Abstract
There is an urgent need to develop uncharged radical precursors to be activated under mild photocatalyzed conditions. 2-Substituted-1,3-oxazolidines (Eox < 1.3 V vs SCE, smoothly prepared from the corresponding aldehydes) have been herein employed for the successful release of tertiary, α-oxy, and α-amido radicals under photo-organo redox catalysis. The reaction relies on the unprecedented C–C cleavage occurring from the radical cation of these heterocyclic derivatives. Such a protocol is applied to the visible-light-driven conjugate radical addition onto Michael acceptors and vinyl (hetero)arenes under mild metal-free conditions.
Keywords: C−C bond cleavage, conjugate addition, metal-free reaction, oxazolidines, photoorganocatalysis
Introduction
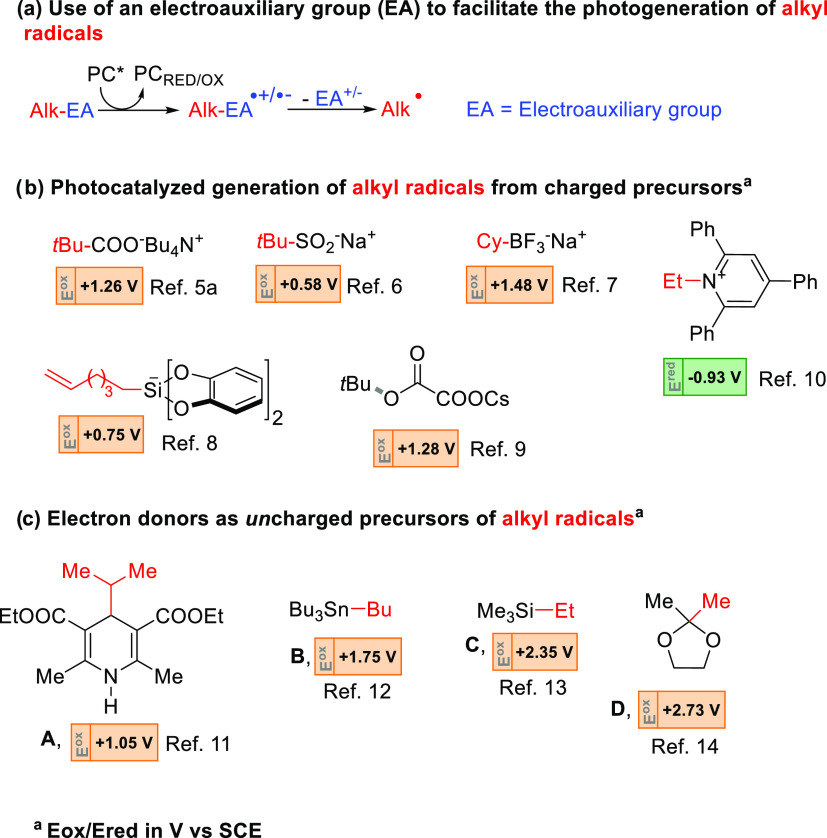
The photochemical/photocatalyzed approach is nowadays the elective method for the generation of ground-state reactive intermediates1 including carbon radicals that can be generated in a mild way using photons as traceless reagents.2 In particular, great attention has been given, in the last decade, to the formation of C(sp3)–C(sp3) bonds via the generation of alkyl radicals,3 and several precursors have been devised3a−3i under tin-free conditions.3e In most cases, the alkyl radical is tethered to an electroauxiliary group (EA)4 that acts as an electron donor/acceptor moiety. Upon photocatalytic oxidation/reduction, an electrofugal/nucleofugal group (EA+/–) is released with the concomitant formation of the alkyl radical (Figure 1a).3e A charged precursor is usually required to facilitate such electron transfer reactions. As shown in Figure 1b, both anionic (e.g., alkyl carboxylates,5 alkyl sulfinates,6 alkyl trifluoroborates,7 bis-catecholato silicates,8 and alkyl oxalates9) or cationic (e.g., Katritzsky’s salt)3g,10 derivatives have been tested.
Figure 1.
(a) Adoption of an electroauxiliary group (EA) to facilitate the generation of alkyl radicals. (b) Main classes of charged precursors used for photocatalyzed alkyl radical formation. (c) Uncharged precursors as electron donors tested for the release of alkyl radicals.
Due to solubility concerns, however, charged radical precursors can be used only in a limited range of solvents. Curiously, the development of uncharged, easily available radical precursors prone to be oxidized under photocatalyzed conditions is less common. In fact, apart from the case of 1,4-dihydropyridine derivatives (e.g., A) that exhibits a low Eox value (1.05 V vs SCE),11 other neutral donors such as tetraalkyl stannanes (B),12 tetraalkyl silanes (C),13 or 2,2-dialkyl 1,3-dioxolanes (D)14 can be activated only under quite prohibitive conditions (Eox up to 2.7 V vs SCE, Figure 1c).
The available literature points out that one of the elective classes for the design of new uncharged electron donors is certainly that of tertiary amines (Eox = 0.83 V vs SCE for triethylamine).5 Formerly, such a class of compounds has been largely employed as sacrificial electron donors in photoredox catalysis to reduce a species (or an intermediate) present in solution.15 Nevertheless, the formation of acidic16 amine radical cations has been extensively employed in synthesis17 for the generation of other valuable reactive intermediates, as sketched in Scheme 1. Indeed, radical cation II often deprotonates to form a nucleophilic α-amino radical III (path b) that may, in turn, undergo oxidation to afford an iminium ion IV (path c)18 that upon the loss of a positively charged group leads to a 1,3-dipole V (path d).18 In rare instances, the α-amino radical is photocatalytically reduced to the corresponding anion VI (path e).19 If the carbons tethered to the nitrogen atom have no hydrogens, deprotonation from the N–H group may take place to give nitrogen-centered radical VII (path f).20 On the other hand, when intermediate II is generated in a tertiary amine that reluctantly loses a proton (e.g., quinuclidine), this species acts instead as an efficient hydrogen atom abstractor (path g).21
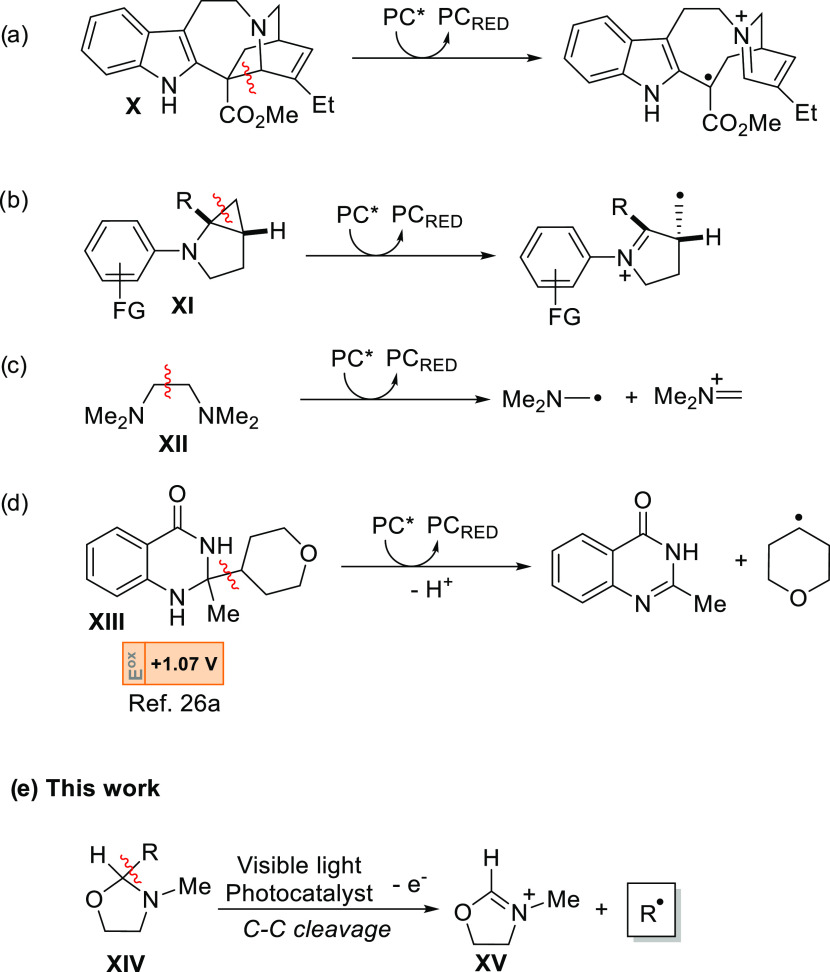
Scheme 1. Intermediates Arising from Photogenerated Amine Radical Cations.
We were intrigued, however, by the possible C–C cleavage to form stable iminium ion IX along with a carbon radical (path h).22 Examples of this cleavage are only rarely reported in the literature and point to the requirement of nitrogen-containing heterocycles as ideal substrates.
The photocatalyzed single-electron oxidation of (+)-catharanthine X indeed induces a C–C bond cleavage in the azabicyclo[2.2.2]oct-5-ene core (Scheme 2a), and the so-modified skeleton of the alkaloid is employed in the preparation of further natural compounds.17a,23 In another instance, the cyclopropyl group in bicyclic cyclopropylamines XI was easily opened upon photocatalyzed oxidative conditions (Scheme 2b).24 The oxidation of tetramethylethanediamine XII led to the generation of an iminium ion and an α-amino radical from the fragmentation of the resulting radical cation (Scheme 2c), but the thus obtained radical was applied exclusively to the polymerization of 2-hydroxyethylacrylate.25 To our knowledge, however, only dihydroquinazolinones (e.g., XIII, Scheme 2d) are used as nitrogen-based heterocycles for the generation of alkyl radicals by a reductive quenching catalytic cycle.26
Scheme 2. Cleavage of a C–C Bond from a Radical Cation of an Amine.
As for the above, a general method to generate (un)substituted alkyl radicals by C–C cleavage from a tertiary amine is so far lacking. We have identified N-methyl oxazolidines (XIV, the nitrogen analogues of dioxolanes) as possible candidates to achieve this goal (Scheme 2e). Indeed, such compounds are oxidized easily (Eox = 1.22 V vs SCE for 2,2,3-trimethyloxazolidine) and act as good electron donors.27 We surmised that the driving force of the cleavage should be the stability of the resulting iminium ion XV. The present approach represents a mild alternative route for the generation of radicals starting from nitrogen-based heterocycles easily prepared from widely available aldehydes. On these premises, we investigated 2-substituted N-methyl oxazolidines for the smooth generation of alkyl radicals to be used in C(sp3)–C(sp3) bond formation, as detailed in the following.
Results and Discussion
Oxazolidines 1a–f have been easily prepared by treating the corresponding aldehydes with 2-(methylamino)ethanol. Related oxazole 1g has been likewise prepared by the reaction of pivalaldehyde and 2-(methylamino)phenol (see the Supporting Information and Scheme S1 for further details). As shown in Table 1 and Figures S2–S8, compounds 1a–g exhibited an oxidation potential in the 0.86–1.35 V (vs SCE) range. The Eox of oxazolidines is quite independent of the presence of the (substituted) alkyl group, whereas the presence of the aromatic ring in oxazole 1g made the oxidation of the heterocycle markedly easier (<1 V vs SCE). These low Eox values allow us to test several (colored) photocatalysts (PCs) for the occurrence of the desired reaction.
Table 1. Measured Oxidation Potentials of 1a–g.
| compound | Eox (V vs SCE) |
|---|---|
| 1a | 1.33 |
| 1b | 1.26 |
| 1c | 1.23 |
| 1d | 1.22 |
| 1e | 1.35 |
| 1f | 1.19 |
| 1g | 0.86 |
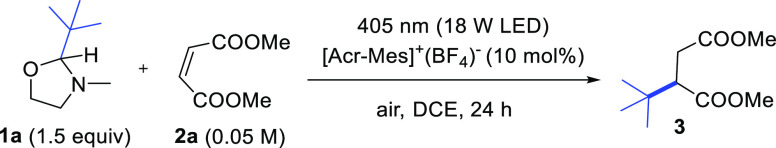
To test our proposal, we then focused on the tert-butylation of dimethylmaleate 2a using N-methyl-2-tertbutyl-oxazolidine 1a. We then embarked on an extensive survey of reaction parameters by varying the PC employed (Ir(III)- and Ru(II)-based complexes as well as photo-organo catalysts), the reaction media, the stoichiometric ratio of the reactants, as well as the influence of oxygen in the reaction (see Table S1 for a detailed description of the experiments). A representative list of control experiments is collected in Table 2.
Table 2. Deviations from the Standard Conditionsa.
| entry | deviations from the standard conditions | 3 (% yield) |
|---|---|---|
| 1 | none | 88 |
| 2 | 4CzIPN (10 mol %), N2 atmosphere | 34 |
| 3 | DCM as the solvent | 52 |
| 4 | MeOH as the solvent | 5 |
| 5 | N2 atmosphere | 71 |
| 6 | no light | 0 |
See Table S1 in the Supporting Information for a detailed optimization of the standard conditions.
Gratifyingly, by adopting the conditions described in Table 1 (entry 1), succinate 3 was isolated in an 88% yield. In detail, we found that the best reaction conditions were as follows: an air-equilibrated DCE solution of 2a (0.05 M) in the presence of 1.5 equiv of 1a, Acr-Mes+ BF4– (10 mol %), irradiated at 405 nm for 24 h (Figure S1). Less satisfactory results were obtained when replacing Acr-Mes+ BF4 (ERED* > 1.88 V vs SCE)28 with 4CzIPN (ERED* > 1.38 V vs SCE28 in MeCN, entry 2) or other metal-free or metal-based PCs (Table S1). The reaction carried out in neat protic solvents (Table 1, entry 4) or in the absence of oxygen (entry 5) led to a decrease in the overall yield. Control experiments confirm the photochemical nature of the process (entry 6). The alkylation yield dropped to 13% when the reaction was carried out in the presence of TEMPO (1 equiv, Table S1, entry 15). The reaction carried out in CD2Cl2 did not show any deuterium incorporation in compound 3 in analogy with the same reaction occurring in DCM (Figures S9 and S10).
The scope of the reaction has been then extended to electron-poor alkenes 2b–h and vinyl heteroarenes 2i and 2j. The results obtained have been depicted in Scheme 3. tert-Butylated derivatives 3–12 have been obtained in good to satisfactory yields. In one case (4), the reaction was repeated on a mmol scale. Allyl-methacrylate 2c was regioselectivity tert-butylated on the electrophilic C=C bond, but ester 5 was isolated in only a 46% yield due to its volatility. The method shows a good tolerance in the presence of different functional groups including esters, nitriles, amides, carbonyls, and even heteroarenes. Similar satisfactory results have been obtained when using oxazolidines 1b–d. In particular, 1b was adopted to incorporate the adamantyl moiety into olefins, and the resulting adducts have been isolated in up to a 91% yield (e.g., for 14). In this case, methanol (20% v/v) was added to completely dissolve 1b. To our delight, we found that the release of substituted alkyl radicals such as α-amido (from 1c) and α-oxy (from glyceraldehyde derivative 1d) led to alkylated products 21–28 in the 43–90% range (Scheme 3). Unfortunately, no alkylation products were detected when 1e and f and aromatic derivative 1g were used as the radical precursors.
Scheme 3. Photoredox Catalyzed Alkylation of Olefins 2a–j.

Reaction carried out on a 1 mmol scale.
Reactions with oxazolidine 1b were carried out in a DCE/MeOH 5:1 mixture for solubility concerns.
This is an appealing approach for the generation of tertiary (e.g., tBu and adamantyl) and α-oxy and α-amido carbon-centered radicals. The reaction took place upon visible light using a commercially available and widely employed organic dye (Acr-Mes+BF4–) as the photoredox catalyst and gives access to a large variety of alkylated compounds, including, among others, β-alkyl-amides, nitriles, and ketones, as well as functionalized nitrogen-based heterocycles via formation of a C(sp3)–C(sp3) bond.
The preparation of 3–20 allows for the introduction of a quaternary carbon in an organic molecule by the forging of a C(sp3)–C(sp3) bond, a topic for which there is great interest in view of all-carbon quaternary scaffolds present in many biologically active compounds.29 Moreover, the adamantylation of olefin is an important strategy to incorporate a moiety able to impart steric bulkiness, chemical inertness, rigidity, and lipophilicity to an organic compound; indeed, several adamantane-based drugs are known to take advantage of these peculiarities.30 The design of catalysts having the adamantane scaffold is also another hot topic.31
As for the above, finding new methods for the formation of tertiary radicals and their application is of utmost importance.3 The photogeneration of these radicals has been only sparsely reported using Barton esters,32N-(acyloxy)phthalimides,33 alkyl N-phthalimidoyl oxalates,34 and alkyl carboxylates.5b Thermal generation of these intermediates involved electrophiles such as alkyl halides35 or alkylsulfones,36 despite that in some cases, the desired C(sp3)–C(sp3) bond formation failed to occur.37
A tentative mechanism for the process illustrated in the present manuscript is proposed in Scheme 4.
Scheme 4. Suggested Mechanism.
Compounds 1a–g are radical precursors having an Eox < 1.3 V vs SCE (Table 1), comparable to that of other uncharged 1,4-dihydropyridine derivatives (Figure 1c).11 The monoelectronic oxidation of 1a–g by the photoexcited acridinium catalyst Acr-Mes+* to give the corresponding radical cations 1a–g•+ is thus feasible (path a). At this stage, an unprecedented C–C cleavage in 1a–d•+ took place, releasing a carbon-centered radical and a stable iminium ion (29+, path b). The peculiar structure of the oxazolidines avoid the possible α-deprotonation at the radical cation stage from position 2 and 4 as well as from the N-Me group to give an α-amino radical (path b’). The driving force of such C–C cleavage is the stability of the tertiary, α-oxy, and α -amido radicals released.
In the case of oxazolidines 1e and f and oxazole 1g, the formation of the corresponding radical cation led to an unproductive alkylation. In the former case, the release of a primary or a secondary radical is expected to be not so favored and competitive paths may operate.15 The structure of compound 1g, however, resembles that of an aniline derivative and may suffer, in analogy with N,N-dialkyl anilines, of competitive deprotonation38 or the reactivity of 1g•+ may not have a role due to the efficient back electron transfer with the reduced form of the PC.39
The alkyl radicals derived from 1a–d•+ are, in turn, trapped by electron-poor olefins or vinyl (hetero)aromatics (path c). Back electron transfer from Acr-Mes• to the adduct radical 30• (path d) followed by protonation (path e) led to the alkylated products while restoring the photoredox catalyst. This agrees with related conjugate radical additions promoted by the acridinium salt.40 A hydrogen atom transfer from the solvent by 30• is safely excluded by the deuteration experiments (see Figures S9–S10). The radical nature of the process is confirmed by the detrimental effect induced by the presence of a radical scavenger (TEMPO, see Table S1).
Conclusions
Summing up, we designed a class of smoothly prepared uncharged precursors for the easy release of alkyl radicals (tertiary, α-oxy, and α-amido) under photoredox catalyzed conditions. This process relies on the unprecedented C–C cleavage in amine radical cations obtained by visible-light irradiation in the presence of commercially available Acr-Mes+ BF4– as a photo-organocatalyst. This approach was exploited for the introduction, among the others, of a quaternary carbon center via C(sp3)–C(sp3) bond formation and for valuable adamantylations.
Acknowledgments
A.L.R. acknowledges European Union’s for the Ph.D. fellowship on the PhotoReAct ITN.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.2c03768.
Materials and methods, experimental procedures, optimization studies, electrochemical measurements, characterization data, NMR spectra, and mass spectrometry data of new compounds (PDF)
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie Grant Agreement No. 956324 (MSCA ITN: PhotoReAct).
The authors declare no competing financial interest.
Supplementary Material
References
- a Albini A.; Fagnoni M.. Photochemically-Generated Intermediates in Synthesis, John Wiley & Sons, Inc: Hoboken, NJ, 2013; p. 380. [Google Scholar]; b Ravelli D.; Protti S.; Fagnoni M. Carbon-Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. 10.1021/acs.chemrev.5b00662. [DOI] [PubMed] [Google Scholar]; c Visible Light Photocatalysis in Organic Chemistry, Stephenson C. R. J.; Yoon T. P.; MacMillan D. W. C., Eds.; Wiley-VCH: Weinheim, Germany, 2018; p. 456. [Google Scholar]; d Chemical Photocatalysis, 2nd ed.; König B., Ed.; De Gruyter, 2020; Vol. 512. [Google Scholar]; e Protti S.; Ravelli D.; Fagnoni M. Designing Radical Chemistry by Visible-Light Promoted Homolysis. Trends Chem. 2022, 4, 305–317. 10.1016/j.trechm.2022.01.009. [DOI] [Google Scholar]
- a Hoffmann N. Photochemical reactions of aromatic compounds and the concept of the photon as a traceless reagent. Photochem. Photobiol. Sci. 2012, 11, 1613–1641. 10.1039/c2pp25074h. [DOI] [PubMed] [Google Scholar]; b Bonfield H. E.; Knauber T.; Lévesque F.; Moschetta E. G.; Susanne F.; Edwards L. J. Photons as a 21st century reagent. Nat. Commun. 2020, 11, 804 10.1038/s41467-019-13988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent reviews on the photogeneration of alkyl radicals see:; a Goddard J. P.; Ollivier C.; Fensterbank L. Photoredox Catalysis for the Generation of Carbon Centered Radicals. Acc. Chem. Res. 2016, 49, 1924–1936. 10.1021/acs.accounts.6b00288. [DOI] [PubMed] [Google Scholar]; b Matsui J. K.; Lang S. B.; Heitz D. R.; Molander G. A. Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development. ACS Catal. 2017, 7, 2563–2575. 10.1021/acscatal.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Roslin S.; Odell L. R. Visible-Light Photocatalysis as an Enabling Tool for the Functionalization of Unactivated C(sp3)-Substrates. Eur. J. Org. Chem. 2017, 2017, 1993–2007. 10.1002/ejoc.201601479. [DOI] [Google Scholar]; d Pitre S. P.; Weires N. A.; Overman L. E. Forging C(sp3)–C(sp3) Bonds with Carbon-Centered Radicals in the Synthesis of Complex Molecules. J. Am. Chem. Soc. 2019, 141, 2800–2813. 10.1021/jacs.8b11790. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Crespi S.; Fagnoni M. Generation of Alkyl Radicals: From the Tyranny of Tin to the Photon Democracy. Chem. Rev. 2020, 120, 9790–9833. 10.1021/acs.chemrev.0c00278. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Niu P.; Li J.; Zhang Y.; Huo C. One-Electron Reduction of Redox-Active Esters to Generate Carbon-Centered Radicals. Eur. J. Org. Chem. 2020, 2020, 5801–5814. 10.1002/ejoc.202000525. [DOI] [Google Scholar]; g Correia J. T. M.; Fernandes V. A.; Matsuo B. T.; Delgado J. A. C.; de Souza W. C.; Paixão M. W. Photoinduced deaminative strategies: Katritzky salts as alkyl radical precursors. Chem. Commun. 2020, 56, 503–514. 10.1039/C9CC08348K. [DOI] [PubMed] [Google Scholar]; h Karmakar S.; Silamkoti A.; Meanwell N. A.; Mathur A.; Kumar Gupta A. Utilization of C(sp3)-Carboxylic Acids and Their Redox-Active Esters in Decarboxylative Carbon-Carbon Bond Formation. Adv. Synth. Catal. 2021, 363, 3693–3736. 10.1002/adsc.202100314. [DOI] [Google Scholar]; i Parida S. K.; Mandal T.; Das S.; Kumar Hota S.; De Sarkar S.; Murarka S. Single Electron Transfer-Induced Redox Processes Involving N-(Acyloxy)phthalimides. ACS Catal. 2021, 11, 1640–1683. 10.1021/acscatal.0c04756. [DOI] [Google Scholar]; j Capaldo L.; Ravelli D.; Fagnoni M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C–H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924. 10.1021/acs.chemrev.1c00263. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Corcé V.; Ollivier C.; Fensterbank L. Boron, silicon, nitrogen and sulfur-based contemporary precursors for the generation of alkyl radicals by single electron transfer and their synthetic utilization. Chem. Soc. Rev. 2022, 51, 1470–1510. 10.1039/D1CS01084K. [DOI] [PubMed] [Google Scholar]
- Yoshida J.-i.; Kataoka K.; Horcajada R.; Nagaki A. Modern Strategies in Electroorganic Synthesis Modern Strategies in Electroorganic Synthesis. Chem. Rev. 2008, 108, 2265–2299. 10.1021/cr0680843. [DOI] [PubMed] [Google Scholar]
- a Roth H. G.; Romero N. A.; Nicewicz D. A. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714–723. 10.1055/s-0035-1561297. [DOI] [Google Scholar]; b Chu L.; Ohta C.; Zuo Z.; MacMillan D. W. C. Carboxylic Acids as A Traceless Activation Group for Conjugate Additions: A Three-Step Synthesis of (±)-Pregabalin. J. Am. Chem. Soc. 2014, 136, 10886–10889. 10.1021/ja505964r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauber T.; Chandrasekaran R.; Tucker J. W.; Chen J. M.; Reese M.; Rankic D. A.; Sach N.; Helal C. Ru/Ni Dual Catalytic Desulfinative Photoredox Csp2-Csp3 Cross-Coupling of Alkyl Sulfinate Salts and Aryl Halides. Org. Lett. 2017, 19, 6566–6569. 10.1021/acs.orglett.7b03280. [DOI] [PubMed] [Google Scholar]
- Yasu Y.; Koike T.; Akita M. Visible Light-Induced Selective Generation of Radicals from Organoborates by Photoredox Catalysis. Adv. Synth. Catal. 2012, 354, 3414–3420. 10.1002/adsc.201200588. [DOI] [Google Scholar]
- Corcé V.; Chamoreau L. M.; Derat E.; Goddard J. P.; Ollivier C.; Fensterbank L. Silicates as Latent Alkyl Radical Precursors: Visible-Light Photocatalytic Oxidation of Hypervalent Bis-Catecholato Silicon Compounds. Angew. Chem., Int. Ed. 2015, 54, 11414–11418. 10.1002/anie.201504963. [DOI] [PubMed] [Google Scholar]
- Nawrat C. C.; Jamison C. R.; Slutskyy Y.; MacMillan D. W. C.; Overman L. E. Oxalates as Activating Groups for Alcohols in Visible Light Photoredox Catalysis: Formation of Quaternary Centers by Redox-Neutral Fragment Coupling. J. Am. Chem. Soc. 2015, 137, 11270–11273. 10.1021/jacs.5b07678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauck F. J. R.; James M. J.; Glorius F. Deaminative Strategy for the Visible-Light-Mediated Generation of Alkyl Radicals. Angew. Chem., Int. Ed. 2017, 56, 12336–12339. 10.1002/anie.201706896. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Bonet Á.; Tellis J. C.; Matsui J. K.; Vara B. A.; Molander G. A. 1,4-Dihydropyridines as Alkyl Radical Precursors: Introducing the Aldehyde Feedstock to Nickel/Photoredox Dual Catalysis. ACS Catal. 2016, 6, 8004–8008. 10.1021/acscatal.6b02786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni M.; Mella M.; Albini A. Radical addition to alkenes via electron transfer photosensitization. J. Am. Chem. Soc. 1995, 117, 7877–7881. 10.1021/ja00135a004. [DOI] [Google Scholar]
- Yoshida J.-i.; Nishiwaki K. Redox Selective Reactions of Organo-Silicon and -Tin Compounds. J. Chem. Soc. Dalton Trans. 1998, 16, 2589–2596. 10.1039/a803343i. [DOI] [Google Scholar]
- Mella M.; Fasani E.; Albini A. Electron Transfer Photoinduced Cleavage of Acetals. A Mild Preparation of Alkyl Radicals. J. Org. Chem. 1992, 57, 3051–3057. 10.1021/jo00037a020. [DOI] [Google Scholar]
- Hu J.; Wang J.; Nguyen T. H.; Zheng N. The chemistry of amine radical cations produced by visible light photoredox catalysis. Beilstein J. Org. Chem. 2013, 9, 1977–2001. 10.3762/bjoc.9.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M.; Wayner D. D. M.; Lusztyk J. Redox and Acidity Properties of Alkyl- and Arylamine Radical Cations and the Corresponding Aminyl Radicals. J. Phys. Chem. A 1996, 100, 17539–17543. 10.1021/jp961286q. [DOI] [Google Scholar]
- a Beatty J. W.; Stephenson C. R. J. Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res. 2015, 48, 1474–1484. 10.1021/acs.accounts.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Morris S. A.; Wang J.; Zheng N. The Prowess of Photogenerated Amine Radical Cations in Cascade Reactions: From Carbocycles to Heterocycles. Acc. Chem. Res. 2016, 49, 1957–1968. 10.1021/acs.accounts.6b00263. [DOI] [PubMed] [Google Scholar]
- Fagnoni M.; Dondi D.; Ravelli D.; Albini A. Photocatalysis for the formation of the C-C Bond. Chem. Rev. 2007, 107, 2725–2756. 10.1021/cr068352x. [DOI] [PubMed] [Google Scholar]
- Murugesan K.; Donabauer K.; Narobe R.; Derdau V.; Bauer A.; König B. Photoredox-Catalyzed Site-Selective Generation of Carbanions from C(sp3)–H Bonds in Amines. ACS Catal. 2022, 12, 3974–3984. 10.1021/acscatal.2c00662. [DOI] [Google Scholar]
- Zhu M.; Zheng N. Photoinduced cleavage of N-N bonds of aromatic hydrazines and hydrazides by visible light. Synthesis 2011, 2011, 2223–2236. 10.1055/s-0030-1260082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo L.; Lafayette Quadri L.; Ravelli D. Photocatalytic hydrogen atom transfer: the philosopher’s stone for late-stage functionalization?. Green Chem. 2020, 22, 3376–3396. 10.1039/D0GC01035A. [DOI] [Google Scholar]
- The generation of alkyl radicals from an amine radical cations has been investigated by computational analyses, see:; Hammerum S.; Norrman K.; Sølling T. I.; Andersen P. E.; Bo Jensen L.; Vulpius T. Competing Simple Cleavage Reactions: The Elimination of Alkyl Radicals from Amine Radical Cations. J. Am. Chem. Soc. 2005, 127, 6466–6475. 10.1021/ja043481l. [DOI] [PubMed] [Google Scholar]
- a Sundberg R. J.; Desos P.; Gadamasetti K. G.; Sabat M. Photoactive C16-C21 Fragmentation of Catharanthine. Tetrahedron Lett. 1991, 32, 3035–3038. 10.1016/0040-4039(91)80680-5. [DOI] [Google Scholar]; b Cocquet G.; Rool P.; Ferroud C. A Catalytic Versus Stoichiometric Electron Transfer Promoted Selective C16-C21 Bond Cleavage of Catharanthine. Tetrahedron Lett. 2001, 42, 839–841. 10.1016/S0040-4039(00)02117-1. [DOI] [Google Scholar]; c Beatty J. W.; Stephenson C. R. J. Synthesis of (−)-Pseudota-bersonine, (−)-pseudovincadifformine, and (+)-Coronaridine Enabled by Photoredox Catalysis in Flow. J. Am. Chem. Soc. 2014, 136, 10270–10273. 10.1021/ja506170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity S.; Zhu M.; Shinabery R. S.; Zheng N. Intermolecular [3 + 2] Cycloaddition of Cyclopropylamines with Olefins by Visible-Light Photocatalysis. Angew. Chem., Int. Ed. 2012, 51, 222–226. 10.1002/anie.201106162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S.; Zhao X.; Wang X.; Liu Q.; Li Z.; Wang D. Z.; Visible-Light-Promoted C–C. Bond Cleavage: Photocatalytic Generation of Iminium Ions and Amino Radicals. Angew. Chem., Int. Ed. 2012, 51, 8050–8053. 10.1002/anie.201202880. [DOI] [PubMed] [Google Scholar]
- a Lv X.-Y.; Abrams R.; Martin R. Dihydroquinazolinones as adaptative C(sp3) handles in arylations and alkylations via dual catalytic C–C bond-functionalization. Nat. Commun. 2022, 13, 2394 10.1038/s41467-022-29984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Li L.; Fang L.; Wu W.; Zhu J. Visible-Light-Mediated Intermolecular Radical Conjugate Addition for the Construction of Vicinal Quaternary Carbon Centers. Org. Lett. 2020, 22, 5401–5406. 10.1021/acs.orglett.0c01724. [DOI] [PubMed] [Google Scholar]
- Fagnoni M.; Protti S.; Ravelli D.; Albini A. Spectroscopic characterization of photo-accumulated radical anions. A Litmus test to evaluate the efficiency of Photoinduced Electron Transfer (PET) processes. Beilstein J. Org. Chem. 2013, 9, 800–808. 10.3762/bjoc.9.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Kim D.; Teets T. S. Photophysical Properties and Redox Potentials of Photosensitizers for Organic Photoredox Transformations. Synlett 2022, 33, 1154–1179. 10.1055/a-1390-9065. [DOI] [Google Scholar]
- a Quasdorf K. W.; Overman L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 2014, 516, 181–191. 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liu Y.; Han S.-J.; Liu W.-B.; Stoltz B. M. Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res. 2015, 48, 740–751. 10.1021/ar5004658. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jamison C. R.; Overman L. E. Fragment Coupling with Tertiary Radicals Generated by Visible-Light Photocatalysis. Acc. Chem. Res. 2016, 49, 1578–1586. 10.1021/acs.accounts.6b00284. [DOI] [PubMed] [Google Scholar]; d Ling T.; Rivas F. All-carbon quaternary centers in natural products and medicinal chemistry: recent advances. Tetrahedron 2016, 72, 6729–6777. 10.1016/j.tet.2016.09.002. [DOI] [Google Scholar]; e Xue W.; Jia X.; Wang X.; Tao X.; Yin Z.; Gong H. Nickel-catalyzed formation of quaternary carbon centers using tertiary alkyl electrophiles. Chem. Soc. Rev. 2021, 50, 4162–4184. 10.1039/D0CS01107J. [DOI] [PubMed] [Google Scholar]
- a De Clercq E. Antiviral agents active against influenza A viruses. Nat. Rev. Drug Discov. 2006, 5, 1015–1025. 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lamoureux G.; Graciela A. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010, 17, 2967–2978. 10.2174/092986710792065027. [DOI] [PubMed] [Google Scholar]; c Wanka L.; Iqbal K.; Schreiner P. R. The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604. 10.1021/cr100264t. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Stockdale T. P.; Williams C. M. Pharmaceuticals that contain polycyclic hydrocarbon scaffolds. Chem. Soc. Rev. 2015, 44, 7737–7763. 10.1039/C4CS00477A. [DOI] [PubMed] [Google Scholar]; e Štimac A.; Sekutor M.; Mlinaric-Majerski K.; Frkanec L.; Frkanec R. Adamantane in Drug Delivery Systems and Surface Recognition. Molecules 2017, 22, 297. 10.3390/molecules22020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew-Francis K. A.; Williams C. M. Catalysts Containing the Adamantane Scaffold. Adv. Synth. Catal. 2016, 358, 675–700. 10.1002/adsc.201500949. [DOI] [Google Scholar]
- Barton D. H. R.; Sas W. The invention of radical reactions. Part XIX. The synthesis of very hindered quinones. Tetrahedron 1990, 46, 3419–3430. 10.1016/S0040-4020(01)81512-X. [DOI] [Google Scholar]
- a Pratsch G.; Lackner G. L.; Overman L. E. Constructing Quaternary Carbons from N-(Acyloxy)phthalimide Precursors of Tertiary Radicals Using Visible-Light Photocatalysis. J. Org. Chem. 2015, 80, 6025–6036. 10.1021/acs.joc.5b00795. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Okada K.; Okamoto K.; Morita N.; Okubo K.; Oda M. Photosensitized decarboxylative Michael addition through N-(acyloxy)phthalimides via an electron-transfer mechanism. J. Am. Chem. Soc. 1991, 113, 9401–9402. 10.1021/ja00024a074. [DOI] [Google Scholar]
- a Lackner G. L.; Quasdorf K. W.; Overman L. E. Direct Construction of Quaternary Carbons from Tertiary Alcohols via Photoredox-Catalyzed Fragmentation of tert-Alkyl N-Phthalimidoyl Oxalates. J. Am. Chem. Soc. 2013, 135, 15342–15345. 10.1021/ja408971t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang Q.; Yue L.; Bao Y.; Wang Y.; Kang D.; Gao Y.; Yuan Z. Oxalates as Activating Groups for Tertiary Alcohols in Photoredox-Catalyzed gem-Difluoroallylation To Construct All-Carbon Quaternary Centers. J. Org. Chem. 2022, 87, 8237–8247. 10.1021/acs.joc.2c00664. [DOI] [PubMed] [Google Scholar]
- a Furst L.; Narayanam J. M. R.; Stephenson C. R. J. Total Synthesis of (+)-Gliocladin C Enabled by Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed. 2011, 50, 9655–9659. 10.1002/anie.201103145. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhu Y.; Zhang L.; Luo S. Asymmetric α-Photoalkylation of β-Ketocarbonyls by Primary Amine Catalysis: Facile Access to Acyclic All-Carbon Quaternary Stereocenters. J. Am. Chem. Soc. 2014, 136, 14642–14645. 10.1021/ja508605a. [DOI] [PubMed] [Google Scholar]
- Nambo M.; Tahara Y.; Yim J. C.-H.; Yokogawa D.; Crudden C. M. Synthesis of quaternary centres by single electron reduction and alkylation of alkylsulfones. Chem. Sci. 2021, 12, 4866–4871. 10.1039/D1SC00133G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T.; Cornella J.; Li C.; Malins L. R.; Edwards J. T.; Kawamura S.; Maxwell B. D.; Eastgate M. D.; Baran P. S. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 2016, 352, 801–805. 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hand R.; Nelson R. F. The Electrochemical Oxidation of N,N-Dimethylaniline. J. Electrochem. Soc. 1970, 117, 1353–1357. 10.1149/1.2407319. [DOI] [Google Scholar]; b Dombrowski G. W.; Dinnocenzo J. P.; Zielinski P. A.; Farid S.; Wosinska Z. M.; Gould I. R. Efficient Unimolecular Deprotonation of Aniline Radical Cations. J. Org. Chem. 2005, 70, 3791–3800. 10.1021/jo047813g. [DOI] [PubMed] [Google Scholar]; c Brown T. A.; Chen H.; Zare R. N. Detection of the Short-Lived Radical Cation Intermediate in the Electrooxidation of N,N-Dimethylaniline by Mass Spectrometry. Angew. Chem., Int. Ed. 2015, 54, 11183–11185. 10.1002/anie.201506316. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Leonori D. Minimization of Back-Electron Transfer Enables the Elusive sp3 CH Functionalization of Secondary Anilines. Angew. Chem., Int. Ed. 2021, 60, 7669–7674. 10.1002/anie.202100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo L.; Riccardi R.; Ravelli D.; Fagnoni M. Acyl Radicals from Acylsilanes: Photoredox Catalyzed Synthesis of Unsymmetrical Ketones. ACS Catal. 2018, 8, 304–309. 10.1021/acscatal.7b03719. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.