Abstract
Streptococcus mutans normally colonizes dental biofilms and is regularly exposed to continual cycles of acidic pH during ingestion of fermentable dietary carbohydrates. The ability of S. mutans to survive at low pH is an important virulence factor in the pathogenesis of dental caries. Despite a few studies of the acid adaptation mechanism of this organism, little work has focused on the acid tolerance of S. mutans growing in high-cell-density biofilms. It is unknown whether biofilm growth mode or high cell density affects acid adaptation by S. mutans. This study was initiated to examine the acid tolerance response (ATR) of S. mutans biofilm cells and to determine the effect of cell density on the induction of acid adaptation. S. mutans BM71 cells were first grown in broth cultures to examine acid adaptation associated with growth phase, cell density, carbon starvation, and induction by culture filtrates. The cells were also grown in a chemostat-based biofilm fermentor for biofilm formation. Adaptation of biofilm cells to low pH was established in the chemostat by the acid generated from excess glucose metabolism, followed by a pH 3.5 acid shock for 3 h. Both biofilm and planktonic cells were removed to assay percentages of survival. The results showed that S. mutans BM71 exhibited a log-phase ATR induced by low pH and a stationary-phase acid resistance induced by carbon starvation. Cell density was found to modulate acid adaptation in S. mutans log-phase cells, since pre-adapted cells at a higher cell density or from a dense biofilm displayed significantly higher resistance to the killing pH than the cells at a lower cell density. The log-phase ATR could also be induced by a neutralized culture filtrate collected from a low-pH culture, suggesting that the culture filtrate contained an extracellular induction component(s) involved in acid adaptation in S. mutans. Heat or proteinase treatment abolished the induction by the culture filtrate. The results also showed that mutants defective in the comC, -D, or -E genes, which encode a quorum sensing system essential for cell density-dependent induction of genetic competence, had a diminished log-phase ATR. Addition of synthetic competence stimulating peptide (CSP) to the comC mutant restored the ATR. This study demonstrated that cell density and biofilm growth mode modulated acid adaptation in S. mutans, suggesting that optimal development of acid adaptation in this organism involves both low pH induction and cell-cell communication.
Streptococcus mutans is an oral bacterium that depends on a “biofilm life-style” for survival and persistence in its natural ecosystem, dental plaque (43). Under appropriate environmental conditions, this bacterium can rapidly produce acid from fermentable dietary carbohydrates and initiate demineralization of the tooth surface (7). S. mutans is therefore considered an important etiological agent of dental caries (34). The environmental conditions encountered by S. mutans in dental biofilms are highly variable, including frequent shifts in pH from above 7.0 to as low as 3.0 during the ingestion of dietary carbohydrates by the host (16). Thus, pH exerts a significant ecological pressure on S. mutans, and its ability to tolerate and grow in low-pH environments is crucial to its survival and pathogenicity.
Considerable evidence indicates that S. mutans has evolved several mechanisms to survive the pH changes encountered in plaque. The best characterized include proton extrusion by proton-translocating F(H+)-ATPase efflux (2, 24) and expulsion of acid end-products (10). Other mechanisms include decreased proton permeability (1), increased synthesis of chaperonins (29), increased expression of the ffh gene involved in targeting of membrane-associated proteins (21), changes in membrane fatty acid composition (52), and up-regulation of DNA repair systems (22, 26).
Studies using continuous cultures first showed that during a shift to acidic pH, survival of S. mutans was enhanced when the external pH was slowly lowered by natural generation of acid end-products as opposed to being quickly dropped by rapid addition of HCl to growing cultures (1, 3, 4, 24). In batch culture, exposure of log-phase cells to a mild or moderately acidic pH (5.0 to 6.0) for 2 h resulted in enhanced survival of a significant proportion of the cell population upon exposure to the lower pH of 3.0 to 3.5 (58). Although these in vitro conditions are extreme, they provide a convenient assay for distinguishing between unadapted and adapted cells. De novo synthesis of proteins is required for the enhanced survival of S. mutans log-phase cells at the low pH (25, 59). This pH-inducible, growth phase- and time-dependent acid resistance has been well characterized in a number of bacteria; it is called the adaptive acid tolerance response (ATR) (18, 20). Although many of the molecular mechanisms of the ATR in S. mutans remain unclear, a signal pH that results in sublethal effects on the cells for sufficient time to allow synthesis of protective proteins appears to be important for induction of the ATR.
In addition to responses to physical and chemical stresses, bacteria are known to regulate diverse physiological processes in a cell density-dependent manner, where secretion of an autoinducer (AI) is detected by neighboring cells that respond by activation of regulons that result in a variety of phenotypic changes (15). Examples include the initiation of bioluminescence in Vibrio fischeri (17), competence development in Streptococcus sp. (37, 41) and Bacillus subtilis (13), biofilm differentiation in Pseudomonas spp. (11, 48), bacteriocin production in Lactococcus spp. (31), conjugal plasmid transfer in Enterococcus faecalis (14), induction of virulence factors in Staphylococcus aureus (30), and stress responses in Escherichia coli (42). Cell density-dependent regulation in these systems appears to follow a common theme, in which a small, self-generated molecule is exported as the signal for intercellular communication, commonly called quorum sensing (14). The best-characterized AIs in gram-positive bacteria are small peptides while in gram-negative bacteria the AIs are typically acylated homoserine lactones. Notably, genomic analyses have recently revealed potential peptide signaling systems that may play a role in cell-cell signaling in gram-negative bacteria (45).
During adherent growth, bacteria can sense their population size via quorum sensing and regulate gene expression and cellular functions as they adopt a biofilm phenotype (49). The changes in physiology and extracellular organization associated with this response have often been equated to multicellular behavior and organization in higher organisms (57). The ability of bacteria to communicate with one another by quorum sensing and behave collectively as a group can provide significant benefits in colonizing a new host, defense against competitors and deleterious environments, cellular differentiation, and species evolution (12).
It is widely accepted that bacteria living in biofilms are more resistant to mechanical, physical, and chemical stresses (9, 32, 48). Since S. mutans normally resides in a biofilm, the ability to withstand acid in this physiological state is likely an important adaptive response. The question of whether acid adaptation involves cell density-dependent events or cell-cell signaling in biofilms has not yet been addressed. Yet, in other bacteria it has been documented that the changes in external pH can significantly influence many physiological parameters, such as energy coupling, ion transport, proton movement, and export of metabolic products, thereby triggering numerous secondary signals (35, 47). In E. coli and Salmonella spp., such signals activate one or more global regulons that modulate expression of multiple gene operons required for acid adaptation (18, 28). Furthermore, during growth at pH 5.0, E. coli can signal acid tolerance to other unadapted cells by secreting a protein-like molecule, termed extracellular induction component (EIC) (54, 55). Although the signal molecule remains unidentified, induction of acid adaptation in E. coli presumably involves cell-cell communication.
We have recently characterized a quorum sensing system in S. mutans that utilizes a 21-amino-acid competence stimulating peptide (CSP) that functions in the induction of genetic competence during growth in biofilms (41). The system is typical of peptide pheromone signal systems in streptococci and involves comC, comD, and comE genes that, respectively, encode the precursor to the CSP, a histidine kinase and a response regulator. This is the first example of a discrete cell-cell signaling mechanism that functions in biofilms of gram-positive bacteria. Since we previously demonstrated that cell-cell signaling via this system elicits phenotypic changes during S. mutans biofilm growth, we initiated the present study to examine the ATR of S. mutans biofilm cells and to determine the effect of cell density on the ability of S. mutans to survive low pH. We also assessed the ability of the CSP to enhance acid tolerance in both wild-type cells and defined mutants defective in CSP production and tested culture filtrates collected from low-pH grown cultures for the ability to confer an acid-tolerant phenotype to unadapted cells.
MATERIALS AND METHODS
Bacterial strains and growth media.
All strains used in this study and their relevant characteristics are listed in Table 1. S. mutans BM71 (wild type) cells were subcultured on Todd-Hewitt agar plates supplemented with 0.3% yeast extract (THYE); the mutants were maintained on THYE agar containing 10 μg of erythromycin/ml. The medium used for assaying acid adaptation in batch cultures was tryptone (1%) yeast extract (0.5%) supplemented with 20 mM glucose (TYG) and 40 mM potassium phosphate/citric acid at desired pH values. For continuous cultures, TYG medium was diluted 4× and supplemented with 0.01% hog gastric mucin (type III; Sigma). In some experiments, glucose in the medium was replaced by an equivalent concentration of sucrose to determine the effect of extracellular polysaccharide on acid tolerance of biofilm cells.
TABLE 1.
Bacterial strains and plasmid used in the study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| S. mutans | ||
| BM71 | Wild type, Ems, Kms | G. H. Bowden, University of Manitoba |
| SMCC-L1 | BM71::pComC-KO, ComC−, Emr, Kms | This study |
| SMCD-L1 | BM71::pComD-KO, ComD−, Emr, Kms | This study |
| SMCE-L1 | BM71::pComD-KO, ComE−, Emr, Kms | This study |
| Plasmid, pDL289b | E. coli/Streptococcus shuttle vector, Kmr | D. J. LeBlanc, Indiana University |
Em, erythromycin; Km, kanamycin.
pDL289 was used as donor DNA to confirm competence phenotype of all the mutants.
We previously demonstrated the presence of the comCDE locus, the genes encoding a quorum sensing system, in six S. mutans strains, including strain BM71 (41). We also used two strategies to construct mutants of comC (SMCC1), comD (SMCD1), and comE (SMCE1) in S. mutans wild-type strain NG8. The same strategy was employed in this study to construct mutants defective in the same genes in S. mutans strain BM71 (41). To make the comC mutant SMCC-L1, we transformed pComC-KO plasmid DNA (pCR-Script harboring an inactivated comC gene; Ampr, Emr) into S. mutans BM71 after the plasmid was linearized by ScaI digestion to disrupt the beta-lactamase gene. Transformed colonies were selected on THYE-erythromycin (10 μg/ml) agar plates and then confirmed by a rapid PCR protocol using the existing primers and strategy as described previously (41). The knockout mutants of comD and comE were, respectively, generated by transforming pComD-KO (pVA8912 harboring a 292-bp internal comD fragment; Emr) and pComE-KO (pVA8912 harboring a 462-bp internal comE fragment; Emr) into S. mutans wild-type strain BM71. Chromosomal DNA was isolated from transformants selected on THYE-erythromycin (10 μg/ml) agar plates to determine the presence of the erm gene at the desired loci by PCR and Southern hybridization as previously described (41). The results confirmed inactivation of the comC, comD, and comE genes with the resultant mutants designated SMCC-L1, SMCD-L1, and SMCE-L1, respectively. Transformation assays (41) demonstrated that all three mutant strains were defective in genetic competence when compared with the parent strain BM71. Genetic competence was restored in strain SMCC-L1 by exogenous addition of the CSP.
Assay for acid adaptation in planktonic batch cultures.
To facilitate measurement of acid adaptation in biofilm cells, S. mutans BM71 cells were first grown in batch culture to assay the innate acid tolerance response in standard log- and stationary-phase cells by using a modification of methods described previously (33, 58). Mid-log-phase cells were obtained by transferring one volume of overnight culture into nine volumes (1:10) of fresh TYG (pH 7.5) and incubated at 37°C in an atmosphere of 5% CO2 for 2 h. These cells were then collected by centrifugation at 8,000 × g for 10 min and resuspended in 2 ml of fresh TYG (pH 5.5) at various cell densities as determined by the optical density at 600 nm (OD600). The cells were induced for acid adaptation by incubation at a “signal pH” of 5.5 for 1 to 3 h at 37°C with 5% CO2. The adapted log-phase cells were then exposed to the killing pH, which was predetermined by incubating unadapted, mid-log phase cells in TYG medium at pHs from 6.0 to 2.0 for 3 h. Stationary-phase cells were prepared by resuspending late-log phase cells in TY medium (tryptone-yeast extract) without glucose. The culture was incubated at 37°C for 2 h to allow the cells to enter stationary phase. Induction of acid adaptation in stationary-phase cells followed a similar procedure to that for log-phase cells except that glucose was omitted. Adaptation of both log- and stationary-phase cells to acidic pH was determined by measuring the ability of the bacteria to survive a killing pH for 3 h. Acid killing was initiated by resuspending cells in the same volume of fresh TYG medium (pH 3.5), and an aliquot of cell suspension was taken immediately from each sample to determine the total viable-cell number at time zero. The cells were then incubated for 3 h at 37°C with 5% CO2, and aliquots were taken at various times to determine the percent survival by viable cell counts.
Acid adaptation of S. mutans biofilms grown in continuous culture.
S. mutans BM71 cells were grown in a chemostat-based continuous flow fermentor for the development of biofilms as previously described (39, 41). Adaptation of biofilm cells to low pH was induced directly in the chemostat by pulsing 40 mM glucose into steady-state cultures and disconnecting the pH control. The culture pH was allowed to drop by the accumulation of acid end-products generated from glucose metabolism. The growth of bacterial cells under this condition was limited by glucose, as determined by assaying residual glucose in the cultures. Initial experiments showed that following the glucose pulse without pH control at a dilution rate of 0.1 h−1, the culture pH dropped rapidly to around pH 5.5 in the first 40 min. The pH continued to decrease for about 6 h until reaching its lowest point of pH 4.92. The pH remained at this level for about 1 to 2 h and slowly returned to about pH 5.95. Adaptation of the cultures was allowed to occur for at least one mean generation time (6.93 h at a dilution rate [D] of 0.1 h−1). Samples were then taken from both the biofilms and the planktonic phase to assess survival of bacterial cells to the killing pH. Control samples (unadapted cells) were also taken from a duplicate culture under pH control (at a constant pH of 7.0) following glucose pulse.
Acid killing of biofilm cells.
Biofilms on glass rods were removed from the chemostat and divided into two groups for acid killing experiments: one group of biofilms was dissociated from the surfaces (dispersed biofilms), and the other group remained attached to the surfaces (intact biofilms). Dissociation of biofilms from surfaces was performed by sonication using the BioSonik IV (Bronwill, Rochester, N.Y.) with a low power output at a setting of 20 for 20 s. This procedure removed >99% of the attached cells as estimated by comparing plate counts and scanning electron micrographs (41). The procedure resulted in dispersion of the majority of aggregates and disruption of chains into single cells, as indicated by light microscopy. Aliquots (200 μl) of the cell suspension were taken from the dissociated biofilm samples to determine the viable cell number at time zero. Total cell numbers in intact biofilms were determined by growing eight rods under identical conditions and using four rods for enumeration following sonication and four rods for the acid killing experiments. Biofilm cells dispersed from, or intact on, each rod were suspended in 2 ml of fresh TYG (pH 3.5) in a 5-ml glass tube and incubated at 37°C with 5% CO2 for 3 h. Following acid killing, intact biofilms were dissociated from the surface by sonication. All samples were serially diluted in 10 mM potassium phosphate buffer (pH 7.2) and plated on THYE agar plates by using a spiral plater (model D; Spiral System Inc., Cincinnati, Ohio). The number of surviving cells was determined by counting colonies after the plates were incubated at 37°C with 5% CO2 for 2 to 3 days. Percent survival was expressed as the number of viable cells after acid killing over the total number of viable cells at time zero when cells were exposed to killing pH (3.5).
Preparation of culture filtrates and induction of acid adaptation.
To determine if low-pH growing cultures contained EICs capable of inducing an ATR, cell-free supernatants were prepared from chemostat cultures of S. mutans wild-type strain BM71 and the comC mutant (SMCC-L1) grown in undiluted TYG medium (3% tryptone, 1% yeast extract, and 20 mM glucose). Cell-free supernatants were collected from chemostat cultures at a dilution rate (D) of 0.2 h−1 at 4 h after a pulse of 40 mM glucose with or without pH control. The cell-free supernatant collected from the culture without pH control (pH 5.2 ± 0.3 [mean ± standard deviation {SD}]) was neutralized to pH 7.0 with KOH and sterilized by passage through a 0.2-μm-pore-size filter (Millipore, Bedford, Mass.), whereas the supernatant collected from the culture with pH control (pH 7.0) was filter sterilized without adjustment of pH. All culture filtrates were then kept at −20°C until use. Some samples were treated either with heat (65°C) for 30 min or with proteinase K (37°C) for 1 h before addition to the cultures. Before their use for induction of the ATR, culture filtrates were added to an equal volume of fresh TYG (pH 7.5) to provide the cells with an energy source. Induction of acid adaptation was initiated by incubating log-phase cells with the culture filtrates for 2 h before exposing them to the killing pH. The effect of culture filtrate concentration on acid adaptation was determined by diluting the filtrates one- to eightfold in TYG.
Acid adaptation in comC, comD, and comE mutants.
The comCDE operon encodes a quorum sensing system that controls the cell density-dependent induction of genetic competence in S. mutans (41). We examined the effects of inactivation of the comC, comD, and comE genes on acid adaptation in S. mutans. Assays for ATR in the mutants were performed by the same methods described above. In addition, we used a 21-amino-acid synthetic signal peptide deduced from the comC gene sequence (41) to determine if the CSP restored acid tolerance in the comC mutant during acid adaptation. The CSP was freshly dissolved in sterile distilled water to a concentration of 1 mg/ml. The solution was then added to the cultures at a final concentration of 2 μg/ml 2 h after inoculation of cells. To determine if secondary signals could augment the activity of the CSP, the pH-adjusted culture filtrates from acid adapted cultures of the comC mutant were used to assay ATR in conjunction with the CSP as described above.
Scanning electron microscopy.
To examine the spatial distribution and biofilm density by scanning electron microscopy, biofilms of different ages were removed, washed once with 10 mM KPO4, and fixed with 2 ml of 3.7% formaldehyde in 10 mM KPO4 buffer overnight. The samples were then dehydrated with a series of alcohol washes (30, 50, 70, 95, and 100%), critical point dried with liquid CO2, mounted, and sputter-coated with gold. The samples were then examined using a scanning electron microscope (model S-2500; Hitachi Instruments, San Jose, Calif.).
RESULTS
pH limit for survival.
To assess the ATR, the pH limit for survival of S. mutans BM71 was first determined by measuring viability after exposure of mid-log phase cells to media at pHs from 2.0 to 6.0 for 3 h. S. mutans BM71 cells grew and maintained 100% viability at pH 6.0 to 5.5, and the cells still survived well at pH 5.0 (91 to 98%). At lower pH, the survival rates rapidly decreased, with 100% of the cells killed after exposure to pH 3.0. Since exposure to pH 3.5 for 3 h could kill over 99.99% of unadapted log-phase cells, we used this pH as the killing pH to assess the ATR in this study.
Cell density modulates induction of acid adaptation.
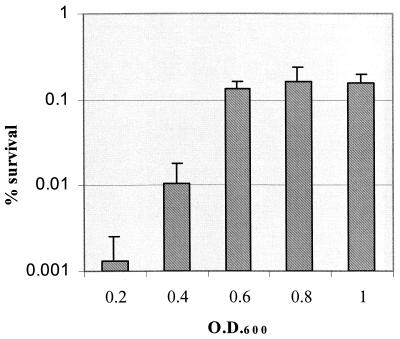
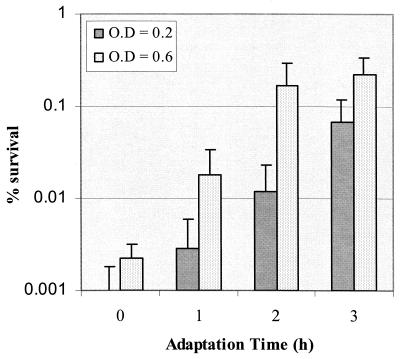
The ability of S. mutans to tolerate exposure to pH 3.5 via pH-induced ATR in log-phase cells was modulated by the population density (Fig. 1). At the higher cell densities (OD600 of 0.6 or higher, approximately 109 cells/ml or more), S. mutans BM71 cells rapidly became adapted to low pH; the induction of adaptation was optimal after 2 h of incubation at pH 5.5 (Fig. 2). Additional exposure (3 h in total) to this signal pH did not further enhance survival of cells to acid challenge. Induction of ATR in log-phase cells at a low cell density (0.2 at OD600, or 106 cells/ml) by exposure to the signal pH for 2 h was insufficient for optimal development of acid adaptation. Increasing the induction time further enhanced acid adaptation, although the magnitude of acid resistance at the low cell density was still lower than that observed with the higher cell density (Fig. 2). In this study, we used the cell density of 0.6 at OD600 for most experiments unless specified. To test whether increased cell density was specific to the adaptation phase, we performed an additional experiment in which S. mutans cells were induced for acid adaptation at pH 5.5 for 2 h at a high density (0.6 at OD600) and then diluted to OD600's of 0.1 and 0.2. These diluted cells and the undiluted sample were exposed to pH 3.5 for 3 h. The results demonstrated that there was no statistical difference in the percentage of survival found between the different groups with T-test (P value of 0.05) (data not shown).
FIG. 1.
Effect of cell density on induction of acid adaptation in S. mutans BM71 log-phase cells. Cells were collected from pH 7.0 cultures and resuspended at various cell densities during the adaptation phase at pH 5.5 for 2 h and were then exposed to the killing pH of 3.5 for 3 h. Results are the mean ± SD of at least three independent cultures.
FIG. 2.
Effects of cell density and adaptation time on induction of acid adaptation by S. mutans BM71. Cells were adapted by incubation in pH 5.5 TYG medium at low (OD600 = 0.2) or high (OD600 = 0.6) density for various times before exposure to the killing pH of 3.5 for 3 h. Results represent the mean number of surviving cells ± SD from at least three independent cultures.
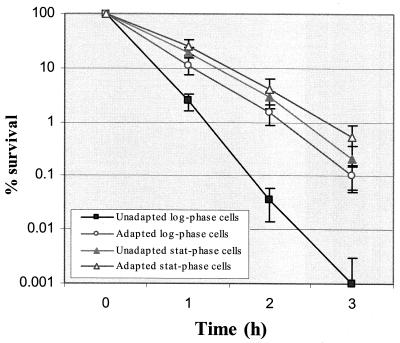
S. mutans BM71 exhibits both log- and stationary-phase ATR.
We tested the ATR of planktonic cells in log and stationary phase for comparison to biofilm-grown cells. The results from batch cultures indicated that survival of S. mutans BM71 to killing pH involved at least two adaptive systems that were dependent of growth phase (Fig. 3). The first system appeared during log phase growth in which preexposure of cells to pH 5.5 for 2 h protected the cells against subsequent killing at pH 3.5. The adapted log-phase cells survived approximately 50- to 100-fold better than the unadapted cells following exposure to the killing pH for 3 h.
FIG. 3.
Survival kinetics of S. mutans BM71 exposed to killing pH of 3.5. Both adapted and unadapted stationary- and log-phase cultures were sampled at various time points after exposure to the killing pH of 3.5. The number of survivors was determined from at least three independent cultures.
Acid protection was also induced by entry into carbon starvation-induced-stationary phase by incubation in medium devoid of glucose as described. These stationary-phase cells had an equivalent or slightly increased resistance to the killing pH 3.5 than the log-phase pH-induced cells (Fig. 3). Further exposure of stationary-phase cells to pH 5.5 for 2 h only slightly enhanced resistance to the killing pH.
Culture filtrate induces log-phase ATR.
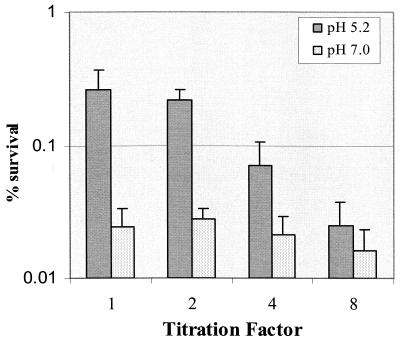
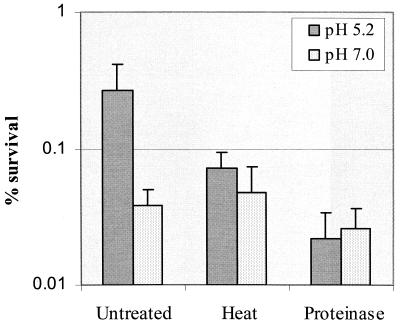
The observation that high cell density promoted induction of acid adaptation (Fig. 1), suggested that S. mutans BM71, during exposure to a low (signal) pH, utilizes a cell density-dependent mechanism to enhance induction of acid adaptation in the population via release of an extracellular signal molecule(s). To test this hypothesis, we collected cell-free supernatants from S. mutans chemostat cultures grown at low pH (5.2 ± 0.3) and assayed acid adaptation conferred by neutralized culture filtrate, as described in Materials and Methods. After incubation in the neutralized culture filtrate for 2 h, log-phase cells of S. mutans BM71 became more resistant to the killing pH than cells incubated in the filtrate collected from the culture at pH 7.0 (Fig. 4). Dilution of the culture filtrate with fresh TYG (pH 7.5) resulted in a titratable decrease in acid resistance, demonstrating that the acid adaptation was dependent on the concentration of the culture filtrate and, consequently, EICs. The culture filtrates at dilution factors of 1:1 and 1:2 revealed no significant difference in the effect on induction of acid adaptation. This suggested that the culture filtrates at both dilutions might represent a saturated concentration of the EIC required for acid adaptation. The EIC(s) in the culture filtrate were probably proteinaceous since heat or proteinase treatment abolished their effect on induction of acid adaptation (Fig. 5).
FIG. 4.
The neutralized culture filtrate collected from the chemostat at pH 5.2 ± 0.3 (mean ± standard deviation) was able to induce a log-phase ATR. The culture filtrate was diluted with fresh TYG medium (pH 7.5) to illustrate a dose-dependent effect. The results are the mean ± SD of at least three independent cultures.
FIG. 5.
Effect of heat or proteinase treatment on induction of acid adaptation in S. mutans BM71 by pH-neutralized culture filtrates collected from pH 5.2 (adapted) and pH 7.0 (unadapted) cultures. The results are the mean ± SD of at least three independent cultures.
Inactivation of comCDE genes results in reduced log-phase ATR.
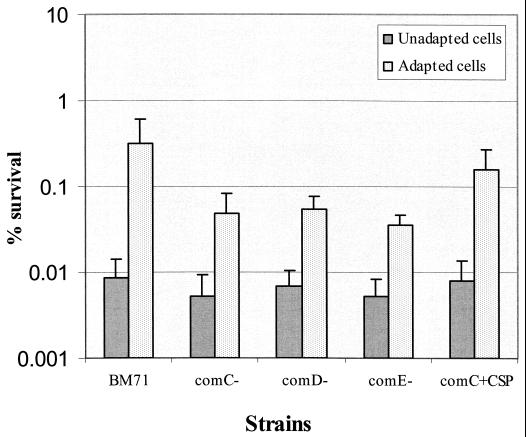
Since the S. mutans comCDE operon encodes a peptide pheromone quorum sensing system that controls the cell density-dependent competence phenotype in S. mutans biofilms (41), we examined the ability of mutants with defects in the signaling system to withstand acid challenge. Disruption of the comC, comD, or comE genes resulted in a diminished log-phase ATR (Fig. 6). Even after exposure to adaptive pH for 2 h, the mutants were still 10-fold more sensitive to the killing pH than the wild-type strain. However, unadapted mutants did not show substantial defects in their ATR relative to the parent strain BM71. Addition of CSP to the culture of the comC mutant during acid adaptation partially restored the wild-type ATR. These results demonstrated that the quorum sensing system plays a role in induction of acid adaptation.
FIG. 6.
Acid tolerance was assayed in mutants defective in comC, comD, and comE, the genes encoding a quorum sensing system essential for cell density-dependent induction of genetic competence in unadapted and adapted S. mutans log-phase grown cells. Addition of CSP into the culture of comC mutant partially restored the wild-type acid tolerance. The results are the mean ± SD of four independent cultures.
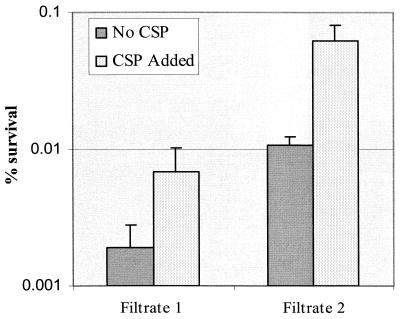
To further test for the presence of secondary molecules that augment the ATR mediated by the signal peptide, we tested the cell-free culture supernatants collected from the comC mutant for their ability to enhance the ATR in the comC mutant in the absence or presence of exogenous CSP. The pH-adjusted culture filtrate from the comC mutant plus the synthetic CSP almost completely restored the log-phase ATR, whereas the CSP or the culture filtrate alone only partially restored the ATR (Fig. 7). The results suggested the presence of a second signal molecule(s) in S. mutans culture supernatant that could augment the ATR.
FIG. 7.
The pH-adjusted culture filtrates collected from comC mutant (SMCC-L1) cultures that were maintained at pH 7.0 (filtrate 1) or pH 5.2 (filtrate 2) were added either individually or with synthetic CSP to unadapted SMCC-L1 cultures before exposure to the killing pH 3.5 for 3 h. The percentage of survivors ± SD was determined from four independent cultures.
Physical characteristics of biofilms formed in chemostat.
To induce acid adaptation in biofilm cells without disrupting the biofilm architecture, we used a chemostat-based biofilm fermentor to establish acid adaptation via acid generated from glucose metabolism of the cells. The cell density of S. mutans biofilms grown under glucose limitation, in terms of total biomass, biofilm thickness, and number of viable cells, is a function of the accumulation time (38). The relative cell densities of biofilms, as represented by the number of viable cells, after glucose pulse with or without pH control are shown in Table 2. Generally, the density of S. mutans BM71 biofilms increased with accumulation time and glucose availability, with or without pH control. Interestingly, the cell number in the biofilms after glucose pulse but without pH control was about twofold higher than that of biofilms grown under pH control. Conversely, the yield of planktonic cells present after the natural pH drop ([8.4 ± 2.8] × 107CFU/ml) was lower than that with pH control ([27.6 ± 6.4] × 107CFU/ml). The results suggested that active multiplication of the planktonic cells after a glucose pulse without pH control was rapidly reduced or even arrested by the acid generated from the culture. In contrast, the biofilms continued to accumulate on the surface during the pH shift, resulting in a three- to fivefold increase in the number of viable cells. When cultures were pulsed with sucrose, S. mutans BM71 cells formed even thicker and denser biofilms than with glucose; extracellular polysaccharide was clearly visible with scanning electron microscopy (data not shown).
TABLE 2.
Viable cell numbers of S. mutans (BM71) biofilms grown at D of 0.1 h−1 following 40 mM glucose pulse with or without pH control
| Culture conditions | Viable cell number (mean ± SD) of:
|
||
|---|---|---|---|
| 12-h biofilms (106/cm2) | 24-h biofilms (106/cm2) | 5-day biofilms (106/cm2) | |
| G-pulse, pH 7.0a | 8.6 ± 3.8 | 21.5 ± 5.3 | 47.4 ± 8.3 |
| G-pulse, pH 5.2b | 26.5 ± 6.8 | 74.5 ± 21.7 | 176.2 ± 29.4 |
Glucose pulse with pH control in which biofilms were taken as unadapted cells.
Glucose pulse without pH control (pH was decreasing down to 4.89) in which biofilms were taken as adapted cells for assaying acid killing.
Acid tolerance of biofilm-grown cells.
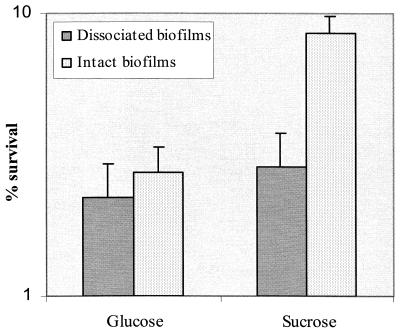
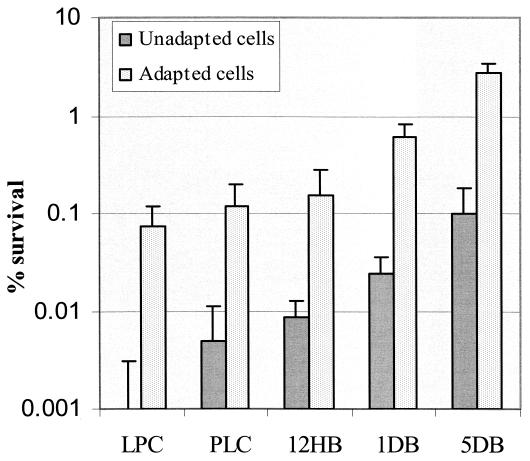
To determine the effects of biofilm integrity on the ability of the cells to withstand acid stress, we compared acid tolerance of S. mutans in both intact and disrupted biofilms. Disruption of structural integrity showed little effect on acid resistance of biofilms grown with glucose as a carbon source (Fig. 8). However, the thicker, intact biofilms formed in sucrose-containing medium showed three- to fivefold more resistance to killing pH than the resuspended biofilm cells (Fig. 8), demonstrating that extracellular polysaccharide enhanced acid resistance of bacteria within biofilms. To minimize the difference in cell numbers in biofilm-grown cells for acid killing experiments, we resuspended the dispersed biofilm-grown cells in TYG medium to a cell density of 0.6 at OD600 prior to exposure to the killing pH. The results indicated that the development of acid adaptation in actively growing biofilms varied with the cell density or thickness of the biofilms regardless of whether the films were dispersed or left intact. Cells from the thicker and denser biofilms had a much higher resistance to the killing pH than the planktonic cells and adapted log-phase cells (Fig. 9). For example, cells recovered from 5-day biofilms that contained the highest cell density per unit area (over 108/cm2) were about 30-fold more resistant to the killing pH than the planktonic cells or even the 12- to 24-h biofilms. The results provided further evidence that biofilms with high cell density facilitated induction of acid adaptation in S. mutans.
FIG. 8.
Effect of biofilm integrity on acid resistance of biofilms. Biofilms were either intact or dissociated from the surfaces by sonication. The biofilms were grown under the condition of either glucose or sucrose as a carbon source as described in Materials and Methods.
FIG. 9.
Acid tolerance response of S. mutans BM71 biofilm cells. LPC, log-phase cells; PLC, planktonic cells; 12HB, 12-h biofilm; 1DB, 1-day biofilm; 5DB, 5-day biofilms. Results are the means ± standard deviation of four cultures.
DISCUSSION
Since dental caries develops in an intermittently acidic environment, one of the most significant virulence properties of cariogenic bacteria like S. mutans is their acid tolerance. Under high-carbohydrate conditions, S. mutans outcompetes some of the most acidogenic and aciduric species in dental plaque (3, 5, 7). The evidence from these studies suggests that induction of acid adaptation in S. mutans is an important virulence factor since its aciduricidy is a feature that sets it apart from a number of other oral bacteria (58). Adaptation of S. mutans to low pH in its natural ecosystem of dental plaque is usually characterized by active transport of carbohydrates, increased glycolytic activity, and an increase in cell number (7, 10, 23, 27, 46).
In planktonic culture, exposure of S. mutans to an extracellular signal pH between 5.5 and 5.0 results in the synthesis of a subset of proteins that enhances its ability to survive low-pH challenges (25, 59). The molecular mechanisms of the S. mutans ATR remain poorly understood, especially during growth in biofilms, where the cells are known to exhibit a phenotype different from that of planktonic cells. Since S. mutans normally encounters acid while living in dense biofilm communities, we proposed that high cell densities of S. mutans may facilitate its survival against low-pH challenges. Testing this hypothesis led us to the unprecedented finding that the ATR interfaces with a density-dependent signaling pathway that also initiates genetic competence.
Since S. mutans relies on a biofilm lifestyle for survival and utilizes a quorum sensing system to modulate genetic competence via a cell density-dependent mechanism (41), we set forth to investigate a possible connection between cell density, biofilm growth, and quorum sensing in the process of acid adaptation by S. mutans. Our results clearly showed that cell density modulated acid adaptation in S. mutans log-phase cells, since S. mutans grown at high cell density established adaptation to the signal pH more rapidly than the cells at the lower density. Similarly, S. mutans cells grown in a high cell density biofilm were more resistant to the killing pH than planktonic-phase cells. In fact, S. mutans cells grown in biofilms not only survived better than the planktonic cells but were also capable of growth at the lower pH following a glucose pulse (Table 2). It is likely that optimal induction of acid adaptation in a population of S. mutans requires a coordinated activity through mechanisms involving both low pH induction and cell density-dependent intercellular communication.
Based on the evidence obtained from this study, we propose that S. mutans, upon exposure to low pH in a growing culture, releases an extracellular signal molecule(s) to enhance induction of acid adaptation in the population. The evidence that neutralized culture filtrates prepared from acid-adapted cells induced a log-phase ATR in cells that had never encountered the signal pH and that the extent of the acid tolerance varied with cell density and concentration (titration) of the culture filtrate strongly supports this hypothesis. The EIC(s) produced during acid adaptation probably acted as a secondary signal to amplify the induction of acid adaptation in the population. Upon detection of falling pH this signal is likely secreted and detected by neighboring cells to activate the ATR to protect them from the impending pH drop, caused by sugar metabolism. In addition, our preliminary results indicated that the extracellular component(s) in the culture filtrate responsible for the induction of acid adaptation were protein-like molecule(s).
We have recently identified and characterized a peptide-mediated quorum sensing system essential for the cell density-dependent induction of genetic competence in S. mutans (41). Since this quorum sensing system functions optimally during biofilm growth, we investigated the role that this system plays in the induction of acid adaptation. This quorum sensing system consists of at least five gene products, including a 21-amino-acid CSP (amino acid sequence, SGSLSTFFRLFNRSFTQALGK) whose precursor is encoded by comC, a histidine kinase sensor protein encoded by comD, and the cognate response regulator encoded by comE. The comCDE genes are located in the same locus and together constitute a signal sensing system for generating and responding to the active CSP. Two other genes required for peptide processing and export, cslAB (comAB), are located on a separate region of the chromosome and encode a CSP-specific secretion system consisting of an ATP-binding cassette transporter cslA (ComA) and its accessory protein cslB (ComB) (50; J. H. Lee, P. C. Y. Lau, Y.-H. Li, M. Meloche, A. J. Cuttichia, R. P. Ellen, and D. G. Cvitkovitch, submitted for publication). Our previous experiments demonstrated that this cell-cell signaling system produced optimal genetic competence when the cells were living in actively growing biofilms. In the present study, we found a connection between the competence signaling pathway and the ATR, suggesting that the CSP may function as an EIC.
The results clearly showed that mutants defective in the comC, comD, or comE genes had a diminished log-phase ATR, although cells exposed to the signal pH still remained more resistant to the killing pH than unadapted cells, suggesting that induction of acid adaptation in S. mutans is regulated by more than one system or signal. To test if a second secreted signal molecule was involved, we collected the cell-free culture supernatants from cultures of acid adapted and unadapted comC mutant SMCC-L1 (unable to produce the CSP) and assayed for the ability of the pH-neutralized filtrate to enhance the ATR in unadapted SMCC-L1 in the presence or absence of exogenous CSP. The results demonstrated that the pH-adjusted culture filtrate collected from the adapted comC mutant did contain a signal molecule(s) that contributed to the overall ATR. In fact, addition of both the pH-adjusted culture filtrate and the synthetic CSP almost completely restored the log-phase ATR in SMCC-L1, demonstrating that CSP and at least one other signal molecule present in the S. mutans culture supernatant contribute to induction of the ATR.
This study also demonstrated that S. mutans BM71 was able to survive low-pH challenge after exposure to a signal pH of 5.5 and was modulated by cell density. This result was consistent with the findings of Svensäter et al. (59), who demonstrated that exposure of log-phase S. mutans to the signal pH (5.0 to 5.5) up-regulated the synthesis of 64 proteins, 25 of them acid specific, although 49 proteins exhibited diminished synthesis upon low-pH exposure. Preliminary studies with S. mutans strain NG8 demonstrated, by comparison of two-dimensional (2-D) gel profiles, that at least 10 identical proteins were induced by both low pH and by the CSP (data not shown). To further support the hypothesis that EIC(s) were involved and we were not simply observing a phenomenon resulting from cells forming physical aggregates, the acid shock at pH 3.5 was repeated with cells at various densities, and no difference in percent survival was observed.
Acid tolerance was also enhanced during stationary phase brought about by carbon starvation. These conditions provided the cells with an equivalent or even slightly higher level of acid resistance than the log-phase ATR. A similar result was observed by Svensäter et al. (59), who showed that carbon starvation in S. mutans resulted in a 7- to 12-fold increase in the resistance to acid killing when compared with the cells grown in medium with 20 mM glucose. More recently, Zhu et al. (60) reported that starvation induced by resuspending S. mutans cells in 40 mM phosphate buffer (pH 7.0) for 24 h resulted in a significant increase in survival of both biofilm and planktonic cells to killing by lactic acid (pH 3.8). In Salmonella spp. and E. coli, this stationary phase acid tolerance system is part of a general stress resistance induced by entry into stationary phase and regulated by an alternative sigma factor, RpoS (18, 36). The system provides cross-protection against a number of other stresses, such as heat, cold, oxidation, high osmolarity, and heavy metals (18, 19, 28). A similar general stress resistance has been identified in several gram-positive bacteria, including Lactococcus lactis (53), Lactobacillus acidophilus (56), and B. subtilis (51), although the molecular mechanism controlling the general stress resistance in gram-positive bacteria remains unclear. We anticipate that starvation-induced acid resistance in S. mutans represents the same general stress resistance observed in other gram-positive organisms. The stationary phase stress resistance mechanism is likely very important for the survival of S. mutans in biofilms since it is believed that the biofilm phenotype is akin to the physiological state that cells exist when in stationary phase (60).
It has been widely reported that bacteria grown as a surface biofilm are more resistant to various stress challenges and antimicrobial agents than planktonic cells (9). The results from our present work and other studies demonstrated that S. mutans grown as a biofilm is more resistant to low-pH challenge than the cells in suspension (60). The underlying mechanisms that lead to increased resistance of biofilms to stress are not well understood. It was originally believed that the increase in resistance was provided to bacterial cells by the physical barrier that exists within biofilms, resulting in diffusion-limiting gradients (8). With S. mutans, however, the physical barrier of biofilms appears to play a limited role in acid diffusion, since use of a proper buffering system in liquid phase provides a relatively good control of biofilm pH, as shown by monitoring the in situ pH of biofilms in an in vitro experimental system (6, 40). Biofilms grown in aquatic environments usually form a 3-D, mushroom-like structure with numerous water channels within the biofilms, which allow various ions and small molecules to diffuse throughout the biofilms (9, 49). The data from this study also showed that intact biofilms grown with glucose as a carbon source did not provide significant physical protection against lethal acid, since cells that were removed from biofilms and were dispersed and resuspended in medium were equally resistant to acid stress, as were cells in intact biofilms. This suggests that the increased acid resistance observed with S. mutans biofilm cells probably resulted from changes in the cells' physiology, mediated in part by extracellular signal molecules. When sucrose was added to the biofilm cultures, there was a marked increase in acid resistance. It is likely that this arises partly from the altered biofilm architecture resulting from accumulation of extracellular polysaccharide formed under these conditions.
Biofilms, particularly thicker biofilms, may provide bacterial cells with a unique environment to fully express their adaptive survival mechanisms. Because of 3-D structures, high cell density, and diffusion barriers, bacterial cells at different locations within a biofilm may not sense the same degree of pH stress simultaneously. The cells that first sense a pH stress may rapidly process the information and pass their secondary signal to the other members of the population through cell-cell signaling systems to initiate a coordinated protective response against potentially lethal acid. Unlike planktonic cells that need to reach a critical concentration of signal molecules and cell density, biofilms can allow signal molecules to accumulate rapidly in the local environment to initiate coordinated activities far more quickly (44, 49). In addition, physiological states of bacterial cells living in a biofilm, in terms of growth rate, growth phase, or metabolic activities, are heterogeneous. This allows the cells to respond to stress in different ways. Apparently, a biofilm population has several advantages since the cells have more time, a sufficient concentration of signal molecules, and high population density to adapt to stress relative to planktonic cells.
We conclude that the ATR in S. mutans has both a log-phase ATR and a general acid resistance system in stationary-phase cells. Population density and cell-cell signaling modulate acid adaptation in S. mutans log-phase cells. Since S. mutans cells grown in high-cell-density biofilms were invariably more resistant to the killing pH than the planktonic cells, we propose that high-cell-density biofilms provide a unique environment for induction of acid adaptation via quorum sensing in S. mutans. The quorum sensing pathway significant for the ATR intersects the regulatory pathway for genetic competence. It is likely that the biofilm growth mode allows S. mutans to utilize multiple mechanisms for survival during low pH stress, and these are dependent on carbohydrate availability, external pH, growth phase, population density, and various coordinated activities.
ACKNOWLEDGMENTS
We thank Marie-Christine Kean for help in preparation of the manuscript.
Our work was supported by PHS grant DE 013230-01 from the National Institute of Dental and Craniofacial Research and grant MT-15431 from the Medical Research Council of Canada and by infrastructure grants from the Canadian Foundation for Innovation and The Ontario Innovation Trust. D.G.C. is supported by a Canada Research Chair and M.N.H. is the recipient of a University of Toronto Open Fellowship and Ontario Graduate Scholarship in Science and Technology.
REFERENCES
- 1.Belli W A, Marquis R E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57:1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender G R, Sutton S V, Marquis R E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986;53:331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowden G H, Hamilton I R. Competition between Streptococcus mutans and Lactobacillus casei in mixed continuous culture. Oral Microbiol Immunol. 1989;4:57–64. doi: 10.1111/j.1399-302x.1989.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 4.Bowden G H, Hamilton I R. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and “S. mitior” growing in continuous culture. Can J Microbiol. 1987;33:824–827. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw D J, McKee A S, Marsh P D. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res. 1989;68:1298–1302. doi: 10.1177/00220345890680090101. [DOI] [PubMed] [Google Scholar]
- 6.Burne R A, Quivey R G, Jr, Marquis R E. Physiologic homeostasis and stress responses in oral biofilms. Methods Enzymol. 1999;310:441–460. doi: 10.1016/s0076-6879(99)10035-1. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson J, Hamilton I R. Metabolic activity of oral bacteria. In: Thylstrup A, Fejerskov O, editors. Textbook of clinical cariology. 2nd ed. Copenhagen, Denmark: Munksgaard; 1994. pp. 71–88. [Google Scholar]
- 8.Characklis W G, Marshall K C. Biofilms. Washington, D.C.: John Wiley & Sons Inc.; 1990. [Google Scholar]
- 9.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 10.Dashper S G, Reynolds E C. pH regulation by Streptococcus mutans. J Dent Res. 1992;71:1159–1165. doi: 10.1177/00220345920710050601. [DOI] [PubMed] [Google Scholar]
- 11.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 12.de Kievit T R, Iglewski B H. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubnau D. Genetic competence in Bacillus subtilis. Microbiol Rev. 1991;55:395–424. doi: 10.1128/mr.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunny G M, Leonard B A. Cell-cell communication in gram-positive bacteria. Annu Rev Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 15.Dunny G M, Winans S C. Cell-cell signaling in bacteria. Washington, D.C.: ASM Press; 1999. p. 367. [Google Scholar]
- 16.Edgar W M, Higham S M. Saliva and the control of plaque pH. In: Edgar W M, O'Mullane D M, editors. Saliva and oral health. 2nd ed. London, United Kingdom: British Dental Association; 1996. pp. 81–94. [Google Scholar]
- 17.Engebrecht J, Silverman M. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic Acids Res. 1987;15:10455–10467. doi: 10.1093/nar/15.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster J W. Low pH adaptation and the acid tolerance response of Salmonella typhimurium. Crit Rev Microbiol. 1995;21:215–237. doi: 10.3109/10408419509113541. [DOI] [PubMed] [Google Scholar]
- 19.Foster J W. Microbial response to acid stress. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: ASM Press; 2000. pp. 99–115. [Google Scholar]
- 20.Foster J W, Hall H K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991;173:5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez J A, Crowley P J, Cvitkovitch D G, Brady L J, Hamilton I R, Hillman J D, Bleiweis A S. Streptococcus mutans ffh, a gene encoding a homologue of the 54 kDa subunit of the signal recognition particle, is involved in resistance to acid stress. Microbiology. 1999;145:357–366. doi: 10.1099/13500872-145-2-357. [DOI] [PubMed] [Google Scholar]
- 22.Hahn K, Faustoferri R C, Quivey R G., Jr Induction of an AP endonuclease activity in Streptococcus mutans during growth at low pH. Mol Microbiol. 1999;31:1489–1498. doi: 10.1046/j.1365-2958.1999.01292.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton I R. Effects of changing environment on sugar transport and metabolism by oral bacteria. In: Reizer J, Peterkofsky A, editors. Sugar transport and metabolism by gram-positive bacteria. New York, N.Y: John Wiley & Sons Inc.; 1987. pp. 94–133. [Google Scholar]
- 24.Hamilton I R, Buckley N D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol. 1991;6:65–71. doi: 10.1111/j.1399-302x.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton I R, Svensäter G. Acid-regulated proteins induced by Streptococcus mutans and other oral bacteria during acid shock. Oral Microbiol Immunol. 1998;13:292–300. doi: 10.1111/j.1399-302x.1998.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 26.Hanna M N, Ferguson R J, Li Y H, Cvitkovitch D G. uvrA is an acid-inducible gene essential for the adaptive response to low pH in Streptococcus mutans. J Bacteriol. 2001;183:5964–5973. doi: 10.1128/JB.183.20.5964-5973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper D S, Loesche W J. Growth and acid tolerance of human dental plaque bacteria. Arch Oral Biol. 1984;29:843–848. doi: 10.1016/0003-9969(84)90015-3. [DOI] [PubMed] [Google Scholar]
- 28.Hengge-Aronis R. The general stress response in Escherichia coli. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: ASM Press; 2000. pp. 161–178. [Google Scholar]
- 29.Jayaraman G C, Penders J E, Burne R A. Transcriptional analysis of the Streptococcus mutans hrcA, grpE and dnaK genes and regulation of expression in response to heat shock and environmental acidification. Mol Microbiol. 1997;25:329–341. doi: 10.1046/j.1365-2958.1997.4671835.x. [DOI] [PubMed] [Google Scholar]
- 30.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleerebezem M, Quadri L E, Kuipers O P, de Vos W M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 32.Kolenbrander P E. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 33.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 34.Kuramitsu H K. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;4:159–176. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- 35.Lazazzera B A. Quorum sensing and starvation: signals for entry into stationary phase. Curr Opin Microbiol. 2000;3:177–182. doi: 10.1016/s1369-5274(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 36.Lee I S, Lin J, Hall H K, Bearson B, Foster J W. The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol Microbiol. 1995;17:155–167. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee M S, Morrison D A. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J Bacteriol. 1999;181:5004–5016. doi: 10.1128/jb.181.16.5004-5016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y H, Bowden G H. Carbon source, environmental pH and accumulation of two species biofilms. J Dent Res. 1996;75:1503. [Google Scholar]
- 39.Li Y H, Bowden G H. Characteristics of accumulation of oral gram-positive bacteria on mucin-conditioned glass surfaces in a model system. Oral Microbiol Immunol. 1994;9:1–11. doi: 10.1111/j.1399-302x.1994.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 40.Li Y H, Chen Y Y, Burne R A. Regulation of urease gene expression by Streptococcus salivarius growing in biofilms. Environ Microbiol. 2000;2:169–177. doi: 10.1046/j.1462-2920.2000.00088.x. [DOI] [PubMed] [Google Scholar]
- 41.Li Y H, Lau P C, Lee J H, Ellen R P, Cvitkovitch D G. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol. 2001;183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Ng C, Ferenci T. Global adaptations resulting from high population densities in Escherichia coli cultures. J Bacteriol. 2000;182:4158–4164. doi: 10.1128/jb.182.15.4158-4164.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marsh P D. Oral ecology and its impact on oral microbial diversity. In: Kuramitsu H K, Ellen R P, editors. Oral bacterial ecology: the molecular basis. Bymondham, Norfolk, United Kingdom: Horizon Scientific Press; 2000. pp. 11–65. [Google Scholar]
- 44.McLean R J, Whiteley M, Stickler D J, Fuqua W C. Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol Lett. 1997;154:259–263. doi: 10.1111/j.1574-6968.1997.tb12653.x. [DOI] [PubMed] [Google Scholar]
- 45.Michiels J, Dirix G, Vanderleyden J, Xi C. Processing and export of peptide pheromones and bacteriocins in Gram-negative bacteria. Trends Microbiol. 2001;9:164–168. doi: 10.1016/s0966-842x(01)01979-5. [DOI] [PubMed] [Google Scholar]
- 46.Minah G E, Solomon E S, Chu K. The association between dietary sucrose consumption and microbial population shifts at six oral sites in man. Arch Oral Biol. 1985;30:397–401. doi: 10.1016/0003-9969(85)90066-4. [DOI] [PubMed] [Google Scholar]
- 47.Olson E R. Influence of pH on bacterial gene expression. Mol Microbiol. 1993;8:5–14. doi: 10.1111/j.1365-2958.1993.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 48.O'Toole G, Kaplan H B, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 49.Parsek M R, Greenberg E P. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci USA. 2000;97:8789–8793. doi: 10.1073/pnas.97.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen F C, Scheie A A. Genetic transformation in Streptococcus mutans requires a peptide secretion-like apparatus. Oral Microbiol Immunol. 2000;15:329–334. doi: 10.1034/j.1399-302x.2000.150511.x. [DOI] [PubMed] [Google Scholar]
- 51.Price C W. Protective function and regulation of the general stress response in Bacillus subtilis and related gram-positive bacteria. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: AMS Press; 2000. pp. 179–197. [Google Scholar]
- 52.Quivey R G, Jr, Faustoferri R, Monahan K, Marquis R. Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol Lett. 2000;189:89–92. doi: 10.1111/j.1574-6968.2000.tb09211.x. [DOI] [PubMed] [Google Scholar]
- 53.Rallu F, Gruss A, Ehrlich S D, Maguin E. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol Microbiol. 2000;35:517–528. doi: 10.1046/j.1365-2958.2000.01711.x. [DOI] [PubMed] [Google Scholar]
- 54.Rowbury R J, Goodson M. An extracellular acid stress-sensing protein needed for acid tolerance induction in Escherichia coli. FEMS Microbiol Lett. 1999;174:49–55. doi: 10.1111/j.1574-6968.1999.tb13548.x. [DOI] [PubMed] [Google Scholar]
- 55.Rowbury R J, Hussain N H, Goodson M. Extracellular proteins and other components as obligate intermediates in the induction of a range of acid tolerance and sensitisation responses in Escherichia coli. FEMS Microbiol Lett. 1998;166:283–288. doi: 10.1111/j.1574-6968.1998.tb13902.x. [DOI] [PubMed] [Google Scholar]
- 56.Sander J W, Venema G, Kok J. Environmental stress responses in Lactococcus lactis. FEMS Microbiol Rev. 1999;23:483–501. [Google Scholar]
- 57.Shapiro J A. Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol. 1998;52:81–104. doi: 10.1146/annurev.micro.52.1.81. [DOI] [PubMed] [Google Scholar]
- 58.Svensäter G, Larsson U B, Greif E C, Cvitkovitch D G, Hamilton I R. Acid tolerance response and survival by oral bacteria. Oral Microbiol Immunol. 1997;12:266–273. doi: 10.1111/j.1399-302x.1997.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 59.Svensäter G, Sjogreen B, Hamilton I R. Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology. 2000;146:107–117. doi: 10.1099/00221287-146-1-107. [DOI] [PubMed] [Google Scholar]
- 60.Zhu M, Takenaka S, Sato M, Hoshino E. Influence of starvation and biofilm formation on acid resistance of Streptococcus mutans. Oral Microbiol Immunol. 2001;16:24–27. doi: 10.1034/j.1399-302x.2001.160104.x. [DOI] [PubMed] [Google Scholar]