Abstract
Among numerous thin film synthesis methods, metalorganic chemical vapor deposition performed in a showerhead reactor is the most promising one for broad use in scalable and commercially adaptable two-dimensional material synthesis processes. Adapting the most efficient monolayer growth methodologies from tube-furnace systems to vertical-showerhead geometries allows us to overcome the intrinsic process limitations and improve the overall monolayer yield quality. Here, we demonstrate large-area, monolayer molybdenum disulphide growth by combining gas-phase precursor supply with unique tube-furnace approaches of utilizing sodium molybdate pre-seeding solution spincoated on a substrate along with water vapor added during the growth step. The engineered process yields a high-quality, 4-inch scale monolayer film on sapphire wafers. The monolayer growth coverage, average crystal size and defect density were evaluated using Raman and photoluminescence spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscopy and scanning transmission electron microscopy imaging. Our findings provide a direct step forward toward developing a reproducible and large-scale MoS2 synthesis with commercial showerhead reactors.
We adapted the most efficient monolayer growth methodologies from tube-furnace systems to the showerhead geometries. A combination of gas-phase precursors, pre-seeding substrate coating and H2O vapor added during growth yields 4-inch monolayer MoS2.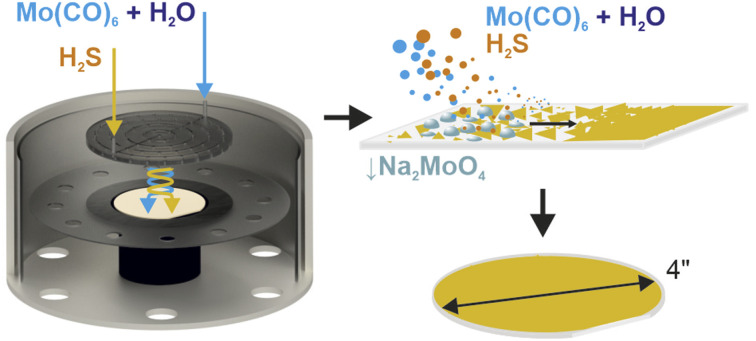
1. Introduction
From the broad family of atomically thin, two-dimensional (2D) materials, molybdenum disulphide (MoS2) has been found particularly interesting in recent years. Being a member of transition metal dichalcogenides (TMDs),1 the unique electronic2,3 and optical properties4 combined with high mechanical flexibility5,6 of MoS2 make it an attractive candidate for a potential new generation of wearable devices,7 flexible sensors8–10 or nanoelectronics2,11 as well as heterostructure research and development.12–20 Therefore, after years of extensive study, the shift of academic focus from small, lab-scale synthesis methods towards prototype applications and scalable processes is inevitable.21–23
One of the most vigorously investigated aspects of scaling up the research and application of MoS2 is large-scale, uniform and high quality material synthesis.24–32 Over the years, numerous efforts to understand, optimize and grow large-area 2D films have been reported.22,33 The most researched and versatile approaches are chemical vapor deposition (CVD)25,30,31,34 and metalorganic chemical vapor deposition (MOCVD) processes,24,32,35,36 both allowing atomic thickness-controlled growth. While CVD is considered the most common and straightforward method due to the use of commonly available powder precursors of sulfur and molybdenum, its metalorganic MOCVD variant is considered to be crucial to achieve the best results and highest reported material quality.24
The most important feature of MOCVD is its high level of repeatability due to the use of gaseous precursors and wide array of tunable process parameters. Recent research showed the potential of MOCVD to obtain high quality monolayer TMD films using hexacarbonyls (e.g. molybdenum hexacarbonyl – Mo(CO)6) as a metal source24,32,35,37 and either diethyl,35,36 dimethyl28 or chalcogenide hydrides as the chalcogen precursor (e.g. hydrogen disulphide H2S24,37). This, combined with a thorough study of the precursor reactions and thermodynamics, gaseous parameters38,39 and added growth promoters using liquid salt solutions28,37,40 proved to be the key to obtaining superior material properties, large crystal size and uniform substrate coverage on large scale substrates.
However, the most promising results in the TMD synthesis field are obtained using the hot-wall, tube furnace geometry. Although a robust and relatively cheap method of 2D material synthesis, tube furnaces do have a significant size limitation prohibiting further fabrication upscaling and industrial adaptation of monolayer MoS2. Promising studies using much more versatile showerhead systems, typically used in the modern semiconductor's industry41 for repeatable, large-scale manufacturing, have demonstrated the capability of MoS2 and other TMD synthesis on a 4-inch wafer scale. Atomic layer deposition (ALD),42,43 molecular beam epitaxy (MBE)44,45 and MOCVD approaches32,46–48 to synthesize TMD monolayers were reported. The material made in such processes however lacks the qualities of tube-furnace monolayers. Due to different furnace geometry (involving a laminar gas flow, shorter distance between the gas inlet and substrate, and heating directly at the substrate susceptor) the most successful tube-furnace methods have not been directly applied with showerhead systems. Instead, thin films reported to date with showerhead reactors show much smaller domain size (from tens of nanometers32,42,43,46,47 to tens of micrometers48) as compared to their tube furnace counterparts, which significantly impact the material properties. The high-temperature decomposition products of organic gaseous precursors tend to contaminate the material with carbonaceous impurities49 and might lead to growth limiting or suppressing phenomena,38,39 and thus need to be precisely controlled. On top of that, a typical process suffers from long deposition times, often reaching tens of hours which makes the grown material expensive to obtain.
In this study we revise and compare the state-of-the-art tube-furnace MOCVD methods of large-scale monolayer MoS2 synthesis: the all-gas-phase process, the use of salt solutions as growth and seeding promoters and the addition of water-vapor are adapted to the showerhead reactor geometry. We optimize these methods and demonstrate for the first time their viability and practical use in an industrial-grade showerhead system. We further engineer the most efficient, combined showerhead MOCVD synthesis approach to achieve uniform, continuous wafer-scale monolayer MoS2 with the largest reported grain sizes to date (>100 μm). Finally, we show and discuss how the material quality, uniformity and coverage can be dramatically improved by adding water vapor to the process gas mixture to eradicate growth-limiting factors such as carbonaceous and organic gas decomposition products. The results are obtained in an industrial-grade MOCVD system and are supported by systematic Raman and photoluminescence (PL) analysis as well as X-ray photoelectron spectroscopy (XPS) and defect density calculations based on a scanning transmission electron microscope (STEM) study. This work provides a direct methodology to widely adapt large-area, monolayer MoS2 synthesis to industrial showerhead systems.
2. Experimental results and discussion
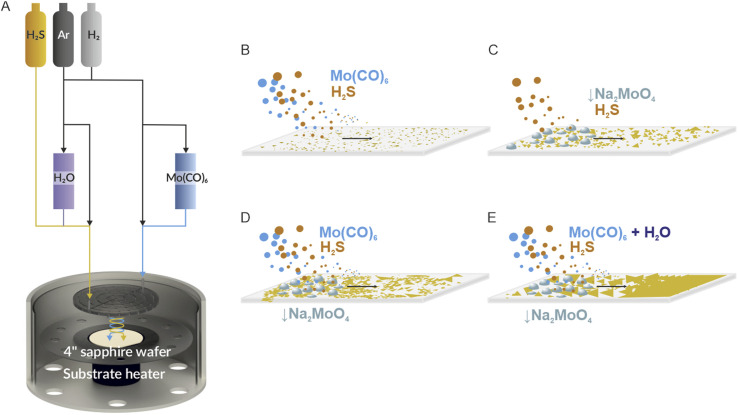
All experiments were performed on a commercial Aixtron BM Novo MOCVD system. The coldwall, showerhead reactor is designed to separate the chalcogen and metal precursor sources preventing the premature occurrence of the MOVCD reactions, before reaching the substrate surface (Fig. 1A) minimizing potential unwanted processes and reactor contamination. Such an adaptation is vital for a controlled and clean process reaction since the decomposition temperature of the gaseous molybdenum precursor used here (Mo(CO)6) is relatively low (250 °C (ref. 50)) and may lead to gas lines and showerhead contamination. All MOCVD growth variations are schematically shown in Fig. 1B–E and are discussed in detail in the following sections. All growth experiments are performed under constant parameters of 960 °C, a pressure of 830 mbar, a growth step time of 150 min and by using a 4-inch c-cut sapphire wafer as a growth substrate. Argon is used as an ambient gas and precursor carrier, whereas H2 is used as an active gas and carbonyl reduction agent. Flow parameters are detailed in Fig. S1† and in the Methods section.
Fig. 1. (A) A schematic of the experimental MOCVD setup with showerhead designed to prevent the chalcogen and metal precursor sources from mixing prematurely before reaching the reactor volume. With the gas-phase only approach (B), a liquid-phase-sulfurization approach (C), hybrid gas + liquid growth method (D) and a H2O assisted growth method with a controlled supply of the moderate amount of H2O vapor (E).
MoS2 growth with gas-phase precursors
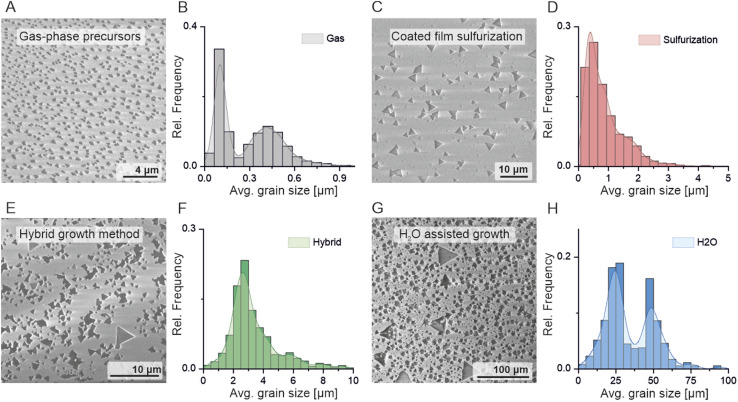
The first investigated MOCVD process pathway for MoS2 growth is gas-phase synthesis (Fig. 1B). It is considered as a benchmark process as it does exploit all tube-furnace method adaptations discussed further. During gas-phase MOVCD growth, all of the precursors are fed into the reactor chamber through the showerhead along with the carrier gas (argon) and additional process gases (hydrogen) necessary for efficient synthesis of the monolayer film. Although a fundamentally scalable and clean synthesis approach, achieving large lateral MoS2 domains in the showerhead system is challenging due to the large nucleation density and low reaction rate.51 The typical crystal size reported in the literature reaches up to 100 nm.32,43,46 In addition, only material thickness (and not single grain size) scales with the process time. In our optimized method, we have surpassed that limit reaching a size of 500 nm of single crystal monolayer material (Fig. 2A and B) with gas-phase precursors only. It is evident that the crystal size is limited by the reaction byproducts24,38,39 causing high temperature etching effects as well as high nucleation rate prohibiting the lateral crystal growth. The average substrate surface is thus composed of large crystals mixed with much smaller MoS2 nuclei, constantly formed and etched during the process (Fig. 2B) by gaseous products of the Mo(CO)6/H2S/H2 chemical reaction.46 On top of that, since the process requires a steady ratio of Mo : S along the entire substrate area, even the smallest flaws or imbalances in the gas feed or showerhead design may cause nonuniform growth conditions leading to uneven crystallization and local growth anomalies. Therefore, achieving stable coverage and substrate-wide uniformity, using this approach, is still challenging and far from the results reported in hotwall MOCVD systems.24
Fig. 2. A comparison of the MoS2 growth yield obtained through different growth approaches. The SEM image analysis of the gas-phase only approach (A) shows small, densely nucleated grain clusters with an average size of 500 nm and large contribution from unripened nuclei (B). A coated-film sulfurization approach yields large, 1 μm crystals which locally reach up to 3–4 μm (C) and (D). The hybrid growth method results in an average of 3 μm single crystals (E) and (F). Finally, with a controlled supply of the moderate amount of H2O vapor the lateral crystal size increases by an order of magnitude (G) and (H) with evident separation between two combined growth regimes (liquid and gas phases) visible in the grain size distribution (H). The presented SEM images were chosen from the substrates' edge to show an av. grain size. Histograms were obtained from 20 separate SEM images from each growth method.
MoS2 growth with coated film sulfurization
Another way of controlling the precursor coverage is to use a liquid source of molybdenum. In this approach, we are spin-coating the substrate with sodium molybdate (Na2MoO4) and sodium chloride (NaCl) water solution (Fig. 1C). Such a combination allows pre-seeding28,35 of the surface with a controlled amount of molybdenum and uniformly coating it with NaCl, widely used in tube furnace MOCVD MoS2 methods for increasing the reaction rate and decreasing the nucleation density leading to large single crystal formation.28,35 The coated wafer is then sulfurized under a H2S gas flow without an external, gaseous source of molybdenum. The material tends to form large single crystals and continuous films, the thickness and coverage of which depend on the spincoated mixture concentration, which controls the local Mo saturation during growth. The Na2MoO4/NaCl sulfurization approach was demonstrated to be efficient for continuous monolayer growth on 1–3 inch substrates;35,36 however the use of the alkali salt solution heavily impacts the final material quality. Intermediary NaCl + MoS2 reaction products52 lead to a fast crystal growth rate but also have been found to remain underneath MoS2 films. This phenomenon, driven by excessive use of NaCl, negatively impacts the epitaxial connection between the crystal and sapphire substrate and may lead to grain boundary defects and uneven grain stitching inherently deteriorating large-area continuous film cohesion and its electronic parameters.52 To avoid this unwanted effect, we have limited the Na2MoO4/NaCl molar concentration to 0.03 M and 0.1 M respectively. This allowed the preservation of the MoS2/sapphire epitaxy and maintenance of high material coverage. In our coldwall, showerhead regime, we note lateral crystal size varying from 0.7 up to 3–4 μm (Fig. 2C and D) with sporadic events of >100 μm single crystals forming continuous films locally. This growth method has an intrinsic self-limiting character due to a fixed amount of Mo coated on the substrate. Tuning the precursor solution molar concentration allows the control over the coverage and nucleation density; however uniformity is problematic on large area substrates. The uneven film thickness inherent to the spin-coating process (especially emphasized in large-scale growth areas) leads to different local Mo : S concentrations during the process. Such a coverage issue is manifested in bilayer, multilayer or blank areas with the presence of unreacted, bulk molybdenum oxide particles on different substrate areas. The sulfurization of the spincoated Na2MoO4/NaCl film is therefore not perfectly adjustable in the case of 4-inch substrates and requires careful molar concentration to balance between preserving epitaxy and promoting continuous film growth to be useable.
MoS2 growth with a hybrid process
Increasing the control over the material's coverage, crystal density and uniformity even further requires finetuning the precursor flux i.e. by combining two precursor sources: adding a gas phase Mo(CO)6 flow to the process with a spincoated mixture of Na2MoO4/NaCl (Fig. 1D) on the substrate. The combined, hybrid method in principle allows the compensation of individual limitations of small grain size present in the gas phase method and self-limiting Mo supply of the spincoated film sulfurization approach. In this case, we consider Na2MoO4/NaCl as a Mo seeding/catalytic agent, leading to the formation of molybdenum oxychlorides, oxysulfides and suboxides28 kickstarting and promoting the film growth under the constant supply of Mo from the Mo(CO)6 gas phase. Indeed, in our experiments the hybrid method yields single crystals of 3 μm average lateral size (Fig. 2E and F) with the largest exceeding 100 μm locally. Adding Mo(CO)6 however reintroduces the carbonyl reaction byproducts from the Mo(CO)6 + H2S + H2 reaction. Further optimization of the process (to improve the single crystal size, uniformity and monolayer coverage) does not intuitively scale with the Mo(CO)6 nor Na2MoO4/NaCl concentration during the growth. The local imbalances of Na2MoO4 and Mo(CO)6 lead to multilayer island formation. We have found that reducing the molar concentration of Na2MoO4 to as low as 0.005 M is enough to effectively pre-seed and nucleate Mo2 and crucial to minimize the multilayer growth under a constant Mo(CO)6 mass flux. As discussed later, in our experiments volatile carbonaceous reaction products (CO2 and CH4, C) do not seem to dope MoS2 nor contaminate the substrate; however they do contaminate the showerhead and MOCVD reactor heavily, significantly suppressing the growth process by releasing carbonaceous products into the gas phase growth environment limiting the reproducibility. Thus, further increase of MoCO6 concentration is unwelcome and counterproductive for obtaining high-quality, large-area films. The scalability of the hybrid approach offers more process parameters and better coverage control; however it still requires significant optimization to be viable on an industrial scale.
Water-assisted large grain growth
The presence of water vapor during the MOCVD reaction is widely considered as unwanted. Uncontrollable moisture can lead to a significant decrease of material quality due to water-induced oxidation and reduction during the growth process.53 However, as was previously reported in tube-furnace, small scale processes, controlled addition of a small amount of water vapor was found to be beneficial due to low oxidizing effects, which assist in the removal of volatile carbonaceous reaction products48,54 and assists in the reduction of molybdenum oxides to suboxides and hydrogen promoting the lateral growth (formulas (1)–(3))
| C + H2O ⇒ CO + H2 | 1 |
| MoO3 + H2 ⇒ MoO3−x + H2O | 2 |
| MoO3−x + (7 − x)2S ⇒ MoS2 + (3 − x)/2SO2 | 3 |
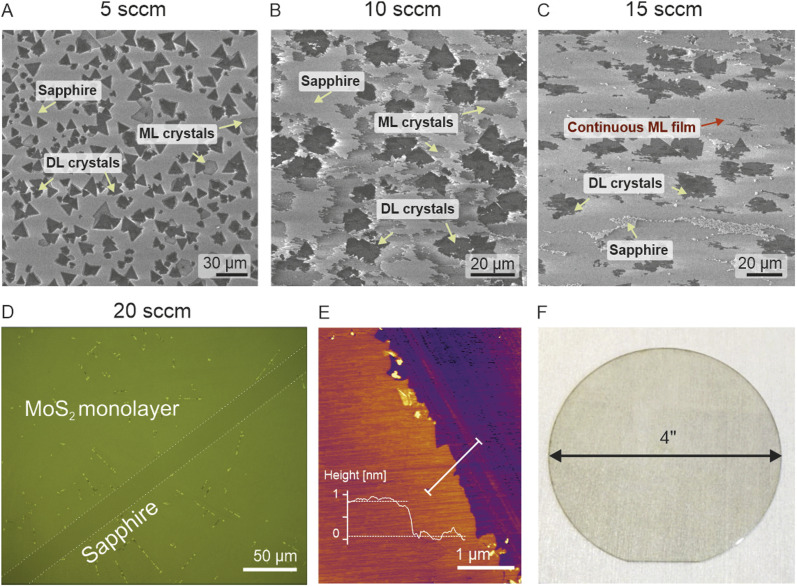
The use of oxidizing materials such as water vapor in an industrial-grade showerhead reactor is in general significant safety concern. However, by maintaining proper safety precautions and source separation we have achieved a water-assisted method combining metalorganic gas supply and promoter film coating (Fig. 1E). We found that a flow of 5 sccm of argon through a room temperature H2O filled bubbler is sufficient to significantly remove impurities and promote monolayer crystals to reach an average lateral size of 25–50 μm (Fig. 2G and H) with the largest locally exceeding 100 μm (Fig. 3) and with a significant increase in continuous film coverage with further increase in the water vapor flow. The monolayer coverage peaked at a H2O vapor flow of 20 sccm (Fig. 3D and E) and its continuity was maintained across a 4-inch substrate area (Fig. 3D) with the exception of the substrate edge (see Fig. S2†). We attribute this dramatic improvement of the crystal size and monolayer coverage to the self-regulating effect of H2O, similar to the growth-etch approach demonstrated in tungsten disulphide (WS2) pulsed MOCVD process.48 Due to gas-flow imbalances coming from subtle showerhead and gas-line imperfections as well as coated film non-uniformity (amplified by the large-area substrate), different Mo(CO)6 + H2S + H2 reaction pathways may occur locally leading to the formation of volatile CH4, C, CO, CO2, COS, and CS2 species in different concentrations.38,46 Water vapor may react with and suppress the formation of excess carbon oxides and sulfides negating the effects of local differences in reaction paths and their negative impact on MoS2 grain lateral growth leading to overall better coverage as shown in Fig. 3D and E.
Fig. 3. SEM images of MoS2 coverage with increasing H2O vapor concentration during the growth process. Coverage of the continuous monolayer (ML) film improves gradually with the increase in the water vapor supply (A)–(C) and peaks at 20 sccm where a continuous ML film without double layer (DL) crystals is observed as shown on panel (D) (optical microscope image of the deliberately scratched MoS2 film). Local AFM analysis confirms the monolayer thickness (E). Material uniformity is maintained over the 4-inch substrate area (F).
Spectroscopic characterization
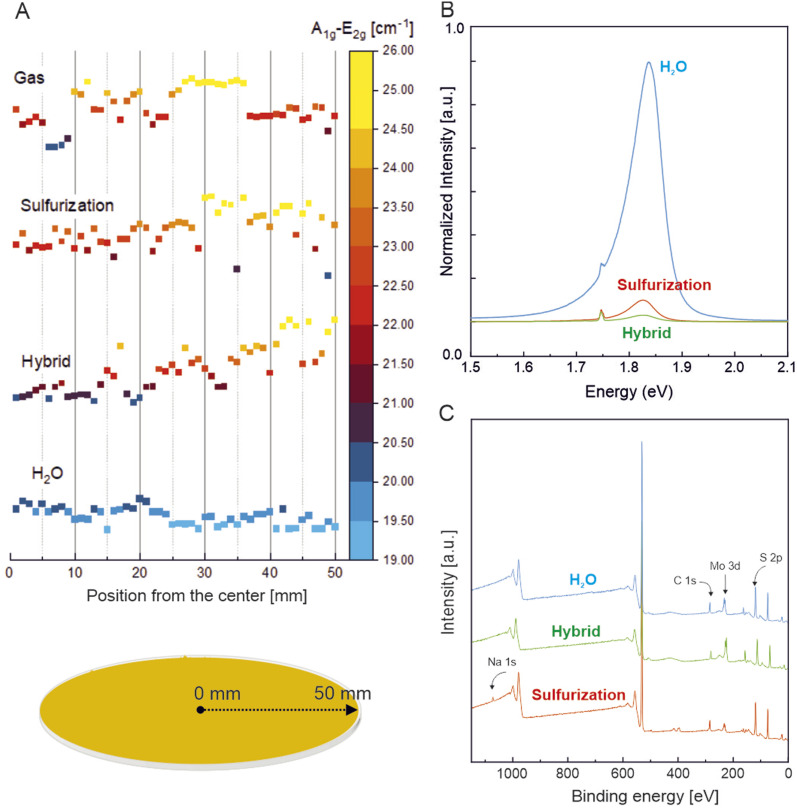
Detailed material characterization was performed to compare the uniformity and crystallinity of MoS2 synthesized by each growth approach. The analysis of the shape of characteristic E2g and A1g Raman peaks from monolayer areas from each method (Fig. S3A†) shows ΔE2g–A1g to be 20.9, 20.8 and 19.5 for sulfurization, hybrid and H2O respectively. The gas-phase growth method proved to be difficult to analyze due to the small grain size and low Raman signal. The full width at half maximum (FWHM) of E2g and A1g of each method is shown in Fig. S3B† and it was found to be narrowest for the H2O assisted approach with an E2g and A1g of 2.6 and 3.4, indicating higher crystallinity and good uniformity. In the case of all substrates, spectra taken from the 1000–1700 cm−1 range showed no distinguishable carbon peaks (Fig. S4†), which suggests that all carbonaceous reaction products remain in the gas phase and do not deposit on the substrate. Raman spectra taken radially from 50 spots on each wafer confirm the self-regulating effect of H2O (Fig. 4A). The separation between E12g and A1g peaks smaller than 20 cm−1 is regarded as a proof of a high-quality monolayer55 film. The positions of Raman peaks in the case of water-assisted growth remain constant which indicates uniformity in thickness and strain compared with other growth methods. Water vapor can promote further oxidation of thick particles from residual Mo oxides and etching of emerging multilayers during the growth process due to local H2 generation (as presented in formulas (1)–(3)). The result is the formation of a continuous monolayer film without thicker, seed particles on a wide area of the 4-inch substrate. Panel 4B shows the PL spectra of MoS2 synthesized with sulfurization (red), hybrid (green) and H2O assisted (blue) methods taken from the monolayer MoS2 sites from each substrate. Significantly higher PL emission from water assisted growth indicates that water improves crystal quality by removing carbon contamination (which is known to quench PL54) and enhances the efficiency of precursor ligand-reduction. The increase in PL intensity signifies a lower impact of grain boundary defects and uneven grain stitching.56 In this case, the obtained Raman and PL spectra are evidence of a high quality, large-area film cohesion obtained with the water-assisted growth method through large crystals and potentially epitaxial grain stitching.
Fig. 4. An average separation of E2g and A1g Raman peaks taken from 50 measurements with a 1 mm interval performed across the radial direction of the wafer (A). Measurements were performed in a line-scan fashion, from the center to the edge of the 4-inch substrate. Comparison of characteristic Raman peak separation shows the radial uniformity of MoS2 synthesised with the water-vapor assisted growth method. Representative PL spectra (B) showing significantly higher PL emission from water assisted growth. A comparison of wide XPS spectra (C) shows no residual Na present with H2O assisted growth. Detailed analysis of Mo 3d and S 2p XPS peaks is shown in ESI Fig. S5.†.
XPS analysis
An XPS measurement was performed to investigate the chemical states and stoichiometric ratio of the grown MoS2. Trace sodium was only detected on liquid-phase sulfurized MoS2 substrates (Fig. 4C and Table S1†) as this process relies on coating the substrate with relatively concentrated Na2MoO4/NaCl solution compared to the hybrid approach (0.03/0.1 M as compared to 0.005/0.1 M). The detailed atomic ratios, molybdenum oxides and sodium contents of each growth method are shown in ESI Table S1.† The analysis of the atomic ratios of each growth method reveals the sulfurization method to yield an ≈2.22 Mo : S ratio, as compared to ≈6.1 for the hybrid and ≈3.4 for the H2O approach. However, compared to other growth methods, the H2O assisted method produces the least oxide states (Fig. S5A and C†), attributed to the Mo6+ ratio. It is explained by water-assisted enhanced reduction of the metal precursor, which results in higher crystallinity. The lack of detected sodium within the measurement limit in the water-assisted method combined with the obtained Raman spectroscopy results discussed in the previous paragraph suggests the high quality of the monolayer material synthesized in this process approach.
STEM imaging
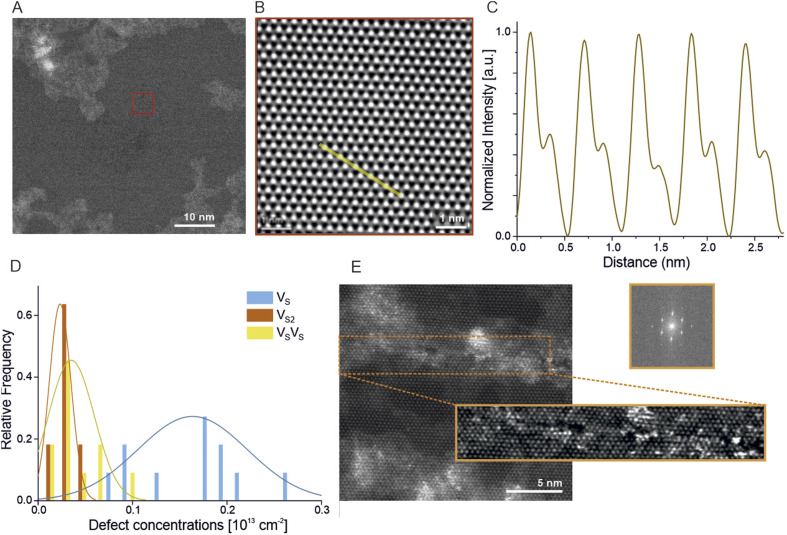
Aberration-corrected STEM imaging was used to image and characterize the structural properties of MoS2 at atomic resolution. Small and large magnified high angle annular dark field (HAADF) images (Fig. 5A and B) show a clean and perfect hexagonal crystal lattice of the monolayer MoS2 film in the 2H phase. Due to the Z-contrast dependency of HAADF, the atomic intensities of Mo and 2S can be distinguished directly from the atomically resolved images (Fig. 5C). In the case of earlier reported exfoliated and MOCVD grown monolayer MoS2, different types of defects have been reported. However, here only sulfur mono- and divacancies can be observed which are very common due to their lower formation energies. Moreover, an electron beam can also induce sulfur defects even at lower acceleration voltages. Thus to extract the intrinsic S defect concentrations, the number of mono- and divacancies can be calculated with respect to the accumulated electron beam dose in an image series.57 Further defect concentration analysis shows a pristine defect concentration of 1.4 × 1013 cm−2 for single sulfur vacancies (VS) and 0.3 × 1013 cm−2 for double sulfur vacancies (V2S) as extrapolated from an E-beam dose rate test (Fig. S6†). A distribution of VS, V2S and sulfur-next-to-sulfur (VSVS) defects taken from 11 STEM images (measured area of 925 nm2) is presented in Fig. 5E. MoS2 synthesized in the water-assisted process does not show any signs of unwanted etching or oxidative damage. Interestingly, the grain boundaries found during imaging are stitched seamlessly (Fig. 5E), demonstrating locally the epitaxial growth mode. Due to the reactive nature of grain boundaries, they tend to attract contamination along it. However, the filtered STEM image (highlighted in orange color) shows no disorientation angle between the stitched crystal areas. The fast Fourier transform (FFT) of the corresponding image shows only one set of hexagonal patterns, further confirming the high-quality growth of MoS2.
Fig. 5. A small magnification STEM image (A) of MoS2 synthesized with the water-assisted method with a large magnification image of the typical MoS2 area (B) (marked in red) with an intensity line-section (C) showing high crystal quality. Typical defect concentration is presented in the bar plot (D) and it is calculated separately for single sulfur VS, double sulfur V2S and sulfur-next-to-sulfur VSVS defect types. Concentrations are calculated from the 16 images taken from different regions. A grain boundary image (E) and its closer inspection show seamless grains stitching (FFT), a sign of local epitaxial growth mode.
3. Conclusion
All experimental evidence points to a strong advantage of combining the hybrid gas and liquid phase growth approaches in a coldwall showerhead system. We have successfully achieved a uniform and continuous MoS2 film over a large scale 4-inch substrate with spincoated Na2MoO4/NaCl solution and gas-phase Mo(CO)6 and H2S by limiting growth reaction imbalances with water vapor introduced during the growth phase. We found that the water regulates the growth reaction by removing carbonaceous products and reduces excess molybdenum oxides. Material characterization confirms the good material quality and no negative impact of using moderate and carefully controlled amounts of H2O.
The synthesized MoS2 showed evidence of epitaxial connection with the sapphire substrate. Our findings provide a direct step forward towards developing scalable, reproducible and large-scale MoS2 synthesis with showerhead reactors and demonstrate the potential of combining key advancements in the field of 2D material growth to overcome the process limitations.
4. Methods
MOCVD growth
All MoS2 synthesis experiments were performed on an Aixtron BM Novo MOCVD system with a coldwall, showerhead, horizontal growth chamber. Prior to growth, 4-inch c-plane sapphire wafers (MTI) were cleaned in IPA, acetone and DI and annealed at 1000 °C for 6 h to obtain an atomically smooth surface. For sulfurization, hybrid and H2O assisted growth methods, a sapphire wafer was additionally treated in 50 W O2 plasma for 5 min to ensure a hydrophilic surface needed for efficient spin-coating of a growth promoter (a solution of Na2MoO4 (Sigma Aldrich, 98.0%) and NaCl (Sigma Aldrich, 99.5%) in DI with concentrations of 0.03 M/0.1 M for sulfurization and 0.005 M/0.1 M for hybrid and H2O). All growth processes started with heating the reactor with a sapphire wafer to 950 °C in 60 min under a constant flow of 300 sccm of high purity argon (Carbagas 99.999%). The growth step started immediately after reaching the set temperature. All substrates were exposed to 20 sccm of hydrogen gas (Vici DBS NM Plus 100 Hydrogen Generator, purity 99.999%) and 60 sccm of H2S (Carbagas, 99.9%). For gas-phase, hybrid and H2O assisted approaches, Mo(CO)6 (Sigma Aldrich 99.9%) was supplied by 150 sccm of Ar gas flowing through a bubbler kept at 10 °C. In the H2O assisted variant, an additional 5–20 sccm of Ar flowing through a DI-filled bubbler at room temperature was introduced. All MoS2 growths were done at 830 mbar with growth steps set to 150 min (although the sulfurization method was completed in only 30 min). After that, the reactor was cooled under ambient H2S and Ar flows. After a growth run, the reactor chamber was thoroughly cleaned and annealed and all the ceramic elements were additionally air-baked to prevent unwanted carbon contamination.
Raman spectroscopy and PL
Raman and PL spectroscopy were done with a Renishaw inVia Confocal Raman Microscope with a 532 nm laser beam at a low power (<0.3 mW) to avoid defect nucleation and substrate damage. A diffraction grating of 3000 mm−1 was used for good spatial resolution.
Atomic force microscopy
Atomic force microscopy was performed on an Asylum Research Cypher AFM system in tapping mode.
SEM imaging
Scanning electron microscopy was done on a Zeiss Merlin SEM with a GEMINI II column. All imaging was done at 1 kV acceleration voltage to minimize substrate charging STEM imaging. The as-grown MoS2 was transferred from the growth substrate using the wet-transfer method36 on 5 × 5 mm Si/SiN TEM grids (Norcada). Scanning transmission electron microscopy imaging was conducted using an aberration-corrected (with a double spherical corrector) FEI Titan Themis TEM 60–300 kV, equipped with a Schottky X-FEG electron source and a monochromator to reduce the effect of chromatic aberrations. To avoid the electron-beam induced knock-on damage, a low acceleration voltage (80 kV) was used for the imaging.58 The electron probe current, C2 aperture size, and beam convergence angle were 25 pA, 50 μm, and 21.2 mrad, respectively. Images were acquired with Velox software (Thermo Fisher Scientific) using a 185 mm camera length, which corresponds to an angular range (49.5–198 mrad) in a HAADF detector. To avoid the sample drift, serial imaging was performed using 512 × 512 pixels with an 8 μs dwell time. The images were aligned and processed using the “double-Gaussian filtering” method in ImageJ.
Author contributions
A. R., A. K. and M. M. conceived and designed the experiments; M. M. synthesized the samples and optimized the process, M. M., H. G. J., Y. Z. and J. Z. developed the process parameters, M. M. performed the optical, SEM, HRTEM, Raman and PL measurements, H. G. J. and M. M. analyzed the data, M. Thakur transferred the substrates on TEM grids, and M. Tripathi obtained and analyzed the STEM images. M. M. wrote the paper, with inputs from all authors; A. K. and A. R. supervised the project; all authors discussed the results and commented on the manuscript.
Conflicts of interest
The authors declare no competing financial interest.
Supplementary Material
Acknowledgments
We thank the Aixtron team for technical assistance. This work was financially supported by the Swiss National Science Foundation (grant no. 200021_192037), the CCMX Materials Challenge grant “Large area growth of 2D materials for device integration”, the European Union's Horizon 2020 research and innovation program under grant agreements No. 785219 and 881603 (Graphene Flagship Core 2 and Core 3) and the European Research Council (grant no. 682332 and 899775).
Electronic supplementary information (ESI) available: One table and six figures. See https://doi.org/10.1039/d2na00409g
References
- Manzeli S. Ovchinnikov D. Pasquier D. Yazyev O. V. Kis A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017;2(8):1–15. doi: 10.1038/natrevmats.2017.33. doi: 10.1038/natrevmats.2017.33. [DOI] [Google Scholar]
- Radisavljevic B. Radenovic A. Brivio J. Giacometti V. Kis A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011;6:147–150. doi: 10.1038/nnano.2010.279. doi: 10.1038/nnano.2010.279. [DOI] [PubMed] [Google Scholar]
- Komsa H.-P. Krasheninnikov A. V. Effects of confinement and environment on the electronic structure and exciton binding energy of MoS2 from first principles. Phys. Rev. B: Condens. Matter Mater. Phys. 2012;86(24):241201. doi: 10.1103/PhysRevB.86.241201. doi: 10.1103/PhysRevB.86.241201. [DOI] [Google Scholar]
- Splendiani A. Sun L. Zhang Y. Li T. Kim J. Chim C.-Y. Galli G. Wang F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010;10(4):1271–1275. doi: 10.1021/NL903868W. doi: 10.1021/NL903868W. [DOI] [PubMed] [Google Scholar]
- Castellanos-Gomez A. Poot M. Steele G. A. Zant H. S. J. v. d. Agraït N. Rubio-Bollinger G. Elastic Properties of Freely Suspended MoS2 Nanosheets. Adv. Mater. 2012;24(6):772–775. doi: 10.1002/ADMA.201103965. doi: 10.1002/ADMA.201103965. [DOI] [PubMed] [Google Scholar]
- Jiang J.-W. Park H. S. Rabczuk T. Molecular dynamics simulations of single-layer molybdenum disulphide (MoS2): Stillinger-Weber parametrization, mechanical properties, and thermal conductivity. J. Appl. Phys. 2013;114(6):064307. doi: 10.1063/1.4818414. doi: 10.1063/1.4818414. [DOI] [Google Scholar]
- Singh E. Singh P. Kim K. S. Yeom G. Y. Nalwa H. S. Flexible Molybdenum Disulfide (MoS2) Atomic Layers for Wearable Electronics and Optoelectronics. ACS Appl. Mater. Interfaces. 2019;11(12):11061–11105. doi: 10.1021/ACSAMI.8B19859. doi: 10.1021/ACSAMI.8B19859. [DOI] [PubMed] [Google Scholar]
- Yoon J. Park W. Bae G. Y. Kim Y. Jang H. S. Hyun Y. Lim S. K. Kahng Y. H. Hong W. K. Lee B. H. Ko H. C. Highly Flexible and Transparent Multilayer MoS2 Transistors with Graphene Electrodes. Small. 2013;9(19):3295–3300. doi: 10.1002/SMLL.201300134. doi: 10.1002/SMLL.201300134. [DOI] [PubMed] [Google Scholar]
- Singh Nalwa H. A review of molybdenum disulfide (MoS2) based photodetectors: from ultra-broadband, self-powered to flexible devices. RSC Adv. 2020;10(51):30529–30602. doi: 10.1039/D0RA03183F. doi: 10.1039/D0RA03183F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JA Rogers Y. H. T. Someya: materials and mechanics for stretchable electronics. Science. 2010;327(5973):1603–1607. doi: 10.1126/science.1182383. doi: 10.1126/science.1182383. [DOI] [PubMed] [Google Scholar]
- Wachter S. Polyushkin D. K. Bethge O. Mueller T. A microprocessor based on a two-dimensional semiconductor. Nat. Commun. 2017;8(1):1–6. doi: 10.1038/ncomms14948. doi: 10.1038/ncomms14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B. Yoon J. Lim S. K. Kim A. R. Kim D.-H. Park S.-G. Kwon J.-D. Lee Y.-J. Lee K.-H. Lee B. H. Ko H. C. Hahm M. G. Chemical Sensing of 2D Graphene/MoS2 Heterostructure Device. ACS Appl. Mater. Interfaces. 2015;7(30):16775–16780. doi: 10.1021/ACSAMI.5B04541. doi: 10.1021/ACSAMI.5B04541. [DOI] [PubMed] [Google Scholar]
- Rathi S. Lee I. Lim D. Wang J. Ochiai Y. Aoki N. Watanabe K. Taniguchi T. Lee G.-H. Yu Y.-J. Kim P. Kim G.-H. Tunable Electrical and Optical Characteristics in Monolayer Graphene and Few-Layer MoS2 Heterostructure Devices. Nano Lett. 2015;15(8):5017–5024. doi: 10.1021/ACS.NANOLETT.5B01030. doi: 10.1021/ACS.NANOLETT.5B01030. [DOI] [PubMed] [Google Scholar]
- Schulman D. S. Arnold A. J. Razavieh A. Nasr J. Das S. The Prospect of Two-Dimensional Heterostructures: A Review of Recent Breakthroughs. IEEE Nanotechnology Magazine. 2017;11(2):6–17. doi: 10.1109/MNANO.2017.2679240. [DOI] [Google Scholar]
- Swain G. Sultana S. Parida K. A review on vertical and lateral heterostructures of semiconducting 2D-MoS2 with other 2D materials: a feasible perspective for energy conversion. Nanoscale. 2021;13(22):9908–9944. doi: 10.1039/D1NR00931A. doi: 10.1039/D1NR00931A. [DOI] [PubMed] [Google Scholar]
- Cai Z. Liu B. Zou X. Cheng H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018;118(13):6091–6133. doi: 10.1021/ACS.CHEMREV.7B00536. doi: 10.1021/ACS.CHEMREV.7B00536. [DOI] [PubMed] [Google Scholar]
- Eda G. Fujita T. Yamaguchi H. Voiry D. Chen M. Chhowalla M. Coherent Atomic and Electronic Heterostructures of Single-Layer MoS2. ACS Nano. 2012;6(8):7311–7317. doi: 10.1021/NN302422X. doi: 10.1021/NN302422X. [DOI] [PubMed] [Google Scholar]
- Liu Y. Weiss N. O. Duan X. Cheng H.-C. Huang Y. Duan X. Van der Waals heterostructures and devices. Nat. Rev. Mater. 2016;1(9):1–17. doi: 10.1038/natrevmats.2016.42. [DOI] [Google Scholar]
- Geim A. Grigorieva I. Van der Waals heterostructures. Nature. 2013;499(7459):419–425. doi: 10.1038/nature12385. doi: 10.1038/nature12385. [DOI] [PubMed] [Google Scholar]
- Sitek J. Plocharski J. Pasternak I. Gertych A. P. Mcaleese C. Conran B. R. Zdrojek M. Strupinski W. Substrate-Induced Variances in Morphological and Structural Properties of MoS2 Grown by Chemical Vapor Deposition on Epitaxial Graphene and SiO2. ACS Appl. Mater. Interfaces. 2020;12:45110. doi: 10.1021/acsami.0c06173. doi: 10.1021/acsami.0c06173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava M. Rao V. R. A Roadmap for Disruptive Applications and Heterogeneous Integration Using Two-Dimensional Materials: State-of the-Art and Technological Challenges. Nano Lett. 2021;21(15):6359–6381. doi: 10.1021/ACS.NANOLETT.1C00729. doi: 10.1021/ACS.NANOLETT.1C00729. [DOI] [PubMed] [Google Scholar]
- Kim S.-Y. Kwak J. Ciobanu C. V. Kwon S.-Y. Recent Developments in Controlled Vapor-Phase Growth of 2D Group 6 Transition Metal Dichalcogenides. Adv. Mater. 2019;31(20):1804939. doi: 10.1002/ADMA.201804939. doi: 10.1002/ADMA.201804939. [DOI] [PubMed] [Google Scholar]
- Wang P. Yang D. Pi X. Toward Wafer-Scale Production of 2D Transition Metal Chalcogenides. Adv. Electron. Mater. 2021;7:2100278. doi: 10.1002/AELM.202100278. doi: 10.1002/AELM.202100278. [DOI] [Google Scholar]
- Kang K. Xie S. Huang L. Han Y. Huang P. Y. Mak K. F. Kim C.-J. Muller D. Park J. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature. 2015;520(7549):656–660. doi: 10.1038/nature14417. doi: 10.1038/nature14417. [DOI] [PubMed] [Google Scholar]
- Yu H. Liao M. Zhao W. Liu G. Zhou X. J. Wei Z. Xu X. Liu K. Hu Z. Deng K. Zhou S. Shi J.-A. Gu L. Shen C. Zhang T. Du L. Xie L. Zhu J. Chen W. Yang R. Shi D. Zhang G. Wafer-Scale Growth and Transfer of Highly-Oriented Monolayer MoS2 Continuous Films. ACS Nano. 2017;11(12):12001–12007. doi: 10.1021/ACSNANO.7B03819. doi: 10.1021/ACSNANO.7B03819. [DOI] [PubMed] [Google Scholar]
- Dumcenco D. Ovchinnikov D. Marinov K. Lazić P. Gibertini M. Marzari N. Sanchez O. L. Kung Y.-C. Krasnozhon D. Chen M.-W. Bertolazzi S. Gillet P. Morral A. F. i. Radenovic A. Kis A. LargeArea Epitaxial Monolayer MoS2. ACS Nano. 2015;9(4):4611–4620. doi: 10.1021/ACSNANO.5B01281. doi: 10.1021/ACSNANO.5B01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. Zou X. Zhang Z. Hong M. Shi J. Chen S. Shu J. Zhao L. Jiang S. Zhou X. Huan Y. Xie C. Gao P. Chen Q. Zhang Q. Liu Z. Zhang Y. Batch production of 6-inch uniform monolayer molybdenum disulfide catalyzed by sodium in glass. Nat. Commun. 2018;9(1):1–10. doi: 10.1038/s41467-018-03388-5. doi: 10.1038/s41467-018-03388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boandoh S. Choi S. H. Park J.-H. Park S. Y. Bang S. Jeong M. S. Lee J. S. Kim H. J. Yang W. Choi J.-Y. Kim S. M. Kim K. K. A Novel and Facile Route to Synthesize Atomic-Layered MoS2 Film for Large-Area Electronics. Small. 2017;13(39):1701306. doi: 10.1002/SMLL.201701306. doi: 10.1002/SMLL.201701306. [DOI] [PubMed] [Google Scholar]
- Boandoh S. Agyapong-Fordjour F. O. T. Choi S. H. Lee J. S. Park J. H. Ko H. Han G. Yun S. J. Park S. Kim Y. M. Yang W. Lee Y. H. Kim S. M. Kim K. K. Wafer-Scale van der Waals Heterostructures with Ultraclean Interfaces via the Aid of Viscoelastic Polymer. ACS Appl. Mater. Interfaces. 2019;11(1):1579–1586. doi: 10.1021/ACSAMI.8B16261. doi: 10.1021/ACSAMI.8B16261. [DOI] [PubMed] [Google Scholar]
- Wang Q. Li N. Tang J. Zhu J. Zhang Q. Jia Q. Lu Y. Wei Z. Yu H. Zhao Y. Guo Y. Gu L. Sun G. Yang W. Yang R. Shi D. Zhang G. Wafer-Scale Highly Oriented Monolayer MoS2 with Large Domain Sizes. Nano Lett. 2020;20(10):7193–7199. doi: 10.1021/ACS.NANOLETT.0C02531. doi: 10.1021/ACS.NANOLETT.0C02531. [DOI] [PubMed] [Google Scholar]
- He T. Li Y. Zhou Z. Zeng C. Qiao L. Lan C. Yin Y. Li C. Liu Y. Synthesis of large-area uniform MoS2 films by substrate-moving atmospheric pressure chemical vapor deposition: from monolayer to multilayer. 2D Materials. 2019;6(2):025030. doi: 10.1088/2053-1583/AB0760. doi: 10.1088/2053-1583/AB0760. [DOI] [Google Scholar]
- Kim T. Mun J. Park H. Joung D. Diware M. Won C. Park J. Jeong S.-H. Kang S.-W. Wafer-scale production of highly uniform two-dimensional MoS2 by metal-organic chemical vapor deposition. Nanotechnology. 2017;28(18) doi: 10.1088/1361-6528/AA6958. doi: 10.1088/1361-6528/AA6958. [DOI] [PubMed] [Google Scholar]
- Sun J. Li X. Guo W. Zhao M. Fan X. Dong Y. Xu C. Deng J. Fu Y. Synthesis Methods of Two-Dimensional MoS2: A Brief Review. Crystals. 2017;7(7):198. doi: 10.3390/CRYST7070198. doi: 10.3390/CRYST7070198. [DOI] [Google Scholar]
- Lin Y.-C. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale. 2012;4(20):6637–6641. doi: 10.1039/c2nr31833d. doi: 10.1039/c2nr31833d. [DOI] [PubMed] [Google Scholar]
- Cun H. Macha M. Kim H. Liu K. Zhao Y. LaGrange T. Kis A. Radenovic A. Wafer-scale MOCVD growth of monolayer MoS2 on sapphire and SiO2. Nano Res. 2019;12(10):2646–2652. doi: 10.1007/S12274-019-2502-9. doi: 10.1007/S12274-019-2502-9. [DOI] [Google Scholar]
- Thakur M. Macha M. Chernev A. Graf M. Lihter M. Deen J. Tripathi M. Kis A. Radenovic A. Wafer-Scale Fabrication of Nanopore Devices for Single-Molecule DNA Biosensing Using MoS2. Small Methods. 2020;4(11):2000072. doi: 10.1002/SMTD.202000072. doi: 10.1002/SMTD.202000072. [DOI] [Google Scholar]
- Kim H. Ovchinnikov D. Deiana D. Unuchek D. Kis A. Suppressing nucleation in metal-organic chemical vapor deposition of MoS2 monolayers by alkali metal halides. Nano Lett. 2017;17(8):5056–5063. doi: 10.1021/acs.nanolett.7b02311. doi: 10.1021/acs.nanolett.7b02311. [DOI] [PubMed] [Google Scholar]
- Dhar S. Kumar V. K. Choudhury T. H. Shivashankar S. A. Raghavan S. Chemical vapor deposition of MoS2 layers from Mo–S–C–O–H system: thermodynamic modeling and validation. Phys. Chem. Chem. Phys. 2016;18(22):14918–14926. doi: 10.1039/C6CP01617K. doi: 10.1039/C6CP01617K. [DOI] [PubMed] [Google Scholar]
- Kumar V. K. Dhar S. Choudhury T. H. Shivashankar S. A. Raghavan S. A predictive approach to CVD of crystalline layers of TMDs: the case of MoS2. Nanoscale. 2015;7(17):7802–7810. doi: 10.1039/C4NR07080A. doi: 10.1039/C4NR07080A. [DOI] [PubMed] [Google Scholar]
- Song J.-G. Ryu G. H. Kim Y. Woo W. J. Ko K. Y. Kim Y. Lee C. Oh I.-K. Park J. Lee Z. Kim H. Catalytic chemical vapor deposition of large-area uniform two-dimensional molybdenum disulfide using sodium chloride. Nanotechnology. 2017;28(46):465103. doi: 10.1088/1361-6528/AA8F15. doi: 10.1088/1361-6528/AA8F15. [DOI] [PubMed] [Google Scholar]
- Feng Z. C., III-Nitride Semiconductor Materials, 2006, 10.1142/P437 [DOI] [Google Scholar]
- Jang Y. Yeo S. Lee H. B. R. Kim H. Kim S. H. Wafer-scale, conformal and direct growth of MoS2 thin films by atomic layer deposition. Appl. Surf. Sci. 2016;365:160–165. doi: 10.1016/J.APSUSC.2016.01.038. doi: 10.1016/J.APSUSC.2016.01.038. [DOI] [Google Scholar]
- Lin J. Monaghan S. Sakhuja N. Gity F. Jha R. K. Coleman E. M. Connolly J. Cullen C. P. Walsh L. A. Mannarino T. Schmidt M. Sheehan B. Duesberg G. S. McEvoy N. Bhat N. Hurley P. K. Povey I. M. Bhattacharjee S. Large-area growth of MoS2 at temperatures compatible with integrating back-end-of-line functionality. 2D Materials. 2020;8(2):025008. doi: 10.1088/2053-1583/ABC460. doi: 10.1088/2053-1583/ABC460. [DOI] [Google Scholar]
- Mortelmans W. Kazzi S. E. Groven B. Mehta A. N. Balaji Y. Gendt S. D. Heyns M. Merckling C. Epitaxial registry and crystallinity of MoS2 via molecular beam and metalorganic vapor phase van der Waals epitaxy. Appl. Phys. Lett. 2020;117(3):033101. doi: 10.1063/5.0013391. doi: 10.1063/5.0013391. [DOI] [Google Scholar]
- Kazzi S. E. Mortelmans W. Nuytten T. Meersschaut J. Carolan P. Landeloos L. Conard T. Radu I. Heyns M. Merckling C. MoS2 synthesis by gas source MBE for transition metal dichalcogenides integration on large scale substrates. J. Appl. Phys. 2018;123(13):135702. doi: 10.1063/1.5008933. doi: 10.1063/1.5008933. [DOI] [Google Scholar]
- Chiappe D. Ludwig J. Leonhardt A. Kazzi S. E. Mehta A. N. Nuytten T. Celano U. Sutar S. Pourtois G. Caymax M. Paredis K. Vandervorst W. Lin D. Gendt S. D. Barla K. Huyghebaert C. Asselberghs I. Radu I. Layer-controlled epitaxy of 2D semiconductors: bridging nanoscale phenomena to wafer-scale uniformity. Nanotechnology. 2018;29(42):425602. doi: 10.1088/1361-6528/AAD798. doi: 10.1088/1361-6528/AAD798. [DOI] [PubMed] [Google Scholar]
- Seol M. Lee M.-H. Kim H. Shin K. W. Cho Y. Jeon I. Jeong M. Lee H.-I. Park J. Shin H.-J. Seol M. Lee M.-H. Kim H. Shin K. W. Cho Y. Jeon I. Jeong M. Lee H.-I. Shin H.-J. Park J. High-Throughput Growth of Wafer-Scale Monolayer Transition Metal Dichalcogenide via Vertical Ostwald Ripening. Adv. Mater. 2020;32(42):2003542. doi: 10.1002/ADMA.202003542. doi: 10.1002/ADMA.202003542. [DOI] [PubMed] [Google Scholar]
- Cohen A. Patsha A. Mohapatra P. K. Kazes M. Ranganathan K. Houben L. Oron D. Ismach A. Growth-Etch Metal-Organic Chemical Vapor Deposition Approach of WS2 Atomic Layers. ACS Nano. 2021;15(1):526–538. doi: 10.1021/ACSNANO.0C05394. doi: 10.1021/ACSNANO.0C05394. [DOI] [PubMed] [Google Scholar]
- Schaefer C. M. Roque J. M. C. Sauthier G. Bousquet J. Hébert C. Sperling J. R. Pérez-Tomás A. Santiso J. Corro E. d. Garrido J. A. Carbon Incorporation in MOCVD of MoS2 Thin Films Grown from an Organosulfide Precursor. Chem. Mater. 2021;33(12):4474–4487. doi: 10.1021/ACS.CHEMMATER.1C00646. doi: 10.1021/ACS.CHEMMATER.1C00646. [DOI] [Google Scholar]
- Mun J. Kim Y. Kang I. S. Lim S. K. Lee S. J. Kim J. W. Park H. M. Kim T. Kang S. W. Low-temperature growth of layered molybdenum disulphide with controlled clusters. Sci. Rep. 2016;6(1):1–7. doi: 10.1038/srep21854. doi: 10.1038/srep21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. Lin J. Huang X. Zhou Y. Chen Y. Xia J. Wang H. Xie Y. Yu H. Lei J. Wu D. Liu F. Fu Q. Zeng Q. Hsu C. H. Yang C. Lu L. Yu T. Shen Z. Lin H. Yakobson B. I. Liu Q. Suenaga K. Liu G. Liu Z. A library of atomically thin metal chalcogenides. Nature. 2018;556(7701):355–359. doi: 10.1038/s41586-018-0008-3. doi: 10.1038/s41586-018-0008-3. [DOI] [PubMed] [Google Scholar]
- Zhang K. Bersch B. M. Zhang F. Briggs N. C. Subramanian S. Xu K. Chubarov M. Wang K. Lerach J. O. Redwing J. M. FullertonShirey S. K. Terrones M. Robinson J. A. Considerations for Utilizing Sodium Chloride in Epitaxial Molybdenum Disulfide. ACS Appl. Mater. Interfaces. 2018;10(47):40831–40837. doi: 10.1021/ACSAMI.8B16374. doi: 10.1021/ACSAMI.8B16374. [DOI] [PubMed] [Google Scholar]
- Boandoh S. Choi S. H. Park J.-H. Park S. Y. Bang S. Jeong M. S. Lee J. S. Kim H. J. Yang W. Choi J.-Y. Kim S. M. Kim K. K. A Novel and Facile Route to Synthesize Atomic-Layered MoS2 Film for Large-Area Electronics. Small. 2017;13(39):1701306. doi: 10.1002/smll.201701306. doi: 10.1002/smll.201701306. [DOI] [PubMed] [Google Scholar]
- Choi S. H. Stephen B. Park J. H. Lee J. S. Kim S. M. Yang W. Kim K. K. Water-Assisted Synthesis of Molybdenum Disulfide Film with Single Organic Liquid Precursor. Sci. Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-02228-8. doi: 10.1038/s41598-017-02228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. From bulk to monolayer MoS2: evolution of Raman scattering. Adv. Funct. Mater. 2012;22(7):1385–1390. doi: 10.1002/adfm.201102111. doi: 10.1002/adfm.201102111. [DOI] [Google Scholar]
- Zhang K. Borys N. J. Bersch B. M. Bhimanapati G. R. Xu K. Wang B. Wang K. Labella M. Williams T. A. Haque M. A. Barnard E. S. Fullerton-Shirey S. Schuck P. J. Robinson J. A. Deconvoluting the Photonic and Electronic Response of 2D Materials: The Case of MoS2. Sci. Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-16970-6. doi: 10.1038/s41598-017-16970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shree S. George A. Lehnert T. Neumann C. Benelajla M. Robert C. Marie X. Watanabe K. Taniguchi T. Kaiser U. Urbaszek B. Turchanin A. High optical quality of MoS2 monolayers grown by chemical vapor deposition. 2D Materials. 2019;7(1):015011. doi: 10.1088/2053-1583/AB4F1F. doi: 10.1088/2053-1583/AB4F1F. [DOI] [Google Scholar]
- Komsa H. P. Kotakoski J. Kurasch S. Lehtinen O. Kaiser U. Krasheninnikov A. V. Two-dimensional transition metal dichalcogenides under electron irradiation: defect production and doping. Phys. Rev. Lett. 2012;109(3):035503. doi: 10.1103/PHYSREVLETT.109.035503/FIGURES/5/MEDIUM. doi: 10.1103/PHYSREVLETT.109.035503/FIGURES/5/MEDIUM. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.