Abstract
Background
The recent outbreak of Human Monkeypox (MPXV) in nonendemic regions of the world is of great concern.
Objective
We aimed to systematically analyze the current epidemiology, clinical presentation, and outcomes of the Monkeypox virus.
Method
Systematic literature was conducted in PubMed, Embase, Google Scholar, and Scopus using predefined MESH terms by using “AND” and “OR.” The following search terms were used: Monkeypox [MeSH] OR “Monkeypox virus” [MeSH] OR “POX” OR “Monkeypox” AND “Outbreak” AND “Outcomes” from December 2019 till 14th June 2022 without restrictions of language.
Results
A total of 1074 (99.90%) patients tested positive for Monkeypox virus through RT‐PCR while 1 (0.09) patient was suspected. There was a gender difference with male predominance (54.23% vs. 45.48%) compared with female patients. Mean age (±SD) of patients was 20.66 ± 16.45 years. The major symptoms were rash (100%), fever (96%), and other important symptoms were upper respiratory symptoms (97%), headache (95%), vomiting (95%), oral ulcers (96%), conjunctivitis (96%) and lymphadenopathy (85%). The average mean duration of treatment was 5 days, while the mean hospitalization duration was 13.3 ± 6.37 days. The outcome of 20 patients was available, 19 of 20 patients recovered fully from monkeypox, however, 1 patient was not able to survive resulting in death.
Conclusion
The recent monkeypox virus outbreak has shown that the virus could transmit in ways that were not previously expected. Further research is needed to understand the possible outcomes and association with humans and their different organ systems.
Keywords: infection, monkeypox virus, outcomes, pathology
The results of this review indicate that recent cases of Monkeypox have been occurring in adults and not only in children. The symptoms were as expected with a predominance of rash, fever, headache, upper respiratory symptoms, and lymphadenopathy. Transmission of the disease can be either by contact with infectious sores, scabs, or body fluids or respiratory droplets, similarly to COVID‐19, therefore, similar preventative measures can prevent the disease from becoming epidemic/pandemic
1. INTRODUCTION
As of the 2nd of June 2022, 780 confirmed cases of Monkeypox have been reported in over 27 nonendemic countries. 1 Figures 1 and 2 show the worldwide distribution of reported Monkeypox cases as of June 8th, 2021, and the daily increase in confirmed Monkeypox cases. Initially believed to be endemic only to Africa, this viral zoonotic illness has grabbed the attention of scientists globally to identify the reason for the rapid spread of the disease and the change in viral behavior. 2 , 3
Figure 1.
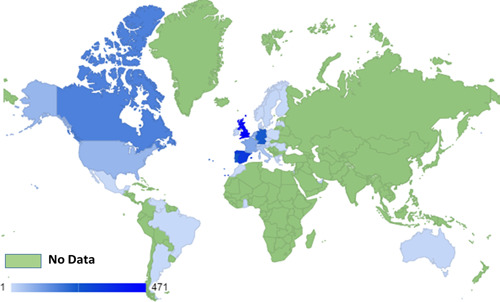
Global picture of Monkeypox confirmed case in nonepidemic countries till 14th of June, 2022. 4
Figure 2.
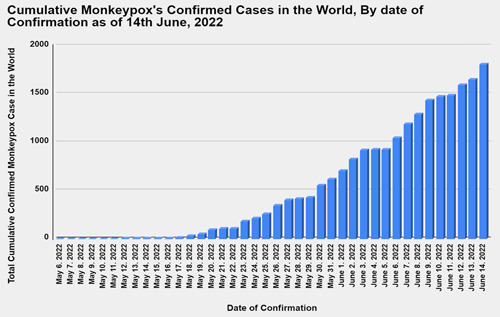
Daily incidence of reported confirmed monkeypox infections up until 14th June 2022. 4
The Human Monkeypox virus (MPXV) is a double‐stranded DNA virus belonging to the Orthopoxvirus genus of the Poxviridae family. 5 Initially, detected in laboratory monkeys in 1958, Monkeypox was thought to transmit from wild animals like rodents or infected people through close contact with bodily fluids. 6 It commonly presents with a 1–4‐day febrile prodrome with headache and fatigue followed by a centrifugal rash ranging from maculopapular, vesicular, or pustular. The spectrum of disease severity is varied, ranging from mild to even fatal with a mortality rate of 1%–10%. 7 The emergence of the disease has been attributed to the waning of the herd immunity of variola or smallpox, which belongs to the same genus as MPXV. 5 With the eradication of smallpox and subsequent discontinuation of the universal smallpox vaccination program in the 1970s, clusters of Monkeypox have been reported in Central and Western Africa, with the most recent outbreak in Nigeria in 2019. 8 Although most affected individuals recover from Monkeypox without complications and treatment, there have been reports of a few fatal cases.
The recent outbreak of Monkeypox in non‐endemic regions of the world is of great concern. Monkeypox detection in individuals with no contact history or travel to endemic areas may suggest an asymptomatic disease state. 3 Furthermore, increasing reported cases of transmission through sexual activity in men who have sex with men (MSM) is also concerning as MPXV was not known to be sexually transmitted. 9 With growing evidence of globally confirmed cases we conducted this very first systematic review to understand better the current epidemiology, pathophysiology, clinical presentation and outcomes of Monkeypox.
2. METHODS
This systematic review was conducted and reported in conformity with the Cochrane and PRISMA (Preferred reporting items for systematic review and Meta‐analysis) 2020 guidelines as described previously. 10 , 11 , 12
2.1. Search strategy
We conducted a systematic literature search in PubMed, Embase, Google Scholar and Scopus using predefined MESH terms by using “AND” and “OR”. The following search terms were used: Monkeypox [MeSH] OR “Monkeypox virus” [MeSH] OR “POX” OR “Monkeypox” AND “Outbreak” AND “Outcomes.”
2.2. Eligibility criteria
Studies were included if they fulfilled the following criteria: patients (of any age) who developed a rash and symptoms with a confirmed diagnosis of Monkeypox via swab or blood test, case reports, case series, prospective or retrospective studies. Studies which involved animal testing, review articles, modeling studies without patients’ data, and studies with smallpox as a diagnosis among the patient population were excluded.
2.3. Study selection
We queried databases from December 2019 till 14th June 2022 without language restriction. The studies were carefully screened and exported to the Endnote 2020 library (X9). Two reviewers (DM and SV) reviewed the titles and abstract. Discrepancies regarding inclusion of studies were arbitrated by the senior author (VJ). The same reviewers also performed the full‐text screening independently to decide which articles fulfilled the inclusion criteria. The senior author arbitrated discrepancies regarding the inclusion of studies.
2.4. Data extraction and statistical analysis
The following data were extracted from the studies: demographic data (age and gender), study design, publication year, study location, patient comorbidities, symptoms, length of hospitalization, duration of management, treatment used, and patient outcomes. Two authors (DM, SM) assembled all available information in a shared Excel® 2019 spreadsheet. For missing, incorrect, or unreported data, the corresponding authors of the respective papers were contacted via email for clarification. Supplementary material related to the main article was also investigated in such cases.
Finally, descriptive statistics were used to summarize the data in this paper. The mean and standard deviation were adopted to describe continuous variables, whereas frequencies and percentages were used for dichotomous data. All statistical analyses were conducted using the software R version 4.1.2 (available at https://cran.r-project.org/)
3. RESULTS
3.1. Study selection
Our initial search identified 438 articles. After removing 195 duplicates, 180 studies were excluded after a review of the title and abstract. 63 studies were sought for retrieval but 35 were not retrieved. A total of 28 studies were reviewed in full‐text form; however, 18 studies were further excluded based on inclusion criteria. A total of 10 studies were included for the review of which 4 were case reports, 13 , 14 , 15 , 16 3 were case series, 17 , 18 , 19 and 3 studies were observational 7 , 20 , 21 (Figure S1).
3.2. Baseline characteristic of included patients
Ten studies were selected for the final review with a total of 1078 patients were identified to have symptoms suggestive of Monkeypox. A slight male predominance was found with (n = 583, 54.23%) male patients, and (n = 489, 45.48%) female patients. Mean age (±SD) of patients was 20.66 ± 16.45 years. Majority of patients (n = 1061, 98.4%) were from the Democratic Republic of Congo (DRC), (n = 10, 0.92% patients were from the United Kingdom), (n = 2, 0.18%) from Central Nigeria and West Africa, and (n = 1, 0.09) from United States of American, Australia, and Singapore (Tables 1, 2).
Table 1.
Baseline patients characteristic of the included studies
| Author | Study design | Year of publication | Country | Sample size | Confirmed cases | Suspected cases | Age, years | Male (M): female (F), n | Monkeypox viral DNA test |
|---|---|---|---|---|---|---|---|---|---|
| Adler et al. 7 | Retrospective observational | 2022 | United Kingdom | 7 | 7 | 0 | 5 patients: 30–40 years; 1 patient: 40–50 years; 1 patient: less than 2 years |
M: 4 F: 3 |
PCR |
| Eseigbe et al. 17 | Case series | 2021 | North Central Nigeria | 2 | 2 | 0 | 20 years, 20 years | 2M | PCR |
| Reynolds et al. 18 | Case series | 2019 | Sierra Leone, West Africa | 2 | 2 | 0 | 11 months; 35 years | 2M | PCR |
| Eltvedt et al. 13 | Case report | 2020 | Democratic Republic of the Congo | 1 | 0 | 1 | 4 years | M | NR |
| Whitehouse et al. 20 | Surveillance data | 2021 | Democratic Republic of the Congo | 1057 | 1057 | 0 | 14 years | M: 568, F: 486, NR: 3 | PCR |
| Ngbolua et al. 21 | Cross sectional study | 2020 | Democratic Republic of the Congo | 3 | 3 | 0 | 13, 7.7 years | 3M | NR |
| Hobson et al. 19 | Case series | 2021 | United Kingdom | 3 | 3 | 0 | NR, NR, 18 months | NR | PCR |
| Yong et al. 15 | Case report | 2020 | Singapore | 1 | 1 | 0 | 38 years | M | PCR |
| Costello et al. 14 | Case report | 2022 | United States | 1 | 1 | 0 | 28 years | M | PCR |
| Hammerschlag et al. 16 | Case report | 2022 | Australia | 1 | 1 | 0 | 30 years | M | PCR |
Table 2.
Summary table of all Monkeypox cases highlighting demographic, symptoms, management, and outcomes
| Variables | n/N (%) |
|---|---|
| N | 1078 |
| Age (mean, SD) | 20.66 ± 16.45 years |
| Male, n (%) | 583 (54%) |
| Female, n (%) | 489 (46%) |
| NR | 6 |
| Country | |
| United Kingdom | 2/10 (20%) |
| Nigeria | 1/10 (10%) |
| Sierra Leone | 1/10 (10%) |
| Democratic Republic of the Congo | 3/10 (30%) |
| Singapore | 1/10 (10%) |
| United States | 1/10 (10%) |
| Australia | 1/10 (10%) |
| Monkeypox viral DNA test | RT‐PCR: 1074/1075 (99.9%) |
| Symptoms | |
| Fever | 1037/1075 (96%) |
| Headache | 1015/1068 (95%) |
| Lymphadenopathy |
905/1070 (85%) (Cervical lymphadenopathy is most common) |
| Upper respiratory symptoms | 1026/1060 (97%) |
| Rash | 1078/1078 (100%) |
| Oral ulcers | 1018/1057 (96%) |
| Vomiting | 1011/1059 (95%) |
| Conjunctivitis | 1017/1058 (96%) |
| Management | |
| Antivirals | 7/11 (63%) |
| Antibiotics | 4/10 (40%) |
| Length of hospitalization (mean) | 13.3 ± 6.37 days |
| Duration of management (mean) | 5 days |
| Outcomes | Recovery: 19/20 (95%), Death: 1/20 (5%) |
3.3. Diagnostic test, symptoms, and clinical findings
A total of 1075 patients, 1074 (99.9%) patients tested positive for Monkeypox virus through RT‐PCR method, while 1 (0.09) patient was suspected. Symptoms were reported in all 10 studies. The major and most significant symptoms were rash 1078/1078 (100%), fever 1037/1075 (96%). Other important symptoms were upper respiratory symptoms 1026/1060 (97%), headache 1015/1068 (95%), vomiting 1011/1059 (95%), oral ulcers 1018/1057 (96%), conjunctivitis 1017/1058 (96%) and lymphadenopathy 905/1070 (85%). Most common lymphadenopathy is cervical lymphadenopathy. The appearance and distribution of rash was variably described in among the included studies with vesicular lesions being the most common presentation. The distribution was most often found to be generalized with an average of 158 lesions distributed over face and trunk also involving the limbs, palms, soles, and genitalia. Table 2 summarizes the symptoms experienced by the patients and the management in Monkeypox.
3.4. Management and patient outcomes
Four studies reported on the management of the patient. The information was available for 11 patients mainly. 4/10 (40%) patients received antibiotics in some form which included intravenous (IV) amoxicillin‐clavulanic acid, ceftriaxone, erythromycin ampicillin‐sulbactam, and antibacterial eye drops. 7/11 (63%) patients received antivirals (brincidofovir, tecovirimat, cidofovir, and acyclovir) and 9/12 (75%) patients underwent supportive treatment with isolation, IV fluids, analgesia, and oxygen therapy. Average duration of treatment was 5 days and mean hospitalization duration was 13.3 ± 6.37 days.
Patient outcomes were reported in 8 studies and for 20 patients.19/20 patients (95%) recovered fully from monkeypox, and 1/20 patient (5%) succumbed to the infection resulting in death (Table 2 and Table S1)
4. DISCUSSION
MPXV was first identified and isolated in 1958 as an outbreak of a pox‐like disease in monkeys in a research facility in Copenhagen, Denmark. 22 It was first identified in humans in 1970 in a 9‐month‐old boy in the DRC who presented with a smallpox‐like disease. 23 Since then, human cases have been increasingly reported in parts of central and western Africa, with numbers exceeding several thousand. 24 Sporadic clusters have also been reported in regions outside of Africa. In 2003, infected domesticated prairie dogs in the Midwestern United States resulted in 53 human cases of Monkeypox. 25 This cluster was identified as the first Monkeypox outbreak outside Africa and was caused by co‐inhabiting imported Gambian giant rats from Ghana. 25 A few cases have also been sporadically reported in Israel, Singapore, United Kingdom, United States, and Canada from 2018 to 2021, all traced to preceding travel history to Nigeria. 26 The exact incidence or prevalence of Monkeypox is unknown due to shortcomings in disease reporting and diagnostic confirmation in rural endemic areas of Africa. The identification of Monkeypox in several non‐African countries in May 2022 has propelled extensive research efforts to further understand disease epidemiology and pathophysiology. 24
4.1. Evolving epidemiology of Monkeypox
Once thought to exclusively affect the pediatric population, this review revealed that Monkeypox is now also seen in adults with mean age of patient 20.66 ± 16.45 years with slight male predominance (54.23%). Similarly, a systematic review by Bunge et al. 6 also noted a similar shift in median age of population affected by MPXV from 4 to 5 years old in the 1970s and 1980s to 10 and 21 years old in the 2000s and 2010s. Over the last five decades, the number of confirmed, probable, and/or potential monkeypox cases has increased by more than tenfold. 6 This is in accordance with the findings of Hoff et al., 27 who concluded that the increase in monkeypox cases in the DRC from 2001 to 2013 was most likely due to significant disease increases rather than increased surveillance, given the monkeypox surveillance system was stable by 2008.
MPXV is classified into two strains: the Congo Basin and the West Africa also called the Central Africa and West Africa clades based upon their geographical segregation. 28 Congo basin clad was found predominantly in central African countries namely DRC, 5 Central African Republic, 29 , 30 and South Sudan 31 whereas the cases from United States, 32 United Kingdom, 33 Israel, 34 Singapore, 15 and the Nigerian epidemic 35 are caused by West African clade. It is believed that West African clades are less virulent and have low transmissibility to humans when compared with Congo basin clades. 28 , 36 The Congo Basin clade virus has been linked to up to 10% mortality in monkeypox cases, whereas the West African clade is associated with fatal results in less than 1% of cases. 37 82.55% of total monkeypox cases reported from the DRC, previously thought to be a geographically limited zoonotic disease, have now spread in 27 other nonendemic countries across the 1 globe. Figure 3 shows the nonendemic countries were Monkeypox was reported as of June 8th, 2021.
Figure 3.
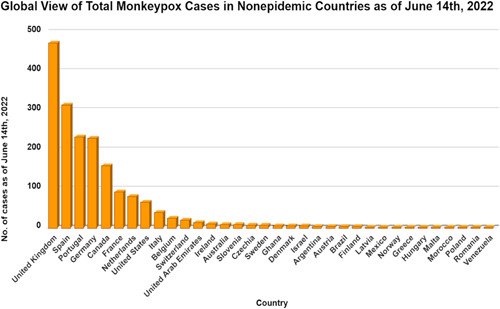
Distribution of the incidence Monkeypox cases globally by country 4
Monkeypox is a zoonotic disease with no known natural reservoir, however, certain rodents (including rope squirrels, tree squirrels, Gambian pouched rats, dormice) and nonhuman primates are known to be naturally susceptible to MPXV. 38 , 39 At present it is unclear if animals outside of the African continent can maintain a zoonotic cycle of MPXV. 38 However, it is thought that reintroduction of MPXV into animals on a regular basis is essential to keep the disease alive in the human population. 40 Despite the role of reservoir species in transmission, research shows that environmental variables such as warm temperature and humid environment have a positive influence on MPXV survival outside a host. 31 , 41 , 42 The sudden uproar of monkeypox cases in numerous nonendemic nations globally during the same period without a known animal reservoir or an established travel history to endemic regions hints towards an undiscovered transmission for an unknown period of time, followed by recent amplifier occurrences.
4.2. Pathophysiology of Monkeypox
MPXV pathophysiology is like that of smallpox. After initial viral entry through the skin or respiratory system, the virus spreads to regional lymph nodes and begins to replicate. The initial signs of viremia appear 3–4 days after infection and the virus further spreads within the lymphatic system. The emergence of fever and clinical symptoms of sickness correlates with the onset of secondary viremia, which occurs between days 8 and 12 following infection. At this stage, the virus has become localized in the oropharyngeal mucosa and small blood vessels of the dermis resulting in the appearance of a rash and myriad of other clinical symptoms. 43 Appearance of a rash in a patient generally indicates the start of the infectious period; however, the Centers for Disease Control and Prevention (CDC) states that a patient can be contagious throughout the prodromal stage. 44 The incubation period is about 12 days long, 45 but it can last up to 21 days. 44
4.3. Clinical features and differential diagnosis of Monkeypox
Overwhelmingly, the clinical features of monkeypox are like those of smallpox. More than 90% patients of monkeypox clinically present with a fever, rash, headache, and upper respiratory symptoms and more than 80% patients have lymphadenopathy, oral ulcers, conjunctivitis, vomiting and diarrhea as noted by this review. Initial symptoms of both smallpox and monkeypox include febrile illness along with the appearance of vesiculopapular rash which is umbilicated and evolves at similar stages of development throughout the illness. 46 Smallpox lesions are deep seated in contrast to superficial lesions of monkeypox. 46 In majority of monkeypox cases, the rash usually starts on the face and spreads out in a centrifugal pattern across the body including oral mucosa, trunk limbs, palms, soles and genitalia. 20 The rash begins with macules, which then develop into papules, vesicles, pustules, and finally crusts. 45 , 47 , 48
Another close differential of monkeypox is chickenpox. Monkeypox rash is differentiated from chickenpox rash by virtue of its slower maturation rate. Chickenpox lesions are more superficial, smaller, and centrally oriented, as opposed to the centrifugal distribution of Monkeypox, and they classically evolve in “crops” over 3–5 days, compared to the typical 12 days for Monkeypox. Presence of lymphadenopathy can help clinicians to differentiate monkeypox from smallpox and chickenpox as well. 45 Numbers of lesions in a monkeypox patient are highly variable, ranging from few to thousands with an average of 158 lesions. Monkeypox generally presents with diffuse multiple skin rash, however, a patient who had direct contact with an infected prairie dog, presented with prodromal symptoms followed by lymphadenopathy and later tested positive for MPXV in serologic testing had only one skin lesion. 49 This makes relying only on clinical presentation of patients for the diagnosis of monkeypox unreliable. For definitive diagnosis of MPXV positive polymerase chain reaction or next generation sequencing in a clinical specimen or isolation of Monkeypox virus in culture from a clinical specimen is required. 50 This increases challenges for physicians in low‐income hospital setups of West and central Africa where monkeypox is endemic. A frequent dilemma physicians face is differentiating Monkeypox from chickenpox in patients with atypical presentation. In low‐income countries, serum anti‐orthopoxvirus IgM antibody tests can be sufficient for diagnosis if the patient has not been exposed to another of the same genus. 50
Common complications of monkeypox include bronchopneumonia, encephalitis, septicemia, corneal scarring, blindness, skin scarring and severe dehydration. 5 Young children, unvaccinated and immunocompromised individuals are at higher risk of poorer outcomes. The case fatality rate varies widely from 0% to 11% depending on the outbreak, but according to the present knowledge the CDC estimates it to be around 10% in Africa. 44
4.4. Spread of Monkeypox in COVID era
Transmission of MPXV is via contact with infected animal, person or materials contaminated with virus. 45 It can be transmitted from animals to humans by infected animal bite or scratch, handling wild game, or using infected animal products. 45 It is now well known that MPXV spreads via large respiratory droplets as well. 45 , 51 It is noteworthy here that MPXV spread between humans is similar to that of coronavirus COVID‐19 hence making it a global health concern. Monkeypox has epidemic potential, according to mathematical modeling of human‐to‐human transmission, with R0 > 1 52 thus raising concerns how infected visitors might serve as index cases for local epidemics. An individual can become infected with MPXV related to sexual or nonsexual contact. Nonsexual contact includes direct contact with the rash, sores, or scabs of infected individuals, contact with objects or surfaces contaminated by infected individuals, or through respiratory droplets or oral fluids of infected individuals. 45 , 53 Spread of MPXV through sexual contact has received considerable attention after evidence from recent studies points towards a higher prevalence of MPXV infection in MSM. A study from the United Kingdom reported 54 MPX cases and noted that all the cases were MSM. 54 Another study from Italy documented four cases of MPX in MSM who reported having unprotected sexual intercourse. 55 A recent review of 121 cases of MPX highlighted that sexual exposure could be attributed to over 91% of the cases, which was supported by the findings of another study that included 528 individuals infected by MPX, which revealed that 98% of the confirmed MPX cases were either gay or bisexual. 56 , 57 Sexual transmission of MPX is an alarming threat to the spread of MPXV, however, more extensive studies confirming the presence of MPX in seminal samples through cell cultures and viral isolation are necessary to ascertain this mode of transmission.
4.5. Recommendations
It is past time for us to begin taking steps to prevent and prepare for epidemics, particularly for viruses that have been identified as substantial human dangers, such as MPXV. Even though it was identified in 1958 and first documented in humans in 1970, no gold standard treatment or vaccine exists. Current treatment guidelines focus on patient isolation, monitoring and treatment of complications and symptomatic treatment. Development of appropriate and effective treatment therapies, as well as active case surveillance, is critical to prevent another global pandemic. Measures for frequent surveillance among suspected animal reservoirs should be implemented to prevent repeated outbreaks. Repeated epidemics may result in a more lethal virus because of genetic recombination hence swift actions by the public health authorities is required. For the last 2 years, Healthcare professionals and the general public are successfully daily practicing airborne precautions and hand hygiene to curb spread of COVID‐19. Similar public education strategies can be used to control the monkeypox epidemic. COVID‐19 pandemic has highlighted the crucial role the research community plays in handling a public health emergency. As seen by the findings in this review, the information about monkeypox disease is still inadequate and fragmented, thus requiring urgent attention of research groups to successfully prevent another pandemic by a deadly virus.
5. CONCLUSION
The recent outbreak of Monkey in over 27 nonendemic countries is of great concern globally, especially following the COVID‐19 pandemic. The results of this review indicate that recent cases of Monkeypox have been occurring in adults and not only in children. The symptoms were as expected with a predominance of rash, fever, headache, upper respiratory symptoms and lymphadenopathy. Transmission of the disease can be either by contact with infectious sores, scabs, or body fluids or respiratory droplets, similarly to COVID‐19, therefore, similar preventative measures can prevent the disease from becoming epidemic/pandemic. Additionally, recent cases in MSM could suggest a sexual transmission of the virus which requires further indepth research to rule out. The findings of this review highlight that the information about monkeypox disease (clinical features and transmission) is still inadequate, thus necessitating further research to successfully prevent the spread of the virus.
AUTHOR CONTRIBUTIONS
Conceptualization: Vikash Jaiswal. Methodology: Vikash Jaiswal. Study Screening and data extraction: Mittal Savaliya. Formal analysis and investigation: Vikash Jaiswal, Dattatreya Mukherjee, Nitya Batra. Images and illustrations work: Mittal Savaliya, Vikash Jaiswal. Writing—original draft preparation: Vikash Jaiswal, Priyanshu Nain, Dattatreya Mukherjee, Amey Joshi, Mittal Savaliya, Angela Ishak, Nitya Batra, Nishan Babu Pokhrel. Writing—review and editing: Vikash Jaiswal, Angela Ishak, Dattatreya Mukherjee, Nishan.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENT
We want to thank Dipansha Maroo for her help during initial phase of manuscript preparation.
Jaiswal V, Nain P, Mukherjee D, et al. Symptomatology, prognosis, and Clinical findings of Monkeypox infected patients during COVID‐19 era: A Systematic‐review. Immun Inflamm Dis. 2022;10:e722. 10.1002/iid3.722
REFERENCES
- 1. World Health Organization . Multi‐country monkeypox outbreak: situation update. World Health Organization. Published 2022. Accessed June 17, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390
- 2. Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. In: Holbrook MR, ed., PLoS Negl Trop Dis. 2019;13(10):e0007791. 10.1371/journal.pntd.0007791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kozlov M. Monkeypox goes global: why scientists are on alert. Nature. 2022;606(7912):15‐16. 10.1038/d41586-022-01421-8 [DOI] [PubMed] [Google Scholar]
- 4. Mathieu E, Dattani S, Ritchie H, Roser M. Monkeypox. Our World in Data. 2022. Accessed June 17, 2022. https://ourworldindata.org/monkeypox
- 5. McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58(2):260‐267. 10.1093/cid/cit703 [DOI] [PubMed] [Google Scholar]
- 6. Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. In: Gromowski G, ed., PLoS Negl Trop Dis. 2022;16(2):e0010141. 10.1371/journal.pntd.0010141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153‐1162. 10.1016/S1473-3099(22)00228-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257. 10.3390/v12111257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vivancos R, Anderson C, Blomquist P, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022;27(22):2200422. 10.2807/1560-7917.ES.2022.27.22.2200422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaiswal V, Ishak A, Peng Ang S, et al. Hypovitaminosis D and cardiovascular outcomes: a systematic review and meta‐analysis. IJC Heart & Vasculature. 2022;40:101019. 10.1016/j.ijcha.2022.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaiswal V, Nepal G, Dijamco P, et al. Cerebral venous sinus thrombosis following COVID‐19 vaccination: a systematic review. J Prim Care Community Health. 2022;13:215013192210744. 10.1177/21501319221074450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eltvedt AK, Christiansen M, Poulsen A. A case report of monkeypox in a 4‐year‐old boy from the DR Congo: challenges of diagnosis and management. Case Rep Pediatr. 2020;2020:8572596. 10.1155/2020/8572596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costello V, Sowash M, Gaur A, et al. Imported monkeypox from international traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022;28(5):1002‐1005. 10.3201/eid2805.220292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yong SEF, Ng OT, Ho ZJM, et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826‐1830. 10.3201/eid2608.191387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammerschlag Y, MacLeod G, Papadakis G, et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 2022;27(22):2200411. 10.2807/1560-7917.ES.2022.27.22.2200411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eseigbe EE, Akude C, Osagie IA, Eseigbe P. Human monkey pox virus infection in Plateau State, North Central Nigeria: a report of two cases. West Afr J Med. 2021;38(12):1242‐1246. [PubMed] [Google Scholar]
- 18. Reynolds MG, Wauquier N, Li Y, et al. Human monkeypox in Sierra Leone after 44‐year absence of reported cases. Emerg Infect Dis. 2019;25(5):1023‐1025. 10.3201/eid2505.180832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hobson G, Adamson J, Adler H, et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021;26(32):2100745. 10.2807/1560-7917.ES.2021.26.32.2100745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whitehouse ER, Bonwitt J, Hughes CM, et al. Clinical and epidemiological findings from enhanced monkeypox surveillance in tshuapa province, democratic republic of the Congo during 2011–2015. J Infect Dis. 2021;223(11):1870‐1878. 10.1093/infdis/jiab133 [DOI] [PubMed] [Google Scholar]
- 21. Ngbolua K‐T‐N, Ngambika GK, Blaise M‐W‐M, et al. First report on three cases of monkey pox in Nord Ubangi Province (Democratic Republic of the Congo). bioex. 2020;2(1):120‐125. 10.33258/bioex.v2i1.117 [DOI] [Google Scholar]
- 22. Magnus P, , von Andersen EK , Petersen KB, Birch‐Andersen A. A pox‐like disease in cynomolgus monkeys. Acta Pathologica Microbiologica Scandinavica. 2009;46(2):156‐176. 10.1111/j.1699-0463.1959.tb00328.x [DOI] [Google Scholar]
- 23. Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in basankusu territory, democratic republic of the Congo. Bull World Health Organ. 1972;46(5):593‐597. [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . Monkeypox. World Health Organization. Published 2022. Accessed June 17 2022. https://www.who.int/health-topics/monkeypox
- 25. Centers for Disease Control and Prevention . Update: Multistate Outbreak of Monkeypox — Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Published 2003. Accessed June 17, 2022. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5227a5.htm
- 26. Moore M, Monkeypox ZF. StatPearls. StatPearls Publishing; 2022. Accessed June 17, 2022. http://www.ncbi.nlm.nih.gov/books/NBK574519/ [Google Scholar]
- 27. Hoff N, Doshi R, Colwell B, et al. Evolution of a disease surveillance system: an increase in reporting of human monkeypox disease in the democratic republic of the Congo, 2001‐2013. IJTDH. 2017;25(2):1‐10. 10.9734/IJTDH/2017/35885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen N, Li G, Liszewski MK, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46‐63. 10.1016/j.virol.2005.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakoune E, Lampaert E, Ndjapou SG, et al. A nosocomial outbreak of human monkeypox in the Central African Republic. Open Forum Infect Dis. 2017;4(4):ofx168. 10.1093/ofid/ofx168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berthet N, Nakouné E, Whist E, et al. Maculopapular lesions in the Central African Republic. The Lancet. 2011;378(9799):1354. 10.1016/S0140-6736(11)61142-2 [DOI] [PubMed] [Google Scholar]
- 31. Formenty P, Muntasir MO, Damon I, et al. Human monkeypox outbreak caused by novel virus belonging to Congo basin clade, Sudan, 2005. Emerg Infect Dis. 2010;16(10):1539‐1545. 10.3201/eid1610.100713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huhn GD, Bauer AM, Yorita K, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742‐1751. 10.1086/498115 [DOI] [PubMed] [Google Scholar]
- 33. Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38):1800509. 10.2807/1560-7917.ES.2018.23.38.1800509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erez N, Achdout H, Milrot E, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980‐983. 10.3201/eid2505.190076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yinka‐Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872‐879. 10.1016/S1473-3099(19)30294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(10):2661‐2672. 10.1099/vir.0.81215-0 [DOI] [PubMed] [Google Scholar]
- 37. World Health Organization . Monkeypox ‐ Democratic Republic of the Congo. World Health Organization. Published 2020. Accessed June 17, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/monkeypox-democratic-republic-of-the-congo
- 38. Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15‐25. 10.1016/s1473-3099(03)00856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monkeypox. Wkly Epidemiol Rec. 2011;86(41):448‐451. [PubMed] [Google Scholar]
- 40. Hutin YJF, Williams RJ, Malfait P, et al. Outbreak of human monkeypox, democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7(3):434‐438. 10.3201/eid0703.017311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomassen HA, Fuller T, Asefi‐Najafabady S, et al. Pathogen‐host associations and predicted range shifts of human monkeypox in response to climate change in Central Africa. In: Khudyakov YE, ed., PLoS ONE. 2013;8(7):e66071. 10.1371/journal.pone.0066071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakazawa Y, Mauldin M, Emerson G, et al. A phylogeographic investigation of African monkeypox. Viruses. 2015;7(4):2168‐2184. 10.3390/v7042168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis . Lab Invest. 2001;81(12):1581‐1600. 10.1038/labinvest.3780373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Centers for Disease Control and Prevention (CDC) . 2022. U.S. Monkeypox Cases. Centers for Disease Control and Prevention (CDC). Published June 16, 2022. Accessed June 17, 2022. https://www.cdc.gov/poxvirus/monkeypox/index.html
- 45. Parker S, Nuara A, Buller RML, Schultz DA. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2(1):17‐34. 10.2217/17460913.2.1.17 [DOI] [PubMed] [Google Scholar]
- 46. Moore ZS, Seward JF, Lane JM. Smallpox. The Lancet. 2006;367(9508):425‐435. 10.1016/S0140-6736(06)68143-9 [DOI] [PubMed] [Google Scholar]
- 47. Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293‐298. 10.1093/infdis/156.2.293 [DOI] [PubMed] [Google Scholar]
- 48. Breman JG. Monkeypox: an Emerging Infection for Humans? Michael Scheld W, Craig WA, Hughes JM, eds., Emerging Infections 4. ASM Press; 2014:45‐67. 10.1128/9781555816971.ch5 [DOI] [Google Scholar]
- 49. Lewis MW, Graham MB, Hammarlund E, Hanifin J, Slifka MK. Monkeypox without exanthem. N Engl J Med. 2007;356(20):2112‐2114. 10.1056/NEJMc062788 [DOI] [PubMed] [Google Scholar]
- 50. Brown K, Leggat P. Human monkeypox: current state of knowledge and implications for the future. TropicalMed. 2016;1(1):8. 10.3390/tropicalmed1010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nolen LD, Osadebe L, Katomba J, et al. Extended human‐to‐human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis. 2016;22(6):1014‐1021. 10.3201/eid2206.150579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grant R, Nguyen LBL, Breban R. Modelling human‐to‐human transmission of monkeypox. Bull World Health Organ. 2020;98(9):638‐640. 10.2471/BLT.19.242347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sah R, Abdelaal A, Reda A, et al. And its possible sexual transmission: where are we now with its evidence? Pathogens. 2022;Aug 17 11(8):924. 10.3390/pathogens11080924 PMID: 36015044; PMCID: PMC9414346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Girometti N, Byrne R, Bracchi M, et al. Epidemiological characteristics and clinical features of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, United Kingdom.: an observational analysis. Lancet Infect. Dis., 2022. 10.1016/S1473-3099(22)00411-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Antinori A, Mazzotta V, Vita S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27:2200421. 10.2807/1560-7917.ES.2022.27.22.2200421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bragazzi NL, Kong JD, Mahroum N, et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J Med Virol. 2022. 10.1002/jmv.27931 [DOI] [PubMed] [Google Scholar]
- 57. Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679‐691. 10.1056/NEJMoa2207323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


