Abstract
Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) and Alzheimer’s disease neuropathologic change (ADNC) are each associated with substantial cognitive impairment in aging populations. However, the prevalence of LATE-NC across the full range of ADNC remains uncertain. To address this knowledge gap, neuropathologic, genetic, and clinical data were compiled from 13 high-quality community- and population-based longitudinal studies. Participants were recruited from United States (8 cohorts, including one focusing on Japanese–American men), United Kingdom (2 cohorts), Brazil, Austria, and Finland. The total number of participants included was 6196, and the average age of death was 88.1 years. Not all data were available on each individual and there were differences between the cohorts in study designs and the amount of missing data. Among those with known cognitive status before death (n = 5665), 43.0% were cognitively normal, 14.9% had MCI, and 42.4% had dementia—broadly consistent with epidemiologic data in this age group. Approximately 99% of participants (n = 6125) had available CERAD neuritic amyloid plaque score data. In this subsample, 39.4% had autopsy-confirmed LATE-NC of any stage. Among brains with “frequent” neuritic amyloid plaques, 54.9% had comorbid LATE-NC, whereas in brains with no detected neuritic amyloid plaques, 27.0% had LATE-NC. Data on LATE-NC stages were available for 3803 participants, of which 25% had LATE-NC stage > 1 (associated with cognitive impairment). In the subset of individuals with Thal Aβ phase = 0 (lacking detectable Aβ plaques), the brains with LATE-NC had relatively more severe primary age-related tauopathy (PART). A total of 3267 participants had available clinical data relevant to frontotemporal dementia (FTD), and none were given the clinical diagnosis of definite FTD nor the pathological diagnosis of frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP). In the 10 cohorts with detailed neurocognitive assessments proximal to death, cognition tended to be worse with LATE-NC across the full spectrum of ADNC severity. This study provided a credible estimate of the current prevalence of LATE-NC in advanced age. LATE-NC was seen in almost 40% of participants and often, but not always, coexisted with Alzheimer’s disease neuropathology.
Keywords: ADRD, Tau, NFT, Nondemented, Oldest-old, Epidemiology, APOE, ROS-MAP, Vantaa 85 +, HAAS, CFAS, CC75C, The 90 + study, ACT, VITA, Nun study, Biobank for aging studies, Mayo clinic study of aging
Introduction
Brain autopsies of persons with documented amnestic dementia often reveal evidence of Alzheimer’s disease neuropathologic change (ADNC) [69], limbic predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) [81], or both. However, the independent and joint prevalence of each of these disorders are unclear. There remain uncertainties about optimal classification of LATE-NC and some individual brains are challenging to categorize, as is the case for other subtypes of neurodegenerative disease [8, 29, 43, 54, 65, 82, 97]. Thus, high-quality data, derived from different geographic locations and including autopsy results, are required to shed light on the prevalence and co-existence of these high-morbidity brain pathologies.
The cardinal diagnostic feature of LATE-NC is TDP-43 pathology–aberrant TDP-43 protein deposits visualized with immunohistochemistry [81]. TDP-43 pathology was discovered in 2006 as the primary pathological hallmark of frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) and amyotrophic lateral sclerosis [84]. However, TDP-43 pathology is now appreciated to occur in many other conditions [19]. Although diagnostic ambiguities still exist in TDP-43 neuropathologic assessments, LATE-NC has distinguishing characteristics including the neuroanatomical distribution of TDP-43 pathology, clinical features, genetic risk factors, and epidemiology [21, 39, 53, 81, 94]. For example, the demographic group most likely to show LATE-NC is persons beyond 85 years of age [81], and, LATE-NC is strongly associated with amnestic dementia, independent of other known brain pathologies [12, 32, 36, 39, 40, 44, 47, 51, 59, 70, 72, 79, 92].
Like LATE-NC, ADNC is prevalent and is associated with amnestic dementia. ADNC and LATE-NC are genetically pleiotropic: the APOE ε4 ADNC risk allele is also associated with increased risk for LATE-NC [3, 28, 44, 118]. LATE-NC and ADNC are often present in the same brains [45, 46, 61, 63], and TDP-43 pathology may co-localize with tau-immunoreactive neurofibrillary tangles (NFTs), a hallmark ADNC lesion [44, 103, 111]. The presence of “mixed” pathologies is important because the clinical manifestations vary with different combinations of pathologies [62]. For example, “pure LATE-NC” is, on average, associated with a less severe clinical phenotype than “pure ADNC”, whereas the common combination (ADNC + LATE-NC) is associated with a more aggressive clinical course than either alone [48, 49, 74, 110, 119].
Despite recent progress, questions persist. Investigators have considered whether TDP-43 pathology in aging is best defined as a subtype of ADNC [43, 117]. While there is heterogeneity in the genetic, pathologic, and clinical features of AD-type dementia [9, 41, 62, 71], there currently are no consensus-based criteria for delineating subtypes of ADNC. Basic related questions include: What is the overall end-of-life frequency of LATE-NC in the brains of older persons? How does the prevalence of LATE-NC vary in different research cohorts? How frequently is LATE-NC seen in brains with no-, low-, intermediate-, or high-severity ADNC, and in those with varying severities of primary age-related tauopathy (PART) [22]?
Addressing questions about the prevalence of different pathologies requires relatively population-representative autopsy cohorts. Dementia clinic- and hospital-based cohorts are invaluable resources for research, but they tend to be substantially enriched for unusual subtypes of dementia [99], early-onset diseases, and genetic risk factors, which limit the generalizability of the findings. While there have been prior reports about LATE-NC from individual research centers, and from various consortia [5, 57, 67], there has not been a prior study bringing together findings from a large number of community-based autopsy cohorts.
In the current study, summary data were gathered related to LATE-NC and ADNC from 13 separate well-established study cohorts with available autopsy information. These cohorts included participants who were mostly recruited without dementia and followed longitudinally to autopsy at research centers in United States (8 cohorts), United Kingdom (2 cohorts), Brazil, Austria, and Finland. Several of the included cohorts can be described as “population-based”, in that the individual donors were recruited from a general population within a geographical boundary in a study design that aimed to recruit from all subgroups within the population (See Supplemental Table 1, online resource). While the cohorts that are not population-based did not use probability-sampling and are not completely generalizable to their target populations, they are likely to be far more representative of the populations from which they were derived than clinic- or hospital-based cohorts. The combined data from multiple research cohorts provided the bases for gaining insights into how commonly LATE-NC is seen at autopsy, with or without comorbid ADNC.
Methods
The main goals of this study were to examine the frequency of LATE-NC at the end of life in community-based research participants and to stratify results by the level of reported ADNC severity. Based on those goals, summary data were requested related to ADNC and LATE-NC from 13 high-quality community-based and population-based cohorts of brain aging and dementia. (The term “community-based” is mostly used from here forward to refer to the present collection of cohorts.) Data were collected from each of the following autopsy cohorts (in alphabetical order): Adult Changes in Thought (ACT) [58]; Brazilian Biobank for Aging Studies (BAS) of the University of Sao Paulo [106]; Cambridge City over-75 s Cohort (CC75C) [16]; Medical Research Council Cognitive Function and Ageing Study (CFAS) [115]; Duke University/University of North Carolina AD Research Center (Duke/UNC-ADRC) [36]; Honolulu Asia-Aging Study (HAAS) [116]; Mayo Clinic Study of Aging (MCSA) [91]; Nun Study[112]; Rush University Religious Orders Study/Memory and Aging Project (ROS-MAP) [10]; University of California Irvine The 90 + Study (The 90 + Study) [50]; University of Kentucky AD Research Center (UKy-ADRC) [98]; Vantaa 85 + Study [52]; and, Vienna Trans-Danube Aging (VITA) study [55]. See Supplemental Table 1, online resource, for more information on each cohort. All study procedures were approved by the respective Institutional Review Boards or Research Ethics Boards. Each participant (or their next of kin if they lacked capacity) provided informed consent for cohort participation. No additional approvals were needed for analysis of the de-identified summary data from each cohort. Many of the research participants were recruited from the community using methods such as local media advertising, health fairs, and presentations to community groups.
The structured data requests sent to a representative of each cohort are shown in Supplemental Table 2, online resource. For the collection of data on ADNC, different pathology-based measures were requested: Braak NFT distribution staging (0–VI scale) [14] performed using anti-phospho-Tau antibodies; CERAD neuritic amyloid plaque density scores (graded as “None”, “Sparse plaques”, “Moderate plaques”, or “Frequent plaques”), which indicate the detected density of neuritic plaques in cerebral cortex [66]; and, Thal Aβ phases (a 0–5 scale based on anatomic distribution of Aβ plaques detected with Aβ immunostaining) [6, 108]. The rationale for incorporating these parameters was that they are all used for determining the presence and severity of ADNC according to current consensus-based criteria [69].
There were differences among the cohorts in the methods of tissue-processing at autopsy, neuropathologic evaluations, and data missingness. See Supplemental Table 3, online resource, for more information about how many participants were included from each cohort. Cohort-specific data format variations were conspicuous in the area of cognitive assessment instruments that were administered to participants. To operationalize global cognitive status, the cohorts used Mini-Mental State Examination (MMSE) [33] scores, except HAAS used the Cognitive Abilities Screening Instrument (CASI) [107], and both the Brazil BAS cohort and MCSA used the Clinical Dementia Rating sum of boxes scores [27]. For the UKy-ADRC, only participants who were recruited while cognitively normal were included and 11 subjects were excluded from the cognitive assessments due to no MMSE scores. For the BAS, participants 50 years or older at death were included and participants were excluded from this cohort with inconsistent clinical information, a post-mortem interval greater than 24 h, or if the brain tissue was incompatible for neuropathological analyses (e.g., cerebrospinal fluid pH < 6.5 or major acute brain lesions including hemorrhages). The Nun Study used MMSE cut points as follows: scores of < 17: dementia; 17–21: mild cognitive impairment (MCI); and, > 21 nondemented. For HAAS, the CASI scores were used at cutoffs > = 74 (normal), 60–73.9 (MCI), or < 60 (dementia). ROS/MAP data on clinical status were missing for 1 subject (0.05%). For The 90 + Study, 14 participants were excluded from the MMSE analyses due to missing scores. For the Duke/UNC-ADRC cohort, participants 90 years or over at death were included in the study. Approximately 70% from this cohort were cognitively normal at recruitment, and 29 participants were excluded from the cognitive assessment due to no MMSE score. For the Vantaa 85 + Study, DSM-IIIR criteria were used to diagnose dementia and MMSE scores were assessed for most participants in the baseline study and follow-ups. For the MCSA, 37 participants did not have the Clinical Dementia Rating sum of boxes scores within 3 years of death.
Cohorts were also queried as to whether they had clinical evaluations during life and corroborating neuropathologic studies that likely would have captured cases of FTD/FTLD-TDP if they were in the cohort. The specific question posed to each autopsy cohort was: how many clear-cut FTD/FTLD-TDP cases were in the cohort? The symptoms of FTD include behavioral disturbances and language problems [53, 89, 104], but variants of these cognitive signs and symptoms (e.g., disinhibition and aphasia) may also occur in Alzheimer’s disease and other dementia disorders, so there was some subjectivity in the clinical diagnosis.
To address whether multiple blinded neuropathologic raters from different institutions would agree with the results of Braak NFT staging, particularly in the context of cases with LATE-NC but lacking substantial ADNC, a multicenter digital pathology study was performed. Brain sections from 10 cognitively impaired individuals were included in this focused study, of which 8 had LATE-NC, 1 had FTLD-TDP, and 1 had severe ADNC. The following slides had been stained for phospho-Tau IHC (PHF-1 antibody [34]): hippocampus at the level of the lateral geniculate nucleus; anterior hippocampus and entorhinal cortex; occipital neocortex (Brodmann Area [BA] 17/18/19); superior and mid-temporal neocortex (BA 21/22); and, middle frontal gyrus (BA 9). Slides were anonymized and then converted to digital format using a Leica/Aperio ScanScope AT2 slide scanner at 40 × resolution. Four separate raters with experience in digital neuropathologic evaluation (coauthors M.D.C., J.D., B.N.D., and J.H.N.) scored the pathologies via internet connection, using either the Aperio ImageScope™ or QuPath open-source software, to derive Braak NFT stages for each case while blinded to other information.
For data analyses, the joint distribution of neuropathologic rating parameters were obtained from each cohort via templated spreadsheets (Supplemental Table 2, online resource). The overall joint distributions were simply summations of each cell in the joint distribution from each cohort. For demographic characteristics (average age at death and sex), a single summary measure was provided by each cohort. To compute the overall summary of age at death and sex distribution, as well as APOE ε4 positivity, cohort-specific results were combined by weighting each cohort by its sample size. The association between APOE ε4 positivity and LATE-NC rate was evaluated using simple meta-regression that ignored sample weights, did not include the VITA cohort (where APOE genotype data were unavailable), and did not factor in APOE genotype data missingness. For the comparisons of Braak NFT stages (PART severity [22]) in Thal Aβ phase = 0 cases (comparing the results with versus without LATE-NC), a Fisher’s exact test was applied to determine statistical significance.
Results
Selected demographic, clinical, genetic, and summary neuropathologic data on included participants from each of the 13 community-based cohorts are shown in Table 1. The total number of included participants was 6196. Subset analyses were performed and the included numbers of subjects from each center for each analysis are provided in Supplemental Table 3, online resource. The median number of research participants included per cohort was 321, with a range of 109–1620 participants per cohort. Mean weighted age of death for all included cohorts was 88.1 years; age ranges for the cohorts was 72.2–97.2 years. Overall, 62.3% of participants were women.
Table 1.
Selected demographic, genetic, clinical, and neuropathologic data on the 13 included cohorts*
| Characteristics | ACT | Brazilian BAS | CC75C | CFAS | Duke/UNC ADRC | HAAS | Mayo/MCSA | Nun study | ROS-MAP | The 90 + study | UKy-ADRC | Vantaa 85 + | VITA | Total or weighted average** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Country | USA | Brazil | UK | UK | USA | USA | USA | USA | USA | USA | USA | Finland | Austria | |
| Sample size | 863 | 625 | 228 | 510 | 109 | 321 | 209 | 382 | 1620 | 402 | 318 | 302 | 307 | 6196 |
| Average age at death, years | 89.0 | 72.2 | 91.5 | 87.0 | 94.1 | 90.3 | 86.6 | 91.1 | 89.9 | 97.2 | 88.4 | 92.4 | 83.2 | 88.1** |
| % Female | 59.4 | 48.7 | 70.6 | 60.4 | 67.0 | 0 | 40.0 | 100 | 69.2 | 69.7 | 63.2 | 83.1 | 58.6 | 62.3%** |
| # Known APOE status | 831 | 306 | 216 | 289 | 109 | 303 | 209 | 369 | 1554 | 371 | 318 | 282 | 0 | 5157 |
| % APOE ε4 allele | 27.4 | 13.0 | 32.5 | 29.0 | 27.6 | 21.8 | 24.9 | 23.0 | 24.9 | 20.8 | 33.6 | 31.6 | N/A | 25.5%** |
| Final cognitive status | ||||||||||||||
| # clinical normal | 308 | 461 | 63 | 189 | 29 | 139 | 142 | 167 | 529 | 114 | 142 | 107*** | 37 | 2427 |
| # clinical MCI/proxy | 17 | 44 | 39 | 10 | 26 | 68 | 44 | 48 | 372 | 113 | 49 | N/A | 11 | 841 |
| # clinical dementia | 381 | 101 | 113 | 275 | 49 | 97 | 22 | 136 | 718 | 174 | 110 | 195 | 26 | 2397 |
| Neuropathologic features | ||||||||||||||
| % neocortical LBs | 12.9 | 3.4 | 4.8 | 3.7 | 3.9 | 6.2 | 6.2 | 6.9 | 14.2 | 9.2 | 13.2 | 14.2 | 4.1 | 9.5%** |
| % Braak stages 0-II | 25.6 | 67.6 | 17.6 | 36.7 | 12.8 | 49.8 | 41.1 | 44.5 | 15.7 | 7.2 | 43.4 | 17.6 | 59.5 | 31.5%** |
| % Braak stages III-IV | 36.3 | 23.9 | 60.8 | 41.5 | 67.0 | 31.2 | 41.6 | 23.6 | 56.0 | 54.0 | 26.7 | 47.2 | 20.3 | 42.0%** |
| % Braak stages V-VI | 38.1 | 8.5 | 21.6 | 21.9 | 20.2 | 19.0 | 17.2 | 31.9 | 28.3 | 38.8 | 29.9 | 35.2 | 20.3 | 26.5%** |
| % LATE-NC**** | 47.9 | 11.1 | 48.7 | 67.7 | 30.3 | 24.7 | 24.9 | 16.2 | 52.2 | 36.1 | 36.0 | 37.1 | 16.9 | 39.4%** |
ACT adult changes in thought [58], BAS Brazilian biobank for aging studies of the University of Sao Paulo [106], CC75C Cambridge city over-75 s cohort [16], CFAS medical research council cognitive function and ageing study [115], Duke/UNC-ADRC Duke/University of North Carolina AD Research Center [36], HAAS Honolulu Asia-aging study [116], MCSA Mayo Clinic study of aging [91], Nun Study[112], ROS-MAP Rush University Religious Orders Study/Memory and Aging Project [10], The 90 + Study University of California Irvine The 90 + Study [50], UKy-ADRC University of Kentucky AD Research Center [98], Vantaa 85 + Study [52], and VITA Vienna Trans-Danube Aging study [55], APOE apolipoprotein E, MCI mild cognitive impairment, LBs lewy bodies, LATE-NC limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes
See text for more details on data missingness and cohort-specific operationalizations
weighted average
number of subjects with no dementia according to the DSMIIIR criteria
these percentages are for cases with full CERAD neuritic plaque data
see Supplemental Table 3, online resource
A chart depicting the clinical features of participants at their last clinical evaluation is shown in Fig. 1 (n = 5665 participants had those data available). Slightly over 40% were judged to be cognitively normal at their last clinical examination, and approximately the same proportion had documented dementia. In the 12 cohorts reporting the parameter, ~ 15% had MCI (See Supplemental Table 3, online resource).
Fig. 1.
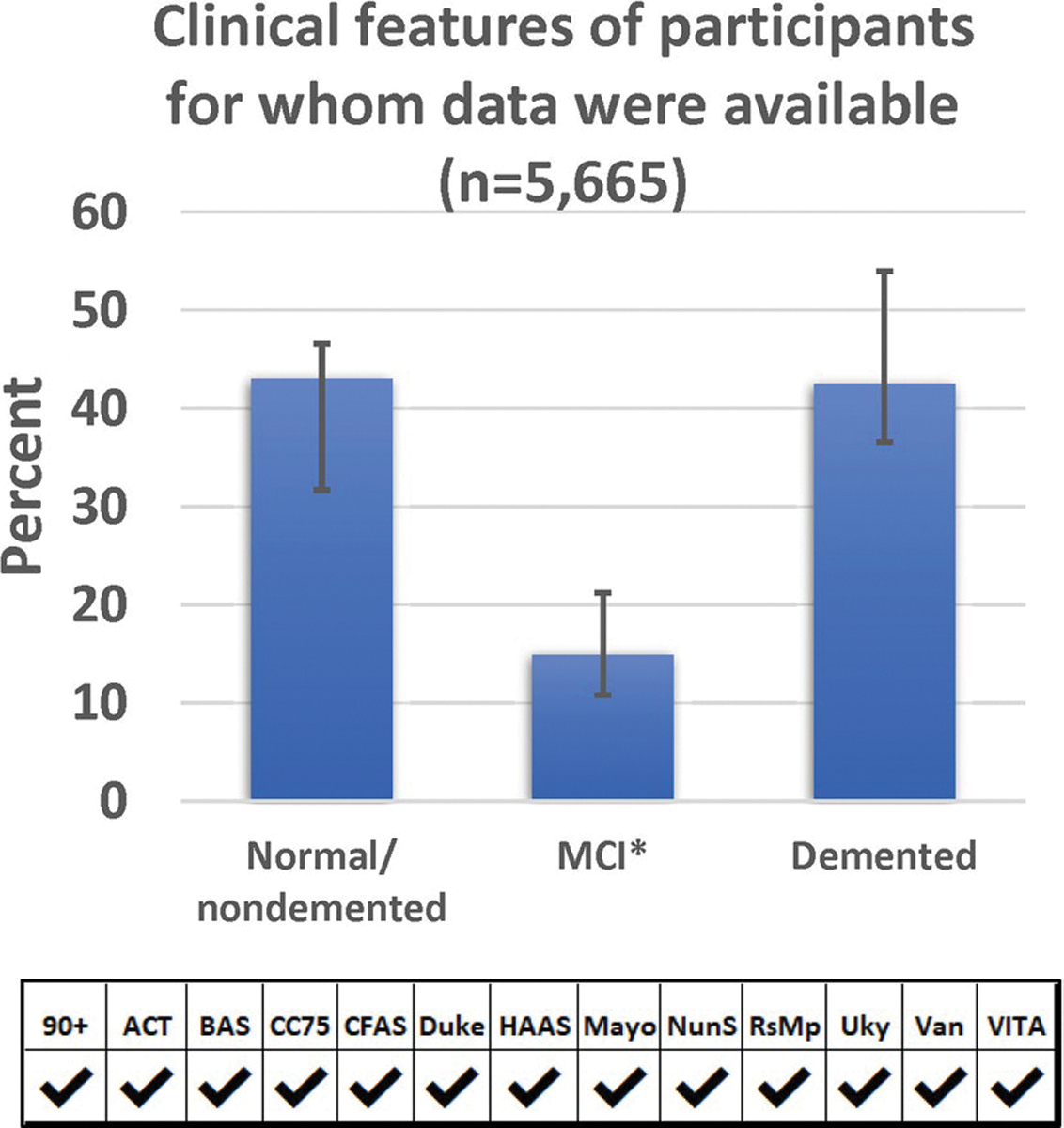
Frequencies of clinical/cognitive features among the included participants. All cohorts had data about whether participants had normal cognition or dementia prior to death, and most (12 cohorts) had some measure for an intermediate clinical status, usually mild cognitive impairment (MCI). The finding of slightly over 40% cognitive normal prior to death is consistent with epidemiologic data of human populations in this age range [21, 60, 86, 90]. The result of each cohort was weighted equally in order to convey the cohort-to-cohort variance. For numbers of participants included from each cohort, see Table 1. Error bars denote 25th and 75th percentiles. *-MCI data were present for all cohorts except Vantaa 85 +
In terms of FTD/FTLD cases, data were only considered from a cohort if FTD cases (clinically) and/or FTLD-TDP cases (pathologically) would likely have been documented in that cohort. Having applied those criteria, data were provided from 9 different cohorts, comprising n = 3267 participants. In this combined subsample, no clinical FTD/FTLD-TDP case was identified (Table 2). Although these participants were evaluated by clinicians, it is conceivable that early FTLD-TDP cases were present but not detected.
Table 2.
Number of cases with definite frontotemporal dementia (FTD) in the nine cohorts where this diagnosis was evaluated (among n = 3267 participants)
| Cohort | Sample size | Number of definite clinical FTD cases identified | Notes |
|---|---|---|---|
|
| |||
| ACT | 863 | 0 | |
| CC75C | 228 | 0 | Ascertained by post-mortem clinical consensus |
| CFAS | 510 | 0 | Ascertained by post-mortem clinical consensus; 2 with “lobar atrophy” |
| Duke ADRC | 109 | 0 | |
| HAAS | 321 | 0 | |
| Mayo/MCSA | 209 | 0 | |
| The 90 + Study (UC Irvine) | 402 | 0 | 2 “possible” bvFTD, 1 turned out to have AD, the other vascular pathology |
| Uky ADRC | 318 | 0 | |
| VITA | 307 | 0 | |
| Total number with clinical workup | 3267 | ||
| Total number of definite clinical FTD cases identified | 0 | ||
APOE ε4 allele genotype data were available from a total of n = 5157 included participants (83.2% of the combined cohort). APOE allele data missingness by cohort is indicated in Table 1. Of the participants with known APOE genotype, 25.5% carried at least one copy of the APOE ε4 allele (range: 13.0–33.6%). In the 12 cohorts with available APOE genotyping, there was a marginal positive association between APOE ε4 allele carrier prevalence and LATE-NC frequency (r2 = 0.36; p = 0.039), indicating that cohorts with higher APOE ε4 prevalence also had higher LATE-NC frequency (Fig. 2a). By contrast, there was no such statistically significant association between LATE-NC frequency with cohorts’ average age, sex (percent female), or percent of included subjects with neocortical Lewy body pathology (Fig. 2b–d).
Fig. 2.
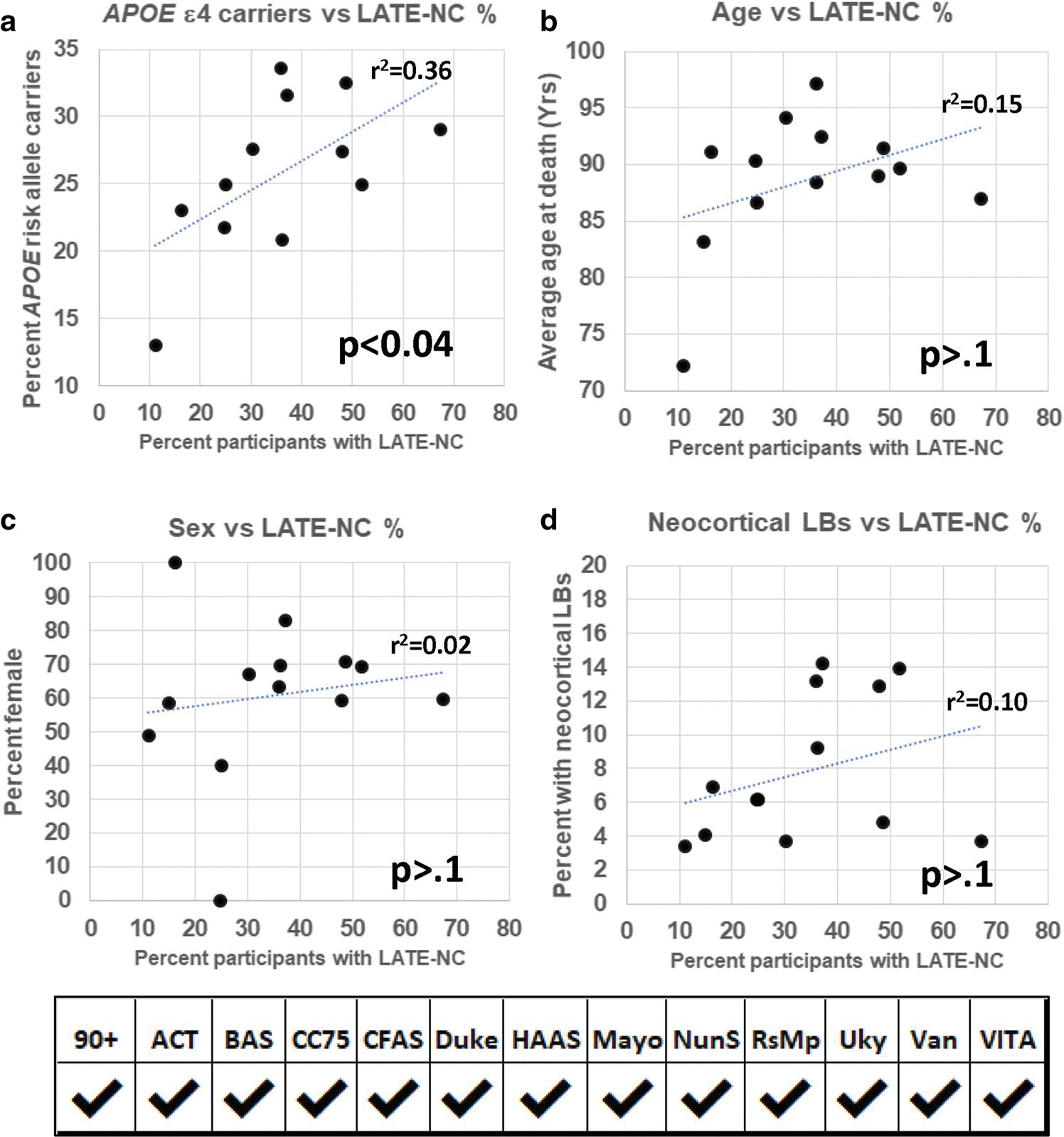
The association between the percentage of included LATE-NC + participants in each cohort (x-axis) with percentages carrying the APOE ε4 allele (a), average age at death (b), sex (percent female (c), and, proportion with neocortical Lewy bodies (LBs), (d) on the y-axes. Each of the autopsy cohorts is indicated by a separate circular marker. The only association that was statistically significant in a simple regression analysis was APOE ε4 carrier frequency rate (a). APOE data were missing from a single cohort; see Table 1 for the numbers of research participants from each contributory cohort
LATE-NC is classified according to a 0–3 stage system, related to the anatomic distribution of TDP-43 pathology [81] and derived from studies that evaluated brains across a broad spectrum of pathologic severity [45, 73]. Cohort neuropathologists applied different antibodies to detect TDP-43 pathology; most cohorts used antibodies against phosphorylated TDP-43 protein (data not shown). Findings in the various subset analyses, stratified by the subsamples evaluated and the LATE-NC results, are depicted in Table 3.
Table 3.
Overall percentage of participants with LATE-NC, stratified by the neuropathologic workups and in the subset of cases with low/no ADNC
| Participants with Braak NFT staging* | Participants with CERAD neuritic amyloid plaque scores* | Participants with Braak NFT stages, Thal Aβ phases, and all LATE-NC stages** | |
|---|---|---|---|
|
| |||
| Number of cohorts providing relevant data | 13 | 13 | 8 |
| Total number of individual participants | 5985 | 6125 | 3803 |
| Overall LATE-NC% in this group | 38.4% | 39.4% | 38.3% |
| Criteria for low/no ADNC | Braak NFT stages = 0-II | CERAD score = “none” | Thal Aβ phase = 0 |
| Number of participants with low/no ADNC | 1883 | 1935 | 787 |
| LATE-NC% in low/no ADNC group | 22.4% | 27.0% | 19.4% |
The full spectrum of ADNC severity was represented in the sample. Among those with known CERAD neuritic plaque scores (n = 6125), 31.6% were classified as CERAD “None”, 17.6% “Sparse plaques”, 28.3% “Moderate plaques”, and 22.5% “Frequent plaques” (Table 4, Fig. 3). In participants with known Braak NFT stage (n = 5985), 31.5% were Braak NFT stages 0-II, 42.0% III/IV, and 26.5% V-VI (Table 4, Fig. 4). As such, approximately 1/4 of participants had severe ADNC.
Table 4.
Joint distribution of LATE-NC positivity with CERAD neuritic amyloid plaque ratings [66] and Braak NFT stages [4, 13]
| Total without LATE-NC | Total with LATE-NC | LATE-NC, % | ||
|---|---|---|---|---|
|
| ||||
| CERAD neuritic amyloid plaque density scores (n = 6125) | None | 1413 | 522 | 27.0 |
| Sparse | 702 | 376 | 34.9 | |
| Moderate | 976 | 759 | 43.7 | |
| Frequent | 621 | 756 | 54.9 | |
| Braak NFT stages (n = 5985) | 0-II | 1461 | 422 | 22.4 |
| III | 812 | 381 | 31.9 | |
| IV | 717 | 604 | 45.7 | |
| V | 492 | 643 | 56.7 | |
| VI | 205 | 248 | 54.7 | |
Fig. 3.
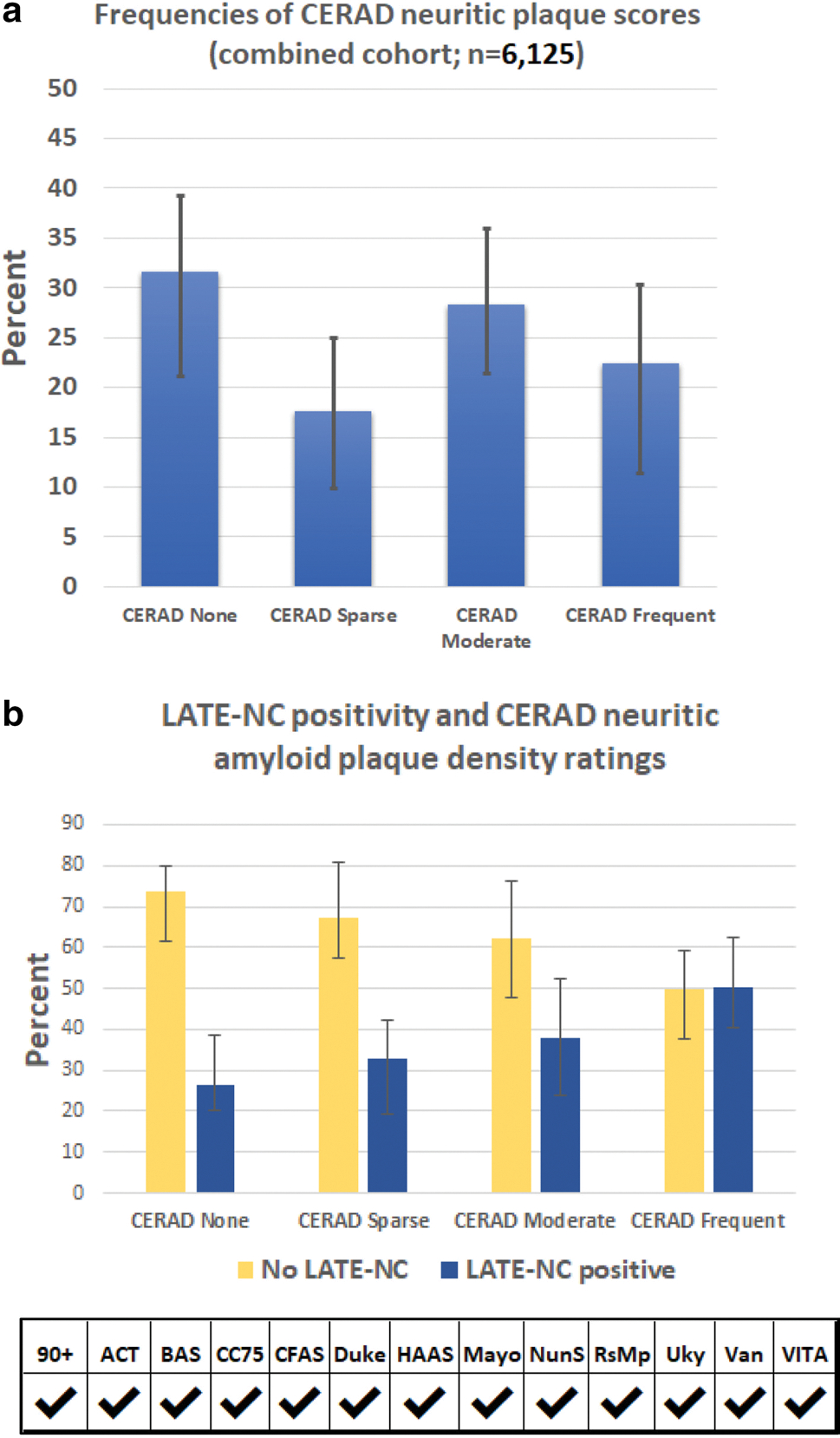
LATE-NC absence or presence, stratified by CERAD neuritic amyloid plaques scores. All LATE-NC stages were combined and the results from each of the cohorts averaged. The frequency of LATE-NC increased with greater neuritic amyloid plaque densities. The distribution of CERAD plaques by frequencies is shown in (a). Note that subgroups with none or minimal ADNC were the most well represented in this combined meta-cohort (see Table 2). Correlation with LATE-NC status is shown in (b). Given the study design differences between cohorts, the results were generally consistent. For these charts, the results of each cohort were weighted equally in order to convey the cohort-to-cohort variance. For exact numbers of participants included from each cohort, see Supplemental Table 3, online resource. Error bars denote 25th and 75th percentiles
Fig. 4.
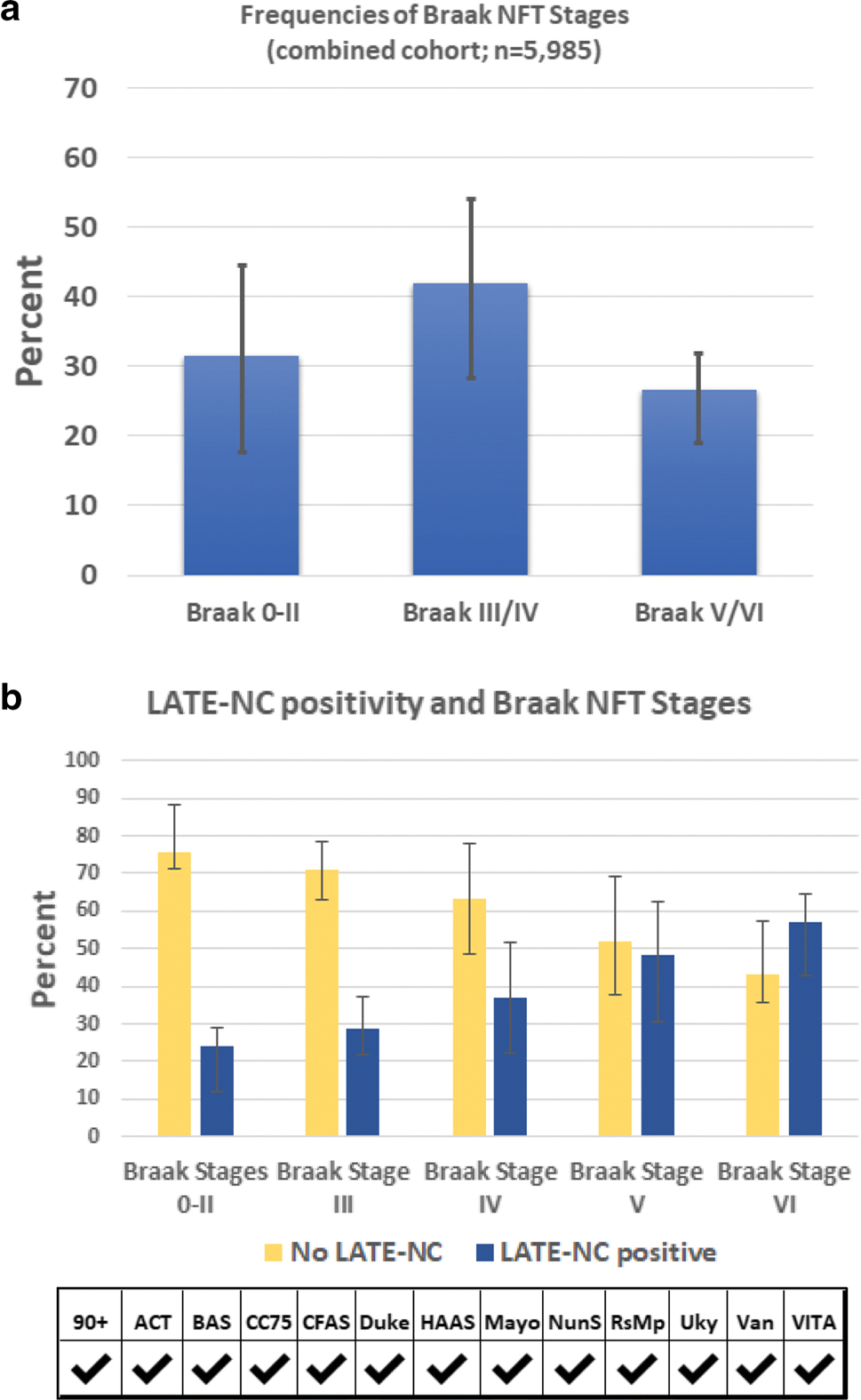
LATE-NC absence or presence, stratified by Braak NFT stages. Here, all LATE-NC stages were combined and the results from each of the cohorts averaged. The distribution of Braak NFT stage groups by frequencies is shown in (a). Correlation with LATE-NC status is shown in (b). The frequency of LATE-NC increased with higher Braak NFT stages. Given the study design differences between cohorts, the results were generally consistent. For these charts the results of each cohort were weighted equally to convey the cohort-to-cohort variance. For exact numbers of participants included from each cohort, see Supplemental Table 3, online resource. Error bars denote 25th and 75th percentiles
In a subset of cases comprising n = 3803 participants, data were available including LATE-NC stages (0–3), Braak NFT stages (0-VI), and Thal Aβ phases (0–5) on each individual subject. The distribution of results stratifying by these parameters is shown in Table 5. Selected findings from those data are presented in chart format in Fig. 5.
Table 5.
Numbers of participants with complete data on LATE-NC stages, Braak NFT stages, and Thal Aβ phases, stratified according to all three pathologic readouts (n = 3803)*
| LATE-NC stage 0 | Braak NFT stages |
Total | |||||||
| 0 | I | II | III | IV | V | VI | |||
|
| |||||||||
| Thal Aβ phases | 0 | 110 | 136 | 176 | 128 | 80 | 4 | 0 | 634 |
| 1 | 18 | 76 | 119 | 130 | 101 | 7 | 1 | 452 | |
| 2 | 16 | 23 | 72 | 54 | 37 | 5 | 2 | 209 | |
| 3 | 7 | 34 | 62 | 130 | 119 | 55 | 8 | 415 | |
| 4 | 2 | 10 | 15 | 58 | 106 | 115 | 17 | 323 | |
| 5 | 0 | 4 | 10 | 23 | 58 | 138 | 68 | 301 | |
| 2334 | |||||||||
|
| |||||||||
| LATE-NC stage 1 | Braak NFT stages |
Total | |||||||
| 0 | I | II | III | IV | V | VI | |||
|
| |||||||||
| Thal Aβ phases | 0 | 4 | 9 | 15 | 22 | 10 | 2 | 0 | 62 |
| 1 | 1 | 8 | 23 | 34 | 31 | 2 | 0 | 99 | |
| 2 | 1 | 2 | 8 | 10 | 7 | 0 | 1 | 29 | |
| 3 | 2 | 7 | 8 | 28 | 40 | 28 | 3 | 116 | |
| 4 | 0 | 1 | 0 | 9 | 48 | 32 | 7 | 97 | |
| 5 | 0 | 0 | 1 | 6 | 19 | 69 | 21 | 116 | |
| 519 | |||||||||
|
| |||||||||
| LATE-NC stage 2 | Braak NFT stages |
Total | |||||||
| 0 | I | II | III | IV | V | VI | |||
|
| |||||||||
| Thal Aβ phases | 0 | 3 | 12 | 9 | 16 | 32 | 3 | 0 | 75 |
| 1 | 1 | 6 | 21 | 22 | 45 | 7 | 1 | 103 | |
| 2 | 0 | 0 | 5 | 22 | 21 | 3 | 1 | 52 | |
| 3 | 0 | 2 | 11 | 20 | 54 | 40 | 7 | 134 | |
| 4 | 0 | 0 | 2 | 10 | 53 | 112 | 11 | 188 | |
| 5 | 0 | 0 | 1 | 6 | 31 | 131 | 61 | 230 | |
| 782 | |||||||||
|
| |||||||||
| LATE-NC stage 3 | Braak NFT stages |
Total | |||||||
| 0 | I | II | III | IV | V | VI | |||
|
| |||||||||
| Thal Aβ phases | 0 | 2 | 2 | 3 | 4 | 5 | 0 | 0 | 16 |
| 1 | 2 | 7 | 5 | 5 | 2 | 0 | 0 | 21 | |
| 2 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 5 | |
| 3 | 0 | 2 | 1 | 6 | 15 | 14 | 1 | 39 | |
| 4 | 0 | 2 | 1 | 2 | 11 | 14 | 3 | 33 | |
| 5 | 0 | 0 | 2 | 2 | 6 | 32 | 12 | 54 | |
| 168 | |||||||||
For the numbers of cases contributory from each cohort, see Supplemental Table 3, online resource
Fig. 5.
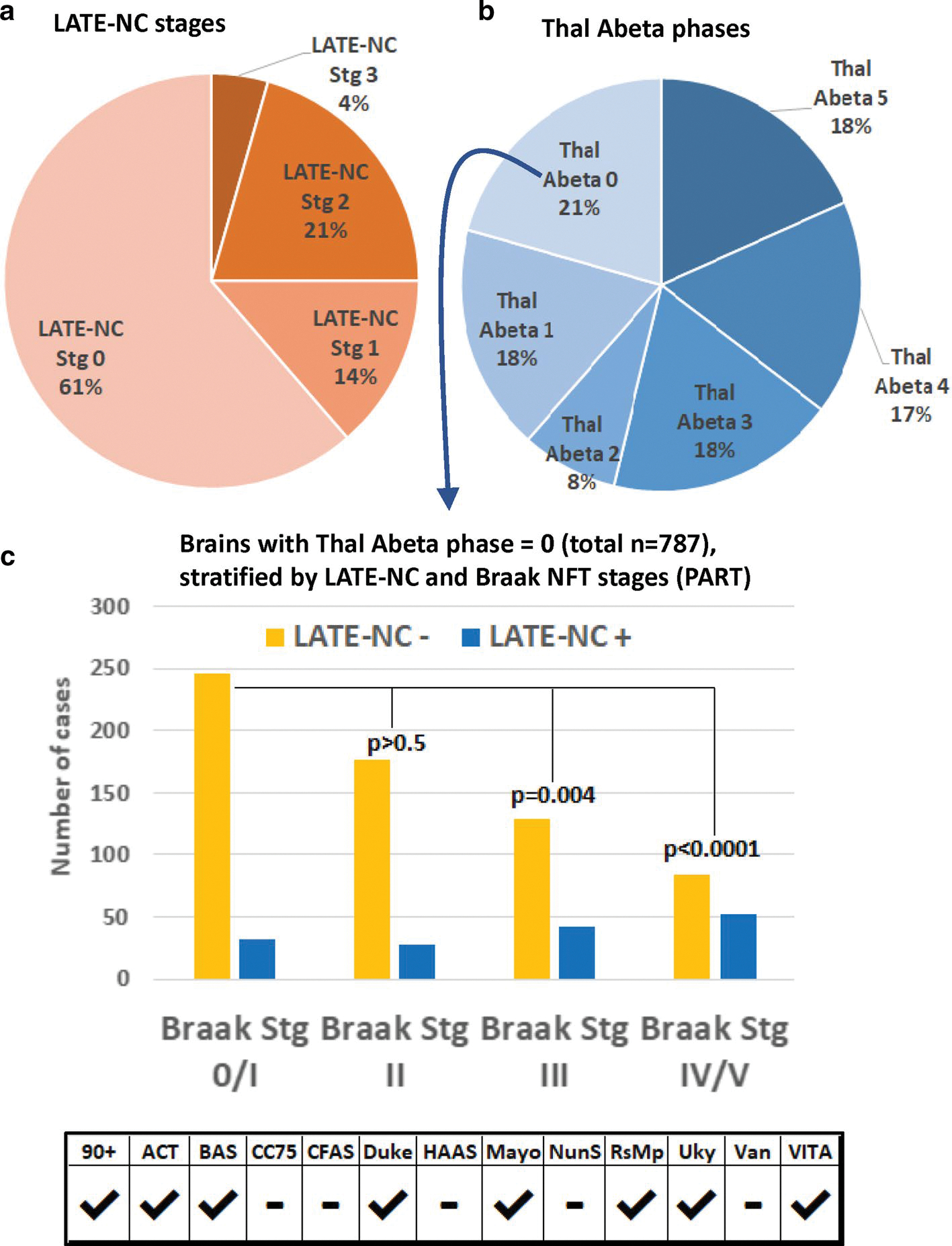
Findings in the 3803 participants with available LATE-NC stage data (a), Thal Aβ phases (b), and Braak NFT staging, which indicate an association between LATE-NC and PART pathology. A pie chart (a) shows the relative frequencies of the different LATE-NC stages. Note that ~ 25% of participants have LATE-NC stage 2 (21% of participants) or stage 3 (4% of participants). A separate pie chart (b) depicts the relative frequencies of different Thal Aβ phases. The bar chart in panel (c) shows the number of cases with Thal Aβ phase = 0, stratified by Braak NFT stages. In these brains lacking Aβ amyloid pathology, the presence of LATE-NC was associated with higher Braak NFT stages (more severe PART pathology). For exact numbers, see Table 5, and for a breakdown of the numbers of participants included from each cohort, see Supplemental Table 3, online resource
Collectively, these data indicated that brains with more severe ADNC were relatively likely to have comorbid LATE-NC. For example, participants with Braak NFT stage 0-II had a 22.4% probability of LATE-NC being diagnosed, whereas those with Braak NFT stage VI had a 54.7% probability of a LATE-NC diagnosis (Table 4, Fig. 3). However, most participants with LATE-NC (61.2%) coincided with Braak NFT stages between 0 and IV (because only ~ 1/4 of participants had severe ADNC). Similar trends were observed for CERAD neuritic amyloid plaque densities (Table 4, Fig. 3), and Thal Aβ phases (Table 5). Although cohort-to-cohort variation was seen, there was broad agreement in findings, as can be appreciated by the 25th–75th percentile error bars in Figs. 3, 4.
Trends could be identified along the full ranges of ADNC and LATE-NC severities. Note that in the Table 5 data, LATE-NC stage 3 brains comprised only 11% of LATE-NC + cases (168 out of 1469), and LATE-NC stage 3 was associated with a high rate of severe ADNC–approximately the same frequency of severe ADNC as seen in LATE-NC stage 2. Furthermore, in brains lacking Aβ amyloid deposition (Thal Aβ phase = 0; n = 787), PART pathology was relatively more severe, i.e. higher Braak NFT stages, in persons with comorbid LATE-NC (Fig. 5).
While LATE-NC tended to be more frequent in more severe ADNC cases, LATE-NC was nonetheless present across all ADNC levels and even in those without ADNC. As shown in Table 3, 1935 participants had “None” neuritic amyloid plaques, and of these, 522 (27.0%) had LATE-NC. In the subset of individuals with known Thal Aβ phase = 0 (i.e. lacking Aβ plaques), 19.4% had LATE-NC, and 11.6% had LATE-NC Stages > 1, a severity of LATE-NC which has been consistently associated with cognitive impairment [18, 70, 73, 74, 78] (Table 5).
To assess how different neuropathologic raters would diagnose Braak NFT staging of LATE-NC cases that lacked severe ADNC, a convenience sample of phospho-Tau immunostained slides was evaluated by four separate blinded neuropathology diagnosticians, using digital pathology over the internet. As expected [4], there was some variance in Braak NFT staging by the raters, but the median rendered Braak NFT stages were within 1 Braak stage of the initial diagnosis in 8/10 cases and within 1.5 Braak stages in all 10 cases (see Supplemental Table 4, online resource).
Summary information on final cognitive status of included participants was requested from each cohort, with the data stratified by Braak NFT stages (bottom of Supplemental Table 2, online resource). These data were a focal-point because Braak NFT staging is the widely gathered ADNC parameter that correlates most robustly with cognitive impairment [80]. Detailed stratified cognitive testing results were not available from VITA, CC75C, and CFAS cohorts and thus were not included in the clinical-pathological analyses. Among the cohorts with accessible information, the cognitive status data were variable from cohort to cohort. There were different cognitive assessment instruments, different intervals of testing, and different workflows used in administering the tests. The nature of these combined summary data precluded statistical testing. However, a recurrent pattern did emerge across the different study groups, despite the many sources of variance and the smaller sample sizes when using data from single cohorts: there was a tendency for cognitive scores to be lower in individuals with LATE-NC, across the full spectrum of ADNC severity in terms of Braak NFT stages (Fig. 6). Some of the implications and context of the present study are presented in Fig. 7.
Fig. 6.
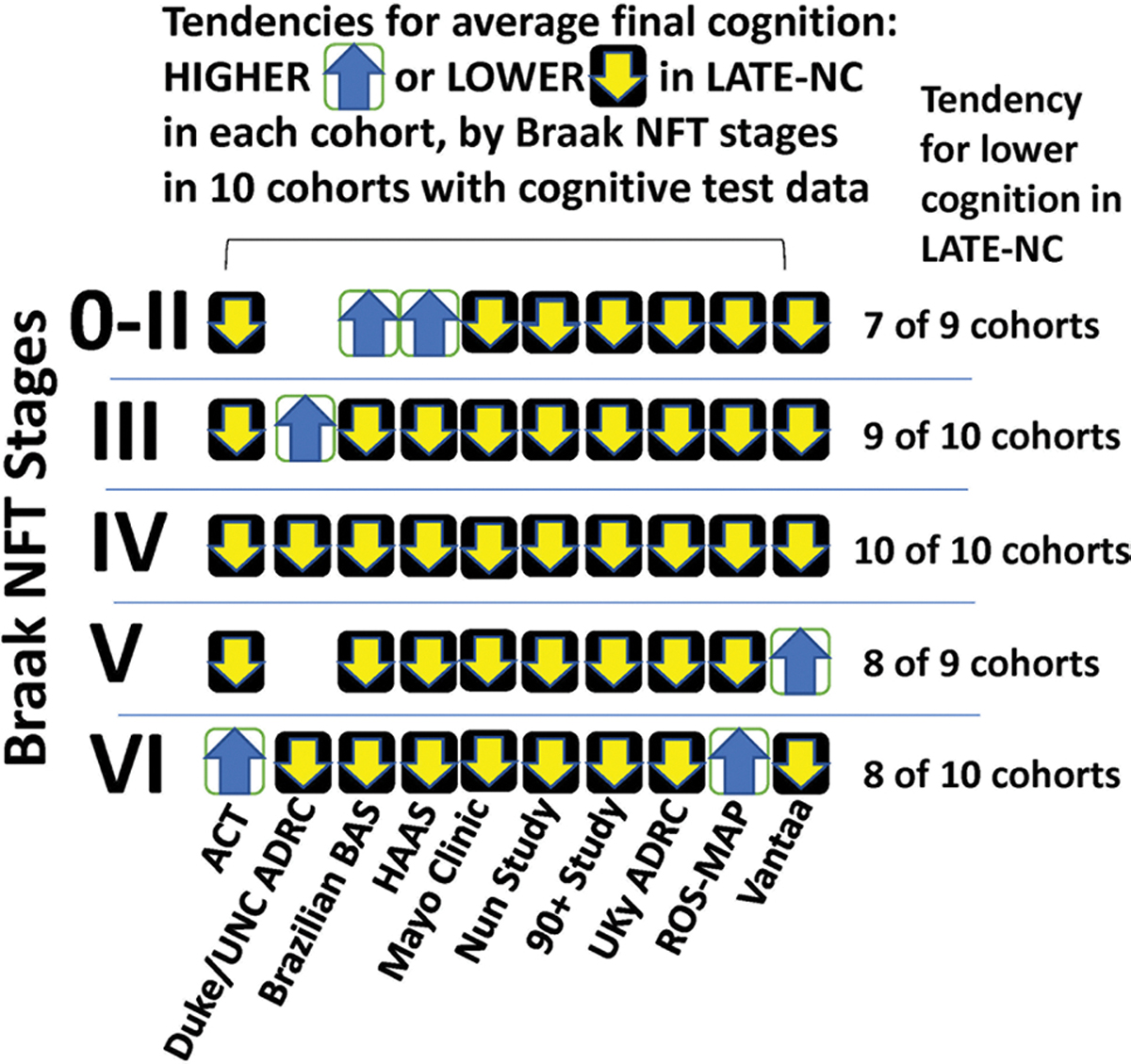
There is a tendency for LATE-NC to be associated with cognitive impairment, across a broad range of Braak NFT stages, in ten community-based cohorts. Data were gathered on cognitive status, stratifying by LATE-NC status and Braak NFT stages. Trends were evaluated from each cohort as to whether the cognitive status tended to be lower in persons with LATE-NC (down-going black arrow) or higher (up-going white arrow) in given Braak NFT stages. To operationalize global cognitive status, final Mini-Mental State Examination scores [33] were used, except HAAS used the Cognitive Abilities Screening Instrument [107] and the Brazil BAS and MCSA cohorts used the Clinical Dementia Rating sum of boxes scores [27]. There was a tendency for participants with LATE-NC to have lower cognition across the full range of Braak NFT stages
Fig. 7.
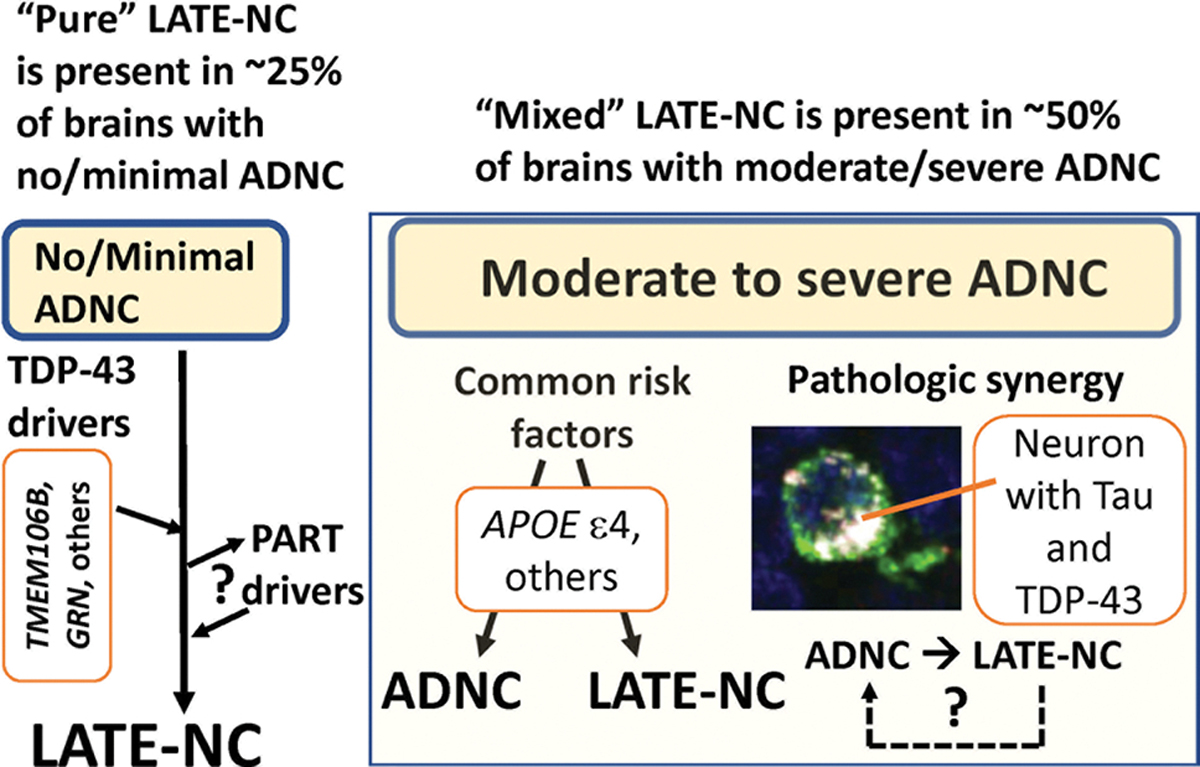
Selected findings and context of the current study. Data were analyzed from participants in 13 high quality community- and population-based cohorts comprising over 6000 individuals followed longitudinally to autopsy. As such, the findings (with appropriate caveats) have broad implications. In participants that had none or minimal ADNC, a substantial proportion (~ 25%) had LATE-NC. This indicates that there are ADNC-independent TDP-43 pathology-driving mechanisms, which probably include gene variants in TMEM106B and GRN [26, 87, 96]. LATE-NC also was associated with more severe PART pathology (and vice versa), indicating pathologic synergy between LATE-NC and PART. Approximately 2/3rd of subjects in advanced age showed moderate or severe ADNC at brain autopsy, in concordance with the published literature [15]. In these individuals, there was a relatively high frequency of LATE-NC: approximately 50% of participants with moderate to severe ADNC had LATE-NC. The “mixed” ADNC-LATE-NC may be driven by pleiotropic genetic factors (e.g., APOE ε4 allele [114]) and there may also be pathologic synergies downstream from genetics. For example, intracellular tauopathy may promote TDP-43 pathology in the same cell [44, 103, 111]. The neuron shown here is stained with immunofluorescence in the hippocampal dentate gyrus, and is immunolabeled green (tau), and red (phospho-TDP-43) with overlap depicted in white [103]
Discussion
Data related to LATE-NC and ADNC were gathered, combined, and analyzed from 13 community-based and population-based longitudinal cohort studies. Overall, almost 40% of autopsied participants had LATE-NC. LATE-NC was relatively common in brains with severe ADNC–approximately half of severe ADNC cases had comorbid LATE-NC. By contrast, approximately one in four brains with no or minimal evidence of ADNC had LATE-NC. PART pathology was relatively more severe in persons with comorbid LATE-NC. There was a tendency for cognitive scores to be worse in persons with LATE-NC, across the full spectrum of ADNC severity. These findings address basic questions about LATE-NC in people with and without comorbid ADNC.
Both the quality and quantity of data were strengths of this study. The community- and population-based study designs of the contributory cohorts included many persons recruited while cognitively normal and followed longitudinally to autopsy. At the last exam before death, clinical features of the combined cohort showed slightly over 40% cognitive normal, and no FTD/FTLD examples were documented. This may underestimate the extent of cognitive impairment experienced, although most of the decedents were assessed in the last year of life. We emphasize that this distribution of clinical findings is in accord with epidemiologic data from human populations of this age group [21, 60, 86, 90]. While no study with autopsies examines all potential subjects, and none is perfectly representative of the variability in human populations across demographic and ethnoracial boundaries, community- and population-based autopsy cohorts are the nearest approximation to a generalizable sample. Each cohort included here has provided the basis for published work related to LATE-NC [3, 32, 36–39, 51, 56, 77, 83, 88, 105]. Aggregating these data into a combined cohort comprising > 6000 people provided new insight into the prevalence of LATE-NC in aging, while also highlighting between-cohort variability.
One way to evaluate recruitment bias in a dementia study is to compare the frequency of APOE ε4 allele among the reported participants with population-based figures. This is especially relevant because APOE ε4 is associated with increased risk for LATE-NC [28, 93, 114]. In most human populations, approximately 25% of individuals carry at least one copy of the APOE ε4 allele [20, 101] (the ε4 prevalence tends to be somewhat higher in Scandinavia [30, 101]). It is notable that 25.5% of the genotyped participants in the current study had at least one APOE ε4 allele. By contrast, in many dementia research cohorts the APOE ε4 prevalence is higher [31]. For example, a recent report on LATE-NC derived from multiple clinic-based cohorts included 495 participants of which 47.4% were APOE ε4 + (and 11.7% had FTD clinical syndrome) [49]. Many dementia studies have even higher APOE ε4 positivity [23]. These studies may provide important insights (some impossible to achieve in community-based cohorts), but the distribution of pathologic findings in such clinic-based cohorts are unlikely to be representative of a broader population.
The current work has important limitations. Although the community-based cohorts encompassed thousands of research participants from five countries on three continents, human populations other than White Caucasians were underrepresented. Prior studies compared LATE-NC between ethnoracially defined groups [72, 77], but more work is required in this area [31, 85].
There were additional challenges in reconciling the LATE-NC data between cohorts. Neuropathologists used study-specific protocols, including non-identical tissue sampling and different antibodies. Some biologic variance is to be expected given the between-cohort differences in age, cognitive status, geography, and birth cohorts. These factors contribute to the wide variability of frequency of detected LATE-NC across the different included cohorts (range 11–63%). However, this inclusive approach, encompassing a range of diagnostic methods rather than one specific proscribed protocol, reflects the broad range of neuropathologic methods that are applied in everyday practice around the world, as well as true differences in frequency of neuropathologic lesions.
Another consideration is that TDP-43 pathology restricted to the amygdala was included to operationalize the presence of LATE-NC. There were undoubtedly LATE-NC false-negatives because the amygdala was not examined in some cases. LATE-NC stage 1 is hypothesized to be an incipient disease stage, analogous to early pathologic stages of AD and Lewy body diseases [76, 80]. As specific examples, Braak NFT stages I-III, Thal Aβ phases 1–2, and Braak Parkinson’s disease stages 1–2 are all common in persons without documented neurological impairment [35, 42, 109]. Among the 3803 brains in the current study where all the LATE-NC stages were known, LATE-NC stage 1 comprised 36% of the LATE-NC cases and may correlate with limited, if any, cognitive manifestations [24, 73–75, 81]. However, the counterpoint is that 25% of the entire cohort had LATE-NC stage > 1, which is associated robustly with cognitive impairment [12, 32, 36, 39, 40, 44, 47, 51, 59, 70, 72, 79, 92].
Beyond the evaluation of LATE-NC, there are other challenges in reconciling neuropathologic data from different cohorts. The various studies had gathered brain donations over decades, and tissue handling methods have changed over time. One may expect imperfect agreement regarding low-Braak NFT stages as uniform staging requires standard sectioning and staining, and neuroanatomical expertise. (LATE-NC has been associated with NFT anatomical distribution that deviates from conventional Braak NFT staging [103].) Indeed, prior studies reported imperfect agreements in ADNC assessments among neuropathologists [4, 68]. This tendency was also evident in our digital pathological study with four separate raters evaluating the same cases using digital pathology over the internet.
An interpretation of the public health implications of this cross-sectional study should consider that the average age at death for included participants was 88.1 years. The frequency of autopsy-confirmed LATE-NC in this study (slightly under 40%), and other findings, does not represent projected population prevalence, but instead are a readout related to persons dying in that age range and agreeing to research brain donation. The study sample coincides with an age group at relatively high risk for LATE-NC [81]. (The role of age as a factor in the relative frequencies of neurodegenerative disorders could not be examined thoroughly in the present study.) It may be argued that the included participants were unusually long-lived persons, considering normative data. For example, the average age of death in the United States during 2020 was 80.5 years for women, and 75.1 for men [2]—slightly older in European cohorts. Yet these averaged longevity calculations included many individuals who died at considerably younger ages. US Social Security Administration actuarial data predict that a woman who lives to age 70 years in the United States has a 32% chance to live until age 90 years, and a 70-year-old man a 21% chance to live until age 90 years [1]. Thus, a substantial proportion of adults will probably survive to the ages of participants included in the current study, with high risk for ADNC and LATE-NC.
This study reported summary information from each cohort rather than individual participant-level data, so regression models and other descriptive statistics were not appropriate for evaluating most of the data. In terms of clinical–pathological correlation, only broad trends were described, because robust statistical testing require a more standardized cognitive assessment format. There are many possible sources of data variability, e.g., additional pathologies, and testing variation between cohorts. Importantly, prior studies have established that LATE-NC is independently associated with cognitive impairment in aging when other factors (e.g., pathologic comorbidities) were considered [12, 36, 39, 70, 79, 92]. Thus, the main contribution of the current study is not clinical–pathological correlation, but instead it is a relatively sound estimate of LATE-NC prevalence in community- and population-based elderly autopsy cohorts across the ADNC severity spectrum.
LATE-NC was more common in brains with comorbid ADNC than in those without ADNC. Specifically, there was a 2- to 2.5-fold enrichment for LATE-NC in persons with severe ADNC versus those lacking ADNC. LATE-NC is not the only pathology that tends to be increased in parallel with ADNC. For example, Lewy body pathology subtypes and cerebrovascular pathologies such as arteriolosclerosis are also relatively prevalent in persons with ADNC [11, 17, 88, 95], as are white matter hyperintensities visualized with neuroimaging [7, 102], and other, rarer, phenomena [25, 64, 100]. The tendency for these brain conditions to coexist with ADNC may be due to shared ‘upstream’ risk factors such as the APOE ε4 allele which is known to be pleiotropic for multiple diseases (see above), or other causes of brain injury. ‘Downstream’ of genetic and other risk factors, one subtype of pathology may directly promote other deleterious changes in the same cells. In particular, TDP-43 pathology often co-occurs with tau pathology in neurons vulnerable to NFT formation, such as in the entorhinal cortex [44, 111]. Conversely, tau inclusions coexist in cells prone to TDP-43 pathology, such as the hippocampal dentate granule neurons, in LATE-NC [103]. The increased severity of PART pathology in cases with LATE-NC in the present study further underscores the tendency for there to be pathologic synergies between tau and TDP-43 pathologies.
Although often comorbid, LATE-NC and ADNC were also seen in brains that lacked the other pathology. It is notable that ~ 75% of participants overall had some detectable ADNC, as shown previously [15, 109]. Thus, the generalization is true that “most people with LATE-NC have ADNC”, yet most old people’s brains without LATE-NC also have ADNC. In this sample with a broad range of pathologies, > 60% of brains with LATE-NC lacked severe ADNC (i.e., had Braak NFT stages 0–IV). Among those with severe ADNC, approximately one-half lacked TDP-43 pathology. These data indicate that LATE-NC is not an integral feature of ADNC. Further support for the idea that LATE-NC and ADNC are distinct disorders come from prior published reports. For example, LATE-NC is an unusual co-pathology (< 10% prevalence) in severe ADNC linked to Down syndrome [113].
There was a substantial subgroup of participants with LATE-NC but with none or very mild ADNC: persons with Braak NFT stages 0-II had a 22.4% probability of LATE-NC whereas persons with “None” neuritic amyloid plaque score had a 26.9% probability of LATE-NC. Remarkably, among 3267 subjects surveyed for the condition, no FTD/FTLD case was identified. Thus, in community dwelling older persons with no or minimal evidence of ADNC, LATE-NC was still common and was not associated with a clinical diagnosis of FTD (in the nine cohorts in which that clinical evaluation was made). It is possible that a handful of FTD/FTLD cases was overlooked. Yet their extreme paucity in such a large combined cohort implies that FTD/FTLD-TDP is very uncommon in community-based cohorts. If the ~ 25% autopsy frequency is considered an estimate, albeit imprecise, of lifetime risk for LATE-NC in persons without ADNC, it can be contrasted with the epidemiologic studies that have found ~ 0.1% lifetime risk for FTLD-TDP [21, 53]. Thus, though there are important intersections between FTLD-TDP and LATE-NC, our results further support the conclusion that LATE-NC should be considered a separate entity from FTD/FTLD.
In summary, the current study found that LATE-NC was a frequent pathology in older brains: ~ 25% of participants overall had LATE-NC stage > 1, which is associated with cognitive impairment. LATE-NC was relatively common in brains with coexisting ADNC, and PART pathology was also relatively more severe in brains with comorbid LATE-NC. However, the presence of LATE-NC or ADNC was neither necessary nor sufficient to predict the presence of the other. Encompassing the full spectrum of ADNC severity, LATE-NC tended to be associated with cognitive impairment. These data are interpreted to indicate that LATE-NC, with or without comorbid ADNC, is highly prevalent and impactful in persons of advanced age.
Supplementary Material
Acknowledgements
We are profoundly grateful to the research participants, caregivers, clinicians, staff, and colleague scientists who contributed to this study.
Funding
We acknowledge National Institutes of Health grants P30 AG072958 (S.-H. J.W.), P30 AG072977 (M.E.F.), K08 AG065463 (M.E.F.), RF1 AG072080 (M.E.F.), K08 AG 065426 (C.S.L), R01 AG038651 (E.L.A.), UF1 AG057707 (T.J.M and L.W), R01AG021055 (CK and MC), P30 AG066519 (UCI ADRC), R01 AG061111 (P.T.N.), R01 AG057187 (P.T.N.), P30 AG072946 (P.T.N.), RF1 NS118584 (M.D.C.), R01 AG054449 (M.E.M.), RF1 AG069052 (J.G.R), P30 AG072972 (UC Davis ADRC), R01 AG062517 (B.N.D.), U19 AG069701 (M.E.M.), K24 AG053435 (L.T.G.), R01AG067482 (J.A.S.), R01AG064233 (J.A.S.), R01AG022018 (J.A.S.), P30AG010161/P30AG072975 (J.A.S.), P30 AG062677 (Mayo ADRC), UF1 NS125417 (R.C.P.), U01 AG006786 (MCSA), R01 AG034676 (REP), P30 AG66509 (UW ADRC), and U19 AG066567 (ACT Study). Academy of Finland (341007) (L.M.); State funding for university-level health research (TYH2020231, TYH2022316) (L.M.); Liv och Hälsa Foundation (L.M.); Rossy Foundation and the Edmond Safra Philanthropic Foundation (G.G.K.); Sao Paulo Research Foundation (FAPESP 06/55318-1, 09/09134-4, 16/24326-0) (C.K.S.); the Nancy and Buster Alvord Endowment (C.D.K.); Alzheimer’s Research UK (ARUK) doctoral studentship (ARUK-PhD2017-34) (R.M.); ARUK-PhD2014-19 (S.R.K.H.). UK Medical Research Council (MRC) (MRC/G9901400, U.1052.00.0013, G0900582). NHMRC Dementia Research Leadership Fellowship NT113567 (H.A.D.K.), Addenbrooke’s Charitable Trust (H.A.D.K., S.H.), Addenbrooke’s Charitable Trust grant 900108 (S.H.), Paul G. Allen Foundation (S.H.), ARUK NSG (S.H.), Alzheimer’s Society 554 (AS-PG-2019b-024) (S.H., J.F.). The Cambridge Brain Bank Laboratory is supported by the National Institute for Health Research, Cambridge Biomedical Research Centre. APOE genotyping from the National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD), which receives government support under a cooperative agreement grant (U24AG021886) awarded by the National Institute on Aging (NIA), were used in this study.
Footnotes
Declarations
Conflict of interest Authors D.W.D., G.G.K., and P.T.N. are members of the Editorial Board of Acta Neuropathologica and J.A. is Editor in Chief of Acta Neuropathologica, but none of the coauthors were involved in the Editorial handling of this article.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00401-022-02444-1.
References
- 1.(2017) https://www.ssa.gov/oact/STATS/table4c6.html. Accessed 14 Dec 2021
- 2.(2021) Provisional life expectancy estimates for January through June, 2020. https://www.cdc.gov/nchs/data/vsrr/VSRR10-508.pdf. Accessed 14 Dec 2021
- 3.Agrawal S, Yu L, Kapasi A, James BD, Arfanakis K, Barnes LL et al. (2021) Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change and microvascular pathologies in community-dwelling older persons. Brain Pathol. 10.1111/bpa.12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H et al. (2008) Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BRAINNEt Europe consortium. Brain Pathol 18:484–496. 10.1111/j.1750-3639.2008.00147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J et al. (2008) Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the brainnet Europe consortium. Acta Neuropathol 115:533–546. 10.1007/s00401-008-0358-2 [DOI] [PubMed] [Google Scholar]
- 6.Alafuzoff I, Thal DR, Arzberger T, Bogdanovic N, Al-Sarraj S, Bodi I et al. (2009) Assessment of beta-amyloid deposits in human brain: a study of the brainnet Europe consortium. Acta Neuropathol 117:309–320. 10.1007/s00401-009-0485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altamura C, Scrascia F, Quattrocchi CC, Errante Y, Gangemi E, Curcio G et al. (2016) Regional MRI diffusion, white-matter hyperintensities, and cognitive function in Alzheimer’s disease and vascular dementia. J Clin Neurol 12:201–208. 10.3988/jcn.2016.12.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachstetter AD, Garrett FG, Jicha GA, Nelson PT (2021) Space-occupying brain lesions, trauma-related tau astrogliopathy, and ARTAG: a report of two cases and a literature review. Acta Neuropathol Commun 9:49. 10.1186/s40478-021-01152-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellenguez C, Kucukali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N et al. (2022) New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet 54:412–436. 10.1038/s41588-022-01024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS (2012) Overview and findings from the religious orders study. Curr Alzheimer Res 9:628–645. 10.2174/156720512801322573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blevins BL, Vinters HV, Love S, Wilcock DM, Grinberg LT, Schneider JA et al. (2020) Brain arteriolosclerosis. Acta Neuropathol. 10.1007/s00401-020-02235-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA et al. (2019) Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol 85:114–124. 10.1002/ana.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357 [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259 [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. 10.1097/NEN.0b013e318232a379 [DOI] [PubMed] [Google Scholar]
- 16.Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH et al. (2009) Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis 18:645–658. 10.3233/JAD-2009-1182 [DOI] [PubMed] [Google Scholar]
- 17.Brenowitz WD, Nelson PT, Besser LM, Heller KB, Kukull WA (2015) Cerebral amyloid angiopathy and its co-occurrence with Alzheimer’s disease and other cerebrovascular neuropathologic changes. Neurobiol Aging. 10.1016/j.neurobiolaging.2015.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buciuc M, Tosakulwong N, Machulda MM, Whitwell JL, Weigand SD, Murray ME et al. (2021) TAR DNA-binding protein 43 is associated with rate of memory, functional and global cognitive decline in the decade prior to death. J Alzheimers Dis 80:683–693. 10.3233/JAD-201166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chornenkyy Y, Fardo DW, Nelson PT (2019) Tau and TDP-43 proteinopathies: kindred pathologic cascades and genetic pleiotropy. Lab Invest 99:993–1007. 10.1038/s41374-019-0196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbo RM, Scacchi R (1999) Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a “thrifty” allele? Ann Hum Genet 63:301–310. 10.1046/j.1469-1809.1999.6340301.x [DOI] [PubMed] [Google Scholar]
- 21.Coyle-Gilchrist IT, Dick KM, Patterson K, Vazquez Rodriquez P, Wehmann E, Wilcox A et al. (2016) Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86:1736–1743. 10.1212/WNL.0000000000002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I et al. (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. 10.1007/s00401-014-1349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crean S, Ward A, Mercaldi CJ, Collins JM, Cook MN, Baker NL et al. (2011) Apolipoprotein E epsilon4 prevalence in Alzheimer’s disease patients varies across global populations: a systematic literature review and meta-analysis. Dement Geriatr Cogn Disord 31:20–30. 10.1159/000321984 [DOI] [PubMed] [Google Scholar]
- 24.Cykowski MD, Arumanayagam AS, Powell SZ, Rivera AL, Abner EL, Roman GC et al. (2022) Patterns of amygdala region pathology in LATE-NC: subtypes that differ with regard to TDP-43 histopathology, genetic risk factors, and comorbid pathologies. Acta Neuropathol. 10.1007/s00401-022-02416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debatin L, Streffer J, Geissen M, Matschke J, Aguzzi A, Glatzel M (2008) Association between deposition of beta-amyloid and pathological prion protein in sporadic Creutzfeldt-Jakob disease. Neurodegener Dis 5:347–354. 10.1159/000121389 [DOI] [PubMed] [Google Scholar]
- 26.Dickson DW, Rademakers R, Nicholson AM, Schneider JA, Yu L, Bennett DA (2015) The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology 85:1354–1355. 10.1212/01.wnl.0000472918.79256.a9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dooneief G, Marder K, Tang MX, Stern Y (1996) The clinical dementia rating scale: community-based validation of “profound” and “terminal” stages. Neurology 46:1746–1749. 10.1212/wnl.46.6.1746 [DOI] [PubMed] [Google Scholar]
- 28.Dugan AJ, Nelson PT, Katsumata Y, Shade LMP, Boehme KL, Teylan MA et al. (2021) Analysis of genes (TMEM106B, GRN, ABCC9, KCNMB2, and APOE) implicated in risk for LATE-NC and hippocampal sclerosis provides pathogenetic insights: a retrospective genetic association study. Acta Neuropathol Commun 9:152. 10.1186/s40478-021-01250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duyckaerts C, Braak H, Brion JP, Buee L, Del Tredici K, Goedert M et al. (2015) PART is part of Alzheimer disease. Acta Neuropathol 129:749–756. 10.1007/s00401-015-1390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewbank DC (2004) The APOE gene and differences in life expectancy in Europe. J Gerontol A Biol Sci Med Sci 59:16–20. 10.1093/gerona/59.1.b16 [DOI] [PubMed] [Google Scholar]
- 31.Filshtein TJ, Dugger BN, Jin LW, Olichney JM, Farias ST, Carvajal-Carmona L et al. (2019) Neuropathological diagnoses of demented hispanic, black, and non-hispanic white decedents seen at an Alzheimer’s disease center. J Alzheimers Dis 68:145–158. 10.3233/JAD-180992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flanagan ME, Cholerton B, Latimer CS, Hemmy LS, Edland SD, Montine KS et al. (2018) TDP-43 neuropathologic associations in the nun study and the honolulu-asia aging study. J Alzheimers Dis 66:1549–1558. 10.3233/JAD-180162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- 34.Greenberg SG, Davies P, Schein JD, Binder LI (1992) Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem 267:564–569 [PubMed] [Google Scholar]
- 35.Halliday GM, Del Tredici K, Braak H (2006) Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J Neural Transm Suppl. 10.1007/978-3-211-45295-0_16 [DOI] [PubMed] [Google Scholar]
- 36.Harrison WT, Lusk JB, Liu B, Ervin JF, Johnson KG, Green CL et al. (2021) Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is independently associated with dementia and strongly associated with arteriolosclerosis in the oldest-old. Acta Neuropathol. 10.1007/s00401-021-02360-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hokkanen SRK, Hunter S, Polvikoski TM, Keage HAD, Minett T, Matthews FE et al. (2018) Hippocampal sclerosis, hippocampal neuron loss patterns and TDP-43 in the aged population. Brain Pathol 28:548–559. 10.1111/bpa.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hokkanen SRK, Kero M, Kaivola K, Hunter S, Keage HAD, Kiviharju A et al. (2020) Putative risk alleles for LATE-NC with hippocampal sclerosis in population-representative autopsy cohorts. Brain Pathol 30:364–372. 10.1111/bpa.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter S, Hokkanen SRK, Keage HAD, Fleming J, Minett T, Polvikoski T et al. (2020) TDP-43 related neuropathologies and phosphorylation state: associations with age and clinical dementia in the Cambridge city over-75s cohort. J Alzheimers Dis: 10.3233/JAD-191093 [DOI] [PubMed] [Google Scholar]
- 40.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA (2016) TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain 139:2983–2993. 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jellinger KA (2022) Recent update on the heterogeneity of the Alzheimer’s disease spectrum. J Neural Transm (Vienna) 129:1–24. 10.1007/s00702-021-02449-2 [DOI] [PubMed] [Google Scholar]
- 42.Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, Cooper GE et al. (2012) Preclinical AD workgroup staging: pathological correlates and potential challenges. Neurobiol Aging 33(622):e621–622. 10.1016/j.neurobiolaging.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josephs KA, Mackenzie I, Frosch MP, Bigio EH, Neumann M, Arai T et al. (2019) LATE to the PART-y. Brain 142:e47. 10.1093/brain/awz224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josephs KA, Murray ME, Tosakulwong N, Weigand SD, Serie AM, Perkerson RB et al. (2019) Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol 137:227–238. 10.1007/s00401-018-1951-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L (2016) Updated TDP-43 in Alzheimer,s disease staging scheme. Acta Neuropathol 131:571–585. 10.1007/s00401-016-1537-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M et al. (2008) Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 70:1850–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM et al. (2014) TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 127:811–824. 10.1007/s00401-014-1269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karanth S, Nelson PT, Katsumata Y, Kryscio RJ, Schmitt FA, Fardo DW et al. (2020) Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol 77:1299–1307. 10.1001/jamaneurol.2020.1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katsumata Y, Abner EL, Karanth S, Teylan MA, Mock CN, Cykowski MD et al. (2020) Distinct clinicopathologic clusters of persons with TDP-43 proteinopathy. Acta Neuropathol 140:659–674. 10.1007/s00401-020-02211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM (2015) Multiple pathologies are common and related to dementia in the oldest-old: the 90+ study. Neurology 85:535–542. 10.1212/WNL.0000000000001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keage HA, Hunter S, Matthews FE, Ince PG, Hodges J, Hokkanen SR (2014) TDP-43 pathology in the population: prevalence and associations with dementia and age. J Alzheimers Dis 42:641–650. 10.3233/JAD-132351 [DOI] [PubMed] [Google Scholar]
- 52.Kero M, Raunio A, Polvikoski T, Tienari PJ, Paetau A, Myllykangas L (2018) Hippocampal sclerosis in the oldest old: a finnish population-based study. J Alzheimers Dis 63:263–272. 10.3233/JAD-171068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knopman DS, Roberts RO (2011) Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci 45:330–335. 10.1007/s12031-011-9538-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kon T, Tomiyama M, Wakabayashi K (2020) Neuropathology of lewy body disease: clinicopathological crosstalk between typical and atypical cases. Neuropathology 40:30–39. 10.1111/neup.12597 [DOI] [PubMed] [Google Scholar]
- 55.Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C et al. (2013) Non-alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 126:365–384. 10.1007/s00401-013-1157-y [DOI] [PubMed] [Google Scholar]
- 56.Latimer CS, Burke BT, Liachko NF, Currey HN, Kilgore MD, Gibbons LE et al. (2019) Resistance and resilience to Alzheimer’s disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathol Commun 7:91. 10.1186/s40478-019-0743-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latimer CS, Keene CD, Flanagan ME, Hemmy LS, Lim KO, White LR et al. (2017) Resistance to Alzheimer disease neuropathologic changes and apparent cognitive resilience in the nun and honolulu-asia aging studies. J Neuropathol Exp Neurol 76:458–466. 10.1093/jnen/nlx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CS, Latimer CS, Henriksen JC, Blazes M, Larson EB, Crane PK et al. (2021) Application of deep learning to understand resilience to Alzheimer’s disease pathology. Brain Pathol 31:e12974. 10.1111/bpa.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez OL, Kofler J, Chang Y, Berman SB, Becker JT, Sweet RA et al. (2020) Hippocampal sclerosis, TDP-43, and the duration of the symptoms of dementia of AD patients. Ann Clin Transl Neurol 7:1546–1556. 10.1002/acn3.51135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L et al. (2013) A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the cognitive function and ageing study I and II. Lancet 382:1405–1412. 10.1016/S0140-6736(13)61570-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2017) TDP-43 pathology in Alzheimer’s disease, dementia with lewy bodies and ageing. Brain Pathol 27:472–479. 10.1111/bpa.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehta RI, Schneider JA (2021) What is Alzheimer’s disease? The neuropathological heterogeneity of clinically defined Alzheimer’s dementia. Curr Opin Neurol 34:237–245. 10.1097/WCO.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 63.Meneses A, Koga S, O’Leary J, Dickson DW, Bu G, Zhao N (2021) TDP-43 pathology in Alzheimer’s disease. Mol Neurodegener 16:84. 10.1186/s13024-021-00503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miklossy J, Steele JC, Yu S, McCall S, Sandberg G, McGeer EG et al. (2008) Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of guam (ALS/PDC). Acta Neuropathol 116:625–637. 10.1007/s00401-008-0439-2 [DOI] [PubMed] [Google Scholar]
- 65.Mimuro M, Yoshida M (2020) Chameleons and mimics: Progressive supranuclear palsy and corticobasal degeneration. Neuropathology 40:57–67. 10.1111/neup.12590 [DOI] [PubMed] [Google Scholar]
- 66.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM et al. (1991) The consortium to establish a registry for Alzheimer’s Disease (CERAD) part II standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486 [DOI] [PubMed] [Google Scholar]
- 67.Mock C, Teylan M, Beecham G, Besser L, Cairns NJ, Crary JF et al. (2020) The utility of the national Alzheimer’s coordinating Center’s database for the rapid assessment of evolving neuropathologic conditions. Alzheimer Dis Assoc Disord 34:105–111. 10.1097/WAD.0000000000000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montine TJ, Monsell SE, Beach TG, Bigio EH, Bu Y, Cairns NJ et al. (2016) Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer’s disease. Alzheimers Dement 12:164–169. 10.1016/j.jalz.2015.07.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al. (2012) National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA et al. (2014) Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol 128:411–421. 10.1007/s00401-014-1302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nag S, Barnes LL, Yu L, Wilson RS, Bennett DA, Schneider JA (2020) Limbic-predominant age-related TDP-43 encephalopathy in black and white decedents. Neurology 95:e2056–e2064. 10.1212/WNL.0000000000010602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA (2018) TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun 6:33. 10.1186/s40478-018-0531-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA et al. (2015) Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 77:942–952. 10.1002/ana.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA (2017) TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology 88:653–660. 10.1212/WNL.0000000000003610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nascimento C, Di Lorenzo Alho AT, Bazan Conceicao Amaral C, Leite REP, Nitrini R, Jacob-Filho W et al. (2018) Prevalence of transactive response DNA-binding protein 43 (TDP-43) proteinopathy in cognitively normal older adults: systematic review and meta-analysis. Neuropathol Appl Neurobiol 44:286–297. 10.1111/nan.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM (2016) Higher prevalence of TDP-43 proteinopathy in cognitively normal asians: a clinicopathological study on a multiethnic sample. Brain Pathol 26:177–185. 10.1111/bpa.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nelson PT (2021) LATE neuropathologic changes with little or no Alzheimer disease is common and is associated with cognitive impairment but not frontotemporal dementia. J Neuropathol Exp Neurol. 10.1093/jnen/nlab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD et al. (2010) Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 20:66–79. 10.1111/j.1750-3639.2008.00244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ et al. (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71:362–381. 10.1097/NEN.0b013e31825018f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K et al. (2019) Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K et al. (2019) Reply: LATE to the PART-y. Brain 142:e48. 10.1093/brain/awz226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson PT, Gal Z, Wang WX, Niedowicz DM, Artiushin SC, Wycoff S et al. (2019) TDP-43 proteinopathy in aging: associations with risk-associated gene variants and with brain parenchymal thyroid hormone levels. Neurobiol Dis 125:67–76. 10.1016/j.nbd.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT et al. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133 [DOI] [PubMed] [Google Scholar]
- 85.Nguyen ML, Huie EZ, Whitmer RA, George KM, Dugger BN (2022) Neuropathology studies of dementia in US persons other than non-hispanic whites. Free Neuropathol. 10.17879/freeneuropathology-2022-3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB et al. (2007) Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29:125–132. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ et al. (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642. 10.1093/hmg/ddn257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahimi J, Kovacs GG (2014) Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther 6:82. 10.1186/s13195-014-0082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF et al. (2007) Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord 21:S14–18. 10.1097/WAD.0b013e31815c3445 [DOI] [PubMed] [Google Scholar]
- 90.Ribeiro FS, de Oliveira Duarte YA, Santos JLF, Leist AK (2021) Changes in prevalence of cognitive impairment and associated risk factors 2000–2015 in Sao Paulo. Brazil BMC Geriatr 21:609. 10.1186/s12877-021-02542-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF et al. (2008) The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30:58–69. 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robinson JL, Corrada MM, Kovacs GG, Dominique M, Caswell C, Xie SX et al. (2018) Non-Alzheimer’s contributions to dementia and cognitive resilience in the 90+ Study. Acta Neuropathol. 10.1007/s00401-018-1872-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C et al. (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141:2181–2193. 10.1093/brain/awy146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robinson JL, Porta S, Garrett FG, Zhang P, Xie SX, Suh E et al. (2020) Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration. Brain 143:2844–2857. 10.1093/brain/awaa219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robinson JL, Richardson H, Xie SX, Suh E, Van Deerlin VM, Alfaro B et al. (2021) The development and convergence of co-pathologies in Alzheimer’s disease. Brain 144:953–962. 10.1093/brain/awaa438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rutherford NJ, Carrasquillo MM, Li M, Bisceglio G, Menke J, Josephs KA et al. (2012) TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology 79:717–718. 10.1212/WNL.0b013e318264e3ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sabbagh MN, Sandhu SS, Farlow MR, Vedders L, Shill HA, Caviness JN et al. (2009) Correlation of clinical features with argyrophilic grains at autopsy. Alzheimer Dis Assoc Disord 23:229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmitt FA, Nelson PT, Abner E, Scheff S, Jicha GA, Smith C et al. (2012) University of kentucky sanders-brown healthy brain aging volunteers: donor characteristics, procedures, and neuropathology. Curr Alzheimer Res 9:724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The Neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 10.3233/JAD-2009-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS (1997) Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 48:959–969. 10.1212/wnl.48.4.959 [DOI] [PubMed] [Google Scholar]
- 101.Singh PP, Singh M, Mastana SS (2006) APOE distribution in world populations with new data from India and the UK. Ann Hum Biol 33:279–308. 10.1080/03014460600594513 [DOI] [PubMed] [Google Scholar]
- 102.Smith CD, Johnson ES, Van Eldik LJ, Jicha GA, Schmitt FA, Nelson PT et al. (2016) Peripheral (deep) but not periventricular MRI white matter hyperintensities are increased in clinical vascular dementia compared to Alzheimer’s disease. Brain Behav. 10.1002/brb3.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith VD, Bachstetter AD, Ighodaro E, Roberts K, Abner EL, Fardo DW et al. (2017) Overlapping but distinct TDP-43 and tau pathologic patterns in aged hippocampi. Brain Pathol 28:264–273. 10.1111/bpa.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strong MJ, Abrahams S, Goldstein LH, Woolley S, McLaughlin P, Snowden J et al. (2017) Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 18:153–174. 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suemoto CK, Ferretti-Rebustini RE, Rodriguez RD, Leite RE, Soterio L, Brucki SM et al. (2017) Neuropathological diagnoses and clinical correlates in older adults in Brazil: a cross-sectional study. PLoS Med 14:e1002267. 10.1371/journal.pmed.1002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suemoto CK, Leite REP, Ferretti-Rebustini REL, Rodriguez RD, Nitrini R, Pasqualucci CA et al. (2019) Neuropathological lesions in the very old: results from a large Brazilian autopsy study. Brain Pathol 29:771–781. 10.1111/bpa.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A et al. (1994) The cognitive abilities screening instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 6:45–58. 10.1017/s1041610294001602 (Discussion 62) [DOI] [PubMed] [Google Scholar]
- 108.Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H (2006) The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowl Environ. 10.1126/sageke.2006.6.re1 [DOI] [PubMed] [Google Scholar]
- 109.Thal DR, Griffin WS, Braak H (2008) Parenchymal and vascular abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer’s disease. J Cell Mol Med 12:1848–1862. 10.1111/j.1582-4934.2008.00411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas DX, Bajaj S, McRae-McKee K, Hadjichrysanthou C, Anderson RM, Collinge J (2020) Association of TDP-43 proteinopathy, cerebral amyloid angiopathy, and lewy bodies with cognitive impairment in individuals with or without Alzheimer’s disease neuropathology. Sci Rep 10:14579. 10.1038/s41598-020-71305-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tome SO, Vandenberghe R, Ospitalieri S, Van Schoor E, Tousseyn T, Otto M et al. (2020) Distinct molecular patterns of TDP-43 pathology in Alzheimer’s disease: relationship with clinical phenotypes. Acta Neuropathol Commun 8:61. 10.1186/s40478-020-00934-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tyas SL, Salazar JC, Snowdon DA, Desrosiers MF, Riley KP, Mendiondo MS et al. (2007) Transitions to mild cognitive impairments, dementia, and death: findings from the nun study. Am J Epidemiol 165:1231–1238. 10.1093/aje/kwm085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wegiel J, Flory M, Kuchna I, Nowicki K, Wegiel J, Ma SY et al. (2022) Developmental deficits and staging of dynamics of age associated Alzheimer’s disease neurodegeneration and neuronal loss in subjects with down syndrome. Acta Neuropathol Commun 10:2. 10.1186/s40478-021-01300-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wennberg AM, Tosakulwong N, Lesnick TG, Murray ME, Whitwell JL, Liesinger AM et al. (2018) Association of apolipoprotein e epsilon4 with transactive response DNA-binding protein 43. JAMA Neurol 75:1347–1354. 10.1001/jamaneurol.2018.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wharton SB, Brayne C, Savva GM, Matthews FE, Forster G, Simpson J et al. (2011) Epidemiological neuropathology: the MRC cognitive function and aging study experience. J Alzheimers Dis 25:359–372. 10.3233/JAD-2011-091402 [DOI] [PubMed] [Google Scholar]
- 116.White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD et al. (2005) Recent clinical-pathologic research on the causes of dementia in late life: update from the honolulu-asia aging study. J Geriatr Psychiatry Neurol 18:224–227. 10.1177/0891988705281872 [DOI] [PubMed] [Google Scholar]
- 117.Wilson AC, Dugger BN, Dickson DW, Wang DS (2011) TDP-43 in aging and Alzheimer’s disease—a review. Int J Clin Exp Pathol 4:147–155 [PMC free article] [PubMed] [Google Scholar]
- 118.Yang HS, Yu L, White CC, Chibnik LB, Chhatwal JP, Sperling RA et al. (2018) Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE epsilon4 haplotype status: a community-based cohort study. Lancet Neurol. 10.1016/S1474-4422(18)30251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu L, Schneider JA, Kapasi A, Bennett DA, Boyle PA (2020) Limbic-predominant age-related TDP-43 encephalopathy and distinct longitudinal profiles of domain-specific literacy. Alzheimer Dis Assoc Disord 34:299–305. 10.1097/WAD.0000000000000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


