Abstract
Background
Allergic bronchopulmonary aspergillosis (ABPA) is an underestimated allergic disease due to Aspergillus fumigatus (AF). The main diagnostic criteria for ABPA rely on the evaluation of immunoglobulin (Ig) E and IgG responses to AF extracts, although these cannot discriminate AF-sensitization from ABPA.
Objectives
To evaluate the performance of cellular functional assays with extract and molecular AF allergens in ABPA.
Methods
A prospective cohort of 67 patients (6 ABPA) was investigated with basophil activation test (BAT) with AF extract. Twelve patients were further investigated for BAT responses to molecular AF components: Asp f 1, Asp f 2, Asp f 3, Asp f 4, and Asp f 6.
Results
BAT with AF extract with an optimized cutoff displayed 100% sensitivity and 77.6% specificity for ABPA diagnosis. Among patients with positive BAT to AF, BAT with Asp f 4 was significantly higher in ABPA patients at 10 ng/mL (mean basophil stimulation index 10.56 in ABPA vs. 1.24 in non-ABPA patients, p = 0.0002).
Conclusion
BAT with AF is a promising diagnostic biomarker in the context of suspected ABPA, which can be further improved with AF molecular allergens, especially Asp f 4.
Keywords: allergic bronchopulmonary aspergillosis (ABPA), basophil activating test (BAT), Aspergillus fumigatus, aspergillus molecular allergens, ex vivo technique
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is an underestimated allergic disease due to the ubiquitous mold Aspergillus fumigatus (AF). ABPA occurs mainly in patients with a chronic pulmonary disease, such as cystic fibrosis (CF), asthma or chronic obstructive pulmonary disease (COPD) (1). Its prevalence reaches 10 and 2% in CF and asthma patients, respectively (2, 3). Up to now, the reason why some AF-sensitized people stay free of subsequent AF-related disease whereas others develop ABPA with irreversible pulmonary lesions remains unknown. The main diagnostic criteria for ABPA, first established in 1977 (4), rely on the evaluation of humoral IgE and IgG responses to AF extracts, which cannot discriminate ABPA from AF-sensitization. Several new diagnostic tools have been evaluated, but none has overcome this limitation (2, 5, 6). More recently, serum immunoglobulin (Ig) E responses to AF molecular components have been proposed, and the combination of specific AF molecular components showed promise for ABPA diagnosis (7–9). Meanwhile, diagnostic criteria based on the evaluation of functional cellular responses against allergens are increasingly cited in international guidelines (10, 11). Performance of the basophil activation test (BAT) with AF extract has been explored (12–16), but its relevance for ABPA diagnosis needs further evaluation. To our best knowledge, AF-induced basophil reactivity has not been deciphered at a molecular level, despite promising results of profiling IgE responses to such molecules. We report here the performance of BAT with AF extract and AF molecular allergens vs. usual diagnostic criteria of ABPA in a prospective cohort.
patients and Methods
Patient Cohort
We assessed adult patients (n = 67) followed at the CF reference care center and at the pulmonology department (Assistance Publique—Hôpitaux de Marseille, France) between January and September 2019. Patients were categorized as ABPA, AF-sensitized (AF-S), or control patients. These categories were defined as follows: ABPA met all the ISHAM criteria (2); AF-S displayed specific IgE (sIgE) to AF (0.1 kUA/L or greater) without fulfilling the ISHAM criteria for ABPA; and patients who were categorized in none of the two previous groups were considered as controls. According to ISHAM criteria, ABPA is made in patients with a lung predisposing condition who display AF sensitization and elevated total IgE levels and at least 2 of the following 3 criteria: (1) detection of AF specific IgG, (2) radiographic abnormalities, (3) total eosinophil count above 500 cells/μL.
Functional Cytometric Tests
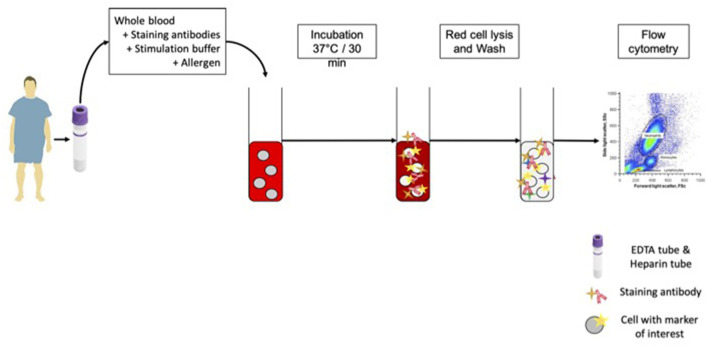
The design of functional cellular assays is illustrated in Figure 1. BAT was firstly performed with AF extract (Bühlmann Laboratories®, Schönenbuch, Switzerland) with the Flow2CAST method (Bühlmann Laboratories®), using CCR3 (CD193) and CD63 as basophil identification and activation markers, following the manufacturer's instructions (17). Upon allergen contact, sensitized basophils degranulate and express CD63 as a cell surface activation marker, measurable by flow cytometry. For in vitro diagnosis, allergen-induced basophil activation is defined as a proportion of CD63+ basophils at least twice higher with the culprit allergen than with the reaction buffer. Positive controls were anti-RFcεI and the bacterial peptide fMLP. Fresh whole blood was incubated with AF extract or controls and staining antibodies for 30 mins at 37°C. After red cell lysis and washes, basophil responses were analyzed by flow cytometry. The proportion of CD63+ unstimulated basophils was lower than 5% in all patients, and no non-responder (anti-FcεRI-induced CD63 expression of 10% or less) was found in this cohort.
Figure 1.
BAT protocol. For each patient in the study, we sampled 1 EDTA tube for BAT. The whole blood was mixed with staining antibodies, stimulation buffer and allergen or controls. After incubation at 37°C, red cell lysis and wash, cells were analyzed on FACS.
Samples with a positive BAT response to AF extract were subsequently assayed with BAT using each of the five following molecular AF: Asp f 1, Asp f 2, Asp f 3, Asp f 4 and Asp f 6 (a gift from Dr Jonas Lidholm, Thermo Fisher Scientific, R&D, Uppsala, Sweden). These molecular AF are the same used in the ImmunoCAP platform. Five concentrations of molecular components from 100 to 0.01 ng/mL by 10-fold dilution were used for each patient.
Flow cytometry was performed with a FACS Canto II platform (Becton Dickinson, Le Pont de Claix, France). At least 200 basophils per sample were acquired for BAT. Data were analyzed using FACS Diva software (TreeStar, Ashland, OR).
Clinical and Laboratory Data
White blood cell count (Sysmex, Villepinte, France) and serum total IgE and specific IgE (sIgE) and IgG (sIgG) to AF extract and molecular components Asp f 1, Asp f 2, Asp f 3, Asp f 4 and Asp f 6 (ImmunoCAP, Thermo Fisher Scientific, Uppsala, Sweden) were part of routine investigations. The quantification thresholds were 0.10 kUA/L for sIgE and 0.01 mgA/L for sIgG (18, 19). The patient's pulmonary function test results were retrieved from medical charts.
Data Analysis
Results were expressed as the basophil or lymphocyte stimulation index (SI), which is the ratio between the level of activation with the allergen and the level of activation with reaction buffer, with a threshold of 2 for allergy diagnosis. Statistical analysis was performed with the R statistical software (20). For BAT, optimal cutoff points were determined with the “OptimalCutpoints” package (21). The Youden index, which defines the maximum potential effectiveness of a biomarker to classify a disease status at a specific cutoff, was also calculated (22). Intertest correlation was estimated using Pearson's correlation coefficient. Mean SI of each group were compared via Wilcoxon's or Kruskal-Wallis tests as appropriate. A two-sided p-value < 0.05 was considered statistically significant.
Ethics Statement
All the experimental protocols were approved by the institutional ethics committee and GDPR commission with the reference number 2019–270. All methods used were carried out in accordance with relevant national guidelines. Written informed consent for participation was obtained for this study in accordance with the national legislation and the institutional requirements (23, 24).
Results
Demography
The cohort was comprised of 20 CF patients, 25 asthmatic patients, 4 COPD and 18 patients with other chronic pulmonary diseases (6 pulmonary arterial hypertension, 4 idiopathic pulmonary fibrosis, 3 emphysema, 2 chronic bronchiectasis, 1 lymphangioleiomyomatosis, 1 idiopathic chronic eosinophilic pneumonia, 1 infectious pneumonitis). The ABPA group was composed of 6 patients, most of them were CF patients (4/6). The AF-S group included 25 patients, especially CF (10) and asthmatic (8) patients. The control group (n = 36) was composed of 7 CF patients, 15 asthmatic patients, 2 COPD and 12 patients with other chronic pulmonary diseases. Demographic data are summarized in Table 1.
Table 1.
Demographic and laboratory data of the study cohort.
| (median +/−5–95 percentile) | ABPA | AF-S | Control | Total | p |
|---|---|---|---|---|---|
| n | 6 | 25 | 36 | 67 | |
| Age (years) | 37.5 (16.3–61.8) |
49.5 (25.0–75.0) |
57.0 (26.7–78.0) |
53.0 (24.3–76.4) |
0.025 |
| Male/Female | 3/3 | 9/16 | 14/22 | 27/40 | 0.797 |
| Cystic fibrosis | 4 | 10 | 7 | 20 | 0.020 |
| Asthma | 1 | 8 | 15 | 25 | 0.514 |
| COPD | 1 | 2 | 2 | 4 | 0.509 |
| Others | 0 | 5 | 12 | 18 | 0.097 |
| Lung transplantation | 2/6 | 6/25 | 14/36 | 22/67 | 0.454 |
| Time since transplantation (years) | 8.6 (8.2–9.1) |
7.7 (0.6–23.6) |
1.7 (0.1–13.2) |
4.5 (0.1–19.7) |
0.294 |
| Bacterial colonization | 3/6 | 9/25 | 5/36 | 17/67 | 0.019 |
| Fungal colonization | 3/6 | 6/25 | 2/36 | 11/67 | 0.005 |
| Total IgE (kIU/L) | 1,132.0 (198.7–5,685.8) |
197.0 (16.6–1,139.0) |
27.5 (2.0–266.3) |
66.7 (3.0–1,413.5) |
<10−3 |
| IgE AF (kUA/L) | 16.8 (0.5–66.3) |
0.4 (0.1–15.8) |
0.05 (0.01–0.09) |
0.10 (0.01–19.9) |
<10−3 |
| IgG AF (mgA/L) | 46.8 (22.0–79.1) |
18.2 (4.9–52.9) |
12.3 (3.1–56.6) |
16.6 (3.4–59.0) |
0.002 |
| Eosinophils (/mm3) | 300 (0–800) |
100 (100–600) |
100 (0–600) |
100 (0–700) |
0.554 |
ABPA, allergic bronchopulmonary aspergillosis; AF, Aspergillus fumigatus; AF-S, Aspergillus fumigatus sensitization; COPD, chronic obstructive pulmonary disease.
BAT With AF Extract Displayed Good Performances for ABPA Diagnosis
BAT dose-response to AF extract showed that 50 ng/mL yielded a maximal response, and was therefore the optimal concentration to be used in further BAT (Supplementary Figure 1). BAT was positive in all ABPA patients (Figure 2). BAT discriminated both ABPA (p = 0.0028) and AF-S patients (p = 0.00023) from controls. However, BAT could not distinguish between ABPA and AF-S patients (p = 0.72). Comparing results in ABPA and the control groups, with the usual SI threshold of 2, BAT with AF extract displayed 100% sensitivity and 65.5% specificity. An optimized SI threshold allowing for the best Youden index was calculated as 6.55. This optimized SI retained 100% sensitivity but specificity increased to 77.6% (Table 2). The area under the curve (AUC) was 0.84 BAT with AF extract and 0.83 for sIgE to AF. Youden index was 0.78 for BAT with the optimized SI cut-off, but only 0.66 for sIgE to AF extract.
Figure 2.
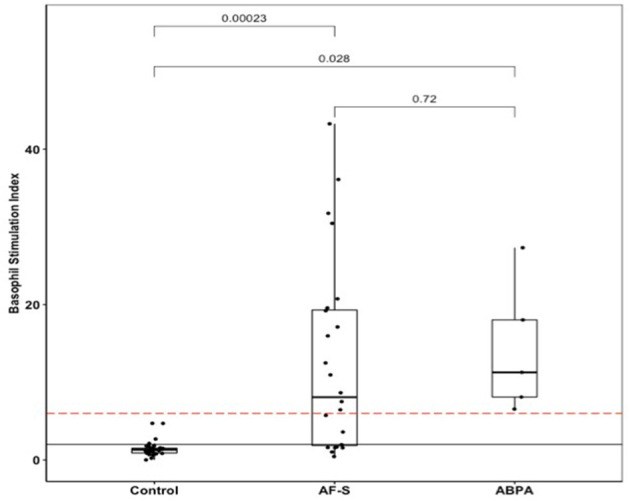
BAT AF in ABPA, AF-sensitized (AF-S), or control patients. The solid black line shows the usual BAT positivity threshold (stimulation index = 2); the dotted red line shows the optimal BAT threshold (stimulation index = 6.55). ABPA, allergic bronchopulmonary aspergillosis; AF, Aspergillus fumigatus; AF-S, Aspergillus fumigatus-sensitized; BAT, basophil activation test.
Table 2.
Performance analysis of total and specific immunoglobulins and cellular functional assays with the optimized cutoff.
| Optimized cutoff |
AUC (5–95 percentile) |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Youden index | |
|---|---|---|---|---|---|---|---|
| Total IgE | 90 | 0.68 (0.54–0.82) |
66.7 | 66.7 | 59.3 | 73.3 | 0.33 |
| IgE AF | 0.24 | 0.83 (0.71–0.94) |
75.0 | 90.9 | 85.7 | 83.3 | 0.66 |
| IgG AF | 27 | 0.69 (0.53–0.84) |
76.2 | 65.5 | 61.5 | 79.2 | 0.42 |
| BAT with AF extract | 6.55 | 0.84 (0.74–0.94) |
100 | 77.6 | 27.8 | 100 | 0.78 |
Optimization consisted in the identification of the cut-off value associated with the best Youden index.
AF, Aspergillus fumigatus; AUC, Area under curve; BAT, Basophil activation test; NPP, Negative predictive value; PPV, Positive predictive value.
BAT With Molecular Components Further Improved BAT Performance
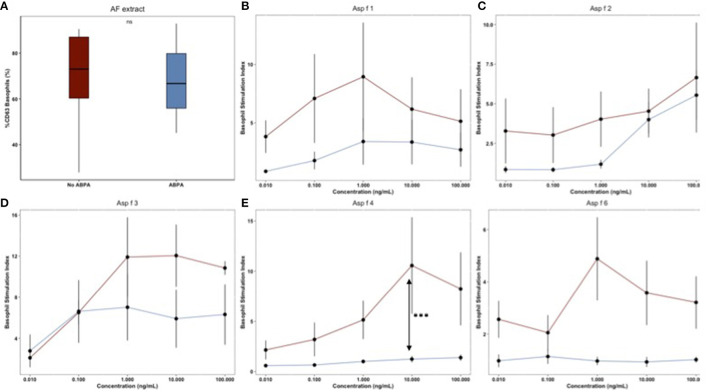
In positive (SI ≥ 2) BAT with AF extract, a dose-response BAT was performed with each of the five AF molecular components. Six AF-S and 6 ABPA patients were tested (Figure 3). There was no significant difference in BAT AF responses for these patients (Figure 3A). The mean responses with each AF molecular component were higher in ABPA patients, but only BAT with Asp f 4 at 10 ng/mL reached the significance level (basophil SI of 10.56 in ABPA group vs. 1.24 in no ABPA group, p = 0.0002) (Figures 3B–E).
Figure 3.
(A–E) Mean BAT responses for AF molecular components: Asp f 1, Asp f 2, Asp f 3, Asp f 4, Asp f 6. Mean (SD) responses for AF extract and each AF molecular component's concentration were calculated for ABPA (n = 6, red lines) and AF sensitized (n = 6, blue lines) patients. Significant difference was calculated at each point.
Matrix Correlation
As illustrated in Figure 4, total IgE were more strongly correlated with sIgE to AF (r = 0.53, p = 0.01) than IgG to AF (r = −0.03, p = 0.001), although sIgE were correlated with sIgG to AF (r = 0.29, p = 0.003). BAT AF was correlated with sIgG (r = 0.45, p < 10−3) and IgE (r = 0.35, p = 0.01) to AF. Tiffeneau index was inversely correlated with the basophil count (r = −0.36, p = 0.03) and sIgE to AF levels (r = −0.35, p = 0.01). The FEV1 (Forced Expiratory Volume in 1 second) was strongly correlated with FVC (Forced Vital Capacity) (r = 0.62, p = < 10−3).
Figure 4.
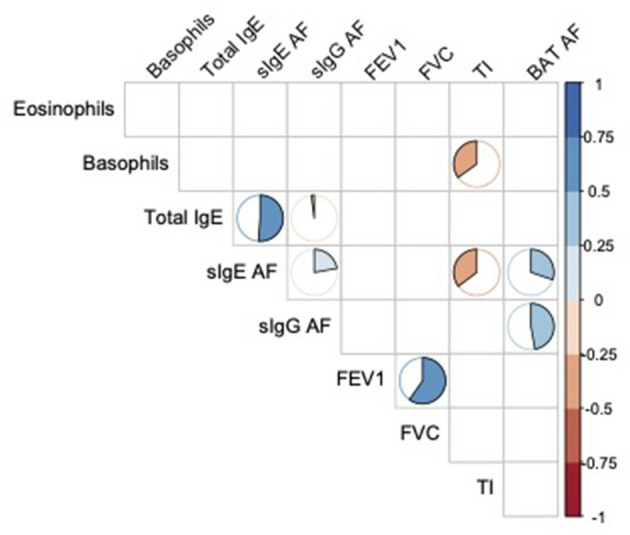
Spearman's correlation matrix of eosinophils and basophils count, total IgE, specific IgE and IgG AF levels, pulmonary function tests results and functional cellular assays. Only significant correlations (a = 0,05) are illustrated by a pie chart. The fill rate of pie chart is proportional to correlation coefficient. AF, Aspergillus fumigatus; FEV1, Forced expiratory volume in 1 sec; FVC, forced vital capacity; TI, Tiffeneau index.
Discussion
Data on BAT as a tool for ABPA diagnosis are scarce, with only five previous studies from our and other teams (12–16). Four of them have focused on CF patients, an underlying disease associated with the highest ABPA incidence (25) and the global conclusion was that BAT could improve ABPA diagnosis in CF patients. The study of Prasad et al. displayed more contrasted results on asthmatic patients. The present study brings further insights, from a clinical viewpoint, into the usefulness of BAT for ABPA diagnosis in asthmatic patients.
First, we demonstrated the absence of a plateau phase in basophil responses to high AF concentrations. This finding suggests that the basophil signaling switch induced by supraoptimal allergen concentrations does occur with AF extract, similarly to experimental conditions of highest response induced by equivalent amounts of allergens and IgE bound to mast cell and basophil FcεRI (26, 27). The bell-shaped curve of AF-induced basophil activation indicates the need for standardized concentrations of AF extract for BAT, and its potential use as a follow-up test through the monitoring of CD-sens and EC-50 (28, 29).
Secondly, we confirmed that BAT with AF extract performs better than humoral markers (total IgE, IgE to AF and IgG to AF) for ABPA diagnosis. With a 100% negative predictive value, BAT could assist with ruling out ABPA in clinical settings.
In order to improve ABPA diagnostic accuracy, we performed BAT with AF molecular components in BAT AF-positive patients from our cohort. The soluble form of ImmunoCAP® antigens was employed. Overall, ABPA patients displayed higher BAT responses to all the molecular components as compared to AF crude extract. Asp f 4 BAT significantly discriminated ABPA from mere AF-sensitization. Serum sIgE to Asp f 4 has been reported as a better discriminant than AF extract and other AF molecular components for ABPA diagnosis in CF patients (9, 30–32). Hence, IgE immunization against Asp f 4 appears as a relevant marker of ABPA pathophysiology. Functions of this protein in Aspergillus sp have not been described yet. Ramachandran et al have studied the structure and immunogenicity of this protein, providing evidence of the key role of C-Terminal cysteine residues for IgE binding (33).
Basophil activation has been correlated with a strong and effective allergic immune activation, lung damage and pulmonary symptoms (34). We speculate that strong BAT responses in ABPA patients are related to their complex molecular sensitization profile (35).
The main limitations of our study were its monocentric design and the relatively small size of the cohort, hence calling for confirmation through larger studies. The main novelty of this study is the evidence of an improved discrimination between AF-S and ABPA, by using AF molecular components. In conclusion, cellular functional assays are easy to implement in the routine clinical laboratory for direct and personalized evaluation of each patient's functional responses to AF extract and proteins. They might thus be the next first-line test for ABPA diagnosis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MM, MG, CG, and JV contributed conception and design of the study. MM, YS, and FM performed the experiments. CC, SR, and MR-G performed the statistical analysis. J-LM wrote the first draft of the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the French National Research Agency under the program Investissements d'avenir, reference ANR-10-IAHU-03, and the Région Provence Alpes Côte d'Azur and European funding FEDER PRIMMI.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Dr. Jonas Lidholm (Thermo Fisher R&D, Uppsala, Sweden) for kindly providing soluble recombinant AF allergens.
Glossary
Abbreviations
- ABPA
Allergic Bronchopulmonary Aspergillosis
- AF
Aspergillus fumigatus
- AUC
Area under the curve
- BAT
Basophil Activation Test
- CF
Cystic fibrosis
- COPD
Chronic Obstructive Pulmonary Disease
- FcεR
Fc epsilon Receptor
- fMLP
Formyl-methionyl-leucyl-phenylalanine
- Ig
Immunoglobulin
- ISHAM
International Society for Human & Animal Mycology
- PHA
Phytohemagglutinin
- SI
Stimulation Index.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2022.898731/full#supplementary-material
References
- 1.Greenberger PA, Bush RK, Demain JG, Luong A, Slavin RG, Knutsen AP. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. (2014) 2:703–8. 10.1016/j.jaip.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy J Br Soc Allergy Clin Immunol. (2013) 43:850–73. 10.1111/cea.12141 [DOI] [PubMed] [Google Scholar]
- 3.Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev Off J Eur Respir Soc. (2011) 20:156–74. 10.1183/09059180.00001011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, Harris KE. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. (1977) 86:405–14. 10.7326/0003-4819-86-4-405 [DOI] [PubMed] [Google Scholar]
- 5.Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis–state of the art: cystic fibrosis foundation consensus conference. Clin Infect Dis Off Publ Infect Dis Soc Am. (2003) 37:S225–264. 10.1086/376525 [DOI] [PubMed] [Google Scholar]
- 6.Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. (2012) 129:280–91; quiz 292–3. 10.1016/j.jaci.2011.12.970 [DOI] [PubMed] [Google Scholar]
- 7.Muthu V, Sehgal IS, Dhooria S, Aggarwal AN, Agarwal R. Utility of recombinant Aspergillus fumigatus antigens in the diagnosis of allergic bronchopulmonary aspergillosis: A systematic review and diagnostic test accuracy meta-analysis. Clin Exp Allergy J Br Soc Allergy Clin Immunol. (2018) 48:1107–36. 10.1111/cea.13216 [DOI] [PubMed] [Google Scholar]
- 8.Muthu V, Singh P, Choudhary H, Sehgal IS, Dhooria S, Prasad KT, et al. Diagnostic cutoffs and clinical utility of recombinant aspergillus fumigatus antigens in the diagnosis of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. (2019) 11:19. 10.1016/j.jaip.2019.08.041 [DOI] [PubMed] [Google Scholar]
- 9.Vitte J, Ranque S, Carsin A, Gomez C, Romain T, Cassagne C, et al. Multivariate analysis as a support for diagnostic flowcharts in allergic bronchopulmonary aspergillosis: a proof-of-concept study. Front Immunol. (2017) 8:1019. 10.3389/fimmu.2017.01019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. (2015) 70:1393–405. 10.1111/all.12698 [DOI] [PubMed] [Google Scholar]
- 11.Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI molecular allergology user's guide. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. (2016) 27:1–250. 10.1111/pai.2016.27.issue-S23 [DOI] [PubMed] [Google Scholar]
- 12.Gernez Y, Waters J, Mirković B, Lavelle GM, Dunn CE, Davies ZA, et al. Blood basophil activation is a reliable biomarker of allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur Respir J. (2016) 47:177–85. 10.1183/13993003.01068-2015 [DOI] [PubMed] [Google Scholar]
- 13.Mirković B, Lavelle GM, Azim AA, Helma K, Gargoum FS, Molloy K, et al. The basophil surface marker CD203c identifies Aspergillus species sensitization in patients with cystic fibrosis. J Allergy Clin Immunol. (2016) 137:436–443.e9. 10.1016/j.jaci.2015.07.045 [DOI] [PubMed] [Google Scholar]
- 14.Katelari A, Tzanoudaki M, Noni M, Kanariou M, Theodoridou M, Kanavakis E, et al. The role of basophil activation test in allergic bronchopulmonary aspergillosis and Aspergillus fumigatus sensitization in cystic fibrosis patients. J Cyst Fibros Off J Eur Cyst Fibros Soc. (2016) 15:587–96. 10.1016/j.jcf.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 15.Michel M, Gomez C, Sereme Y, Gouitaa M, Chartier C, Blanchard P, et al. Evaluation of cellular responses for the diagnosis of allergic bronchopulmonary mycosis: a preliminary study in cystic fibrosis patients. Front Immunol. (2020) 10:3149. 10.3389/fimmu.2019.03149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad KT, Muthu V, Sehgal IS, Dhooria S, Singh P, Sachdeva MUS, et al. The utility of the basophil activation test in differentiating asthmatic subjects with and without allergic bronchopulmonary aspergillosis. Mycoses. (2020) 63:588–95. 10.1111/myc.13081 [DOI] [PubMed] [Google Scholar]
- 17.Hemmings O, Kwok M, McKendry R, Santos AF. Basophil activation test: old and new applications in allergy. Curr Allergy Asthma Rep. (2018) 18:77. 10.1007/s11882-018-0831-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal R, Aggarwal AN, Garg M, Saikia B, Chakrabarti A. Cut-off values of serum IgE (total and A. fumigatus -specific) and eosinophil count in differentiating allergic bronchopulmonary aspergillosis from asthma. Mycoses. (2014) 57:659–63. 10.1111/myc.12214 [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R, Dua D, Choudhary H, Aggarwal AN, Sehgal IS, Dhooria S, et al. Role of Aspergillus fumigatus-specific IgG in diagnosis and monitoring treatment response in allergic bronchopulmonary aspergillosis. Mycoses. (2017) 60:33–9. 10.1111/myc.12541 [DOI] [PubMed] [Google Scholar]
- 20.R Foundation for Statistical Computing . R: A language environment for statistical computing [Internet]. Vienna, Austria: R Development Core Team; (2005). Available online at: http://www.R-project.org [Google Scholar]
- 21.López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez C, Gude-Sampedro F. OptimalCutpoints: an r package for selecting optimal cutpoints in diagnostic tests. J Stat Softw. (2014) 61:1–36. 10.18637/jss.v061.i08 [DOI] [Google Scholar]
- 22.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J Biom Z. (2008) 50:419–30. 10.1002/bimj.200710415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LOI n° 2012-300 du 5 mars 2012 . Relative aux recherches impliquant la personne humaine. 2012-30 (2012). [Google Scholar]
- 24.Décret n° 2016-1537 du 16 novembre 2016 . relatif aux recherches impliquant la personne humaine. 2016-1537 Nov 16. (2016). [Google Scholar]
- 25.Muldoon EG, Strek ME, Patterson KC. Allergic and noninvasive infectious pulmonary aspergillosis syndromes. Clin Chest Med. (2017) 38:521–34. 10.1016/j.ccm.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 26.Huber M. Activation/Inhibition of mast cells by supra-optimal antigen concentrations. Cell Commun Signal CCS. (2013) 11:7. 10.1186/1478-811X-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidelberger M, Kendall FE. A quantitative theory of the precipitin reaction : iii. the reaction between crystalline egg albumin and its homologous antibody. J Exp Med. (1935) 62:697–720. 10.1084/jem.62.5.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos AF, Douiri A, Bécares N, Wu S-Y, Stephens A, Radulovic S, et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. (2014) 134:645–52. 10.1016/j.jaci.2014.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson SGO, Lilja G, Hallberg J, Nopp A. A clinical follow-up of omalizumab in routine treatment of allergic asthma monitored by CD-sens. Immun Inflamm Dis. (2018) 18:95. 10.1002/iid3.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmann S, Nikolaizik WH, Schöni MH, Blaser K, Crameri R. Differential IgE recognition of recombinant Aspergillus fumigatus allergens by cystic fibrosis patients with allergic bronchopulmonary aspergillosis or Aspergillus allergy. Eur J Immunol. (1998) 28:1155–60. [DOI] [PubMed] [Google Scholar]
- 31.Casaulta C, Flückiger S, Crameri R, Blaser K, Schoeni MH. Time course of antibody response to recombinant Aspergillus fumigatus antigens in cystic fibrosis with and without ABPA. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. (2005) 16:217–25. 10.1111/j.1399-3038.2005.00262.x [DOI] [PubMed] [Google Scholar]
- 32.Knutsen AP, Hutcheson PS, Slavin RG, Kurup VP. IgE antibody to Aspergillus fumigatus recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy. (2004) 59:198–203. 10.1046/j.1398-9995.2003.00310.x [DOI] [PubMed] [Google Scholar]
- 33.Ramachandran H, Banerjee B, Greenberger PA, Kelly KJ, Fink JN, Kurup VP. Role of C-terminal cysteine residues of Aspergillus fumigatus allergen Asp f 4 in immunoglobulin E binding. Clin Diagn Lab Immunol. (2004) 11:261–5. 10.1128/CDLI.11.2.261-265.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kauffman HF, Tomee JFC. Inflammatory cells and airway defense against aspergillus fumigatus. Immunol Allergy Clin North Am. (1998) 18:619–40. 10.1016/S0889-8561(05)70025-4 [DOI] [Google Scholar]
- 35.Vitte J, Romain T, Carsin A, Gouitaa M, Stremler-Le Bel N, Baravalle-Einaudi M, et al. Aspergillus fumigatus components distinguish IgE but not IgG4 profiles between fungal sensitization and allergic broncho-pulmonary aspergillosis. Allergy. (2016) 71:1640–3. 10.1111/all.13031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.