Abstract
SARS-CoV-2 triggers a dysregulated innate immune system activation. As the mevalonate pathway (MVP) prevents the activation of inflammasomes and cytokine release and regulates endosomal transport, compromised signaling could be associated with the pathobiology of COVID-19. Prior transcriptomic studies of host cells in response to SARS-CoV-2 infection have not reported to date the effects of SARS-CoV-2 on the MVP. In this study, we accessed public data sets to report in silico investigations into gene expression. In addition, we proposed candidate genes that are thought to have a direct association with the pathogenesis of COVID-19, and which may be dependent on signals derived from the MVP. Our results revealed dysregulation of genes involved in the MVP. These results were not found when investigating the gene expression data from host cells infected with H3N2 influenza virus, H1N1 influenza virus, or respiratory syncytial virus. Our manually curated gene set showed significant gene expression variability in A549 cells infected with SARS-CoV-2, as per Blanco-Melo et al. data set (GSE147507). In light of the present findings, SARS-CoV-2 could hijack the MVP, leading to hyperinflammatory responses. Prompt reconstitution of this pathway with available agents should be considered in future studies.
Keywords: autophagy, COVID-19, endosomes, inflammation, mevalonate, SARS-CoV-2, statins, trained immunity
1 |. INTRODUCTION
In March 2020, a pandemic was declared after SARS-CoV-2 spread throughout the world, disrupting global health and economy. The rapid viral transmission has led to more than 40 million cases worldwide with over one million deaths as of November 2020.1 This novel virus has 79.5% similarity with the previous SARS-CoV. Although both viruses possess a spike protein able to bind ACE2 on the host cell, a unique furin cleavage site in the S protein was found in SARS-CoV-2, that allows for more efficient entry and infection.2 During severe infection, prominent lymphopenia and elevation of pro-inflammatory cytokines occur.3 Dysregulation of the innate immune system with macrophagic activation are characteristic of fulminant COVID-19, and have been attributed to lack of conventional dendritic cell type 1 (cDC1) maturation and presentation of HLA-DR.4,5
Innate immune activation is, at least in part, controlled by products of the mevalonate synthesis pathway (MVP). Intermediaries in this pathway enable prenylation of small GTPases. The downstream GTPases constitutively activate the PI3K cascade, which, in turn, represses the synthesis of toll-like receptor (TLR)-induced pro-inflammatory cytokines.6 Absence of geranylgeranylation of the GTPases triggers a hyperinflammatory environment, characterized by TLR-mediated constitutive release of pro-inflammatory cytokines derived from inflammasome activation.6 Therefore, it should be considered that the disruption of genes involved in the MVP as a consequence of SARS-CoV-2 infection could facilitate the clinical landscape seen in COVID-19.
Recently, a retrospective study suggested that statin use could improve the clinical outcome of patients admitted to the ICU.7 We previously proposed the use of aminobisphosphonates as stimulants of trained immunity, which could be utilized as prophylactics for COVID-19.8 Bisphosphonates block farnesyl diphosphate synthase (FDPS), an essential step for prenylation and cholesterol synthesis, promoting γδ T-cell expansion and modulating membrane expression in innate immune cells.
SARS-CoV-2 is internalized via autophagy-mediated endocytic pathways. Human coronaviruses replicate in the interior of double-membrane endosomes, preventing the activation of RIG1-like receptors.9 This may account for the 5-day latency period of SARS-CoV-2 infection.10 In addition, farnesylation intermediaries promote replication of other RNA viruses such as HIV-1 via GTPases.11 Hence, the MVP may determine the host response at major steps involved in the pathogenesis of COVID-19.
In this in silico study, we analyze publicly available gene expression data sets derived from models of SARS infection, and propose how the MVP could be involved in the pathogenesis of COVID-19. Data could suggest that defective expression of MVP intermediaries inhibits autophagy and prompts tissue inflammation following SARS-CoV-2 infection. Disease severity could be reduced by efficient and early reconstitution of this pathway5 or by use of MVP modulators.7,8
2 |. METHODS
In this in silico study, we analyzed publicly available gene expression data sets. For studying host response to SARS-CoV or SARS-CoV-2, data were accessed and obtained from Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under the terminology “SARS.”
Data analysis was done using R v.3.3.6 and v.4.0.2.
The following data sets were analyzed: GSE147507, GSE1739, GSE17400, GSE150392, GSE5972. A data set derived from host response to H3N2 influenza infection was found by literature search,12 and then accessed and analyzed (GSE135069).
For data sets containing raw counts, normalization was done using the function DGElist() from the “edgeR” package. Normalized counts were scaled with the scale() function.
The getBM() function from biomart package was used for platform to gene ID conversion.
The packages “ggsci” and “cowplot” were applied for computing the barplots generated in Figure 1.
FIGURE 1.
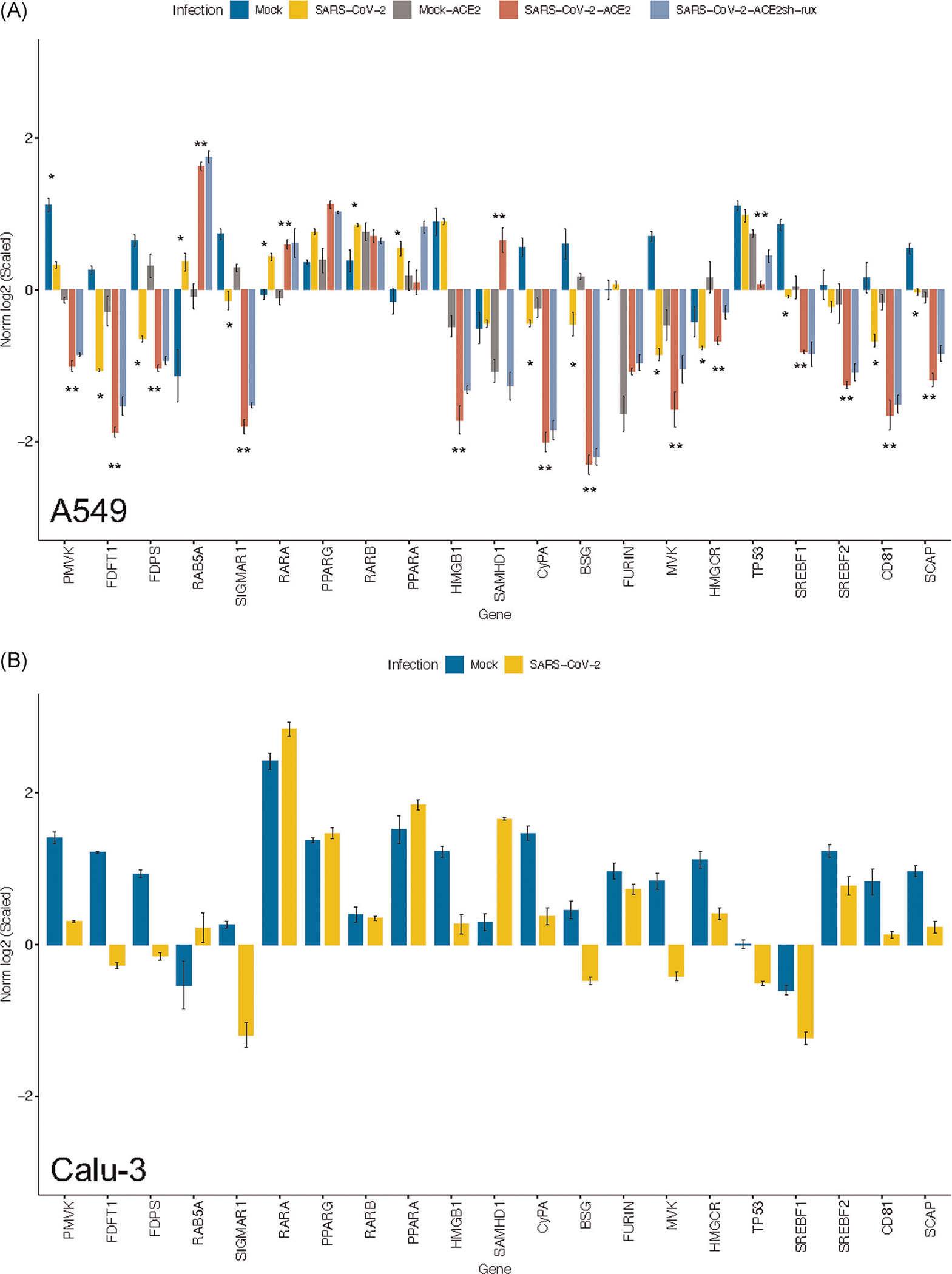
Dysregulation of candidate genes as a consequence of SARS-CoV-2 infection. Gene expression data were obtained from Blanco-Melo et al. (GSE147507). Candidate genes involved in the pathogenesis of COVID-19 were represented as scaled values. Gene expression was analyzed from (A) A549 and (B) Calu-3 cells. *p < .05 for Mock versus SARS-CoV-2; **p < .05 for Mock-ACE2 versus SARS-CoV-2-ACE2
The gene selection process for this manuscript was twofold. First, genes from the gene ontology (GO) signature prenyltransferase activity were retrieved from https://www.gsea-msigdb.org/, and used to analyze differences in expression of genes involved in the MVP. Then, a manually curated gene signature was developed based on our prior results, existing literature, and hypothesis generation. The data set GSE147507 was used for the analysis of gene expression in Figure 1, as it allowed the exploration of diverse cell lines, and the observation of the influence of ACE2 transduction in the host response to SARS-CoV-2.
Heatmaps were plotted using the ComplexHeatmap package. Heatmaps were plotted on a scaled format. The gene list represented was retrieved from the GO prenyltransferase activity.
The package ggpubr was used to compute statistical analyses and bar plotting. The default Wilcoxon t test analysis was computed to compare scaled expression values using the function stat_compare_means().
3 |. RESULTS
3.1 |. Expression variability in the MVP triggered by coronavirus infection but not by other respiratory viruses
The MVP controls innate immunity through constitutive signaling of downstream metabolites derived from mevalonic acid.6,13 To ascertain if the response to SARS infection could be due, at least in part, to direct disbalance of this pathway induced by coronavirus, we performed gene expression analyses of several genes involved in prenylation. Genes were part of the gene set GO prenyltransferase activity, available at https://www.gsea-msigdb.org/.
We first explored the gene expression profile derived from peripheral blood mononuclear cells (PBMCs), which were derived from patients who were admitted after being diagnosed with SARS during the 2003 epidemic.14 Gene expression profiling of this data set (GSE1739) revealed significant downregulation of the rate-limiting step in cholesterol synthesis, HMGCR (p < .05). PGGT1B, FNTA, and COX10 were also significantly downregulated (p < .005), indicating decreased prenylation of Rab and Ras groups upon coronavirus infection, as well as a possibly decreased Heme A synthesis (Figure S1A,B).
Next, we investigated a data set from Yoshikawa et al. (GSE17400).15 In this study, RNA was obtained from 2B4 (Calu-3 clone) cells infected with SARS-CoV or Dhori virus (DOHV), the latter orthomyxovirus used as control. Gene expression at 48-h post infection with a multiplicity of infection (MOI) of 0.1 revealed partial downregulation of genes such as FNTA (Ras farnesylation), RABGGTA, RABGGTB, PTAR (involved in transferring geranylgeranyl groups to cysteine at the C-terminus of Rab), and DHDDS (elongation of cis-prenyl chains for the production of dolichol), with upregulation of FNTB (Ras farnesylation), GGPS1 (geranylation of Rab GTPases) and COX10 (p = .1; Figure S1C,D).
A recent study published the transcriptomic response to SARS-CoV-2 by different cellular hosts, which included NHBE cells, A549, and Calu-3 cells.16 The strain USA-WA1/2020 was utilized. Gene expression profiling of this data set revealed downregulation of MVK, FDPS, and FDFT1 in SARS-CoV-2-infected samples. In A549 cells, downregulation was further induced by ACE2 transduction and an increased MOI (from 0.2 to 2). Human parainfluenza virus type 3 (HPIV3), influenza avian virus (IAV), and respiratory syncytial virus (RSV) were also used to infect A549 cells. Interestingly, no gene expression variability was seen after infection with these respiratory viruses, suggesting specific response to SARS-CoV-2 (Figure S2A,B).
To further expand our analysis, we accessed gene expression data from Sharma et al. (GSE150392).17 In this study, induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) were infected (SARS-CoV-2-USA-WA1/2020) for 72 h at an MOI of 0.1. Gene expression profiling revealed a trend in downregulation of FDFT1 (cholesterol synthesis), GGPS1 (geranylation of Rab GTPases), COQ2 (mitochondrial redox carrier), FDPS (synthesis of farnesyl diphosphate), COX10, FNTB (Ras prenylation), and MVK (Figure S3A,B).
To ascertain if the aforementioned dysregulation of the MVP during COVID-19 is shared by influenza infection, we analyzed the gene expression from tumor-free lung explants infected with the H3N2 virus (strain A/Panama/2007/1999; GSE135069). Using this data set, no differences in expression were found for any of the main genes involved in the MVP (Figure S4).
The summary of these analyses suggests that compromise of the MVP upon COVID-19 infection may account for the rare complications that occur in severe infections.
3.2 |. Inactivation of the MVP could be initiated by decreased SCAP/SREBF
Sterol regulatory binding proteins (SREBFs) are transcription factors that regulate lipid biosynthesis pathways, such as double-membrane vesicle formation and protein palmitoylation, both of which are critical for viral replication. Increased SREBF facilitates the propagation of different viruses, including MERS-CoV and influenza.18 SREBFs are modulated by the endoplasmic reticulum SREBF cleavage-activating protein (SCAP).19 SREBFs activate the MVP mainly through FDPS, although SREBF1 does so more potently.20
It has been shown that when lipid biosynthesis is stopped after TLR4 stimulation, SREBF1 increases to provide for compensatory lipid synthesis and provide strong anti-inflammatory signals that aid in the resolution of initial pro-inflammatory reaction.21 However, SREBF1 expression has also been reported to be involved in the direct activation of inflammasomes. On the contrary, SREBF2 is involved promoting autophagy.19
Gene expression analysis from GSE147507 revealed downregulation in SCAP, SREBF1, and SREBF2, which could constitute the initial step for decreasing the metabolic fate of the MVP (Figure 1). In this context, it appears that low SREBFs may limit viral entry and replication, but it is less clear whether it might also be involved in reduced control of pro-inflammatory events. Even though SREBF1 directly activates the NLRP inflammasome, its downregulation also involves decreasing the activity of the MVP. This effect could affect autophagosome clearance and secondary induction of pro-inflammatory events. Hence, further studies are required that investigate the connection between SREBF, mevalonate, and direct SARS-CoV-2 infection. Paradoxically, a recent study reported the opposite effect,22 where authors found elevated levels of SREBF2 in PBMCs from patients hospitalized for COVID-19, and its blockade seemed to prevent cytokine storm. It might also be of note to consider that depending on the disease stage, the consequences of SREBF inhibition might differ.
3.3 |. Host-derived autophagic responses derived from the MVP are reprogrammed in SARS-CoV-2 infection
Cellular entry of SARS-CoV-2 occurs through endolysosomal pathways,23 which are controlled by prenylated Rab GTPases.24 The presence of a furin cleavage site in the S protein leads to early viral endosomal fusion, which is RAB5-mediated (a prenylated small GTPase). As opposed to other coronaviruses, due to the presence of furin, late endosomal formation and lysosomal protease action are not needed for fusion. Downregulation of RAB5 is associated with a decrease in viral replication.23 In addition, although RAB5 modulation does not alter lysosomal function, it can suppress autophagy by activating mTOR. Analysis of expression data derived from a GSE147507 showed that RAB5 was upregulated in A549 and Calu-3 cells infected with SARS-CoV-2. RAB5 upregulation was enhanced by inhibition of IFN1β response with ruxolitinib (Figure 1). This suggests that proteins involved in viral internalization such as furin, basigin, or ACE2 may selectively enhance expression and geranylgeranylation of specific Rab proteins.
Autophagosome and lysosome fusion takes place as part of the autophagic clearance. For this process to occur, SIGMAR1 expression is needed. Decreased SIGMAR1 leads to the accumulation of autophagosomes and degradation of mitochondria within the cytoplasm.25 Activation of SIGMAR1 may also reduce dendritic cell-induced pro-inflammatory differentiation of Th1 and Th17 cells with decreased cytokine release.26 In addition, SRERBF1 and HMGCR are decreased more than two- and sixfold, respectively, upon knockout of SIGMAR1.27 Abrogation of SIGMAR1 expression after SARS-CoV-2 infection could be closely associated with perturbation of MVP activity. As expected, we found downregulation of SIGMAR1 in SARS-CoV-2-infected A549 and Calu-3 cells (Figure 1), further supporting the notion that SARS infection involves halting multiple steps surrounding cholesterol synthesis and prenylation.
3.4 |. A known restrictor of viral replication, SAMHD1, is upregulated in SARS-CoV-2
SAMHD1 acts as a primary restriction factor for viral replication during HIV infection in mononuclear cells.28 SAMHD1 expression is upregulated during IFN1-dependent responses in certain cell types via IRF3.29 SAMHD1 interacts with the tetraspanin CD81 to promote HIV reverse transcription, and colocalizes to early endosomes when CD81 is downregulated.30 CD81 constitutes a high-affinity binding receptor for hepatitis C virus (HCV), in conjunction with DC-SIGN, a mediator of SARS-CoV-2 entry in the dendritic cell.31 CD81 relies on the activity of the cholesterol synthesis pathway. It has been shown to mediate endosomal entry of HCV, suggesting a critical role of endosome formation on viral entry and replication.32
Analysis of A549 and Calu-3 cells from GSE147507 demonstrated downregulation of CD81 during SARS-CoV-2 infection, which was unaffected by IFN1β inhibition in A549 cells. SAMHD1 was upregulated in SARS-CoV-2-infected A549-ACE2 cells. This response was reverted with ruxolitinib. An upregulation of SAMHD1 was also seen in Calu-3 cells (Figure 1). Herein, low CD81 levels could be strongly linked to upregulation of SAMHD1, providing clues for innate control of viral transcription during acute infection.
3.5 |. Downregulation of CyPA and Basigin may be involved with an immune-deficient environment and self-limited virion entr
Cyclophilin A (PPIA), is an intra- and extracellular signaling mediator of chemotaxis that catalyzes the isomerization of the Pro211 residue in Basigin (BSG), also known as CD147. BSG facilitates SARS-CoV-2 cell entry.33 PPIA is also a known host receptor of cyclosporin A, an interleukin-2 inhibitor. Importantly, mevalonate blockade-dependent IFNγ release does not occur in the absence of IL-2,34 suggesting that lack of PPIA expression could explain poor IFNγ responses. PPIA also acts as an HIV replication enhancer after binding to the capsid and preventing its inactivation by TRIM5α.35 It also mediates liver fibrosis during HBV and HCV infection.36 On the contrary, BSG expression and its glycosylation are decreased after statin use, a mechanism that seems to be dolichol-dependent37 (Figure 1). Remarkably, the association between BSG expression and statin use could explain the improvement in the clinical resolution of COVID-19 seen among patients on statin use. This would further suggest that if given early on, statins could reduce the onset and severity of symptoms.
Therefore, we sought to analyze the expression of these proteins in GSE147507. We found that both PPIA and BSG were downregulated upon SARS-CoV-2 infection, both in A549 and Calu-3 cells. We speculate that downregulation of BSG may take place due to internalization of the receptor and posttranslational repression of BSG transcription. Downregulation of this gene could also be related to limited entry of more virions. Downregulation of PPIA could therefore be associated with the immunosuppressive environment that is found in patients with COVID-19.
3.6 |. Unbalanced systemic inflammation in COVID-19 could be characterized by lack of HMGB1 expression
HMGB1 is involved in monocyte maturation (mainly antigen-presenting cells) and resolution of inflammation.38 It has been reported that γδ T lymphocytes, which are activated by isopentenyl pyrophosphate, upregulate HMGB1 in monocytes. Aminobisphosphonates upregulate HMGB1 and enhance its secretion by the mononuclear cell.39 Innate memory can be induced by disease-associated molecular patterns (DAMPs) in a way by which sterile inflammation following tissue damage remains under control. In this regard, HMGB1 promotes trained immunity by acting as a DAMP. At the extracellular level, HMGB1 release helps in preventing cytokine damage following cell injury if bound to C1q, but can become pro-inflammatory in its free form.40 Therefore, HMGB1 expression could constitute a determinant of disease outcome in COVID-19. In addition, HMGB1 promotes the formation of autophagosomes when translocated to the cytoplasm from the nucleus.41 In this regard, HMGB1 depletion induced by SARS-CoV-2 could be involved in blunting autophagy and promoting pro-inflammatory events.
HMGB1 closely interacts with p53, forming a balanced regulation between apoptosis and survival. In normal conditions, their expression is mutually exclusive.42 p53 prevents viral replication; however, during SARS-CoV infection, downregulation of p53 occurs, enhancing viral replication.43 In the present analysis, we found HMGB1 to be downregulated following viral inoculation (Figure 1). The expression p53 was, in fact, downregulated as well in infected A549-ACE2 cells compared with their control. The effects of the downregulation of both genes need to be further elucidated.
3.7 |. Retinoid receptors could modulate trained immunity during SARS-CoV-2 infection
RAR and PPAR are heterodimeric partners of the retinoid X receptor (RXR) family, whose activity is regulated by the presence of retinoic acid (RA) or/and all-trans-retinoic acid (ATRA).44
RA maintains an immune tolerant state in dendritic cells by preventing induced expansion of Th17 cells.45 ATRA is involved in FoxP3+ Treg differentiation in the intestinal mucosa, hence participating in intestinal immune tolerance against pathogens and preventing pro-inflammatory events.46 RXR stimulation also promotes neutrophile expansion.47 ATRA attenuates airway inflammation by downregulating transcription factors that control Th2 and Th17 differentiation in a model of experimental asthma.48 In addition, RAR-α is necessary for CD4+ T-cell activation.45 ATRA constitutes a potent activator of RAR-α, and ATRA-induced differentiation may strongly rely upon farnesylation activity,49 which involves the MVP. Hence, the influence of prenylation in signaling derived from retinoid receptors may strongly influence immune cell maturation. Retinoids have also the ability to enhance IFN-1 responses, and have been proposed to be used as targets for COVID-19.50 However, host response in terms of retinoid receptor gene expression needs to be more extensively studied in the context of the SARS-CoV-2 pandemic.
Inhibition of the MVP with lycopene upregulates expression of RA receptor isoforms RAR-α and RAR-β in breast cancer cells.51 When upregulated, RARA and ATRA are capable of inhibiting chaperone-mediated autophagy, a process consisting of the translocation of cytosolic proteins that are delivered to the surface of lysosomes by hsc70, and then translocated into the lysosomal lumen by LAMP-2A.52
Another important partner of RXR is PPARγ, which is stimulated endogenously by farnesyl pyrophosphate in adipocytes.53 It has been shown that upon HMGCR knockdown, PPARG expression increases whereas PPARA expression is barely modified. Statins, in fact, reduce the expression of PPARG. HMGCR deficiency is also linked to severe hyperglycemia and apoptosis.54 However, data suggest that PPARγ also seems to increase autophagy to attenuate inflammatory responses.55
Given that RXRs might constitute a tight linkage between the MVP and lymphocyte maturation, we investigated the expression of these genes in GSE147507. We found significant upregulation of RARA upon SARS infection in A549, which was not blunted by IFN1β inhibition. A smooth increase was appreciated in Calu-3 cells. In addition, PPARG was barely upregulated in A549 cells after infection. Inhibition of IFN1β on infected cells appeared to further upregulate expression of both PPARA and PPARG.
Hence, RAR-α and PPARγ could also participate in the pro-inflammatory response of COVID-19, although their exact roles will need to be deciphered.
4 |. DISCUSSION
The response of the innate immune system to SARS-CoV-2 infection can be exuberant and unspecific, questioning how immune training could have been perturbed by COVID-19. In this study, we investigated the effects of SARS-CoV-2 on the MVP. None or few studies to date have reported the potential implications of mevalonate-dependent signals involved in direct SARS-CoV-2 infection. It is worth mentioning that while some studies have shown that SARS-CoV-2 does not infect A549 cells,56 these are widely used as they only require the expression of an ACE2 plasmid to allow viral entry and replication.16,56,57
In this study, we evaluated the transcriptomic response to COVID-19 in silico. We focused on mevalonate, as it constitutes a major orchestrator for autophagy-mediated monocyte differentiation and immune surveillance, and because alteration of trained immunity constitutes a causal trigger of dysregulated inflammatory response seen in COVID-19.4,58 The JAK–STAT pathway is involved in the pathogenic response to SARS-CoV-2, which could be stimulated by virus-induced IL-6 secretion. Secretion of this cytokine, as well as IL-1β and TNF-α, happen to be further enhanced by the interaction of the p38/MAPK pathway and NFκB. Excessive IL-6 is also correlated with lack of viral clearance, lack of T-CD4+ differentiation, and poor IFN response. Activation of MyD88 through TLR 3 and TLR 7/8 could also be involved in the degradation of IκB and activation of NFκB.59 Although these competing pathways are worth considering, it is beyond the scope of this manuscript.
Our analyses suggest that nonspecific inflammatory responses seen with COVID-19 are likely due to acutely blunted autophagy and induced immune suppression derived from abruptly decreased activity in the MVP. Even though our results are unsupported by direct experimentation, they may propose new experimental lines of investigation. There are multiple other cellular effects downstream of the MVP that could as well be considered, such as the generation of lipid rafts and by prenylation of other GTP-binding proteins not discussed in the present manuscript, but whose downregulation could compromise cell function, such as phosphorylation of PI3K/Akt/mTOR or MEK/Erk, pathways, which have already been discussed elsewhere.60,61 Nevertheless, we identify and bring together critical mediators of viral response that could be reliant on upstream MVP activity. These genes had not previously been discussed in conjunction.
First, we investigated the response of PBMCs to coronavirus infection. In this case, we identified the downregulation of important genes in the MVP upon infection of SARS-CoV. In vitro infectivity and metabolic response to SARS was also seen with lung epithelial cells (Calu-3), even with an MOI of 0.1. Further downregulation of some of the genes involved in the MVP was seen when higher MOIs were utilized in A549 cells (MOI 2). Primarily, MVK, FDFT1, and FDPS were downregulated. MVK is a key early enzyme involved in the pathway. In hyper-IgD syndrome, an inborn error of metabolism where this enzyme is deficient, a 10% decrease can cause severe attacks of sterile inflammation, whose clinical scenario may sometimes improve upon mevalonic acid supplementation.62 Nonetheless, inflammatory responses of this syndrome are not seen by only inhibiting the prenylation of Rabs.63 Inflammasome inhibitors might be considered candidates for refractory cases.64 FDPS and FDFT1 constitute downstream enzymes that may be responsible for cholesterol synthesis and prenylation of small GTPases (Figure 2). FDPS, in particular, constitutes the target for aminobisphosphonates, which were previously proposed by our group to be inducers of innate immune response and prophylactic agents against SARS-CoV-2.8 A recent study has suggested that PBMCs derived from COVID-19-infected patients possess increased MVP activity based on increased SREBF-2 expression. These results are validated by expression data (GSE5972) from PBMCs derived from SARS-infected patients in 2003, who during the post-ventilator use phase of disease experienced upregulation in genes involved in the MVP (Figure S5).65 It is important to note that the samples from this study were not matched. It is hypothetical, but likely, that this signature may account for noninfected, activated PBMCs responding to SARS infection.
FIGURE 2.
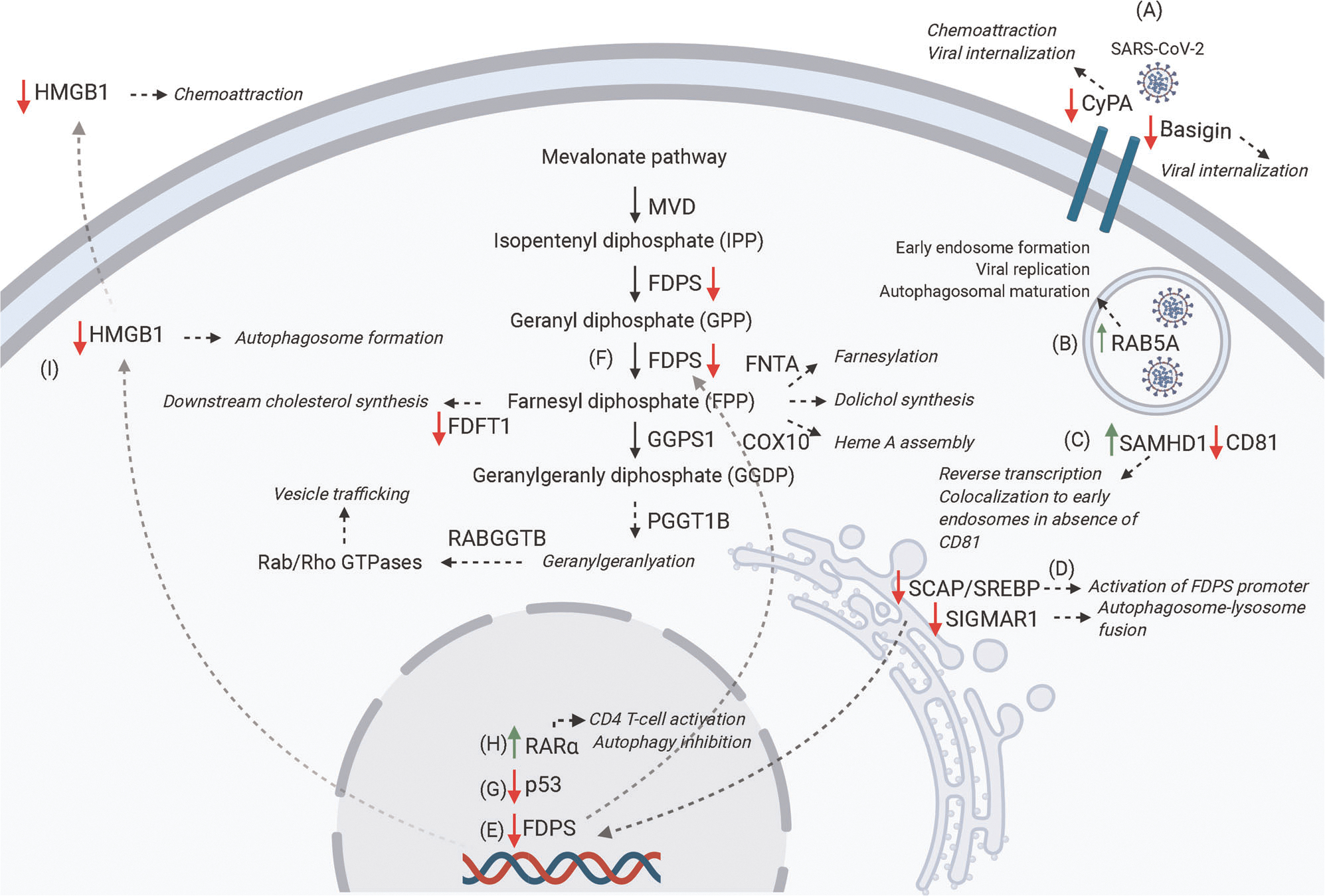
Schematic representation of proposed genes involved in SARS-CoV-2 pathogenesis based on GSE147507. (A) SARS-CoV-2 infection promotes downregulation of the membrane receptors CyPA and Basigin, both of which are involved in viral internalization. CyPA also contributes to chemotaxis. (B) SARS-CoV-2 upregulates Rab5A, which is involved in early endosome formation, viral replication, and autophagosomal maturation. (C) SARS-CoV-2 upregulates SAMHD1, which colocalizes to endosomes when CD81 expression is decreased. SAMHD1 is also involved in the reverse transcription of HIV. (D) SARS-CoV-2 downregulates SCAP/SREBP, which modulates the mevalonate pathway through activation of FDPS promoter (E). SARS-CoV-2 downregulates FDPS and FDFT1. These genes are involved in downstream geranyl/farnesylation and cholesterol synthesis, respectively (F). SARS-CoV-2 downregulates p53, which helps in preventing SARS-CoV replication (G). SARS-CoV-2 upregulates RAR-α, which is involved in CD4 T-cell activation and autophagy inhibition (H). SARS-CoV-2 downregulates HMGB1, which promotes autophagosome formation when present in the cytoplasm and has chemotactic activity when released extracellularly (I) (figure created with BioRender.com)
The fact that gene expression variability was not seen upon infection of either IAV, RSV, or HPIV3 (as per GSE147507) points to a more specific, coronavirus-related metabolic reprogramming, perhaps a provocative event could occur upon binding of the Spike protein to ACE2, furin, or BSG/CyPA. Additionally, it has been shown that as opposed to influenza or other common respiratory viruses, SARS-CoV-2 does not trigger the activation of interferon-stimulating genes. Rather, SARS-CoV-2 induces the release of chemokine ligands (CXCL), as well as IL-6 and IL-1.16 It is possible that polymorphisms in the S protein of SARS viruses lead to enhanced endosomal viral uptake mediated by RAB5 (Figure 2). Early endosomal latency would account for several days of asymptomatic prodrome. Viral interaction with BSG’s extracellular domain may potentially occur early on during the disease.
Studies have reported an increased incidence of nonobstructive ST-elevation myocardial infarction with high variability of presentation.66 New-onset heart conduction abnormalities and myocarditis also constitute a hallmark of the current pandemic.67,68 Our data on hiPSC-CMs suggests that direct viral infection of myocardial cells might diminish the expression of critical genes in the MVP. Downregulation of some of these genes may ultimately account for decreased autophagy and enhanced inflammasome activation in these tissues. We propose this to be a mechanism of myocardial injury during the course of the infection, which needs to be taken into consideration.
SARS-CoV-2 appears to decrease the expression of SCAP/SREBP based on in silico data. This could account as the main mechanism for downregulation of the MVP, primarily MVK, FDFT1, and FDPS. SCAP/SREBP signaling could be halted as a consequence of SIGMAR1 downregulation, although the opposite could also be held true (Figure 2). Nevertheless, this reciprocal cascade of events seems to inevitably lead to the accumulation of intracellular mitophagosomes and autophagosomes that are accompanied by deficient IFN1β and prominent cytokine secretion in an immune-adaptive-deficient microenvironment.
Infected cells could be HMGB1-deficient, as suggested in the present study. Inactivation of HMGB1 during the latent phase could remain an important cause for the immune-deficient environment seen in COVID-19. Lack of HMGB1 may prevent effective autophagy, dendritic cell maturation, and Th1 activation. Aminobisphosphonates upregulate surface expression of HMGB1 on γδ T cells.39 The fact that SARS-CoV-2 drastically diminishes the expression of HMGB1 further supports that aminobisphosphonates could be candidates for prophylactic treatment against COVID-19.8 Importantly, HMGB1 secretion has been shown to be suppressed after statin use in models of vascular inflammation.69 The signaling mechanisms linking HMGB1 expression and the MVP, however, still remain to be elucidated. Nevertheless, activation of both HMGB1 and the MVP is highly dependent upon expression of p53,70,71 whose expression also appeared to be decreased in SARS (Figure 2).43
Finally, the expression of nuclear retinoid receptors seems to increase after SARS-CoV-2 infection (Figure 2). This could decrease sensitivity to retinoids, and therefore, infected immature cells could lack the ability for differentiation. On the contrary, increased RAR-α could enhance sterile neutrophilia in the context of poor MVP signaling. However, this study is limited to the generation of hypotheses based on reported data, so that these biological observations may be experimentally tested.
This study has limitations. First, the manuscript is limited to the analysis of gene expression derived from publicly deposited data sets. It does not provide with an experimental methodology that supports the main results. The interpretation of these results is also speculative but intended to generate a testable hypothesis. Nevertheless, the current clinical knowledge of COVID-19 disease further is in line with our postulates.
In summary, this study suggests that SARS-triggered inflammatory response might be mediated, at least in part, by the MVP. Agents able to modulate this pathway might be considered as potential treatments to prevent acute illness and its complications.
Supplementary Material
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
DATA AVAILABILITY STATEMENT
The data sets used in this study are openly available in Gene Expression Omnibus: GSE17400, GSE1739, GSE147507, GSE135069, GSE150392, GSE5972.
REFERENCES
- 1.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard.
- 2.Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117(21):11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou R, To KKW, Wong YC, et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53:864–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akula MK, Shi M, Jiang Z, et al. Control of the innate immune response by the mevalonate pathway. Nat Immunol. 2016;17(8):922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang XJ, Qin JJ, Cheng X, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32(2):176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brufsky A, Marti JLG, Nasrazadani A, Lotze MT. Boning up: amino-bisphophonates as immunostimulants and endosomal disruptors of dendritic cell in SARS-CoV-2 infection. J Transl Med. 2020;18(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prentice E, Jerome WG, Yoshimori T, Mizushima N, Denison MR. Coronavirus replication complex formation utilizes components of cellular autophagy. J Biol Chem. 2004;279(11):10136–10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020; 172(9):577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amet T, Nonaka M, Dewan MZ, et al. Statin-induced inhibition of HIV-1 release from latently infected U1 cells reveals a critical role for protein prenylation in HIV-1 replication. Microbes Infect. 2008;10(5):471–480. [DOI] [PubMed] [Google Scholar]
- 12.Matos AR, Wunderlich K, Schloer S, et al. Antiviral potential of human IFN-α subtypes against influenza A H3N2 infection in human lung explants reveals subtype-specific activities. Emerg Microbes Infect. 2019;8(1):1763–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekkering S, Arts RJW, Novakovic B, et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell. 2018;172(1–2):135–146. [DOI] [PubMed] [Google Scholar]
- 14.Reghunathan R, Jayapal M, Hsu LY, et al. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol. 2005;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshikawa T, Hill TE, Yoshikawa N, et al. Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection. PLOS One. 2010;5(1):e8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Garcia G Jr., Wang Y, et al. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med. 2020;1(4):100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan S, Chu H, Chan JFW, et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat Commun. 2019;10(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology—divergent pathophysiology. Nat Rev Endocrinol. 2017;13(12):710–730. [DOI] [PubMed] [Google Scholar]
- 20.Amemiya-Kudo M, Shimano H, Hasty AH, et al. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res. 2002;43(8):1220–1235. [PubMed] [Google Scholar]
- 21.Oishi Y, Spann NJ, Link VM, et al. SREBP1 contributes to resolution of pro-inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 2017;25(2):412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee W, Ahn JH, Park HH, et al. COVID-19-activated SREBP2 disturbs cholesterol biosynthesis and leads to cytokine storm. Signal Transduct Target Ther. 2020;5(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkard C, Verheije MH, Wicht O, et al. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLOS Pathog. 2014;10(11):e1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langemeyer L, Frohlich F, Ungermann C. Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol. 2018;28(11):957–970. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Shen H, Li J, Guo LW. SIGMAR1/Sigma-1 receptor ablation impairs autophagosome clearance. Autophagy. 2019; 15(9):1539–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajnavolgyi A P4–246: activation of the Sigma-1 receptor by specific ligands inhibits human inflammatory dendritic cell functions and effector T-lymphocyte responses. Alzheimer’s Association International Conference 2014;10(4S):P876–P. [Google Scholar]
- 27.Tsai SY, Rothman RK, Su TP. Insights into the Sigma-1 receptor chaperone’s cellular functions: a microarray report. Synapse. 2012;66(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laguette N, Sobhian B, Casartelli N, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474(7353):654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Zhan Y, Zhou Y, et al. Interferon regulatory factor 3 is a key regulation factor for inducing the expression of SAMHD1 in antiviral innate immunity. Sci Rep. 2016;6:29665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocha-Perugini V, Suárez H, Álvarez S, et al. CD81 association with SAMHD1 enhances HIV-1 reverse transcription by increasing dNTP levels. Nat Microbiol. 2017;2(11):1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brufsky A, Lotze MT. DC/L-SIGNs of hope in the COVID-19 pandemic. J Med Virol. 2020;92:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81(1):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke Wang WC, Yu-Sen Zhou, Jian-Qi Lian, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. Biorxiv. 2020. [Google Scholar]
- 34.Gruenbacher G, Gander H, Nussbaumer O, Nussbaumer W, Rahm A, Thurnher M. IL-2 costimulation enables statin-mediated activation of human NK cells, preferentially through a mechanism involving CD56+ dendritic cells. Cancer Res. 2010;70(23):9611–9620. [DOI] [PubMed] [Google Scholar]
- 35.Selyutina A, Persaud M, Simons LM, et al. Cyclophilin A prevents HIV-1 restriction in lymphocytes by blocking human TRIM5α binding to the viral core. Cell Rep. 2020;30(11):3766–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naoumov NV. Cyclophilin inhibition as potential therapy for liver diseases. J Hepatol. 2014;61(5):1166–1174. [DOI] [PubMed] [Google Scholar]
- 37.Sasidhar MV, Chevooru SK, Eickelberg O, Hartung HP, Neuhaus O. Downregulation of monocytic differentiation via modulation of CD147 by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. PLOS One. 2017;12(12):e0189701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81(1):84–91. [DOI] [PubMed] [Google Scholar]
- 39.Ciucci A, Gabriele I, Percario ZA, Affabris E, Colizzi V, Mancino G. HMGB1 and cord blood: its role as immuno-adjuvant factor in innate immunity. PLOS One. 2011;6(8):e23766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soloff AC, Lotze MT. A peaceful death orchestrates immune balance in a chaotic environment. Proc Natl Acad Sci USA. 2019;116(46):22901–22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorgulho CM, Romagnoli GG, Bharthi R, Lotze MT. Johnny on the spot-chronic inflammation is driven by HMGB1. Front Immunol. 2019;10:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livesey KM, Kang R, Vernon P, et al. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012;72(8):1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma-Lauer Y, Carbajo-Lozoya J, Hein MY, et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc Natl Acad Sci USA. 2016;113(35):E5192–E5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: new insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008;582(1):32–38. [DOI] [PubMed] [Google Scholar]
- 45.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204(8):1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duong V, Rochette-Egly C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim Biophys Acta. 2011;1812(8):1023–1031. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Zhang Y, Liu Q, Zhong W, Xia Z. All-trans retinoic acid attenuates airway inflammation by inhibiting Th2 and Th17 response in experimental allergic asthma. BMC Immunol. 2013;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gueddari-Pouzols N, Duriez P, Chomienne C, Trussardi A, Jardillier JC. Interaction between mevalonate pathway and retinoic acid-induced differentiation. J Biomed Biotechnol. 2001;1(3):108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trasino SE. A role for retinoids in the treatment of COVID-19? Clin Exp Pharmacol Physiol. 2020;47(10):1765–1767. [DOI] [PubMed] [Google Scholar]
- 51.Chalabi N, Delort L, Satih S, Dechelotte P, Bignon YJ, Bernard-Gallon DJ. Immunohistochemical expression of RARα, RARβ, and Cx43 in breast tumor cell lines after treatment with lycopene and correlation with RT-QPCR. J Histochem Cytochem. 2007;55(9):877–883. [DOI] [PubMed] [Google Scholar]
- 52.Anguiano J, Garner TP, Mahalingam M, Das BC, Gavathiotis E, Cuervo AM. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol. 2013;9(6):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goto T, Nagai H, Egawa K, et al. Farnesyl pyrophosphate regulates adipocyte functions as an endogenous PPARγ agonist. Biochem J. 2011;438(1):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeh YS, Jheng HF, Iwase M, et al. The mevalonate pathway is indispensable for adipocyte survival. iScience. 2018;9:175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assumpcao JAF, Magalhaes KG, Correa JR. The role of pparγ and autophagy in ROS production, lipid droplets biogenesis and its involvement with colorectal cancer cells modulation. Cancer Cell Int. 2017;17:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harcourt J, Tamin A, Lu X, et al. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg Infect Dis. 2020;26(6):1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mossel EC, Huang C, Narayanan K, Makino S, Tesh RB, Peters CJ. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J Virol. 2005;79(6):3846–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Netea MG, Giamarellos-Bourboulis EJ, Domínguez-Andrés J, et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 Infection. Cell. 2020;181(5):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Battagello DS, Dragunas G, Klein MO, Ayub ALP, Velloso FJ, Correa RG. Unpuzzling COVID-19: tissue-related signaling pathways associated with SARS-CoV-2 infection and transmission. Clin Sci (Lond). 2020;134(16):2137–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramaiah MJ. mTOR inhibition and p53 activation, microRNAs: the possible therapy against pandemic COVID-19. Gene Rep. 2020;20:100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cai Y, Liu Y, Zhang X. Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J Virol. 2007;81(2):446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stabile A, Compagnone A, Napodano S, Raffaele CG, Patti M, Rigante D. Mevalonate kinase genotype in children with recurrent fevers and high serum IgD level. Rheumatol Int. 2013;33(12):3039–3042. [DOI] [PubMed] [Google Scholar]
- 63.Jurczyluk J, Munoz MA, Skinner OP, et al. Mevalonate kinase deficiency leads to decreased prenylation of Rab GTPases. Immunol Cell Biol. 2016;94(10):994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeyaratnam J, Frenkel J. Management of mevalonate kinase deficiency: a pediatric perspective. Front Immunol. 2020;11:1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cameron MJ, Ran L, Xu L, et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81(16):8692–8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med. 2020;382(25):2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crotti L, Arbelo E. COVID-19 treatments, QT interval, and arrhythmic risk: the need for an international registry on arrhythmias. Heart Rhythm. 2020;17:1423–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu M, Yu Y, Jiang H, et al. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(−/−) mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol Sin. 2013;34(6):830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moon SH, Huang CH, Houlihan SL, et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell. 2019;176(3):564–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang R, Zhang Q, Zeh HJ 3rd, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clin Cancer Res. 2013;19(15):4046–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used in this study are openly available in Gene Expression Omnibus: GSE17400, GSE1739, GSE147507, GSE135069, GSE150392, GSE5972.


