Abstract
Mitofusin (MFN) 1 and MFN2 are dynamin GTPase family mitochondrial proteins that mediate mitochondrial fusion requiring MFN conformational shifts, formation of macromolecular complexes on and between mitochondria, and GTP hydrolysis. Damaging MFN2 mutations cause an untreatable, largely pediatric progressive peripheral neuropathy, Charcot-Marie-Tooth (CMT) disease type 2A. We used small molecule allosteric mitofusin activators that promote MFN conformations favoring fusion to interrogate the effects of MFN2 conformation and GTPase activity on MFN2-mediated mitochondrial fusion and motility in vitro. We translated these findings in vivo by defining dose-dependent pharmacodynamic and disease-modifying effects of mitofusin activators in murine CMT2A. MFN2 catalytic GTPase activity and MFN2 conformational switching are essential for mitochondrial fusion, but the two processes are separate and dissociable. We report the first concentration-response relationships for mitofusin activators to stimulate mitochondrial transport through CMT2A neuronal axons, which is similar to their stimulation of mitochondrial fusion. In CMT2A mice, intermittent (daily short acting) and sustained (twice daily long acting) mitofusin activation were equally effective in reversing neuromuscular degeneration. Moreover, acute dose-dependent pharmacodynamic effects of mitofusin activators on mitochondrial transport through CMT2A neuronal axons anticipated those for long-term reversal of neurodegenerative phenotypes. A crossover study showed that CMT2A neuronal deficits recurred after mitofusin activators are discontinued, and revealed that CMT2A can be ameliorated by mitofusin activation even in old (>74 week) mice. These data add to our understanding of mitochondrial dysfunction induced by a CMT2A MFN2 GTPase mutation and provide additional information supporting the approach of pharmacological mitofusin activation in CMT2A.
SIGNIFICANCE
This study interrogated the roles of MFN2 catalytic activity and allosteric activation on impaired mitochondrial fusion and neuronal transport as they impact an untreatable peripheral neuropathy caused by MFN2 mutations, Charcot-Marie-Tooth disease type 2A. The results mechanistically link mitochondrial fusion and motility to the relaxed MFN2 protein conformation and correction of mitochondrial abnormalities to in vivo reversal of neurodegeneration in murine CMT2A.
Introduction
Charcot-Marie-Tooth (CMT) disease type 2A is a largely pediatric peripheral neuropathy caused in most instances by mutations of the nuclear gene encoding a mitochondrial fusion protein, mitofusin (MFN) 2 (Züchner et al., 2004; Verhoeven et al., 2006). Although other genetic causes have been reported (Zhao et al., 2001), the majority of CMT2A cases are produced by loss-of-function MFN2 mutations with autosomal dominant transmission or that arise spontaneously; rarely, autosomal recessive inheritance of CMT2A caused by MFN2 mutations has been reported (Tazir et al., 2013; Stuppia et al., 2015). Although the >100 implicated MFN2 mutations confer some heterogeneity in disease onset and progression (Verhoeven et al., 2006; Feely et al., 2011; Bombelli et al., 2014; Pipis et al., 2020), neuromuscular degeneration typically manifests in the “toddler” period of age 3–5, progresses during childhood and adolescence, and then stabilizes in young adults, producing life-long disability. Because there has been no way of correcting mutational MFN2 dysfunction, this disease remains untreatable and is managed with supportive measures.
The consequences of mitofusin dysfunction in CMT2A (Palau et al., 2009; Wolf et al., 2019) have traditionally been interrogated through overexpression of wild-type or mutant human MFN cDNAs or genetic ablation/suppression of endogenous mouse mitofusin (Mfn)1 or Mfn2. The recent development of small molecule mitofusin activators made it possible to investigate mitofusin functioning without experimentally perturbing Mfn1 or Mfn2 levels. To date, three chemical classes of mitofusin activators have been described: triazolureas (Rocha et al., 2018; Zacharioudakis et al., 2022), phenylhexanamides (Dang et al., 2020, 2021), and derivatives of the natural compound piperine (Zhang et al., 2022). All of these small molecule mitofusin activators chemically mimic amino acid side chains that determine mitofusin protein conformation. Thus, when mitofusin activators bind to mitofusins, they compete with endogenous peptide-peptide interactions that normally maintain a folded MFN structure unfavorable for mitochondrial fusion (Franco et al., 2016). As a consequence, mitofusins acquire a relaxed protein conformation that facilitates mitochondrial fusion and motility. This pharmaceutical mechanism of activation mimics phosphorylation-induced MFN conformational changes during natural homeostatic regulation (Li et al., 2022). Mitofusin activators exhibit a high degree of selectivity (>3 orders of magnitude) for mitofusins compared with other drug targets (Rocha et al., 2018; Dang et al., 2020, 2021), suggesting that they can be useful additions to the research armamentarium.
The prototype triazolurea mitofusin activators, collectively called Chimeras (Rocha et al., 2018), underwent rapid first-pass hepatic elimination and were therefore not useful in vivo (Dang et al., 2020). However, the equipotent phenylhexanamide, trans-MiM111, possessed in vivo pharmacokinetic properties suitable for studying MFN2 dysfunction in mice (Dang et al., 2020). An initial feasibility study of daily intramuscular trans-MiM111 in murine CMT2A reversed functional, histologic, and neuroelectrophysiological signs of neuromuscular degeneration (Franco et al., 2020). This study employed a “burst activation” approach in which mitofusin activator plasma levels were therapeutic during only ∼8 hours of every 24, and the presumed disease-relevant pharmacodynamic effect (increased mitochondrial motility in CMT2A mouse sciatic nerve axons) was completely lost between daily doses (Franco et al., 2020).
The above cited proof-of-concept study for mitofusin activation in murine CMT2A raises questions whose answers may determine whether preclinical development is possible or warranted. First, although transient mitofusin activation was beneficial, might sustained mitofusin activation be superior? Second, is the duration of benefit from mitofusin activation in CMT2A temporary, or does it extend beyond the treatment period? And finally, how does mitofusin activator dose relate to reversal of mitochondrial dysmotility in peripheral nerves and improved in vivo neuromuscular function? Here, we take advantage of a recently described longer-acting phenylhexanamide mitofusin activator, N-[(1s,4s)-4-Hydroxycyclohexyl] (1R,2R)-2-(3-phenylpropyl) cyclopropanecarboxamide (CPR1-B) (Dang et al., 2021), to further interrogate mechanisms of mutant MFN2 dysfunction in CMT2A and better define the relationships between mitofusin activator pharmacodynamics and disease response.
Materials and Methods
Mouse Lines
All experimental procedures were approved by the Washington University in St. Louis, School of Medicine Animal Studies Committee; IACUC protocol number 19-0910 Exp:12/16/2022. Rosa-STOP-mMFN Thr105Met (T105M) mice (C57BL/6 Gt(ROSA)26 Sortm1 (CAG-MFN2*T105M)Dple/J) from The Jackson Laboratory (Bar Harbor, Maine; Stock No: 025322) were crossed to HB9-Cre mice (B6.129S1-Mnx1tm4(cre)Tmj/J) from The Jackson Laboratory (Stock No: 006600) to generate motor neuron-targeted MFN2 T105M mice as described (Franco et al., 2020). For in vivo longitudinal studies, mice were randomized to treatment groups by Lihong Zhang, and experiments performed by Antonietta Franco, who was blind to treatment status. Male and female mice were randomized to treatment without regard to sex. Because there were no differences between CMT2A male and female mice prior to or after completing 8 weeks of mitofusin activator treatment (Supplemental Fig. 1), males and females were combined for analyses of in vivo phenotypes.
Chemicals and Reagents
Viral Vectors
Viral constructs used human adenovirus Type5 (dE1/E3). Cells were transduced at a multiplicity of infection (MOI) of 50 using: adenovirus β-galactosidase (Vector Biolabs Cat#: 1080), adenovirus Mito-Ds-Red2 (Signagen Cat#: 12259), or adenovirus Cre-recombinase (Vector Biolabs Cat#: 1794); adenovirus (Ad)-MFN2 wild-type (WT) and MFN2 T105M (Franco et al., 2020) were synthesized at Vector Biolabs.
Antibodies and Stains
Mouse monoclonal anti-mitofusin 2 was from AbCAM (Cat#: ab56889; 1:1000 dilution), rabbit polyclonal anti-cytochrome c oxidase subunit 4 (COX-IV) was from AbCAM (Cat#: ab16056; 1:1000 dilution), mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase was from AbCAM (Cat#: ab8245; 1:3000 dilution), α-bungarotoxin Alexa-Fluor 594 was from ThermoFisher (Cat#: B12423; 0.5 μg/ml), Alexa-Fluor 488 goat anti-rabbit was from ThermoFisher (Cat#: A11008; 1:400 dilution), fluorescein-conjugated wheat germ agglutinin was from Invitrogen (Cat#: W834; _1:50 dilution), MitoTracker Orange was from Thermo Fisher (Cat#: M7510), tetramethylrhodamine ethyl ester was from Thermo Fisher (Cat#: T669), and Hoechst nuclear stain was from Thermo Fisher (Cat#: H3570).
Chemical Compounds
Trans-MiM111 and CPR1-B (Dang et al., 2020, 2021) were initially obtained from Mitochondria in Motion, Inc. under terms of an Material Transfer Agreement, and later resynthesized at WuXi Apptech. Chimera C (Rocha et al., 2018; Dang et al., 2020) was resynthesized at Paraza Pharma. Each small molecule mitofusin activator was maintained as a 10 mM stock in DMSO. For in vivo administration the final vehicle was 10% DMSO/90% [70% water/30% 2-hydroxypropyl-β-cyclodextrin; Sigma Cat#: 332607]. Mitofusin activating peptide MP1(Franco et al., 2016) was synthesized at ThermoFisher.
Cultured Cells
Mfn2 null and Mfn1/Mfn2 double null murine embryonic fibroblasts (MEFs) were purchased from American Type Culture Collection (ATCC Manassas, Virginia, CRL-2993 and CRL-2994, respectively) and cultured under standard conditions as described (Dang et al., 2020).
Dorsal root ganglion (DRG) neurons were isolated from ∼8-week-old MFN2 T105M flox-stop transgenic mice and cultured as described (Franco et al., 2020). After 5 days in culture, DRGs were transduced with adenovirus-Cre to induce MFN2 T105M expression and with adenovirus-mitoRFP to label neuronal mitochondria. Mitochondrial motility was assessed 48 hours thereafter by time-lapse video confocal imaging (Franco et al., 2020) after overnight treatment with trans-MiM111 or CPR1-B (100 nM) or vehicle (DMSO).
Live Cell Mitochondrial Imaging in Mfn2 Null MEFs
Concentration-dependent fusogenic effects of mitofusin activators were assayed in MitoTracker Orange-stained live cells independently by two investigators in different laboratories using separate techniques and equipment.
Mitochondrial aspect ratio was measured by Antonietta Franco using confocal microscopy of individual MEFs exactly as described (Rocha et al., 2018). Briefly, images were acquired at room temperature on a Nikon Ti Confocal microscope using either a 60X1.3 NA oil immersion objective or 10X0.3 NA dry objective. Cells were maintained in Krebs-Henseleit buffer (138 NaCl, 3.7 nM KCL, 1.2 n M KH2PO4, 15 nM Glucose, 20 nM HEPES pH: 7.2–7.5, and 1mM CaCl2) during imaging. MitoTracker Orange was excited with the 549 nm laser and emission monitored at 590 nm. Mitochondrial aspect ratio was quantified using Image J software.
The % of Mfn2 null MEFs with filamentous mitochondria (Detmer and Chan, 2007) was measured by Lihong Zhang using a Keyence BZ-X800 Fluorescence Microscope with automatic image acquisition (excitation 549 nm, emission 590 nm; eight 40x images per well) of 12-well plates. The proportion of cells with filamentous mitochondria in each image, corrected for vehicle, was indexed to the same value for 1 μM trans-MiM111.
Interpolated D-R curves from both methods were generated using Prism software (version 9) and the data reported as EC50 (nM) and Emax (% trans-MiM111) with 95% CI.
Mitochondrial Motility Studies
Mitochondria of cultured DRG neurons mitochondria were labeled with Adeno-mitoDsRed2 for 48h (excitation: 561 nm; emission: 585 nm). Mitochondria of sciatic nerves mitochondria were labeled ex vivo with tetramethylrhodamine ethyl ester (200nM). Mitochondrial movement was tracked in cultured neurons or explanted sciatic nerves using time-lapse confocal imaging (0.2 frames/s) at 37°C on a Nikon A1Rsi Confocal Microscope with the 40x oil objective. Kymographs and quantitative data were generated using an Image-J velocity measurement tool plug-in as described (Rocha et al., 2018).
GTPase Assay
Mfn1/Mfn2 double-null MEFs were transduced with adenoviral vectors encoding wild-type MFN2, MFN2 T105M, or β-galactosidase (β-Gal) at a MOI of 50. 72 hours later mitochondria were isolated (Frezza et al., 2007) and total mitochondrial GTPase activity assayed using 100 μg of mitochondria protein in triplicate for each condition and components of the GTPase-Glo assay kit from Promega (#V7681; Madison, WI, USA) following the manufacturer’s protocol. In some studies, mitofusin activators Chimera or trans-MiM 111 (1 μM) or their DMSO vehicle were included in the assay. Luminescence was quantified on a Promega GloMax Luminometer. MFN2-specific GTPase activity is GTPase activity in MFN2 expressing cells minus that for β-Gal expressing cells.
Mitofusin Conformation Forster Resonance Energy Transfer Assay
Wild-type and T105M MFN2 were engineered with Cerulean fluorescent protein fused to the amino terminus and Venus fluorescent protein fused to the carboxyl terminus. Mfn1/Mfn2 double-null MEFs were transduced for 48 hours with adenoviri expressing the Forster resonance energy transfer (FRET)-MFN2 proteins (50 MOI) for 48 hours, the cells harvested, and mitochondria isolated (Frezza et al., 2007). The FRET assay is described in detail in Dang et al. (2020). Briefly, 65 µg of mitochondrial protein in 100 µl 10mM Tris-MOPS (pH 7.4), 10mM EGTA/Tris, 200mM sucrose was added to each well of a polystyrene 96-well assay plate (Costar CAT: 3916). Mitofusin agonist peptide MP-1 (5 μM), a small molecule mitofusin activator (1 μM), or their respective vehicles (water or DMSO) were added for 4 hours at room temperature with gentle shaking. Data were acquired on a Tecan Safire II multi-mode plate reader as follows: FRET – Excitation 433 nm, Emission 528 nm; Cerulean – Excitation 433 nm, Emission – 475 nm. FRET signals were normalized to their respective cerulean signals.
Western Blotting
Protein electrophoresis and immunoblotting used standard techniques. Briefly, whole cell lysates were size-separated on 10% polyacrylamide mini-gels at 30 milliamps for ∼1 hour at room temperature before transferring to nylon membranes. After blocking for 1 hour with 5% milk, primary antibodies against Mfn2 (1:500, Abcam ab56889) or glyceraldehyde-3-phosphate dehydrogenase (1:3000, Abcam ab8245) were added for 1 hour. Immunoreactive proteins were visualized using a horseradish peroxidase linked anti-mouse IgG from Cell Signaling Technology (1:3000, cs7076) and imaged on a LI-COR Odyssey detection system.
Evaluation of Mouse CMT2A Phenotype
HB9-Cre/MFN2 T105M flox-stop mice were aged to 50 weeks to develop the complete CMT2A phenotype (Franco et al., 2020). Rotarod and neurophysiological tests of sciatic nerve/tibialis muscle function were performed at baseline and 4-week intervals thereafter as described (Franco et al., 2020). For studies comparing trans-MiM111 to CPR1-B and the crossover sub study, baseline evaluation of Rotarod latency and compound muscle action potential (CMAP) amplitude was followed by randomization of 23 mice to receive, by oral gavage, trans-MiM111 (50 mg/kg once daily), CPR1-B (60 mg/kg twice daily) or daily vehicle (10% DMSO/90% [30% hydroxypropyl-β-cyclodextrin] in water). The trans-MiM111/CPR1-B comparison study was terminated after 8 weeks, at which time 5 of the 10 trans-MiM111 treated mice, the 5 CPR1-B treated mice, and 3 of the 8 vehicle-treated mice were euthanized by anesthesia overdose and tissue specimens obtained for histologic studies.
The remaining 9 mice (4 trans-MiM111 treated and 5 vehicle treated) continued in a crossover study. Treatments were discontinued for a 16-week washout period, and the treatment groups crossed over for 8 additional weeks. One mouse (subsequently determined to be in the trans-MiM111 treatment group) was euthanized for rectal prolapse unrelated to the study; these data were not included in the analysis.
Histologic Studies
Tibialis nerves and gastrocnemius muscles were isolated and prepared for frozen sections. For histology and immunohistology, nerves were fixed in 4% paraformaldehyde for 2 hours, transferred to 30% sucrose in PBS overnight (4°C), embedded in optimal cutting temperature medium (OCT, Tissue-TEK Cat: 4583), and stored at -80°C until frozen sectioned. Gastrocnemius muscles were fixed in Acetone for 10 minutes (room temperature), washed 3 times with PBS, treated for 15 minutes in 10% Triton-X in PBS, and washed twice more with PBS. 10% goat serum was added for 15 minutes followed by Alexa Fluor 594 conjugated α-bungarotoxin overnight to stain acetyl choline receptors. Mitochondria were stained with anti-COX IV. Skeletal myocyte sarcolemma was visualized using fluorescein-conjugated wheat germ agglutinin. Mitochondrial area within neuromuscular synapses was calculated as the number of green/red pixels using Image J.
Data Presentation and Statistical Analyses
Data are reported as mean ± S.E.M., mean ± S.D., or mean with 95% confidence intervals as specified. Two-group comparisons used Student’s t test; multigroup comparisons used one-way ANOVA except for time course-by-treatment comparisons that used two-way ANOVA. Post hoc ANOVA analyses used Tukey’s test to obtain individual statistical comparisons. P < 0.05 was considered significant.
Results
Equal Responsiveness of Mitochondrial Fusion and Transport to Mitofusin Activation
The appellative function of mitofusins is initiation and promotion of mitochondrial fusion (Santel and Fuller, 2001; Dorn, 2019). Accordingly, the adverse effects of mitofusin mutants and the benefits of mitofusin activators have been described almost exclusively in terms of mitochondrial fusion, most commonly as the observed change in organelle length or aspect ratio (length/width) (Detmer and Chan, 2007; Dang et al., 2022). However, accumulating evidence underscores a critical role for mitochondrial dysmotility in some neurodegenerative diseases, including CMT2A (Baloh et al., 2007; De Vos et al., 2008; Sheng and Cai, 2012; Dorn, 2020a; Schiavon et al., 2021). Indeed, mitochondrial dysmotility in neuronal axons may be a biomarker for neurodegeneration in this disease, and improved mitochondrial motility is postulated to predict therapeutic response (Rocha et al., 2018; Franco et al., 2020; Dang et al., 2022). Because mitofusin activator dose-response relations for mitochondrial motility have not been defined, we compared concentration-dependent effects of two pharmaceutically acceptable mitofusin activators, trans-MiM111 and CPR1-B (Fig. 1), on mitochondrial fusion and motility.
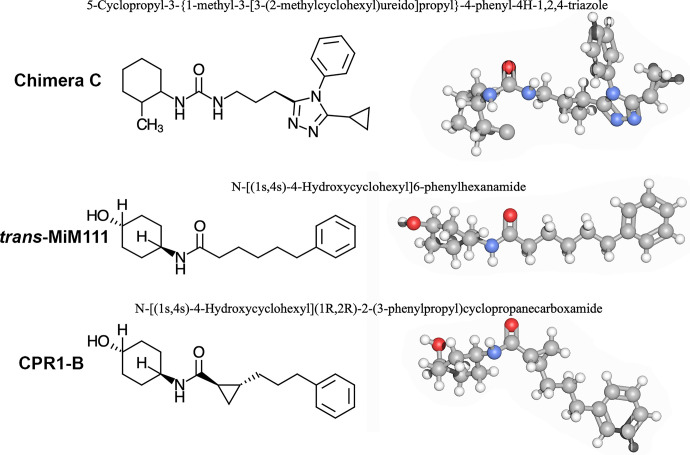
Fig. 1.
Mitofusin activators used in these studies. (top) Chimera C is the prototype for first generation triazolurea class of mitofusn activators that are not suitable for in vivo studies. (Middle) trans-MiM111 and (lower) CPR1-B are second generation phenylhexanamide mitofusin activators that have stereoisomer-specific activity and can be used in vivo.
Increased mitochondrial fusion manifests as mitochondrial elongation in mouse embryonic fibroblasts (MEFs) lacking Mfn2, in which mitochondria are atypically short or “fragmented” at baseline (Chen et al., 2003). Mitochondrial elongation can be assayed either as the increase in proportion of cells having predominantly filamentous mitochondria (Detmer and Chan, 2007) or as the increase in mitochondrial aspect ratio (organelle length/width) within individual cells (Dang et al., 2022). Here, fusogenic activities of MiM111 and CPR1 were similar when measured by either method (Fig. 2, A and B; Table 1). As reported (Dang et al., 2020, 2021), both MiM111 and CPR1 exhibited stereoisomer-specific fusogenic activity (Fig. 2A).
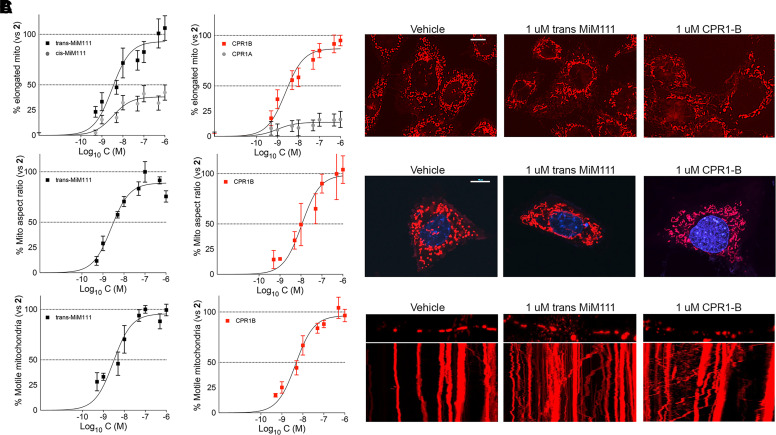
Fig. 2.
Mitofusin activator concentration-response relationships for mitochondrial fusion and motility. (A) Fusogenicity measured as the proportion of Mfn2 null MEFs having a preponderance of elongated filamentous mitochondria. Comparisons to each compound’s less active stereoisomer are shown. (B) Fusogenicity measured as mitochondrial aspect ratio (organelle length/width) in Mfn2 null MEFs. (C) Mitochondrial motility measured as % motile mitochondria in neuronal processes of MFN2 T105M expressing murine DRGs. Means ± S.E.M. of 3 experiments with 8 (A), ∼15 (B), or 4–5 (C) replicates. Representative images are shown to the right; mitochondria are stained red. Scale bars are 10 um. In Figs. 2 and 4 trans-MiM111 is black and CPR1-B is red.
TABLE 1.
Calculated data from Fig. 2 showing comparative activities of small molecule mitofusin activators for mitochondrial fusion versus mitochondrial motility
Fusogenicity was measured in Mfn2 null MEFs. Motility was measured in MFN2 T105M expressing mouse DRGs. Data are means (95% confidence interval). All concentration-response relations were indexed to independent trans-MiM111 studies.
| Compound | Fusogenicity | Motility | ||||
|---|---|---|---|---|---|---|
| % filamentous mito | Mito aspect ratio | % motile mito | ||||
| EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | |
| cis-MiM111 | NA | 38 (32–45) | ND | ND | ND | ND |
| trans-MiM111 | 3.6 (2.1–6.0) | 93 (82–104) | 3.2 (2.1–4.7) | 89 (82–96) | 3.3 (2.0–5.3) | 96 (86–105) |
| CPR1-A | NA | 14 (8.2–19.7) | ND | ND | ND | ND |
| CPR1-B | 3.3 (2.0–5.1) | 87 (79–95) | 4.9 (3.4–6.9) | 96 (89–104) | 11.2 (5.8–21) | 99 (82–115) |
NA, not applicable; ND, not determined.
The same compounds were evaluated for their effects on mitochondrial motility in dorsal root ganglion (DRG) neurons derived from mice expressing the human CMT2A MFN2 T105M mutation (Franco et al., 2020). This mutant is representative of the majority of CMT2A mutations located within the MFN2 GTPase domain (Feely et al., 2011); mitochondrial motility is severely depressed in neuronal processes of these DRG neurons (Franco et al., 2020). As shown in Fig. 2C, concentration-dependent effects of trans-MiM111 and CPR1-B to increase mitochondrial motility in MFN2 T105M neuronal processes paralleled their induction of mitochondrial elongation in fibroblasts (Fig. 2C, compare with Fig. 2, A and B). EC50 and Emax values are in Table 1. Similar mitofusin activator concentration-response relationships for mitochondrial fusion and transport strengthen the proposition that these processes are coregulated by mitofusins (Baloh et al., 2007; Schiavon et al., 2021).
Dissociation of MFN2 GTPase Activity and Allosteric Activation
Mitofusins are dynamin-family GTPases that undergo conformational changes to physically link mitochondria and promote fusion of their outer membranes (Santel and Fuller, 2001; Detmer and Chan, 2007; Knott et al., 2008; Franco et al., 2016; Dorn, 2020b, 2022). However, it is unclear how MFN GTPase activity relates to MFN protein conformation. This question has both scientific and clinical relevance as a majority of CMT2A-linked MFN2 mutations occur within the MFN2 GTPase domain (Feely et al., 2011) and are postulated to impair catalytic GTPase activity.
We considered that a combined genetic and pharmacological approach could help inform a more complete mechanistic understanding of how mitofusin-mediated mitochondrial fusion is impacted by mitofusin GTPase activity and protein conformation. Wild-type MFN2 or CMT2A mutant MFN2 T105M were expressed in murine embryonic fibroblasts (MEFs) lacking both Mfn1 and Mfn2, thus avoiding confounding effects of endogenous mitofusins; adenovirus carrying β-Gal was the negative control, reflecting responses in the absence of any mitofusin target (Fig. 3A). Compared with β-Gal expressing mitofusin null cells, WT MFN2 evoked mitochondrial elongation by increasing organelle fusion (Fig. 3B) (Detmer and Chan, 2007; Dang et al., 2022). By contrast, mitochondria of cells expressing MFN2 T105M remained severely fragmented, indicating that this CMT2A mutant has little or no intrinsic fusogenic activity (Fig. 3B). Representative triazolurea (Chimera C) and phenylhexanamide (trans-MiM111) mitofusin activators did not induce mitochondrial elongation in β-Gal expressing mitofusin null cells that lack their protein targets (i.e., absence of both Mfn1 and Mfn2), nor did they correct absence of intrinsic fusogenicity in cells expressing CMT2A mutant MFN2 T105M (Fig. 3B).
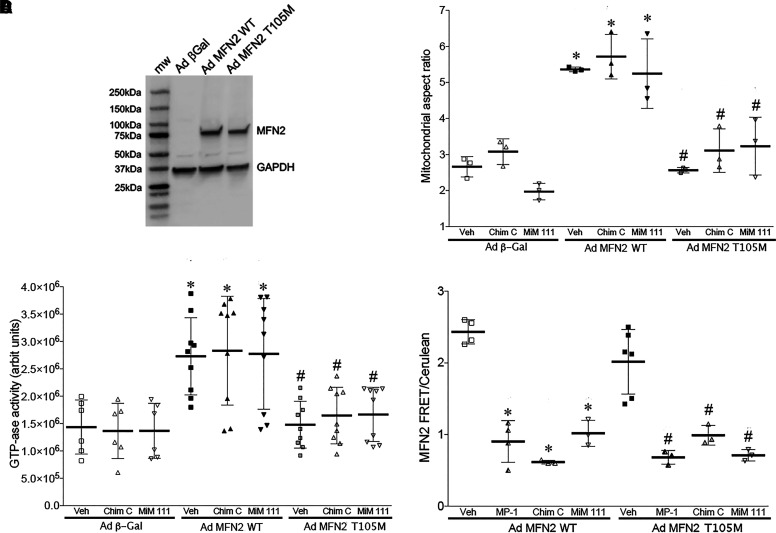
Fig. 3.
Physiochemical abnormalities of CMT2A mutant MFN2 T105M. (A) Immunoblot showing adenoviral-mediated expression of WT and mutant T105M MFN2 in Mfn1/Mfn2 double knockout MEFs. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is loading control. (B) Fusogenicity measured as the increase in mitochondrial aspect ratio after adenoviral transduction and treatment with Chimera C (Chim C) or trans-MiM111 (MiM111). Adeno β-Gal is negative control for MFN2 expression. * = P < 0.05 versus same treatment of Ad β-Gal; # = P < 0.05 versus same treatment of Ad MFN2 WT (2-way ANOVA). (C) GTPase activities after adenoviral transduction and treatment with Chimera C (Chim C) or trans-MiM111 (MiM111). * = P < 0.05 versus same treatment of Ad β-Gal; # = P < 0.05 versus same treatment of Ad MFN2 WT (2-way ANOVA). (D) Conformational relaxation of WT (left) and T105M (right) MFN2 assessed by FRET. Mini-peptide 1 (MP-1) is positive control mitofusin agonist peptide. * = P < 0.05 versus Veh for same MFN2. (B–D) Each marker represents a separate experiment performed with ∼20 (B) or 3 (C, D) replicates.
Absence of mitochondrial fusogenicity stimulated by MFN2 T105M recapitulates dysfunction reported for the MFN2 K109A mutant (4 amino acids distal in the GTPase domain) engineered to be GTPase defective (Detmer and Chan, 2007). Yet, a previous report described increased (not decreased as expected) GTP hydrolysis with T105M substitution into a fragmentary MFN2 protein lacking amino acids 1-21 and 401-705 (Li et al., 2019). We posited that deletion of almost half of the MFN2 protein might have produced an artificial result, for example, by removing MFN2 from its physiologic membrane insertion site and perturbing its orientation and/or oligomerization. Moreover, normal mitochondrial mechanisms that regulate GTPase activity were not present in the published study design. Here, we compared GTP hydrolysis by MFN2 WT and T105M expressed as intact proteins in mitofusin null cells, permitting evaluation of the complete proteins in their natural state. When MFN2 GTPase activity is assayed in situ, adenovirally expressed WT MFN2 accounts for approximately half of the total mitochondrial GTPase activity (Fig. 3C). By comparison, MFN2 T105M contributed no GTPase activity (Fig. 3C). We further observed that mitofusin activators did not affect mitochondrial GTPase activity under any of the experimental conditions (Fig. 3C). Thus, the CMT2A MFN2 GTPase domain mutation T105M abrogates enzymatic GTPase activity, which can account for its inability to promote mitochondrial fusion that requires GTP hydrolysis (Cohen and Tareste, 2018).
As introduced above, mitofusins are believed to undergo one or more conformational transitions that enable transorganelle tethering and membrane fusion. Multiple models have been proposed envisioning different MFN structures, topologies, and multimeric complexes (reviewed in Cohen and Tareste, 2018; Dorn, 2020b, 2022). Although the precise structures remain uncertain, MFN2 conformational shifts have been reliably and reproducibly measured using Forster resonance energy transfer (FRET) of recombinantly expressed MFN2 proteins engineered with amino- and carboxyl-terminal chromophores (Franco et al., 2016; Dang et al., 2020). In these assays a mitofusin activating peptide, MP1 (Franco et al., 2016), induces a relaxed conformation indicated by a lower FRET signal (increased distance between amino and carboxyl termini). Here, we assayed conformational switching induced by small molecule mitofusin activators in comparison with MP1. As previously reported (Dang et al., 2020, 2021), trans-MiM111 and CPR1-B promoted a relaxed WT MFN2 conformation (Fig. 3D, left). Thus, conformational switching induced by mitofusin activator stereoisomers paralleled their stimulation of mitochondrial fusion and motility.
To our knowledge, the impact of a CMT2A MFN2 mutation on conformational switching has not been described using intact proteins in situ. We observed that MFN2 T105M shifted to the relaxed/active conformation in response to MP1, trans-MiM111, and CPR1-B in a manner identical to WT MFN2 (Fig. 3D, right). Thus, MFN2 T105M is not impaired in its ability to change conformation. Since MFN2 T105M is GTPase defective (Fig. 3C), this finding dissociates mitofusin conformational shifting from GTP hydrolysis. The ability of mitofusin activators to act allosterically and promote the relaxed/active MFN2 conformation in GTPase-deficient MFN2 T105M mutant further dissociates these two processes.
Nonsuperiority of Sustained Versus Burst Mitofusin Activation for Reversing Murine CMT2A
The above results indicate that trans-MiM111 and CPR1-B have similar effects on mitochondrial fusion and motility in vitro and that they promote the relaxed/active MFN2 conformation without affecting catalytic GTPase activity. However, these two mitofusin activators have different in vivo pharmacokinetic and pharmacodynamic properties: trans-MiM111 is short acting and induces transient “burst” mitofusin activation when administered once daily, whereas CPR1-B has emerged as a longer acting compound that can provide sustained mitofusin activation (Dang et al., 2020, 2021). We took advantage of these features to test the hypothesis that sustained mitofusin activation would provide a more rapid or complete CMT2A response than burst activation. Because both mitofusin activators reportedly have acceptable oral bioavailability (50%–70%) (Dang et al., 2020, 2021), we also took the opportunity to determine the efficacy of oral mitofusin activator administration in this CMT2A model.
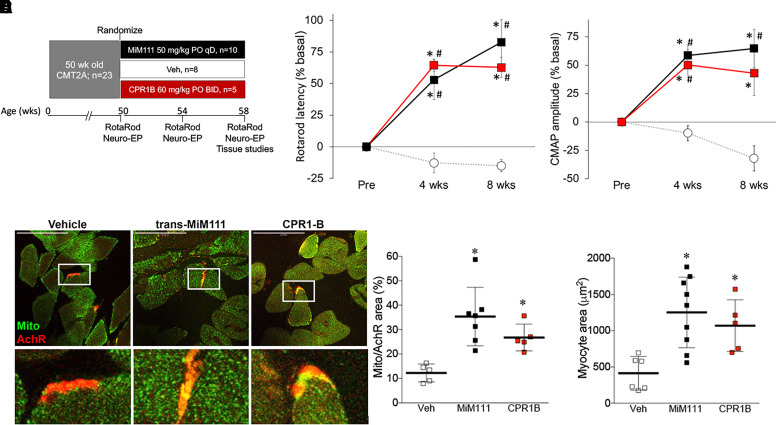
Neuromuscular degeneration in MFN2 T105M mice manifests functionally as an impaired ability to remain on a rotating cylinder (Rotarod latency) and electrophysiologically as decreased hind-limb compound muscle action potentials (CMAP) measured from sciatic nerves and tibialis muscles. Juvenile MFN2 T105M mice appear normal, but by 50 weeks of age Rotarod latency and CMAP amplitude decreased to approximately 50% of normal controls (Franco et al., 2020). Here, 50-week-old MFN2 T105M mice recapitulating these features were randomized to vehicle treatment, burst mitofusin activation with trans-MiM111 (50 mg/kg given orally once daily), or sustained mitofusin activation with CPR1-B (60 mg/kg given orally twice daily) (Fig. 4A). Sustained and burst mitofusin activation improved RotaRod latency and increased CMAP amplitude to the same extent after both 4 and 8 weeks of treatment (Fig. 4, B and C). Likewise, both mitofusin activator treatment protocols restored neuronal innervation of lower limb muscles measured as the density of neuromuscular junctions and mitochondrial residency within neuromuscular synapses, which are characteristically reduced in this CMT2A model (Franco et al., 2020) (Fig. 4, D and E). Thus, in vivo disease mitigation in MFN2 T105M CMT2A mice was similar after transient (trans-MIM111) versus sustained (CPR1-B) MFN activation.
Fig. 4.
Sustained and burst mitofusin activation reverse neuromuscular degeneration in murine CMT2A. (A) Schematic depiction of experimental design. Once daily MiM111 confers transient mitofusin activation (Black); twice daily CPR1-B confers sustained mitofusin activation (red). (B, C) Improvement in neuromuscular function of 50-week-old MFN2 T105M CMT2A mice after mitofusin activation. (B) Rotarod latency; (C) hindlimb neuroelectrophysiological study CMAP amplitude. Results for each mouse are reported as % change in baseline. * = P < 0.05 versus baseline; # = P < 0.05 versus vehicle at same time. (D) Mitochondria (green, labeled with anti-COX IV) residency within acetylcholine receptors at neuromuscular synapses (AchR; red, labeled with Alexa Fluor conjugated α-bengarotoxin). Group quantitative data are to the right. (E). Tibialis muscle myocyte cross sectional area. (D, E) * = P < 0.05 versus vehicle (ANOVA); each marker represents a different mouse.
Restoration of Mitochondrial Motility in CMT2A Mice Anticipates Reversal of Neuromuscular Dysfunction
Phenotypic reversal after mitofusin activation in CMT2A mice is thought to accrue from enhanced mitochondrial fusion and improved transport of axonal mitochondria to neuromuscular synapses (Dorn, 2021). The results reported in Fig. 2 above revealed similar concentration-dependent mitofusin activator effects on mitochondrial fusion and neuronal transport in vitro. However, it is not known how these in vitro findings translate to in vivo reversal of CMT2A-associated neuromuscular degeneration. To provide clarity on this issue we assayed dose-dependent effects of mitofusin activation on mitochondrial motility in CMT2A sciatic nerve axons (a disease-relevant pharmacodynamic endpoint) and compared the acute pharmacodynamic response to chronic effects on Rotarod and CMAP phenotype testing. Because there were no differences between trans-MiM111 and CPR1-B in vitro or in vivo (vide supra), these experiments were performed only with trans-MiM111.
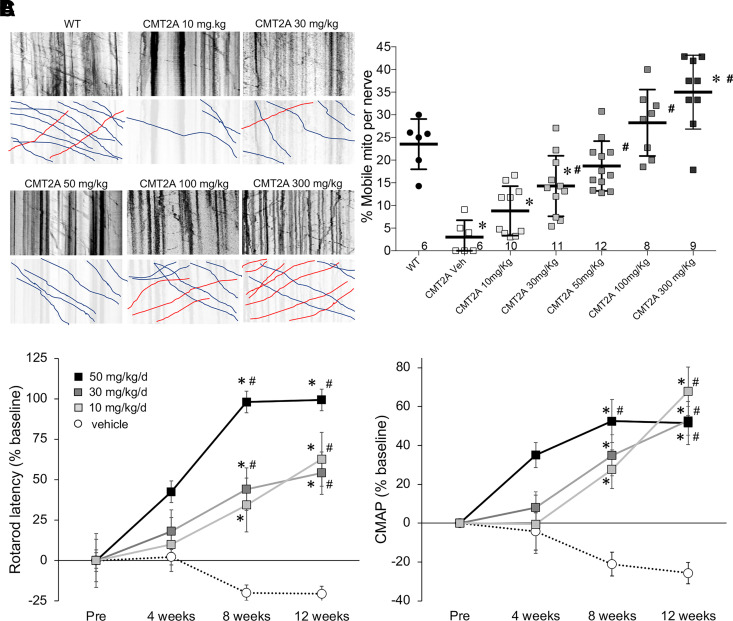
First, we defined the dose-dependent in vivo effects of trans-MiM111 on mitochondrial transport in CMT2A mice. The mitofusin activator was administered by oral gavage to naïve (i.e., no previous mitofusin activator treatment) MFN2 T105M CMT2A mice. Six hours thereafter the mice were sacrificed and their sciatic nerves placed in short-term organ culture for time-lapse confocal imaging of axonal mitochondria (Rocha et al., 2018). Approximately ∼25% of axonal mitochondria are motile in a normal mouse sciatic nerve, and this value is reduced to <5% in CMT2A mice (Fig. 5A) (Franco et al., 2020). The 50 mg/kg dose of trans-MiM111 used in the disease reversal study (see Fig. 4) increased mitochondrial motility to the normal range (Fig. 5A). The minimum dose tested, 10 mg/kg, significantly improved mitochondrial motility to approximately half the normal value (Fig. 5A), whereas the highest dose (300 mg/kg) increased mitochondrial motility to approximately 1.5 times normal (Fig. 5A).
Fig. 5.
Dose-dependent effects of mitofusin activation on mitochondrial motility and neuromuscular integrity in CMT2A mice. (A) Mitochondrial motility in CMT2A mouse sciatic nerve axons. (left) Representative kymographs showing motile mitochondria in neuronal axons 6 hours after indicated oral dosing of MiM111; mitochondria appear black. Raw data on top; bottom panels are retracings emphasizing motile mitochondria (blue is antegrade and red is retrograde transport). (right) Quantitative group mean mitochondrial motility data. Each marker represents a different neuronal axon from 2–3 mice per condition. * = P < 0.05 versus WT, # = P < 0.05 versus vehicle by ANOVA. (B and C) Effects of orally administered MiM111 given once daily on CMT2A mouse Rotarod latency (B) and CMAP amplitude (C). * = P < 0.05 versus baseline, # = P < 0.05 versus vehicle at same time point, by 2-way ANOVA.
Next, we determined the impact of trans-MiM111 daily dose on the CMT2A mouse neurodegenerative phenotype. As in Fig. 4, CMT2A mice were aged to 50 weeks to develop the full-blown disease and then treated with oral trans-MiM111 at 10, 30, or 50 mg/kg/d (the highest trans-MiM111 dose for chronic studies was limited to 50 mg/kg/d by compound solubility). Rotarod latency and CMAP amplitude were measured after 4, 8, and 12 weeks of treatment. Both metrics of neuromuscular degeneration were improved at all drug doses, but the treatment duration required for improvement was inversely related to drug dose (Fig. 5, B and C). Thus, the MFN2 T105M CMT2A mouse model exhibits analogous dose-dependent effects of mitofusin activation on the acute pharmacodynamic endpoint of mitochondrial motility and on long-term phenotype reversal.
CMT2A Recurs after Withdrawing Mitofusin Activators
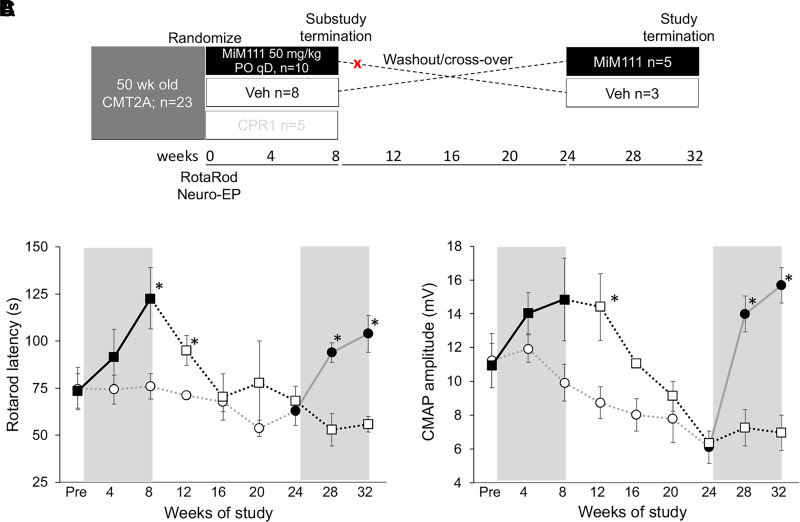
Gradual dying-back of peripheral neurons due to impaired delivery of healthy mitochondria to distal neurons is a postulated mechanism for neuropathy progression in CMT2A (Dorn, 2021). Accordingly, we envisioned that correcting mitochondrial dysfunction through mitofusin activation involves enhanced delivery of healthy mitochondria to neuronal growth buds and synapses, thus contributing to neuronal repair and regeneration. Because mitofusin activation does not correct intrinsic dysfunction of MFN2 T105M (vide supra), fusion/fission dynamics are seemingly corrected through activation of endogenous nonmutant Mfn1 and Mfn2. For this reason, we considered that mitofusion activation might have to be maintained for long-term disease amelioration and tested this notion in a randomized, blinded crossover study of oral trans-MiM111 in CMT2A MFN2 T105M mice (Fig. 6A). As in previous CMT2A mouse cohorts treated with trans-MiM111 [(Franco et al., 2020); vide supra], both functional and electrophysiological metrics of neuromuscular integrity improved within weeks of mitofusin activator treatment (Fig. 6, B and C). However, when the mitofusin activator was discontinued, neuromuscular dysfunction recurred over an 8 week period (Fig. 6, B and C). Remarkably, therapeutically naïve 74-week-old CMT2A mice in the crossover study design responded to mitofusin activation with improved exercise capacity and neuroelectrophysiological integrity comparable to the 50-week-old mice (Fig. 6, B and C). Thus, removal of the mitofusin activator is associated with disease recurrence, but even older CMT2A mice responded to mitofusin activation with marked improvement in the disease phenotype.
Fig. 6.
CMT2A recurs after mitofusin activation is withdrawn in MFN2 T105M mice. (A) Schematic depiction of experimental design for this substudy, which was a continuation of the experiment depicted in Fig. 4. Red X indicates where one mouse was withdrawn due to an unrelated medical issue. (B and C) Rotarod latency (B) and CMAP amplitude (C) results. Bold lines indicate MiM111 treatment group within gray background treatment periods. * = P < 0.05 versus corresponding vehicle group (t test).
Discussion
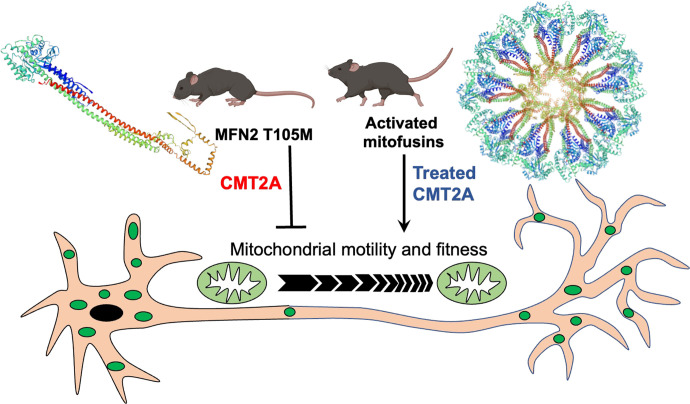
Mitochondrial fusion mediated by mitofusin GTPases is essential to organelle fitness and function (Chen and Chan, 2005). Interrupting mitochondrial fusion by combined genetic ablation of Mfn1 and Mfn2 in the mouse nervous system, skeletal muscle, or heart adversely impacts homeostasis of those tissues (Chen et al., 2007, 2010, 2011; Kasahara et al., 2013). By contrast, interrupting mitochondrial motility through germ-line genetic ablation of the Miro1 mitochondrial transport adaptor protein preferentially affects the neurologic system (Nguyen et al., 2014; López-Doménech et al., 2016). These and other findings support a paradigm where mitochondrial fusion is broadly important to cellular health, but mitochondrial motility and transport have particular importance to long neurons (Sheng and Cai, 2012). Consistent with this idea, the progressive peripheral neuropathy CMT2A, caused in most instances by mutations of MFN2, has been variously attributed to defective mitochondrial fusion, motility, or both (Frank, 2006; Herzig and Martinou, 2008; Chen and Chan, 2009; Dorn, 2020a). Here, we used recently developed research platforms and new reagents to interrogate the consequences of mutational MFN2 dysfunction on mitochondrial fusion and motility and to better understand how mitofusin activation can reverse CMT2A-associated mitochondrial pathology and neuromuscular degeneration. We found that: 1) MFN2 GTPase activity and conformational shifting are independent and mechanistically unrelated; 2) mitochondrial fusion and motility are enhanced by small molecule mitofusin activators over a similar range of drug concentrations, and improved mitochondrial motility in CMT2A neuronal axons predicts improved neuromuscular phenotype; and 3) in vivo mitofusin activation must be maintained over weeks, but need not be sustained throughout the day, to reverse and protect against neurodegeneration in murine CMT2A. The preclinical results are summarized in Fig. 7.
Fig. 7.
Schematic depiction of current study preclinical findings. CMT2A mutant MFN2 T105M is GTPase defective and therefore exhibits impaired fusion despite normal conformational shifting (unfolded monomeric configuration shown). Reduced motility of mitochondria (green ovals) through MFN2 T105M expressing neurons, together with loss of reparative mitochondrial fusion, diminishes delivery of healthy mitochondria to neuronal termini. The consequence is neuromuscular degeneration in CMT2A mice. Burst and sustained mitofusin activation of endogenous normal MFN1 and MFN2 enables MFN-MFN oligomerization (putative toroid structure shown) and restores mitochondrial motility and respiratory fitness, thereby reversing limb denervation and normalizing neuromuscular function.
MFN2 and MFN1 physically connect mitochondria across cytosolic space and mediate the critical initial steps of reparative mitochondrial fusion (Chen and Chan, 2005). Uniquely, MFN2 can act as a mitochondrial receptor for Parkin in PINK1-Parkin mediated mitophagy (Chen and Dorn, 2013; Li et al., 2022). Finally, MFN2 (and possibly MFN1) can positively affect mitochondrial motility through incompletely understood mechanisms (Misko et al., 2010). Multifunctionality of MFN2 complicates interpretation of the many published studies that relied upon gene ablation or mRNA suppression to modulate MFN2 function, which we eschewed.
MFN2 tasking is thought to be determined by its binding to different protein partners (Dorn, 2020b; Dorn and Dang, 2022). Thus, MFN-MFN interactions mediate mitochondrial fusion, MFN-Parkin pairing mediates mitophagy, and MFN-Miro pairing likely regulates mitochondrial transport. MFN2 protein partnering is, in turn, determined by the phosphorylation status of 3 MFN2 PINK1 kinase sites: T111, S378, and S442. Phosphorylation of T111 and S442 promote Parkin binding and mitophagy (Chen and Dorn, 2013; Li et al., 2022), whereas S378 phosphorylation does not affect mitophagy but instead acts as an “on-off switch” for mitochondrial fusion (Li et al., 2022). MFN2 S378 is located in the so-called “zipper domain” (MFN2 367-384) that regulates protein conformation via internal peptide-peptide interactions (Franco et al., 2016; Rocha et al., 2018). S378 de-phosphorylation increases the probability of MFN2 “unzipping” into a more relaxed protein conformation that favors mitochondrial fusion. The mitofusin activating peptide MP1 (Franco et al., 2016) and the peptidomimetic small molecule mitofusin activators used herein (Rocha et al., 2018; Dang et al., 2020, 2021) act by disrupting the intramolecular S378-regulated peptide-peptide interaction, thereby inducing a change in conformation that is more conducive to both mitochondrial fusion and motility.
The role of GTP hydrolysis as a determinant of mitofusin macromolecular structure has been studied using partial proteins in solution (Misko et al., 2012; Cao et al., 2017; Yan et al., 2018; Li et al., 2019), but whether GTP hydrolysis controls mitofusin conformation in situ remained unclear. Our data show that GTPase activity, which is abrogated by the MFN2 T105M mutation, is dispensable for conformational shifting. Furthermore, allosteric mitofusin activators induced a relaxed MFN conformation without affecting GTP hydrolysis. Thus, GTP hydrolysis is necessary for mitochondrial fusion but does not regulate and is not impacted by allosteric mitofusin activation.
Of the many putative functions for MFN2, its modulation of mitochondrial transport is probably the least understood. Multiple groups have observed impaired mitochondrial motility in CMT2A neurons (Baloh et al., 2007; Misko et al., 2012; Franco et al., 2020; Mou et al., 2021). Baloh suggested that MFN2 modulates transport by interacting with Miro proteins (Misko et al., 2010). The current observations that mitofusin activator concentration-response relationships for mitochondrial fusion and transport are virtually identical suggest that a relaxed mitofusin conformation is conducive to both mitochondrial fusion and transport. Likewise, parallel dose-response relationships for trans-MiM111 to enhance mitochondrial motility in therapeutically naïve CMT2A mouse sciatic nerve neurons and to reverse neurodegeneration in CMT2A mice supports any therapeutic approach that can restore mitochondrial motility in this condition (Dorn, 2021). It is notable that a low trans-MIM111 dose (10 mg/kg/d), which only partially corrected mitochondrial dysmotility in CMT2A sciatic nerves, was capable of improving neuromuscular degeneration in the mice, although over a longer time period than higher doses. One question raised by these results is whether mitofusin activation might also help correct mitochondrial dysmotility in other neurodegenerative conditions such as amyotrophic lateral sclerosis (Ligon et al., 2005; De Vos et al., 2007).
Mitofusin activators were only recently described and, to our knowledge, are the only small molecules that directly enhance mitochondrial fusion and transport. Multiple mitofusin activator chemical backbones have been described (Rocha et al., 2018; Dang et al., 2021; Zhang et al., 2022), each of which chemically mimic MP1, a MFN2-derived peptide that competitively inhibits peptide-peptide interactions that determine MFN1 and MFN2 folded versus unfolded tertiary structure (Franco et al., 2016; Rocha et al., 2018). Competing peptide MP1 and small molecules that mimic MP1 critical peptide side chains (Rocha et al., 2018) promote MFN extended or unfolded conformations, thereby evoking mitochondrial fusion and motility. This mechanism of action has been independently validated for the commercially available compound “MASM7” (Zacharioudakis et al., 2022), which was renamed from, but is chemically identical to, previously described Chimera parent compound “B01” (Rocha et al., 2018). Importantly, each of the mitofusin activators employed herein exhibits identical fusogenic activity for MFN1 and MFN2 (i.e., in Mfn2 null and Mfn1 null cells, respectively), and lacks fusogenic activity in cells lacking mitofusins (i.e., Mfn1/Mfn2 double null cells) (Fig. 3B; Ad-βGal transduced) (Dang et al., 2020, 2021; Franco et al., 2020).
Our study comparing oral administration of a shorter- versus a longer-acting mitofusin activator demonstrated equivalent phenotype reversal, supporting the sufficiency of intermittent or “burst” mitofusin activation in this pathophysiology. If one accepts the idea that delivery of healthy mitochondria to and removal of damaged mitochondrial from neuromuscular junctions is central to CMT2A reversal, then nonsuperiority of continuous versus burst mitofusin activation can be explained. Mitochondria at neuronal termini generate ATP that fuels neuromuscular signaling and neuronal repair (Sheng and Cai, 2012). Accordingly, impaired transport of mitochondria to neuromuscular junctions contributes to neuronal degeneration because healthy mitochondria can’t replace damaged mitochondria where they are needed, and damaged mitochondria accumulate with cytotoxic consequences. However, the “mitochondrial shuttle system” does not have to run continuously. If mitochondrial senescence takes weeks, but intermittent replacement can be achieved daily, then neuronal function and repair should be maintained with intermittent or burst activation. Our data showing that mitochondrial transport can be pushed to supraphysiological levels by very high mitofusin activator doses raises the question of whether “more” is necessarily “better” in this context. Because mitochondria being actively transported are not doing their proper work generating ATP to maintain local neuronal metabolism, we think that the therapeutic goal should be a normal level of mitochondrial motility.
Our study has limitations. First, we focused on a single CMT2A mutation, MFN2 T105M. We selected this mutant for detailed study in part because it is an exemplar for many other MFN2 amino terminal GTPase domain mutations that are the most prevalent cause of CMT2A. Additionally, complementary in vitro and in vivo experimental platforms are available for this particular CMT2A mutation. It is comforting that similar mitochondrial dysfunction has been characterized and responsiveness to mitofusin activation demonstrated in patient fibroblasts and reprogrammed motor neurons carrying this and several other CMT2A mutations (Franco et al., 2020; Dang et al., 2022). Nevertheless, our in vitro mechanistic results indicating that MFN2 GTPase activity has no effect on MFN2 conformational switching and the observed in vivo parallels between dose-dependent mitofusin activator pharmacodynamic effects on mitochondrial motility and neurodegenerative phenotype reversal will need to be tested for CMT2A MFN2 mutations that are not GTPase-defective, especially within the MFN2 hydrophobic core and carboxyl terminus. A second limitation is our exclusive use of murine cells and mouse models to relate in vitro mitochondrial pathology in live cells with in vivo neuromuscular phenotype. This is necessary until mitofusin activators are approved for human use. However, published results studying the same MFN2 T105M mutation in human CMT2A patient dermal fibroblasts (Dang et al., 2022) and reprogrammed neurons (Franco et al., 2020) suggest that the types of findings we report here are translatable across species.
Acknowledgments
G.W.D. is the Philip and Sima K. Needleman-endowed Professor at Washington University in St. Louis and a past Scholar-Innovator awardee of the Harrington Discovery Institute. Studies with trans-MiM111 and CPR1-B were performed under terms of an MTA between Mitochondria in Motion, Inc. and Washington University in St. Louis.
Abbreviations
- β-Gal
β-galactosidase
- CMAP
compound muscle action potential
- CMT
Charcot-Marie-Tooth
- CPR1-B
N-[(1s,4s)-4-Hydroxycyclohexyl] (1R,2R)-2-(3-phenylpropyl) cyclopropanecarboxamide
- DRG
dorsal root ganglion
- FRET
Forster resonance energy transfer
- MEF
murine embryonic fibroblast
- MFN
human mitofusin
- Mfn
mouse mitofusin
- MOI
multiplicity of infection
- trans-MiM111
N-(trans-4-Hydroxycyclohexyl)-6-phenylhexanamide
- WT
wild-type
Authorship Contributions
Participated in research design: Molinoff, Dorn.
Conducted experiments: Franco, Dang, Zhang.
Contributed new reagents or analytic tools: Dorn.
Performed data analysis: Franco, Dang, Zhang, Dorn.
Wrote or contributed to the writing of the manuscript: Dorn.
Footnotes
This work was supported by National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grant R42-NS115184] (to G.W.D.), the National Heart, Lung, and Blood Institute [Grant R35-HL135736] (to G.W.D.), the Muscular Dystrophy Association [Grant 628906] (to G.W.D.), and by the Harrington Discovery Institute at University Hospitals in Cleveland, Ohio (to P.B.M. and G.W.D.). G.W.D. is an inventor on patents that cover the use of small molecule mitofusin activators to treat neurodegenerative diseases and is the founder of Mitochondria in Motion, Inc., a Saint Louis based biotech R&D company that aims to enhance mitochondrial trafficking and fitness in neurodegenerative diseases.
The other authors declare no competing interests.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Baloh RH, Schmidt RE, Pestronk A, Milbrandt J (2007) Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci 27:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombelli FStojkovic TDubourg OEchaniz-Laguna ATardieu SLarcher KAmati-Bonneau PLatour PVignal OCazeneuve C, et al. (2014) Charcot-Marie-Tooth disease type 2A: from typical to rare phenotypic and genotypic features. JAMA Neurol 71:1036–1042. [DOI] [PubMed] [Google Scholar]
- Cao YLMeng SChen YFeng JXGu DDYu BLi YJYang JYLiao SChan DC, et al. (2017) MFN1 structures reveal nucleotide-triggered dimerization critical for mitochondrial fusion. Nature 542:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC (2005) Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14 Spec No. 2:R283–289. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum Mol Genet 18:R169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130:548–562. [DOI] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dorn GW 2nd (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu Y, Dorn GW 2nd (2011) Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109:1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Tareste D (2018) Recent insights into the structure and function of Mitofusins in mitochondrial fusion. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang X, Walton EK, Zablocka B, Baloh RH, Shy ME, Dorn GW 2nd (2022) Mitochondrial phenotypes in genetically diverse neurodegenerative diseases and their response to mitofusin activation. Cells 11:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang X, Williams SB, Devanathan S, Franco A, Fu L, Bernstein PR, Walters D, Dorn GW 2nd (2021) Pharmacophore-based design of phenyl-[hydroxycyclohexyl] cycloalkyl-carboxamide mitofusin activators with improved neuronal activity. J Med Chem 64:12506–12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang X, Zhang L, Franco A, Li J, Rocha AG, Devanathan S, Dolle RE, Bernstein PR, Dorn GW 2nd (2020) Discovery of 6-phenylhexanamide derivatives as potent stereoselective mitofusin activators for the treatment of mitochondrial diseases. J Med Chem 63:7033–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJChapman ALTennant MEManser CTudor ELLau KFBrownlees JAckerley SShaw PJMcLoughlin DM, et al. (2007) Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet 16:2720–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, Grierson AJ, Ackerley S, Miller CC (2008) Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci 31:151–173. [DOI] [PubMed] [Google Scholar]
- Detmer SA, Chan DC (2007) Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol 176:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW (2021) Mitofusin activation enhances mitochondrial motility and promotes neuroregeneration in CMT2A. Neural Regen Res 16:2201–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DornGW, 2nd (2019) Evolving concepts of mitochondrial dynamics. Annu Rev Physiol 81:1–17. [DOI] [PubMed] [Google Scholar]
- Dorn GW 2nd (2020a) Mitofusin 2 dysfunction and disease in mice and men. Front Physiol 11:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DornGW, 2nd (2020b) Mitofusins as mitochondrial anchors and tethers. J Mol Cell Cardiol 142:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW 2nd (2022) Neurohormonal connections with mitochondria in cardiomyopathy and other diseases. Am J Physiol Cell Physiol 323:C461–C477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW 2nd, Dang X (2022) Predicting mitochondrial dynamic behavior in genetically defined neurodegenerative diseases. Cells 11:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feely SM, Laura M, Siskind CE, Sottile S, Davis M, Gibbons VS, Reilly MM, Shy ME (2011) MFN2 mutations cause severe phenotypes in most patients with CMT2A. Neurology 76:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco ADang XWalton EKHo JNZablocka BLy CMiller TMBaloh RHShy MEYoo AS, et al. (2020) Burst mitofusin activation reverses neuromuscular dysfunction in murine CMT2A. Elife 9:e61119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AKitsis RNFleischer JAGavathiotis EKornfeld OSGong GBiris NBenz AQvit NDonnelly SK, et al. (2016) Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature 540:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S (2006) Dysregulation of mitochondrial fusion and fission: an emerging concept in neurodegeneration. Acta Neuropathol 111:93–100. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2:287–295. [DOI] [PubMed] [Google Scholar]
- Herzig S, Martinou JC (2008) Mitochondrial dynamics: to be in good shape to survive. Curr Mol Med 8:131–137. [DOI] [PubMed] [Google Scholar]
- Kasahara A, Cipolat S, Chen Y, Dorn GW 2nd, Scorrano L (2013) Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science 342:734–737. [DOI] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9:505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Dang X, Franco A, Dorn GW 2nd (2022) Reciprocal regulation of mitofusin 2-mediated mitophagy and mitochondrial fusion by different PINK1 phosphorylation events. Front Cell Dev Biol 10:868465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJCao YLFeng JXQi YMeng SYang JFZhong YTKang SChen XLan L, et al. (2019) Structural insights of human mitofusin-2 into mitochondrial fusion and CMT2A onset. Nat Commun 10:4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, LaMonte BH, Wallace KE, Weber N, Kalb RG, Holzbaur EL (2005) Mutant superoxide dismutase disrupts cytoplasmic dynein in motor neurons. Neuroreport 16:533–536. [DOI] [PubMed] [Google Scholar]
- López-Doménech G, Higgs NF, Vaccaro V, Roš H, Arancibia-Cárcamo IL, MacAskill AF, Kittler JT (2016) Loss of dendritic complexity precedes neurodegeneration in a mouse model with disrupted mitochondrial distribution in mature dendrites. Cell Rep 17:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30:4232–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko AL, Sasaki Y, Tuck E, Milbrandt J, Baloh RH (2012) Mitofusin2 mutations disrupt axonal mitochondrial positioning and promote axon degeneration. J Neurosci 32:4145–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Y, Dein J, Chen Z, Jagdale M, Li XJ (2021) MFN2 deficiency impairs mitochondrial transport and downregulates motor protein expression in human spinal motor neurons. Front Mol Neurosci 14:727552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TTOh SSWeaver DLewandowska AMaxfield DSchuler MHSmith NKMacfarlane JSaunders GPalmer CA, et al. (2014) Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci U S A 111:E3631–E3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palau F, Estela A, Pla-Martín D, Sánchez-Piris M (2009) The role of mitochondrial network dynamics in the pathogenesis of Charcot-Marie-Tooth disease. Adv Exp Med Biol 652:129–137. [DOI] [PubMed] [Google Scholar]
- Pipis MFeely SMEPolke JMSkorupinska MPerez LShy RRLaura MMorrow JMMoroni IPisciotta C, et al. (2020) Natural history of Charcot-Marie-Tooth disease type 2A: a large international multicentre study. Brain 143:3589–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AGFranco AKrezel AMRumsey JMAlberti JMKnight WCBiris NZacharioudakis EJanetka JWBaloh RH, et al. (2018) MFN2 agonists reverse mitochondrial defects in preclinical models of Charcot-Marie-Tooth disease type 2A. Science 360:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114:867–874. [DOI] [PubMed] [Google Scholar]
- Schiavon CR, Shadel GS, Manor U (2021) Impaired mitochondrial mobility in Charcot-Marie-Tooth disease. Front Cell Dev Biol 9:624823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q (2012) Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci 13:77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuppia G, Rizzo F, Riboldi G, Del Bo R, Nizzardo M, Simone C, Comi GP, Bresolin N, Corti S (2015) MFN2-related neuropathies: clinical features, molecular pathogenesis and therapeutic perspectives. J Neurol Sci 356:7–18. [DOI] [PubMed] [Google Scholar]
- Tazir M, Bellatache M, Nouioua S, Vallat JM (2013) Autosomal recessive Charcot-Marie-Tooth disease: from genes to phenotypes. J Peripher Nerv Syst 18:113–129. [DOI] [PubMed] [Google Scholar]
- Verhoeven KClaeys KGZuchner SSchroder JMWeis JCeuterick CJordanova ANelis EDe Vriendt EVan Hul M, et al. (2006) MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain 129:2093–2102. [DOI] [PubMed] [Google Scholar]
- Wolf C, Zimmermann R, Thaher O, Bueno D, Wüllner V, Schäfer MKE, Albrecht P, Methner A (2019) The Charcot-Marie Tooth disease mutation R94Q in MFN2 decreases ATP production but increases mitochondrial respiration under conditions of mild oxidative stress. Cells 8:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Qi Y, Huang X, Yu C, Lan L, Guo X, Rao Z, Hu J, Lou Z (2018) Structural basis for GTP hydrolysis and conformational change of MFN1 in mediating membrane fusion. Nat Struct Mol Biol 25:233–243. [DOI] [PubMed] [Google Scholar]
- Zacharioudakis EAgianian BKumar Mv VBiris NGarner TPRabinovich-Nikitin IOuchida ATMargulets VNordstrøm LURiley JS, et al. (2022) Modulating mitofusins to control mitochondrial function and signaling. Nat Commun 13:3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dang X, Franco A, Zhao H, Dorn GW (2022) Piperine derivatives enhance fusion and axonal transport of mitochondria by activating mitofusins. Chemistry (Easton) 4:655–668. [Google Scholar]
- Zhao CTakita JTanaka YSetou MNakagawa TTakeda SYang HWTerada SNakata TTakei Y, et al. (2001) Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell 105:587–597. [DOI] [PubMed] [Google Scholar]
- Züchner SMersiyanova IVMuglia MBissar-Tadmouri NRochelle JDadali ELZappia MNelis EPatitucci ASenderek J, et al. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36:449–451. [DOI] [PubMed] [Google Scholar]