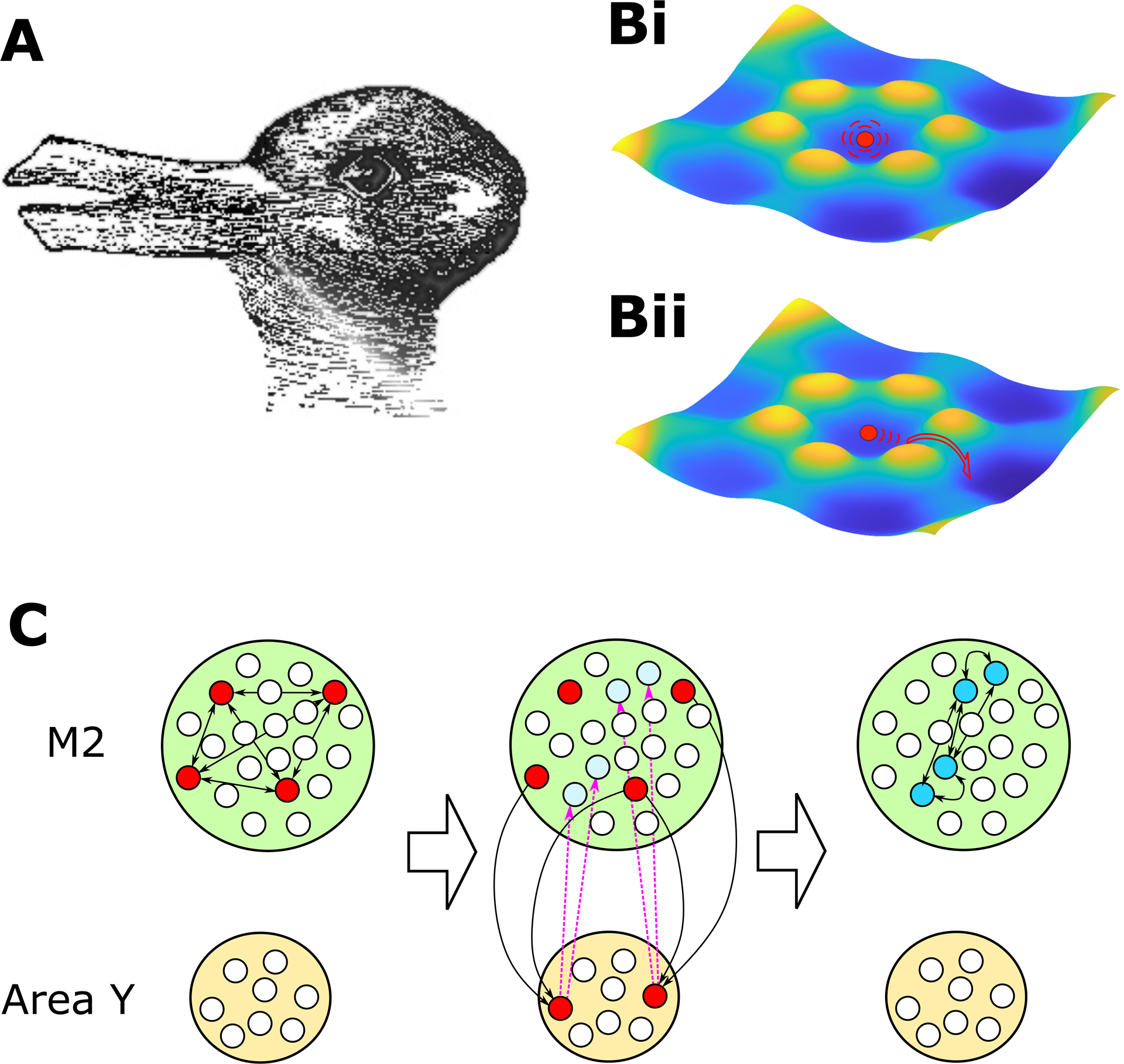
A. A constant stimulus can evoke two distinct percepts, each of which must be maintained by a distinct pattern of neural activity. The image of a leftward-facing duck or rightward-facing rabbit (whose ears are the duck’s beak) is attributed to American psychologist Joseph Jastrow (Jastrow, 1899). B. Representation of a system with multiple attractor states, where basins (dark blue) represent relatively stable patterns of activity, and the axes can be thought of as combinations of firing rates of multiple neurons. When the system is in one activity state (solid red circle), if noise fluctuations are of equal amplitude across all neurons (B1), the next transition could occur in any direction, not necessarily across the lowest saddle to the desired next attractor state. If instead, the noise fluctuations are directed (B2), then the transition can be to a predetermined pattern of firing rates even while the timing of the transition is highly variable. C. A network model (Recanatesis et al., 2022), shows how appropriate coupling of neurons in M2 to an auxiliary circuit (Area Y) leads to the observed activity state changes in M2. Solid red circles indicate activity in Pattern 1. Blue circles indicate activity in Pattern 2, the next state in the sequence. Arrows indicate a key subset of connections at each stage. Left: When an activity pattern is established in M2 it is maintained by reciprocal connections within M2. Center: It immediately excites neurons in Area Y which return highly fluctuating input (magenta, dashed connections) that “prime” the next activity pattern in M2 (light blue). Right: Sometime later a random fluctuation is sufficient to establish that subsequent pattern in M2 (solid blue circles).
Analysis of neural activity as a sequence of relatively stable activity patterns with more rapid transitions between them goes back to the 1990s (Seidemann et al., 1996, Gat et al., 1997). Those authors used Hidden Markov modeling (HMM) to extract distinct activity states from frontal cortical areas of animals preparing motor responses in a task for which the timing of an action was important. HMM is the method of choice, because it produces the optimal fit of the neural activity as a function of time separately in each trial to a set of unique activity patterns, which are also optimized to the data. The benefit of fitting each trial’s time-variation independently of that in other trials is essential for systems in which the state transitions occur at different times on different trials. If you were to look at Figure 1A again, the times following stimulus onset when you perceive either the rabbit or the duck will differ from the first viewing time. If we were to average your neural activity across such trials, we would lose the distinct neural representations of each percept and the rapid transitions between those representations (Miller, 2016). HMM allows us to avoid such a confound.
Prior work has shown how the variability of neural spike trains inherent in the awake brain can lead to variable timing in a sequence of state transitions, even in the face of a constant stimulus (Miller and Katz, 2010). However, such a result required a carefully manufactured network possessing a small number of distinct activity states. In networks that possess many discrete activity states and are not finely tuned to produce the desired result, a problem arises in producing reliable sequences. When the neural activity is in one state, there is a necessary transition to the next state in the sequence, but there are many alternative states to choose from. When activity is fluctuating randomly, even though the desired transition can be far more likely than any other, errors will abound because there are so many alternatives to choose from (Figure 1Bi). If the level of fluctuating noise is reduced to make the “best” transition more likely, then either transitions become too infrequent, or the process becomes deterministic without the observed variability in timing.
In this issue of Neuron (Recanatesis et al., 2022), the authors find reliable sequences of variably timed states extracted by HMM, and show that such data can be accounted for if noise fluctuations are directed toward the next state in the sequence (Figure 1Bii). Importantly, their hypothesis that noise is not independent and uncorrelated across neurons, but more directional—so, for example, neurons with increased rate in the next state in a sequence have a greater amplitude of activity fluctuations than other neurons—is validated by their analysis of the spike trains. Moreover, the authors devise and simulate a neural architecture, in which the activity in a connected auxiliary circuit (Area Y in Figure 1C), returns state-dependent fluctuating input back to M2, the area where they record neural spike trains and find state sequences. The authors suggest Area Y, whose activity determines the next state, could be within the basal ganglia, an area important for action selection. Any context- or history-dependence of activity in the auxiliary circuit should enable the learning and directing of multiple overlapping or non-Markovian sequences (see (Cone and Shouval, 2021)).
The timing of self-initiated actions relative to preceding neural activity, initially via a rising “readiness potential” has received considerable attention in the field of neurophilosophy (Libet et al., 1983). Recanatesis et al. show that multiple state transitions precede the first action in a series, providing further grist to the discussion about the timescale of neural activity’s impact on unforced actions. Meanwhile the transitions found to be more closely time-locked to actions support the growing literature on their importance for behavior (Sadacca et al., 2016).
References
- CONE I & SHOUVAL HZ 2021. Learning precise spatiotemporal sequences via biophysically realistic learning rules in a modular, spiking network. Elife, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAT I, TISHBY N & ABELES M 1997. Hidden markov modeling of simultaneously recorded cells in the associative cortex of behaving monkeys. Network: Computational and Neural Systems, 8, 297–322. [Google Scholar]
- JASTROW J 1899. The mind’s eye. Popular Science Monthly, 54, 299–312. [Google Scholar]
- LIBET B, GLEASON CA, WRIGHT EW & PEARL DK 1983. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain, 106 (Pt 3), 623–42. [DOI] [PubMed] [Google Scholar]
- MILLER P 2016. Itinerancy between attractor states in neural systems. Curr Opin Neurobiol, 40, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER P & KATZ DB 2010. Stochastic transitions between neural states in taste processing and decision-making. J Neurosci, 30, 2559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORENO-BOTE R, RINZEL J & RUBIN N 2007. Noise-induced alternations in an attractor network model of perceptual bistability. J Neurophysiol, 98, 1125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECANATESIS S, PEREIRA U, MURAKAMI M, MAINEN Z & MAZZUCATO L 2022. Metastable attractors explain the variable timing of stable behavioral action sequences. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADACCA BF, MUKHERJEE N, VLADUSICH T, LI JX, KATZ DB & MILLER P 2016. The Behavioral Relevance of Cortical Neural Ensemble Responses Emerges Suddenly. J Neurosci, 36, 655–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIDEMANN E, MEILIJSON I, ABELES M, BERGMAN H & VAADIA E 1996. Simultaneously recorded single units in the frontal cortex go through sequences of discrete and stable states in monkeys performing a delayed localization task. J Neurosci, 16, 752–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


