Abstract
Aims
To investigate the effectiveness of a 3-year worksite lifestyle intervention on cardiovascular metrics and to study whether outcomes are influenced by baseline subclinical atherosclerosis (SA) by non-invasive imaging.
Methods and results
A randomized controlled trial was performed to compare a lifestyle intervention with standard of care in asymptomatic middle-aged subjects, stratified by SA. The intervention consisted of nine motivational interviews during the first year, followed by three further sessions between Years 1 and 3. The primary outcome was the change in a pre-specified adaptation of the Fuster-BEWAT score (Blood pressure, Exercise, Weight, Alimentation, and Tobacco) between baseline and follow-up Years 1–3. A total of 1020 participants (mean age 50 ± 4 years) were enrolled, of whom 510 were randomly assigned to the intervention and 510 to the control group. The baseline adapted Fuster-BEWAT score was 16.2 ± 3.7 points in the intervention group and 16.5 ± 3.5 points in the control group. At Year 1, the score improved significantly in intervention participants compared with controls [estimate 0.83 (95% CI 0.52–1.15) points]. However, intervention effectiveness decreased to non-significant levels at Year 3 [0.24 (95% CI –0.10 to 0.59) points]. Over the 3-year period, the intervention was effective in participants having low baseline SA [0.61 (95% CI 0.30–0.93) points] but not in those with high baseline SA [0.19 (95% CI –0.26 to 0.64) points].
Conclusion
In middle-aged asymptomatic adults, a lifestyle intervention was associated with a significant improvement in cardiovascular health and behavioural metrics. The effect attenuated after 1 year as the intensity of the intervention was reduced.
Trial registration
ClinicalTrials.gov (NCT02561065).
Keywords: Lifestyle intervention, Subclinical atherosclerosis, Cardiovascular, Randomized controlled trial
Structured Graphical Abstract
Structured Graphical Abstract.
Left pannel: Summary of the design and primary outcome of the TANSNIP-PESA trial. Right upper pannel: Worksite lifestyle intervention components. Right lower pannel: Primary outcome results.
BEWAT, Blood pressure, Exercise, Weight, Alimentation, and Tobacco; CVD, cardiovascular disease; PESA, Progression of Early Subclinical Atherosclerosis; TANSNIP, Trans-Atlantic Network to study Stepwise Non-invasive Imaging as a tool for CVD Prognosis and prevention; SA, subclinical atherosclerosis.
See the editorial comment for this article ‘Primary lifestyle intervention: the challenge of making a difference’, by Christian Torp-Pedersen et al., https://doi.org/10.1093/eurheartj/ehac376.
Introduction
Cardiovascular disease (CVD) is the leading healthcare burden in the world and is a major contributor to reduced quality of life.1 In addition to causing premature deaths and morbidity, CVD also presents an overwhelming economic burden for society and healthcare systems.2 There is therefore an urgent need for preventive strategies to tackle this worrisome and growing public health problem.3 A large part of the CVD burden is attributable to modifiable, lifestyle-related risk factors, such as smoking, low physical activity (PA), high sedentary time, and poor dietary pattern.1,4–6 Lifestyle interventions, whether individual or population-based, are among the recommended strategies for cardiovascular (CV) health promotion and risk factor control.3,7,8 However, evidence supporting the benefit of such interventions, particularly in the context of primary prevention, is limited.9–12 The workplace is a promising setting for the implementation of CVD prevention programmes, and several worksite health promotion strategies have been tested, focusing on improving PA, dietary pattern, sedentary behaviour, or smoking status.13–18 Nevertheless, most of these studies are relatively small trials with short-term intervention programmes and follow-ups and their effectiveness has been mixed. Opinion in the field has thus recognized the need for evidence from larger high-quality randomized controlled trials (RCTs).12–14
The Progression of Early Subclinical Atherosclerosis (PESA) is an ongoing prospective cohort study examining imaging, biological, and behavioural parameters associated with the presence and progression of early subclinical atherosclerosis (SA) in mid-life.19,20 Working with a PESA sub-cohort, here, we conducted an RCT to assess whether a 3-year worksite lifestyle intervention was able to improve CV health metrics. The primary objective was to assess the effectiveness of the lifestyle intervention on a CVD risk and lifestyle score over 3 years. Additionally, we hypothesized that awareness of the SA burden would lead to more favourable changes in those participants having a high baseline SA burden by creating a greater sense of urgence about changing their behaviour, in order to slow down the progression of their already existing SA disease (awareness of the disease as the motivational factor). Our secondary objective was to evaluate whether the effectiveness of the lifestyle intervention differed between participants with either a high or a low baseline SA burden (evaluated with multi-territorial non-invasive imaging modalities).
Methods
Study design
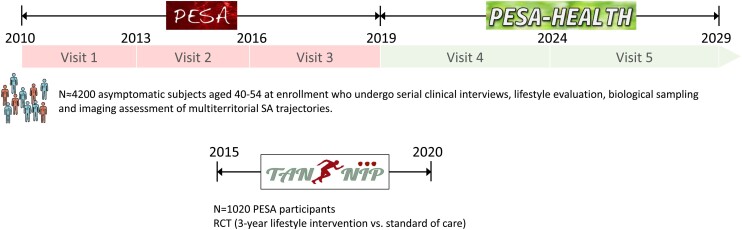
The design and rationale of the TANSNIP (Trans-Atlantic Network to study Stepwise Non-invasive Imaging as a tool for CVD Prognosis and prevention)-PESA study have been published previously.21 Briefly, the study is an RCT including middle-aged asymptomatic subjects from the PESA cohort in Spain in whom SA was assessed by non-invasive imaging of the carotid, iliofemoral, and aortic arteries using vascular ultrasound as well as by coronary artery calcification with computed tomography (CT)19,20 (Figure 1). The study was approved by the Instituto de Salud Carlos III (ISCIII) ethics committee, and all eligible participants gave written informed consent. An overview of the PESA design, together with the Consolidated Standards of Reporting Trials (CONSORT) checklist can be found in the Supplementary material online, Appendix.
Figure 1.
Schematic representation and timeline of the PESA and PESA-HEALTH studies and the present TANSNIP-PESA RCT nested within the PESA cohort. PESA, Progression of Early Subclinical Atherosclerosis; RCT, randomized controlled trial; SA, subclinical atherosclerosis; TANSNIP, Trans-Atlantic Network to study Stepwise Non-invasive Imaging as a tool for CVD Prognosis and prevention.
Participant selection
All PESA participants (N = 4184, aged 40–54 years at inclusion) were screened to participate in this RCT, and those showing interest in joining were scheduled for a first visit with the study nurse to give written informed consent and to undergo baseline assessments. Participants were included in the study if they had completed all baseline TANSNIP-PESA assessments (wearing an activPAL PA monitor for 7 consecutive days, providing a blood sample, and completing the study questionnaires) and had valid imaging results from PESA for stratification into the low or high SA study subgroups.21 It was a requirement to have undergone all baseline evaluations before randomization in order to avoid the baseline questionnaire responses to be influenced by treatment allocation. High SA was defined as being in the highest plaque thickness tertile on vascular ultrasound and/or having any coronary artery calcification on cardiac CT.19 Low SA was defined as being in the two lowest plaque thickness tertiles on vascular ultrasound and having no calcified coronary atherosclerosis [coronary artery calcium score (CACS) = 0]. All study participants were informed of their atherosclerosis burden on written reports.
Additionally to the general exclusion criteria for the PESA study (previous CVD, cancer, or any other disease expected to shorten life span or influence protocol adherence),19 subjects with no plaque burden, a normal body mass index (BMI, 18.5–25 kg/m2),22 and a healthy lifestyle were also excluded from this RCT. This criterion was set in order to include participants who had at least one non-ideal CV behaviour to ensure certain room for improvement.
Randomization
After completing baseline measurements, participants were randomized 1:1 to receive the lifestyle intervention at their workplace or standard care (usual care at the discretion of their occupational physician and other primary care providers). Randomization was performed using stratified computerized fixed blocks with SA burden as the stratification variable and a block size of 10. Group allocation was performed by the study nurse.
Procedures
The lifestyle intervention was systematically designed using a socioecological approach and has been described in detail elsewhere.21 In summary, the intervention had three objectives: to increase daily PA, reduce sedentary time, and promote a healthier (i.e. Mediterranean) dietary pattern. The intervention consisted of 12 individual motivational interview sessions delivered over a 3-year period (nine motivational interview sessions during the first year, followed by three sessions in Years 1–3). Also, participants allocated to the intervention arm received a wrist-worn PA tracker and were offered to have a sit–stand workstation installed at their workplace.
Motivational interviewing is an individual-centred counselling style aimed at eliciting behavioural change that has shown to improve adherence to lifestyle intervention programmes in different contexts.23–25 In TANSNIP-PESA, the participant and interviewing psychologist agreed on a plan of action that was developed thereafter. The first seven motivational interview sessions (each lasting 1 h) were structured around personalized lifestyle and behavioural change modules (PA, sedentary behaviour, dietary pattern, and smoking) and took place every second week during the first 3 months after inclusion [see detailed timing and content of the lifestyle intervention as well as the TIDieR (Template for Intervention Description and Replication) guidelines in the Supplementary material online, Appendix]. Sessions 8–12 (30-min duration) focused on the maintenance of healthy behaviours acquired through the programme and took place at 5, 10, 16, 22, and 30 months. Sessions were delivered by three psychologists trained in motivational interviewing and who received expert feedback on their adherence to motivational interviewing techniques every 6 months.
During Session 2, participants received a Fitbit Flex PA tracker to facilitate PA self-monitoring and goal setting. Around the time of Session 3, participants in the intervention arm were also offered an Ergotron sit–stand workstation. This device was on request installed in participants’ workspaces, allowing them to alternate between sitting and standing while working at their desk (further information in the Supplementary material online, Appendix).
Outcomes
The primary outcome measure was the change in a pre-specified adapted version of the Fuster-BEWAT score between baseline and Years 1–3. The Fuster-BEWAT score is a composite measurement consisting of Blood pressure (BP), Exercise, Weight, Alimentation, and Tobacco. This score is a simple, non-invasive tool for monitoring lifestyle behaviours and CVD risk,26 and has been internally validated against the ideal CV health score in the PESA cohort, and externally validated in the Northeast China Rural Cardiovascular Health study population.27,28 Moreover, this tool has been used to assess outcomes after lifestyle interventions across diverse populations such as parents or caregivers of children from a socioeconomically disadvantaged community in the New York City, or low–middle-income individuals from the island of Grenada.26,29,30 For the current TANSNIP-PESA study, an adapted version of the original Fuster-BEWAT score was pre-established as the primary outcome.21 The reasons to adapt the original score were to enable the detection of relevant changes in the PESA cohort (a homogeneous physically active population with overall low–intermediate CVD risk20) and to incorporate sedentary time, which is one of the three target behaviours of the TANSNIP-PESA intervention. The adapted Fuster-BEWAT score consists of BP, Exercise (objectively measured PA and sedentary time), Weight (BMI), Alimentation (fruit and vegetable consumption), and Tobacco. Scoring of each of the components is shown in Supplementary material online, Table S1. The score ranges from 0 (poor CV health) to 24 (ideal CV health) and is the sum of the scores for the individual components (0–4 points each).
Secondary outcomes included the original Fuster-BEWAT score, individual components of the adapted score, anthropometric measurements, blood biomarkers, psychosocial measures, and work-related outcomes. Separate analyses will assess the cost-effectiveness and feasibility of the programme, as well as the degree of compliance with the intervention by process evaluation at the following three levels: the participant, the psychologist, and other involved stakeholders.
Each participant received four worksite medical follow-up visits: at baseline, and at 1–3 years. Each visit included anthropometric assessments (i.e. body height, body weight, and waist circumference), BP measurement, placement of an activPAL PA monitor, drawing a fasting blood sample, and completion of the study questionnaires. These visits took place at the Banco Santander Headquarters or at the CNIC facilities in Madrid. Outcomes were assessed by PESA technicians or nurses, who were not blinded to participant allocation arm. Nevertheless, all assessments were performed according to previously described standardized protocols (details on data collection are provided in the Supplementary material online, Appendix).19,21 Blinding to allocation arm was likewise not possible for participants, the TANSNIP-PESA coordinator, or the intervention psychologists. Study statisticians were blinded to the allocation arm until the data were analysed [see Statistical Analysis Plan (SAP) in the Supplementary material online, Appendix].
PA and sedentary time were objectively measured with an activPAL activity monitor (PAL Technologies Limited, Glasgow, UK), which was attached during the visit to the participant’s thigh, worn for at least 7 consecutive full days, and then returned to the TANSNIP staff via the Bank’s internal mail system. ActivPAL data were post-processed to calculate objective PA (number of steps/day) and sedentary time [defined as any waking behaviour characterized by an energy expenditure ≤1.5 metabolic equivalents of task (METs) while in a sitting, reclining, or lying posture]. Dietary pattern, including fruit and vegetable consumption, was measured using the Mediterranean Diet Adherence Screener (MEDAS) score from the PREDIMED trial.31 Tobacco consumption was assessed with a self-report questionnaire. Other self-reported behavioural and psychosocial parameters were collected through online questionnaires completed after each check-up. Cardiometabolic biomarkers were measured in fasting blood samples. A more detailed description of the procedures is included in the Supplementary material online, Appendix.
Statistical analysis
Using a stratified analysis and assuming a different treatment effect between low and high SA participants, the study was powered to detect between-group relative changes in the adapted Fuster-BEWAT score of 6.1% in the low SA subgroup and 10.9% in the high SA subgroup (low SA participants were expected to have a smaller treatment effect, whereas high SA participants were expected to have a larger treatment effect). This required 590 and 260 participants in the low and high SA subgroups, respectively (with 80% power and two-sided α=0.05), and within these subgroups in a 1:1 ratio for the intervention and control groups.21 All effectiveness analyses were performed according to the intention-to-treat principle. Data are expressed as mean (standard deviation) of non-missing values for continuous variables and as frequencies and percentages for categorical variables. For the primary outcome analysis, measurements at each follow-up time point were analysed longitudinally by repeated linear mixed model analysis. The regression model included the outcome variable measured at the different follow-up measurements adjusted for the baseline value of the outcome. A random intercept at the individual level was added to deal with correlated observations within the individual.32 Interaction tests in the primary outcome model were performed to determine whether the intervention effectiveness was not homogeneous by age or gender.
Furthermore, we carried out an intention-to-treat analysis of the primary outcome (adapted Fuster-BEWAT score) after multiple imputation for missing data by the monotone imputation method including all randomized participants with valid data at baseline, as pre-established in the SAP. The adapted Fuster-BEWAT score values at follow-ups were set as imputed dependent variables. The following covariates were used for the imputation approach: age (continuous variable), sex (binary variable), risk group (binary variable), intervention group (binary variable), and baseline adapted Fuster-BEWAT score (continuous variable). Missing data were assumed to be missing at random. Complete covariates information was available for all randomized enrolled participants. The number of iterations was set at 50.
Secondary outcomes were analysed with linear mixed effect regression models (or logistic models where applicable), where the outcome was the intention-to-treat dependent variable, and where the group (intervention vs. standard care) was modelled as an independent variable, and values were adjusted to the baseline outcome value. The 3-year follow-up was prioritized to ensure a high retention rate. Due to logistical reasons (extension of the inclusion period that resulted in overlapping yearly follow-up visits and lack of capacity to perform these visits simultaneously), the percentage of participants with missing data at the second-year follow-up was higher than expected. For this reason, the SAP pre-established that it would be inadequate to evaluate Year-2 measurements separately, as an independent outcome. However, information about outcomes at Year 2 was included in the repeated measures analysis. All analyses included the total study population, stratified by SA as pre-specified. Between-group comparisons were reported as estimated mean differences with a 95% confidence interval (CI) and P-value. For all analyses, a two-tailed significance level of P < 0.05 was considered statistically significant. To control Type I error, and to correct for multiple testing in reporting the effect on individual components of the primary outcome,33 the level of statistical significance was adjusted for the number of comparisons (Bonferroni correction). All analyses were performed with SPSS version 23.0 (for multiple imputation) or 24.0. The signed SAP is included on the Supplementary material online, Appendix. The trial is registered at ClinicalTrials.gov (NCT02561065).
Results
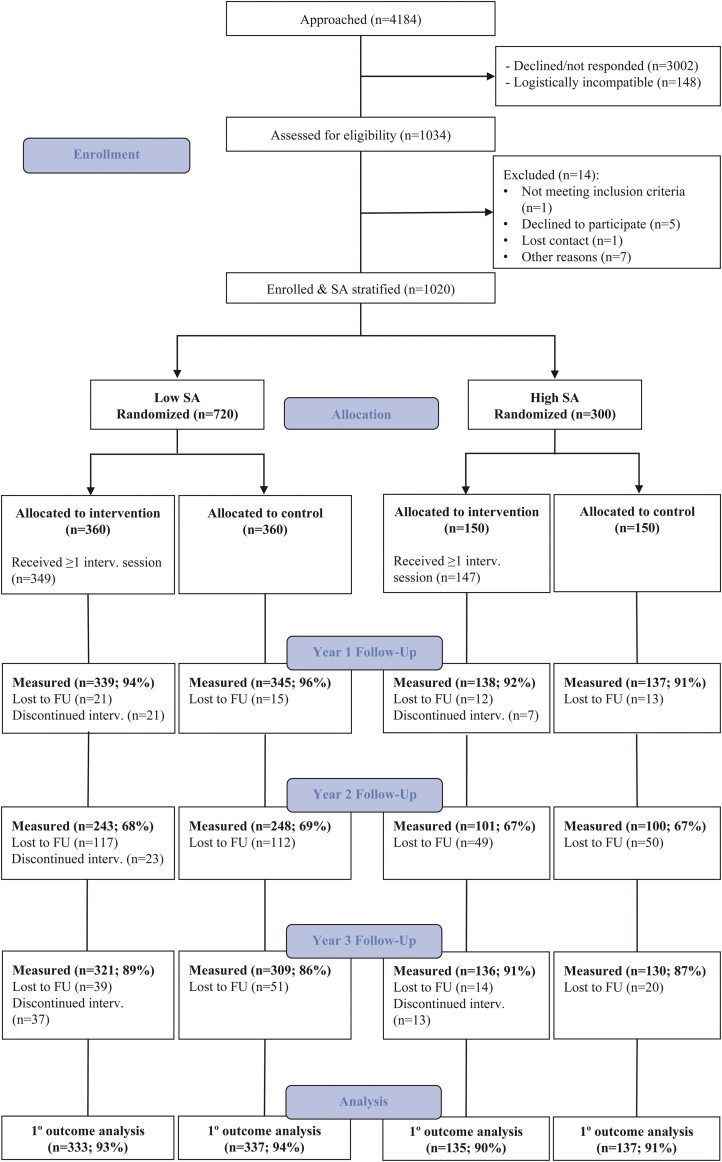
A total of 1034 participants from the PESA cohort were assessed for eligibility for the TANSNIP-PESA RCT between May 2015 and February 2017. Of them, 1020 fulfilled all inclusion criteria and had no exclusion criteria, and 720 were in the low and 300 in the high SA subgroup. After signing the informed consent and completing all baseline assessments, participants were randomized to the intervention or control arm. In total, 959 participants (94.0%) completed the Year-1 follow-up assessment, 692 (67.8%) the Year-2 assessment, and 896 (87.8%) the Year-3 assessment. For the repeated measure analysis of the primary outcome, 942 participants (92.4%) were included. A summarized CONSORT flowchart is shown in Figure 2 (see the extended version in Supplementary material online, Figure S1). Compliance with the three elements of the intervention was evaluated in each of the 12 motivational interview sessions, and the results are shown in Supplementary material online, Figure S2. In brief, attendance to motivational interviews was high (91% of the intervention group over the 12 sessions), as was Fitbit use (81%). In contrast, on average only 37% of participants used the sit–stand workstation over the 3-year period.
Figure 2.
Summarized CONSORT flowchart. CONSORT, Consolidated Standards of Reporting Trials; FU, follow-up; int, intervention; SA, subclinical atherosclerosis.
Baseline demographic characteristics are presented in Table 1 for the total population and for the low and high SA subgroups. Mean age at inclusion was 49.9 ± 3.9 years, and 30.9% of the participants were women. Compared with low SA participants, the high SA subgroup had a worse CV risk profile at baseline, i.e. they were significantly older, higher percentage were male, they had lower educational level, and higher values for BP, BMI, glucose and lipid profile as well as poorer baseline adapted Fuster-BEWAT score than their low SA counterparts (see Supplementary material online, Table S2). In the high SA subgroup, the mean CACS was 40.8 ± 135.7 Agatston Units, and 2D-vascular ultrasound revealed atherosclerotic plaques in the abdominal aorta, carotid arteries, and iliofemoral arteries of 51.0%, 56.0%, and 80.0% of participants, respectively. Low SA participants were free of calcified coronary artery disease at baseline by definition,19,20 but atherosclerotic plaques were present by ultrasound imaging in 16.3%, 21.5%, and 30.1% for the abdominal aorta, carotid arteries, and iliofemoral arteries, respectively. At baseline, the study population had a low-intermediate prevalence of classical CV risk factors (approximately 10% of participants had hypertension, 15% were obese, and nearly 20% were smokers). Participants were physically active (40% exceeded 10 000 steps/day), but sedentary time was high (nearly 70% above 9.5 h/day) (see Supplementary material online, Figure S3).
Table 1.
Baseline characteristics of the TANSNIP-PESA study population
| Total sample (n = 1020) | Low SA (n = 720) | High SA (n = 300) | |||||
|---|---|---|---|---|---|---|---|
| Total sample (n = 1020) | Intervention (n = 510) | Control (n = 510) | Intervention (n = 360) | Control (n = 360) | Intervention (n = 150) | Control (n = 150) | |
| Age (years) | 49.9 (3.9) | 50.0 (3.9) | 49.9 (3.9) | 49.4 (3.7) | 49.2 (3.7) | 51.3 (3.9) | 51.7 (3.8) |
| Female sex | 315 (30.9%) | 154 (30.2%) | 161 (31.6%) | 122 (33.9%) | 130 (36.1%) | 32 (21.3%) | 31 (20.7%) |
| Marital status | |||||||
| Married/Defacto | 771 (75.8%) | 379 (74.3%) | 392 (77.3%) | 265 (73.6%) | 278 (77.4%) | 114 (76.0%) | 114 (77.0%) |
| Other | 246 (24.2%) | 131 (25.7%) | 115 (22.7%) | 95 (26.4%) | 81 (22.6%) | 36 (24.0%) | 34 (23.0%) |
| Working hours per week | 39.7 (1.2) | 39.7 (1.0) | 39.6 (1.3) | 39.7 (1.0) | 39.5 (1.5) | 39.7 (1.0) | 39.8 (0.9) |
| Education level | |||||||
| Without University degree | 161 (15.8%) | 94 (18.4%) | 67 (13.1%) | 58 (16.1%) | 42 (11.7%) | 36 (24.0%) | 25 (16.9%) |
| With University degree | 856 (84.2%) | 416 (81.6%) | 440 (86.9%) | 302 (83.9%) | 317 (88.3%) | 114 (76.0%) | 123 (83.1%) |
Data are mean (SD) or N (%).
PESA, Progression of Early Subclinical Atherosclerosis; SA, subclinical atherosclerosis; TANSNIP, Trans-Atlantic Network to study Stepwise Non-invasive Imaging as a tool for CVD Prognosis and prevention.
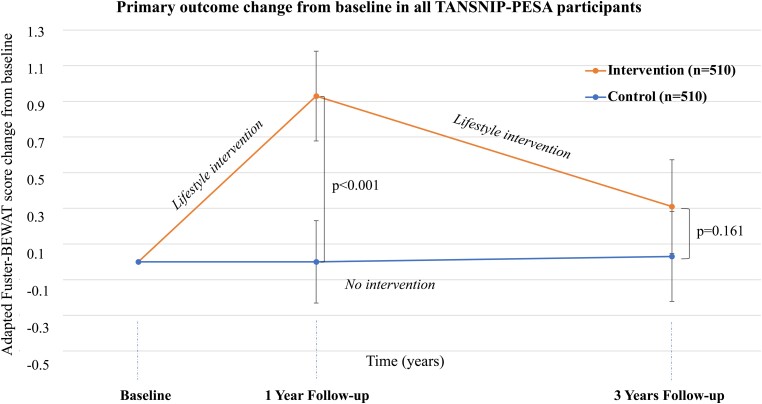
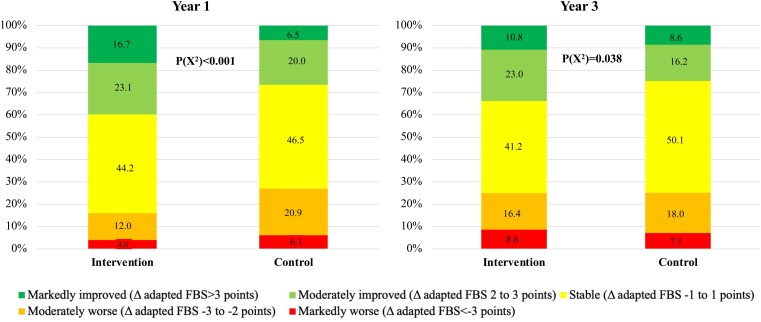
Compared with the control group, the intervention group showed a significant improvement in the primary outcome measure (adapted Fuster BEWAT score) over the 3-year period, indicating a healthier CV status. The overall repeated measures effect over the 3-year period was 0.49 (95% CI 0.23–0.75) points in favour of the intervention group (Table 2). The effect of the intervention on the adapted Fuster-BEWAT score was maximal and statistically significant at Year 1 [estimate 0.83 (95% CI 0.52–1.15) points] and decreased to non-significant levels at Year 3 [estimate 0.24 (95% CI –0.10 to 0.59) points] (Table 2 and Figure 3). To better illustrate the within-group temporal changes in adapted Fuster-BEWAT score, Figure 4 presents change categories in adapted Fuster-BEWAT score for the intervention and control groups at Years 1 and 3.
Table 2.
Changes and between-group comparisons in the adapted Fuster-BEWAT score for all TANSNIP-PESA participants and the low and high SA subgroups
| Intervention | Control | Intervention effect | ||||
|---|---|---|---|---|---|---|
| N | mean (SD) | N | mean (SD) | Estimate (95% CI) | P-value | |
| Adapted Fuster-BEWAT score (all participants) (0–24) | ||||||
| Baseline | 425 | 16.2 (3.7) | 445 | 16.5 (3.5) | – | – |
| Year 1 | 425 | 17.1 (3.6) | 445 | 16.5 (3.5) | 0.83 (0.52–1.15)a | <0.001 |
| Year 3 | 408 | 16.6 (3.7) | 395 | 16.5 (3.6) | 0.24 (−0.10 to 0.59)a | 0.161 |
| Overall time trend | 468 | – | 474 | – | 0.49 (0.23–0.75)b | <0.001 |
| Adapted Fuster-BEWAT score (low SA participants) (0–24) | ||||||
| Baseline | 307 | 16.5 (3.7) | 320 | 16.7 (3.4) | – | – |
| Year 1 | 307 | 17.4 (3.6) | 320 | 16.7 (3.4) | 1.01 (0.63–1.39)a | <0.001 |
| Year 3 | 289 | 17.0 (3.5) | 272 | 16.7 (3.5) | 0.34 (−0.07 to 0.76)a | 0.105 |
| Overall time trend | 333 | – | 337 | – | 0.61 (0.30–0.93)b | <0.001 |
| Adapted Fuster-BEWAT score (high SA participants) (0–24) | ||||||
| Baseline | 118 | 15.4 (3.7) | 125 | 16.0 (3.6) | – | – |
| Year 1 | 118 | 16.2 (3.6) | 125 | 16.2 (3.6) | 0.38 (−0.20 to 0.95)a | 0.195 |
| Year 3 | 119 | 15.7 (3.8) | 123 | 16.0 (4.0) | 0.03 (−0.57 to 0.63)a | 0.927 |
| Overall time trend | 135 | – | 137 | – | 0.19 (−0.26 to 0.64)b | 0.405 |
Fuster-BEWAT score and estimate mean difference are expressed in points.
For the outcome analysis at Years 1 and 3, estimates were calculated using linear mixed effect regression models.
For the overall time trend, estimates were calculated with repeated measures regression models.
BEWAT, Blood pressure, Exercise (objectively measured PA and sedentary time), Weight (BMI), Alimentation (fruit and vegetable consumption), and Tobacco; PESA, Progression of Early Subclinical Atherosclerosis; SA, subclinical atherosclerosis; TANSNIP, Trans-Atlantic Network to study Stepwise Non-invasive Imaging as a tool for CVD Prognosis and prevention.
Figure 3.
Change in the primary outcome at baseline, 1-year follow-up, and 3-year follow-up in all TANSNIP-PESA participants. The line-plot represents mean change (dots) for the adapted Fuster-BEWAT score at different follow-up times relative to values at the initial screening for intervened participants (orange) and controls (blue). P-values as derived from between-group differences of Fuster-BEWAT score change at each follow-up assessment from baseline using linear mixed effect regression models. The score ranges from 0 (poor cardiovascular health) to 24 (ideal cardiovascular health) and is derived from the sum of the individual components (0–4 points each). Adapted Fuster-BEWAT score, Blood pressure, Exercise (PA and sedentary time), Weight (BMI), Alimentation (fruit and vegetable consumption), and Tobacco (smoking habit); PESA, Progression of Early Subclinical Atherosclerosis; TANSNIP, Trans-Atlantic Network to study Stepwise Non-invasive Imaging as a tool for CVD Prognosis and prevention.
Figure 4.
Distribution of participants according to their degree of absolute change in the adapted Fuster-BEWAT score at Year 1 (left panel) and Year-3 follow-up (right panel) in both study treatment arms. The adapted Fuster-BEWAT score ranges from 0 (poor cardiovascular health) to 24 (ideal cardiovascular health) and is derived from the sum of the individual components (0–4 points each). Participants were classified into five categories according to their change in the adapted Fuster-BEWAT score from baseline: Markedly improved (dark green: a >3 point change); moderately improved (light green: a 2–3 point change); stable (yellow: a –1 to 1 point change); moderately worse (orange: a –2 to –3 point change) and markedly worse (red: a >–3 point change). BEWAT, Blood pressure, Exercise (objectively measured PA and sedentary time), Weight (BMI), Alimentation (fruit and vegetable consumption), and Tobacco.
Over the 3-year period, the intervention was effective in participants with a low baseline SA [overall effect 0.61 (95% CI 0.30–0.93) points] but not in those with a high baseline SA [overall effect 0.19 (95% CI –0.26 to 0.64) points] (Table 2). As in the total population, the intervention effect in the low SA subgroup was highest at the first-year follow-up and attenuated at Year 3. No significant intervention effect was observed at either time point in the high SA subgroup (Table 2). Since there was no interaction for the primary outcome between the intervention arm and age or gender (P = 0.596 and P = 0.592, respectively), further stratified analyses by these factors were not performed.
After using a multiple imputation technique for missing data, the intention-to-treat analysis of the primary outcome showed consistent findings with the non-imputed dataset. The overall intervention effect was in favour of the intervention group with a borderline statistical significance [overall effect 0.40 (95% CI–0.02 to 0.82) points, P = 0.064]. Similar to the non-imputed results, the intervention was effective over the 3-year period in those participants with a low baseline SA [overall effect 0.54 (95% CI 0.07–1.01) points, P = 0.024] but not in those with high baseline SA [overall effect 0.07 (95% CI –0.70 to 0.49) points, P = 0.855].
Results of individual adapted Fuster-BEWAT score components are shown in Table 3. There were significant between-group differences in favour of the intervention group in PA, sedentary time, and fruit and vegetable consumption. All these effects were attenuated and were no longer different at the 3-year follow-up assessment. These between-group treatment effect differences in the adapted Fuster-BEWAT score behavioural components were also present (and were of higher magnitude) in the low SA subgroup (Table 4). The high SA group showed no statistically significant effect for these outcomes at either Year 1 or Year 3 follow-up. No significant changes were found in BMI or the number of cigarettes smoked/day.
Table 3.
Changes and between-group comparisons in the individual components of the adapted Fuster-BEWAT score for all TANSNIP-PESA participants
| Intervention | Control | Intervention effect | ||||
|---|---|---|---|---|---|---|
| N | mean (SD) | N | mean (SD) | Estimate (95% CI) | P-value | |
| Systolic Blood Pressure (mmHg) | ||||||
| Baseline | 477 | 115.6 (13.6) | 481 | 113.6 (12.2) | – | – |
| Year 1 | 477 | 116.6 (12.9) | 481 | 116.6 (12.3) | −1.36 (−2.47 to −0.25) | 0.017 |
| Year 3 | 451 | 119.3 (14.8) | 434 | 118.2 (13.8) | −0.85 (−2.15 to 0.44) | 0.197 |
| Diastolic Blood Pressure (mmHg) | ||||||
| Baseline | 477 | 77.6 (9.5) | 481 | 76.0 (8.4) | – | – |
| Year 1 | 477 | 72.3 (8.9) | 481 | 72.0 (8.4) | −0.83 (−1.59 to −0.08) | 0.031 |
| Year 3 | 451 | 75.4 (10.2) | 434 | 74.8 (9.8) | −0.92 (−1.85 to 0.02) | 0.055 |
| Physical activity (activPAL) (steps/day) | ||||||
| Baseline | 459 | 9480 (2743) | 465 | 9452 (2874) | – | – |
| Year 1 | 459 | 10241 (3319) | 465 | 9712 (3139) | 508 (182 to 833) | 0.002 |
| Year 3 | 424 | 10079 (3166) | 408 | 9806 (3249) | 222 (−137 to 580) | 0.226 |
| Sedentary time (activPAL) (h/day) | ||||||
| Baseline | 459 | 10.6 (1.3) | 465 | 10.5 (1.3) | – | – |
| Year 1 | 459 | 10.5 (1.3) | 465 | 10.4 (1.3) | −0.21 (−0.34 to −0.07) | 0.003 |
| Year 3 | 424 | 10.1 (1.3) | 408 | 10.1 (1.5) | −0.05 (−0.22 to 0.12) | 0.563 |
| BMI (kg/m2) | ||||||
| Baseline | 476 | 26.3 (3.6) | 482 | 26.3 (3.9) | – | – |
| Year 1 | 476 | 26.2 (3.7) | 482 | 26.2 (3.9) | −0.06 (−0.21 to 0.09) | 0.435 |
| Year 3 | 450 | 26.6 (3.8) | 436 | 26.4 (4.0) | 0.07 (−0.11 to 0.25) | 0.451 |
| Fruit and vegetable consumption (servings/day) | ||||||
| Baseline | 462 | 3.3 (1.6) | 467 | 3.2 (1.6) | – | – |
| Year 1 | 462 | 3.4 (1.6) | 467 | 3.1 (1.6) | 0.23 (0.08–0.38) | 0.003 |
| Year 3 | 456 | 3.3 (1.6) | 438 | 3.2 (1.5) | 0.05 (−0.11 to 0.20) | 0.558 |
| Smoking (units/day) | ||||||
| Baseline | 474 | 1.7 (5.1) | 474 | 1.3 (4.3) | – | – |
| Year 1 | 474 | 1.5 (4.7) | 474 | 1.4 (4.1) | −0.13 (−0.50 to 0.24) | 0.498 |
| Year 3 | 457 | 1.2 (4.3) | 438 | 1.3 (4.2) | −0.31 (−0.64 to 0.03) | 0.074 |
| Original BEWAT Score (0–15) | ||||||
| Baseline | 429 | 10.4 (2.5) | 450 | 10.7 (2.4) | – | – |
| Year 1 | 429 | 10.9 (2.4) | 450 | 10.7 (2.4) | 0.39 (0.18–0.60) | <0.001 |
| Year 3 | 410 | 10.6 (2.6) | 402 | 10.4 (2.5) | 0.19 (−0.04 to 0.42) | 0.11 |
For the outcome analysis at Years 1 and 3, estimates were calculated using linear mixed effect regression models.
BEWAT, Blood pressure, Exercise (objectively measured PA and sedentary time), Weight (BMI), Alimentation (fruit and vegetable consumption), and Tobacco; BMI, body mass index; PESA, Progression of Early Subclinical Atherosclerosis; SA, subclinical atherosclerosis; TANSNIP, Trans-Atlantic Network to study Stepwise Non-invasive Imaging as a tool for CVD Prognosis and prevention.
Table 4.
Changes and between-group comparisons in the individual components of the adapted Fuster-BEWAT score in the low and high SA subgroups
| Intervention | Control | Intervention effect | ||||
|---|---|---|---|---|---|---|
| N | mean (SD) | N | mean (SD) | Estimate (95% CI) | P-value | |
| Low SA subgroup | ||||||
| Systolic Blood Pressure (mmHg) | ||||||
| Baseline | 339 | 114.8 (14.0) | 344 | 112.4 (12.1) | – | – |
| Year 1 | 339 | 115.8 (12.6) | 344 | 115.3 (11.7) | −1.06 (−2.35 to 0.23) | 0.105 |
| Year 3 | 316 | 118.6 (15.0) | 305 | 116.8 (13.6) | −0.32 (−1.88 to 1.25) | 0.692 |
| Diastolic Blood Pressure (mmHg) | ||||||
| Baseline | 339 | 76.9 (9.8) | 344 | 75.2 (8.4) | – | – |
| Year 1 | 339 | 71.7 (9.0) | 344 | 71.4 (8.2) | −0.91 (−1.78 to −0.05) | 0.039 |
| Year 3 | 316 | 74.7 (10.4) | 305 | 73.8 (9.7) | −0.65 (−1.77 to 0.47) | 0.255 |
| Physical activity (activPAL) (steps/day) | ||||||
| Baseline | 326 | 9354 (2674) | 334 | 9456 (2803) | – | – |
| Year 1 | 326 | 10083 (2907) | 334 | 9623 (3077) | 530 (170 to 890) | 0.004 |
| Year 3 | 301 | 10038 (3179) | 284 | 9575 (2988) | 472 (63 to 881) | 0.024 |
| Sedentary time (activPAL) (h/day) | ||||||
| Baseline | 326 | 10.6 (1.3) | 334 | 10.4 (1.3) | – | – |
| Year 1 | 326 | 10.3 (1.3) | 334 | 10.4 (1.3) | −0.229 (−0.39 to −0.07) | 0.004 |
| Year 3 | 301 | 10.1 (1.3) | 301 | 10.1 (1.5) | −0.07 (−0.27 to 0.13) | 0.489 |
| BMI (kg/m2) | ||||||
| Baseline | 338 | 25.8 (3.4) | 345 | 26.1 (4.0) | – | – |
| Year 1 | 338 | 25.7 (3.5) | 345 | 26.1 (4.1) | −0.09 (−0.26 to 0.09) | 0.333 |
| Year 3 | 314 | 26.0 (3.6) | 307 | 26.3 (4.1) | 0.00 (−0.22 to 0.22) | 0.988 |
| Fruit and Vegetable consumption (servings/day) | ||||||
| Baseline | 330 | 3.3 (1.6) | 334 | 3.3 (1.5) | – | – |
| Year 1 | 330 | 3.4 (1.6) | 334 | 3.1 (1.6) | 0.28 (0.09–0.46) | 0.003 |
| Year 3 | 320 | 3.3 (1.6) | 308 | 3.2 (1.5) | 0.08 (−0.106 to 0.265) | 0.400 |
| Smoking (units/day) | ||||||
| Baseline | 337 | 1.4 (4.4) | 351 | 1.3 (3.9) | – | – |
| Year 1 | 337 | 1.2 (4.5) | 351 | 1.4 (4.1) | −0.28 (−0.69 to 0.14) | 0.192 |
| Year 3 | 321 | 0.9 (3.9) | 308 | 1.2 (3.7) | −0.31 (−0.70 to 0.08) | 0.113 |
| Original BEWAT Score (0–15) | ||||||
| Baseline | 309 | 10.7 (2.5) | 324 | 10.8 (2.4) | – | – |
| Year 1 | 309 | 11.2 (2.4) | 324 | 10.8 (2.3) | 0.45 (0.20–0.71) | 0.001 |
| Year 3 | 290 | 10.9 (2.5) | 279 | 10.5 (2.5) | 0.30 (0.02–0.58) | 0.038 |
| High SA subgroup | ||||||
| Systolic Blood Pressure (mmHg) | ||||||
| Baseline | 138 | 117.7 (12.3) | 137 | 116.6 (11.9) | – | – |
| Year 1 | 138 | 118.6 (13.3) | 137 | 119.7 (13.0) | −1.98 (−4.15 to 0.18) | 0.072 |
| Year 3 | 135 | 120.9 (14.2) | 129 | 120.9 (14.2) | −2.09 (−4.42 to 0.24) | 0.079 |
| Diastolic Blood Pressure (mmHg) | ||||||
| Baseline | 138 | 79.4 (8.4) | 137 | 78.1 (8.0) | – | – |
| Year 1 | 138 | 73.8 (8.7) | 137 | 73.5 (8.8) | −0.63 (−2.15 to 0.90) | 0.421 |
| Year 3 | 135 | 77.1 (9.6) | 129 | 77.4 (9.5) | −1.52 (−3.23 to 0.19) | 0.080 |
| Physical activity (activPAL) (steps/day) | ||||||
| Baseline | 133 | 9790 (2890) | 131 | 9438 (3059) | – | – |
| Year 1 | 133 | 10627 (4148) | 131 | 9942 (3294) | 400 (−298 to 1097) | 0.260 |
| Year 3 | 123 | 10179 (3144) | 124 | 10337 (3738) | −355 (−1076 to 367) | 0.334 |
| Sedentary time (activPAL) (h/day) | ||||||
| Baseline | 133 | 10.7 (1.3) | 131 | 10.8 (1.1) | – | – |
| Year 1 | 133 | 10.4 (1.4) | 131 | 10.5 (1.3) | −0.14 (−0.41 to 0.13) | 0.307 |
| Year 3 | 123 | 10.2 (1.4) | 124 | 10.2 (1.5) | 0.00 (−0.32 to 0.32) | 0.994 |
| BMI (kg/m2) | ||||||
| Baseline | 138 | 27.6 (3.9) | 137 | 26.5 (3.5) | – | – |
| Year 1 | 138 | 27.5 (4.0) | 137 | 26.5 (3.3) | 0.04 (−0.28 to 0.379) | 0.800 |
| Year 3 | 136 | 27.9 (3.9) | 129 | 26.8 (3.6) | 0.27 (−0.06 to 0.60) | 0.114 |
| Fruit and Vegetable consumption (servings/day) | ||||||
| Baseline | 132 | 3.2 (1.5) | 133 | 3.2 (1.6) | – | – |
| Year 1 | 132 | 3.2 (1.7) | 133 | 3.1 (1.5) | 0.12 (−0.16 to 0.40) | 0.402 |
| Year 3 | 136 | 3.1 (1.5) | 130 | 3.1 (1.5) | −0.03 (−0.33 to 0.26) | 0.840 |
| Smoking (units/day) | ||||||
| Baseline | 137 | 2.7 (6.5) | 139 | 1.4 (5.1) | – | – |
| Year 1 | 137 | 2.3 (5.2) | 139 | 1.2 (4.2) | 0.35 (−0.40 to 1.09) | 0.359 |
| Year 3 | 136 | 2.0 (4.9) | 130 | 1.5 (5.0) | −0.28 (−0.94 to 0.38) | 0.405 |
| Original BEWAT Score (0–15) | ||||||
| Baseline | 120 | 9.8 (2.5) | 126 | 10.5 (2.5) | – | – |
| Year 1 | 120 | 10.3 (2.3) | 126 | 10.6 (2.4) | 0.23 (−0.17 to 0.63) | 0.264 |
| Year 3 | 120 | 9.8 (2.4) | 123 | 10.2 (2.5) | −0.08 (−0.49 to 0.32) | 0.689 |
For the outcome analysis at Years 1 and 3, estimates were calculated using linear mixed effect regression models.
BEWAT, Blood pressure, Exercise (objectively measured PA and sedentary time), Weight (BMI), Alimentation (fruit and vegetable consumption), and Tobacco; BMI, body mass index; PESA, Progression of Early Subclinical Atherosclerosis; SA, subclinical atherosclerosis; TANSNIP, Trans-Atlantic Network to study Stepwise Non-invasive Imaging as a tool for CVD Prognosis and prevention.
Additionally, we found a borderline-significant between-group effect on total cholesterol levels at Year 3 in favour of the intervention [–4.05 (95% CI –8.00 to –0.10) mg/dL] (Table 5). In the low SA subgroup, between-group differences in favour of the intervention were significant in total and low-density lipoprotein (LDL) cholesterol levels at Year 3 and borderline-significant in triglyceride levels at Years 1 and 3 (see Supplementary material online, TableS3). High SA participants showed no significant between-group differences in blood biomarkers (see Supplementary material online, Table S4).
Table 5.
Laboratory measurements for all participants
| Intervention | Control | Intervention effect (between-group differences) | ||||
|---|---|---|---|---|---|---|
| N | mean (SD) | N | mean (SD) | Estimate (95% CI) | P-value | |
| Baseline | ||||||
| Glucose, mg/dL | 464 | 92.4 (11.1) | 469 | 92.0 (14.8) | – | – |
| Total cholesterol, mg/dL | 464 | 188.9 (31.0) | 469 | 189.5 (30.3) | – | – |
| LDL, mg/dL | 461 | 115.4 (27.1) | 466 | 115.5 (26.8) | – | – |
| HDL, mg/dL | 464 | 55.7 (12.8) | 469 | 55.9 (13.2) | – | – |
| Triglycerides, mg/dL | 464 | 91.6 (53.1) | 469 | 96.3 (71.7) | – | – |
| Year 1 | ||||||
| Glucose, mg/dL | 464 | 90.7 (13.4) | 469 | 90.1 (13.3) | 0.45 (−0.97 to 1.86) | 0.535 |
| Total cholesterol, mg/dL | 464 | 188.0 (31.2) | 469 | 189.5 (33.5) | −1.24 (−4.88 to 2.40) | 0.504 |
| LDL, mg/dL | 461 | 117.2 (26.4) | 466 | 118.0 (28.4) | −0.80 (−3.80 to 2.20) | 0.602 |
| HDL, mg/dL | 464 | 53.3 (13.0) | 469 | 52.7 (12.4) | 0.74 (−0.57 to 2.04) | 0.267 |
| Triglycerides, mg/dL | 464 | 89.5 (51.7) | 469 | 96.9 (63.4) | −5.22 (−11.64 to 1.19) | 0.111 |
| Year 3 | ||||||
| Glucose, mg/dL | 437 | 88.8 (17.1) | 423 | 87.4 (11.6) | 0.98 (−0.80 to 2.76) | 0.281 |
| Total cholesterol, mg/dL | 437 | 196.6 (32.3) | 423 | 200.8 (33.4) | −4.05 (−8.00 to −0.10) | 0.044 |
| LDL, mg/dL | 434 | 122.0 (29.1) | 414 | 125.2 (29.7) | −3.31 (−6.84 to 0.24) | 0.067 |
| HDL, mg/dL | 437 | 54.8 (14.2) | 423 | 54.9 (13.7) | −0.11 (−1.56 to 1.34) | 0.882 |
| Triglycerides, mg/dL | 437 | 100.0 (63.3) | 423 | 107.6 (88.7) | −5.14 (−14.39 to 4.11) | 0.276 |
For the outcome analysis at Years 1 and 3, estimates were calculated using linear mixed effect regression models.
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The results presented above were generally supported by behavioural results from the activPAL PA monitor and questionnaires, showing improvements in the first year and attenuation at Year 3 (see Supplementary material online, Table S5). No significant changes were seen in the anthropometric variables. Psychosocial and work-related results showed a few non-significant effects at Year 1 in the expected direction, most notably a reduction in work absenteeism in favour of the intervention group (see Supplementary material online, Table S6). The findings presented above were further supported by the results for the other secondary outcomes in low and high SA participants (see Supplementary material online, Tables S7–S10). Supplementary material online, Table S11 shows the number of missing data for the primary endpoint at each follow-up visit.
Discussion
In this RCT, conducted in an asymptomatic middle-aged population at low–intermediate CV risk, the most important result was that a 3-year intensive worksite intervention was able to produce a modest improvement in lifestyle behaviour and overall CV health. However, intervention effectiveness peaked 1 year after baseline and attenuated at the Year-3 evaluation (Structured Graphical Abstract). In light of the motivational interview scheme used (one session every second week for the first 3 months followed by booster sessions spaced every 5–8 months), one possible explanation for the observed attenuation of intervention effectiveness is that maintenance of lifestyle improvements beyond the first year requires sustained and more intensive behavioural support over time, similar to preventive pharmacological approaches that are maintained chronically. Second, the intervention was effective in participants having low baseline SA, but counterintuitively not in those with high SA. From a health promotion perspective, one may argue that prevention efforts should be increased for those subjects with high SA.
Comparison with other studies
The pattern of transient favourable changes after a lifestyle intervention that tend to dilute over time is consistent with the existing literature. For instance, the Fifty–Fifty trial was a multicentre RCT conducted in Spain testing the effect of a peer-group intervention in adults with at least one CV risk factor.26 In this trial, the intervention produced a significant improvement in the Fuster-BEWAT score at the first-year follow-up compared with the control group. However, the residual beneficial effect in the intervention group vs. the control group was negligible at 4-year follow-up.34 FAMILIA, the Grenada Heart Project and the Look AHEAD trial,29,30,35 among others, are recent large-sized lifestyle intervention trials that have failed to demonstrate a benefit of different lifestyle interventions in either participants, or patients with Type 2 diabetes, thus illustrating the difficulty to obtain significant and sustained lifestyle changes through coaching in adulthood.
Different intervention effect between subclinical atherosclerosis groups
Our stratified analysis shows a stronger treatment effect in the low SA subgroup and a smaller, non-significant effect in the high SA subgroup. This counterintuitive finding conflicts with our initial hypothesis that the high SA group would be more receptive to the intervention, based on our expectation that the higher atherosclerotic plaque burden in this group would foster a greater sense of urgency about lifestyle changes. We do not have a clear explanation for this finding. Analysis of the baseline differences among our two study subgroups showed that high SA participants had a less favourable CV risk profile than the low SA subgroup (see Supplementary material online, Table S2). One possibility is that the high SA group had been struggling with a healthy lifestyle for a longer period and the intervention was not intensive enough to resolve those struggles. From this perspective, the chances to improve would be higher in the low SA subgroup (since the baseline motivation towards CV health was probably higher to begin with). Another possibility is that differences on the implementation process and/or adherence to individual intervention components between the low and high SA subgroups may have influenced the intervention effect. Our ongoing (pre-specified) process evaluation21 will provide further insight into the level of implementation and barriers for adoption of the TANSNIP-PESA intervention and will explore the association between the degree of implementation and changes in participants’ outcomes, especially with regard to the high and low SA subgroups. In line with our results, one previous RCT found a limited value of motivational interviewing for CVD risk reduction and PA increase in individuals at high baseline CVD risk.36 What remains to be elucidated and should be tested in future trials is whether high SA participants would benefit from more aggressive interventional strategies, including a higher frequency lifestyle intervention or eventually direct early initiation of pharmacological approaches. TANSNIP-PESA process evaluation will analyse in depth the lessons learned from the participants who made the greatest (and smallest) improvement that may be useful to design these future interventions. Other intervention approaches such as group-based dynamics, use of behavioural psychology game theory or population-based approaches with a focus on changing workplace culture and behavioural norms might be alternative intervention strategies to be considered. Overall, our results reinforce the concept that the CVD-prevention programmes need to start early, at ages when subclinical disease is presumably less advanced, and that this support needs to be sustained throughout life.
Clinical implications of this study and future research
The TANSNIP-PESA intervention was able to modestly improve PA, sedentary, and dietary behaviours after 1 year, and these lifestyle improvements had a small and transitory effect on CV risk factor reduction. In our low SA subgroup, blood cholesterol changes did not appear immediately after lifestyle improvements but appeared later, and the effect was sustained in the Year-3 evaluation (see Supplementary material online, Table S3). Our results suggest that sustainability may depend on the maintenance of the coaching support although this issue should be further investigated. Given that atherosclerosis (the underlying cause of most CVD) is a chronic disorder with a very long asymptomatic period, it is also logical to think that behavioural strategies aiming to reduce this problem should be applied recurrently, otherwise their beneficial effect on individuals will disappear once the support is withdrawn, as happened in TANSNIP-PESA and other lifestyle intervention RCTs.34
Although the absolute reduction in the primary outcome observed in our study may seem small, it is known that prevention strategies aimed at a population level often offer little benefit to each participating individual, but much to the population as a whole; ‘where a little means a lot’.37 We plan to further assess the effect of the TANSNIP-PESA intervention on SA initiation and/or progression using the extensive phenotyping available from the ongoing PESA imaging studies.20
Strengths and limitations of this study
Strengths of the current trial include the large sample, the prolonged intervention and follow-up periods for the study of lifestyle-change maintenance, the high retention rates at Years 1 and 3 and the objective measurement of outcomes such as PA and sitting time with accelerometry. Nesting of TANSNIP-PESA within the PESA cohort provides a perfect opportunity for further comprehensive assessments of the relationship between lifestyle behavioural changes and SA. Similarly, cost-effectiveness analyses will be undertaken using data from subsequent PESA follow-ups.
The PESA cohort is a homogeneous, highly educated, and quite physically active population, with overall low–intermediate CVD risk. This limits the generalizability of the results to the general population. Although this RCT was conducted in Spain, the baseline adherence to a Mediterranean dietary pattern was only moderate, giving plenty of room for dietary improvement. Due to logistical issues, almost one-third of the study population did not undergo follow-up measurements at Year 2. Therefore, Year 2 data were only included in the repeated measures analysis and were not analysed separately, as indicated in the SAP (see Supplementary material online, Appendix). However, the Year-3 retention rate was 87.8%. There were no significant differences between baseline Fuster-BEWAT score values of those participants with and without missing value on the outcome at Year 2 (data not shown). Due to the study design, neither participants, intervention providers, nor data collection staff could be blinded to the allocation arm. Nevertheless, all study measurements followed standardized procedures, and many outcomes relied on objective parameters not subject to self-report bias (such as laboratory tests or accelerometer assessments). An interaction test was not conducted to fully confirm differences between SA subgroups (adhering to our SAP, see Supplementary material online, Appendix). Finally, the laboratory analyses were performed on frozen samples at baseline and Year 1, whereas fresh samples were analysed at Years 2 and 3. This may have generated some intra-individual longitudinal variation. However, any systematic bias would be the same for the control and intervention groups.
Conclusion
This RCT provides evidence of beneficial effects of a lifestyle intervention on lifestyle behaviours and CV health in asymptomatic low–intermediate risk middle-aged adults. Intervention effects on lifestyle attenuated over time, suggesting that sustained effects need a more intense intervention beyond the first year. The intervention was especially effective in participants with low SA burden whereas subjects with high SA might need extra support or alternative approaches for behaviour change.
Authors’ contributions
I.G.-L was involved in investigation, methodology, project administration, writing of the original draft. H.P.v.d.P. was involved in conceptualization, methodology, supervision, writing, reviewing, and editing of the article. J.M.F.A. and F.v.N were involved in data curation, formal analysis, investigation, methodology, writing, reviewing, and editing of the article. J.M.C.V. was involved in conceptualization, methodology, project administration, supervision, writing, reviewing, and editing of the article. A.J.v.d.B. was involved in conceptualization, methodology, supervision, writing, reviewing, and editing. X.R. was involved in formal analysis, methodology, supervision, writing, reviewing, and editing. A.F.-O. was involved in project administration, resources, supervision, writing, reviewing, and editing. J.C. was involved in conceptualization, investigation, methodology, writing, reviewing, and editing. J.M.v.D. was involved in conceptualization, methodology, data curation, formal analysis, writing, reviewing, and editing. J.M.M. was involved in project administration, resources, supervision, writing, reviewing, and editing. B.I. was involved in conceptualization, methodology, project administration, resources, supervision, writing, reviewing, and editing. W.v.M. was involved in conceptualization, methodology, supervision, writing, reviewing, and editing.V.F. was involved in conceptualization, funding acquisition, methodology, supervision, writing, reviewing, and editing.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank all TANSNIP-PESA participants for their invaluable participation in this trial. Evelyn Cárdenas Marín and Antonio Quesada Navidad were outstanding in the coordination of the RCT and the overall development of the project. The authors also acknowledge the work of Carolina Rojas Murcia and Maria Isabel Martínez Castro, who provided top quality motivational interview sessions; Sergio Cárdenas and Jesús Molina for their continuous IT support; Magdalena López for logistics within Santander Bank; Marta Cortés-Canteli for helpful discussions and all the PESA nutrition, biobank, and imaging technicians and CNIC nurses for their enthusiastic contributions. Simon Bartlett provided English editing. The authors also thank Ergotron Inc. for providing partial support for the acquisition of the sit-stand workstations used in the intervention programme.
Contributor Information
Ines Garcia-Lunar, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; CIBER Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; Cardiology Department, University Hospital La Moraleja, Madrid, Spain.
Hidde P van der Ploeg, Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
Juan Miguel Fernández Alvira, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain.
Femke van Nassau, Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
Jose Maria Castellano Vázquez, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; Centro Integral de Enfermedades Cardiovasculares (CIEC), Hospital Universitario Monteprincipe, Grupo HM Hospitales, Madrid, Spain.
Allard J van der Beek, Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
Xavier Rossello, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; CIBER Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; Cardiology Department, Health Research Institute of the Balearic Islands (IdISBa), Hospital Universitari Son Espases, Palma, Spain.
Antonio Fernández-Ortiz, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; CIBER Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; Instituto Cardiovascular, Hospital Clínico San Carlos, IdISSC, Madrid, Spain.
Jennifer Coffeng, Dutch Institute of Employee Benefits Schemes (UWV), Amsterdam, The Netherlands.
Johanna M van Dongen, Department of Health Sciences, Faculty of Science, Vrije Universiteit Amsterdam, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Jose Maria Mendiguren, Banco de Santander, Madrid, Spain.
Borja Ibáñez, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; CIBER Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; Cardiology Department, IIS-Hospital Universitario Fundación Jiménez Díaz, Madrid, Spain.
Willem van Mechelen, Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands.
Valentin Fuster, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; Cardiovascular Institute, Mount Sinai Heart at Icahn School of Medicine, New York, NY, USA.
Funding
TANSNIP-PESA is funded by Fundación Centro Nacional de Investigaciones Cardiovasculares (CNIC) Carlos III through an Investigator-initiated Study grant to Icahn School of Medicine from AstraZeneca. The PESA study is co-funded by the CNIC and Banco Santander. The study also received funding from the Instituto de Salud Carlos III (PI15/02019) and the European Regional Development Fund (ERDF) ‘A way to make Europe’. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia e Innovación (MCIN), and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (grant CEX2020-001041-S funded by MICIN/AEI/10.13039/501100011033. The study funders were not involved in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Data Availability
Will individual participant data be available (including data dictionaries)? Yes.
What data in particular will be shared? Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices).
What other documents will be available? Study protocol, statistical analysis plan, informed consent form.
When will data be available? Immediately following publication; no end date.
With whom? Investigators whose proposed use of the data has been approved by the TANSNIP-PESA scientific and publications committee and by the PESA scientific committee.
For what types of analyses? To achieve the aims of the approved proposal.
By what mechanism will data be made available? Proposals should be directed to pesa-h@cnic.es. To gain access, data requestors will need to sign a data access agreement.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, et al. European society of cardiology: cardiovascular disease statistics 2019. Eur Heart J 2020;41:12–85. [DOI] [PubMed] [Google Scholar]
- 3. Gupta R, Wood DA. Primary prevention of ischaemic heart disease: populations, individuals, and health professionals. Lancet 2019;394:685–696. [DOI] [PubMed] [Google Scholar]
- 4. Zhang YB, Chen C, Pan XF, Guo J, Li Y, Franco OH, et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ 2021;373:n604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet 2017;390:2643–2654. [DOI] [PubMed] [Google Scholar]
- 6. Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med 2016;375:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–337. [DOI] [PubMed] [Google Scholar]
- 9. Sisti LG, Dajko M, Campanella P, Shkurti E, Ricciardi W, de Waure C. The effect of multifactorial lifestyle interventions on cardiovascular risk factors: a systematic review and meta-analysis of trials conducted in the general population and high risk groups. Prev Med 2018;109:82–97. [DOI] [PubMed] [Google Scholar]
- 10. Alageel S, Gulliford MC, McDermott L, Wright AJ. Multiple health behaviour change interventions for primary prevention of cardiovascular disease in primary care: systematic review and meta-analysis. BMJ Open 2017;7:e015375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jorgensen T, Jacobsen RK, Toft U, Aadahl M, Glumer C, Pisinger C. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ 2014;348:g3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev 2011;1:CD001561. [DOI] [PubMed] [Google Scholar]
- 13. Shrestha N, Grgic J, Wiesner G, Parker A, Podnar H, Bennie JA, et al. Effectiveness of interventions for reducing non-occupational sedentary behaviour in adults and older adults: a systematic review and meta-analysis. Br J Sports Med 2019;53:1206–1213. [DOI] [PubMed] [Google Scholar]
- 14. Shrestha N, Kukkonen-Harjula KT, Verbeek JH, Ijaz S, Hermans V, Pedisic Z. Workplace interventions for reducing sitting at work. Cochrane Database Syst Rev 2018;6:CD010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gysan DB, Millentrup S, Albus C, Bjarnason-Wehrens B, Latsch J, Gohlke H, et al. Substantial improvement of primary cardiovascular prevention by a systematic score-based multimodal approach: A randomized trial: The PreFord-Study. Eur J Prev Cardiol 2017;24:1544–1554. [DOI] [PubMed] [Google Scholar]
- 16. Cahill K, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database Syst Rev 2014;2:CD003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rongen A, Robroek SJW, van Lenthe FJ, Burdorf A. Workplace health promotion: a meta-analysis of effectiveness. Am J Prev Med 2013;44:406–415. [DOI] [PubMed] [Google Scholar]
- 18. Hutchinson AD, Wilson C. Improving nutrition and physical activity in the workplace: a meta-analysis of intervention studies. Health Promot Int 2012;27:238–249. [DOI] [PubMed] [Google Scholar]
- 19. Fernandez-Ortiz A, Jimenez-Borreguero LJ, Penalvo JL, Ordovas JM, Mocoroa A, Fernandez-Friera L, et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J 2013;166:990–998. [DOI] [PubMed] [Google Scholar]
- 20. Ibanez B, Fernandez-Ortiz A, Fernandez-Friera L, Garcia-Lunar I, Andres V, Fuster V. Progression of early subclinical atherosclerosis (PESA) study: JACC focus seminar 7/8. J Am Coll Cardiol 2021;78:156–179. [DOI] [PubMed] [Google Scholar]
- 21. Coffeng JK, van der Ploeg HP, Castellano JM, Fernandez-Alvira JM, Ibanez B, Garcia-Lunar I, et al. A 30-month worksite-based lifestyle program to promote cardiovascular health in middle-aged bank employees: Design of the TANSNIP-PESA randomized controlled trial. Am Heart J 2017;184:121–132. [DOI] [PubMed] [Google Scholar]
- 22. Rossello X, Fuster V, Oliva B, Sanz J, Fernandez Friera LA, Lopez-Melgar B, et al. Association between body size phenotypes and subclinical atherosclerosis. J Clin Endocrinol Metab 2020;105:3734–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lundahl B, Moleni T, Burke BL, Butters R, Tollefson D, Butler C, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns 2013;93:157–168. [DOI] [PubMed] [Google Scholar]
- 24. Stonerock GL, Blumenthal JA. Role of counseling to promote adherence in healthy lifestyle medicine: strategies to improve exercise adherence and enhance physical activity. Prog Cardiovasc Dis 2017;59:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burgess E, Hassmen P, Welvaert M, Pumpa KL. Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity: a systematic review and meta-analysis. Clin Obes 2017;7:105–114. [DOI] [PubMed] [Google Scholar]
- 26. Gomez-Pardo E, Fernandez-Alvira JM, Vilanova M, Haro D, Martinez R, Carvajal I, et al. A comprehensive lifestyle peer group-based intervention on cardiovascular risk factors: the randomized controlled fifty-fifty program. J Am Coll Cardiol 2016;67:476–485. [DOI] [PubMed] [Google Scholar]
- 27. Fernandez-Alvira JM, Fuster V, Pocock S, Sanz J, Fernandez-Friera L, Laclaustra M, et al. Predicting subclinical atherosclerosis in low-risk individuals: ideal cardiovascular health score and Fuster-BEWAT score. J Am Coll Cardiol 2017;70:2463–2473. [DOI] [PubMed] [Google Scholar]
- 28. Wang HY, Dou KF, Sun YX. Fuster-BEWAT score versus cardiovascular health score to predict subclinical target organ damage: Insights from a large-scale Asian population. Eur J Prev Cardiol 2020;27:2292–2295. [DOI] [PubMed] [Google Scholar]
- 29. Latina J, Fernandez-Jimenez R, Bansilal S, Sartori S, Vedanthan R, Lewis M, et al. Grenada Heart Project-Community Health ActioN to EncouraGe healthy BEhaviors (GHP-CHANGE): A randomized control peer group-based lifestyle intervention. Am Heart J 2020;220:20–28. [DOI] [PubMed] [Google Scholar]
- 30. Fernandez-Jimenez R, Jaslow R, Bansilal S, Diaz-Munoz R, Fatterpekar M, Santana M, et al. Different lifestyle interventions in adults from underserved communities: the FAMILIA trial. J Am Coll Cardiol 2020;75:42–56. [DOI] [PubMed] [Google Scholar]
- 31. Martinez-Gonzalez MA, Garcia-Arellano A, Toledo E, Salas-Salvado J, Buil-Cosiales P, Corella D, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 2012;7:e43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Twisk J, Bosman L, Hoekstra T, Rijnhart J, Welten M, Heymans M. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun 2018;10:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pocock SJ, Rossello X, Owen R, Collier TJ, Stone GW, Rockhold FW. Primary and secondary outcome reporting in randomized trials: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:827–839. [DOI] [PubMed] [Google Scholar]
- 34. Fernandez-Alvira JM, Fernandez-Jimenez R, de Miguel M, Santos-Beneit G, Bodega P, Hill CA, et al. The challenge of sustainability: long-term results from the Fifty-Fifty peer group-based intervention in cardiovascular risk factors. Am Heart J 2021;240:81–88. [DOI] [PubMed] [Google Scholar]
- 35. Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ismail K, Bayley A, Twist K, Stewart K, Ridge K, Britneff E, et al. Reducing weight and increasing physical activity in people at high risk of cardiovascular disease: a randomised controlled trial comparing the effectiveness of enhanced motivational interviewing intervention with usual care. Heart 2020;106:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rose G. Sick individuals and sick populations. Int J Epidemiol 2001;30:427–432. discussion 33-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Will individual participant data be available (including data dictionaries)? Yes.
What data in particular will be shared? Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices).
What other documents will be available? Study protocol, statistical analysis plan, informed consent form.
When will data be available? Immediately following publication; no end date.
With whom? Investigators whose proposed use of the data has been approved by the TANSNIP-PESA scientific and publications committee and by the PESA scientific committee.
For what types of analyses? To achieve the aims of the approved proposal.
By what mechanism will data be made available? Proposals should be directed to pesa-h@cnic.es. To gain access, data requestors will need to sign a data access agreement.