Abstract
Purpose of review
Patients with neurogenic orthostatic hypotension (OH) frequently have hypertension in the supine position (sHTN). We review the controversies surrounding the need and safety of treating sHTN in patients with OH.
Recent findings
The presence of sHTN complicates the management of OH because treatment of one can worsen the other. New approaches have been developed to treat OH without worsening sHTN by preferentially improve standing blood pressure, such as medications that harness the patient’s residual sympathetic tone like pyridostigmine and atomoxetine, and devices such as an automated abdominal binder that targets the inappropriate splanchnic venous pooling causing OH. There is a reluctance to treat sHTN for fear of increasing the risks of falls and syncope associated with OH, thought to be more immediate and dangerous than the late complications of organ damage associated with sHTN. This, however, does not take into account that nighttime sHTN induces natriuresis, volume loss, and begets daytime orthostatic hypotension. It is possible to treat sHTN in ways that reduce the risk of worsening OH. Furthermore, novel approaches, such as the use of local heat can control nighttime sHTN, reduce nocturia and improve OH.
Summary
Although continued progress is needed, recent findings offer hope that we can treat nocturnal sHTN with the goal of improving daytime OH, strongly suggesting the controversy whether to treat or not sHTN.
Keywords: orthostatic hypotension, supine hypertension, optimal treatment, pathophysiology
1. Introduction
Orthostatic hypotension is defined as a sustained reduction of systolic blood pressure (SBP) of at least 20 mmHg, or of diastolic blood pressure (DBP) of 10 mmHg within 3 minutes of standing or at least 60-degree head-up tilt [1]. OH has a prevalence of approximately 5–6% in the general population, it increases significantly with aging, and is up to 50% in nursing homes and 68% in hospitalized elderly [2, 3]. OH significantly impairs quality of life [4], is associated with a 2.6-fold increased risk of falls [5], and is an independent risk factor of mortality [6]. The most severe forms of OH are seen in neurodegenerative disorders of the autonomic nervous system, due to the deposit of alpha-synuclein that impair neural pathways involved in cardiovascular control either in the central nervous system forming glial cytoplasmic inclusions (multiple system atrophy, MSA) or in the periphery forming Lewy bodies (Parkinson’s disease, PD and pure autonomic failure, PAF) [1].
Although the disabling nature of symptoms associated with OH dominate the clinical picture, impairment of cardiovascular autonomic regulation also leads to an increase of BP in the supine position [7, 8]. Supine hypertension (sHTN), defined by recent consensus statements as a SBP of at least 140 mmHg and/or DBP of at least 90 mmHg [7] can appear de novo or as the result of preexisting essential hypertension. It has been estimated that more than 50% of patients with OH [9], and approximately 34–46% of patients with MSA or PD [10, 11], may suffer sHTN. It is likely, however, that the actual prevalence of sHTN might be higher because sHTN is often overlooked by both patients and clinicians, in part because autonomic failure patients can remain asymptomatic even when blood pressure is very high [8]. The diagnosis of OH and sHTN can be easily made by measuring supine and upright BP and HR. However, these are rarely performed in the clinic. Only seated BP is routinely measured, and it is normal in most patients. Home BP measurements and 24-hour ambulatory BP monitoring can also help in the diagnosis [7, 12].
The presence of supine hypertension complicates the management of OH because treatment of one can worsen the other. It has been argued that management of OH should have priority over treatment of sHTN because the short-term risk of falls and syncope [13] associated with OH is greater than the long-term consequences of end-organ damage associated with sHTN, particularly in patients with reduced life expectancy. This view, however, does not take into account that supine hypertension can worsen orthostatic hypotension by inducing pressure diuresis. In this review we will discuss the hemodynamic mechanisms and pathophysiology of sHTN, including the concept of pressure diuresis. We can then apply these concepts to the management of OH with therapies that preferentially improve OH without worsening sHTN, and new treatment options for sHTN that may improve OH. Development and validation of these novel approaches will be transformative, solving the argument whether or not to treat sHTN in patients with OH.
2. Pathophysiology of the hypertension of autonomic failure
Long-acting pressor agents used in the treatment of OH, most notably fludrocortisone, can induce sHTN. Supine hypertension can also be due to the worsening of preexisting essential hypertension because of loss of baroreflex buffering capacity characteristic of autonomic failure. Finally, sHTN can arise de novo, as part of the impairment in autonomic cardiovascular regulation. Hemodynamically, sHTN is driven by an increase in systemic vascular resistance [14]. The pathophysiology of this paradoxical vasoconstriction differs depending on the underlying cause of autonomic failure. In patients with MSA, central autonomic pathways are impaired but efferent noradrenergic fibers are preserved, as evidenced by normal or only slight reduced plasma norepinephrine [15]. In these patients, ganglionic blockade with trimethaphan, by eliminating this residual sympathetic tone, dramatically reduces supine blood pressure to normotensive levels [16, 17]. Thus, in MSA patients sHTN is driven by residual sympathetic tone.
In patients with peripheral forms of autonomic failure, such as PAF and PD, efferent autonomic fibers degenerate, as reflected by low plasma norepinephrine. Residual sympathetic tone may still contribute to sHTN in mildly affected patients, but in patients with severe forms of the disease sHTN is unresponsive to trimethaphan [17] suggesting that sympathetically independent mechanisms are involved in its genesis. The driving force of the increased systemic vascular resistance in peripheral forms of autonomic failure is not clear. Nitric oxide mechanisms are not only intact in these patients but can even contribute to their OH [18], and potentiation of nitric oxide-induced vasodilation can be used to treat their sHTN [18, 19]. Supine hypertension also responds to blockade of angiotensin receptors with losartan [20] and mineralocorticoid MR receptors with eplerenone [21], suggesting that these mechanisms contribute to sHTN.
2. Treating OH Without Worsening sHTN
Ideally, therapies for OH would selectively improve upright BP without increasing supine BP. Most pressor agents used in OH, however, increase both supine and upright BP, and some increase supine BP more than standing. Fludrocortisone is an example of the latter and whenever possible it should be avoided in patients with sHTN because it has a poorer safety profile than midodrine, particularly in patients with heart failure [22]. Midodrine also can worsen sHTN but the risks can be reduced by having patients avoid the supine posture for 3–4 hours after each dose. Newer therapies like atomoxetine [23] and droxidopa [24] may have a lower risk of worsening sHTN but comparative studies are not available. Oral water bolus can also provide an effective approach to improve upright BP [25] because its effect is short-lived enough that supine posture can be avoided.
Pyridostigmine, a cholinesterase inhibitor that blocks the hydrolysis of acetylcholine in the synaptic cleft can be used to treat OH by preferentially increasing BP while standing without worsening sHTN (Table 1) [26–28]. The theoretical basis of its use is that it facilitates neurotransmission at the level of the autonomic ganglia, thus amplifying the increase in sympathetic tone that occurs on standing. It is particularly useful in milder patients with sufficient “sympathetic reserve” but may not benefit severely affected patients. For this type of patients, yohimbine is a better alternative [29]. Yohimbine is an alpha-2 antagonist, pharmacologically opposite to clonidine; it increases central sympathetic outflow and potentiates the release of norepinephrine from postganglionic noradrenergic fibers [28].
Table 1.
Advances in pharmacologic and nonpharmacologic treatments for OH and sHTN.
| Mechanism of action | Recommended dosage | Outcomes | |
|---|---|---|---|
| Treatment of OH that do not worsen sHTN | |||
| Pyridostigmine [55] | Cholinesterase inhibitor, facilitates autonomic ganglia neurotransmission | 30–60 mg, orally, up to 3 times/day | improve OH without worsening supine hypertension |
| Automated inflatable abdominal binder [30] | Target splanchnic venous pooling | servo-controlled venous compression (40 mm Hg) | Ameliorates OH without aggravating supine hypertension. As effective as midodrine (SBP increase: 10 mm Hg). |
| Treatment of nocturnal sHTN with short-acting antihypertensives | |||
| Captopril [20] | angiotensin-converting enzyme (ACE) inhibitor | 50 mg, orally, at bedtime | no definite effect on supine blood pressure |
| Clonidine [46] | Central alpha-2 agonist | 0.1mg, orally, with dinner | SBP decrease: 29 mm Hg |
| Eplerenone [21] | mineralocorticoid receptor antagonist | 50 mg, orally, at bedtime | SBP decrease: 32 mm Hg |
| Sildenafil [19] | Phosphodiesterase inhibitor, potentiates nitric oxide | 25 mg, orally, at bedtime | SBP decrease: 24 mm Hg |
| Losartan [20] | angiotensin II receptor antagonist | 50 mg, orally, at bedtime | SBP decrease: 32 mm Hg |
| Nifedipine [56] | calcium channel blocker | 30 mg extended-release, orally, at bedtime or 10 mg short-acting. | SBP decrease: 37 mm Hg, but increase nocturnal natriuresis (30 mg short-acting nifedipine). |
| Nitroglycerin patch [9] | Vasodilator, nitric oxide donor | 0.1 mg/h patch at bedtime, remove in the morning | SBP decrease: 36 mm Hg |
| Treatment of nocturnal sHTN that can improve daytime OH | |||
| Head-up and bed tilt [7, 48, 57] | induce venous pooling below the level of the heart, reduce renal hyper-perfusion and reduce pressure diuresis | raise head by tilting the whole bed (10 degrees) or by using pillows (30 degrees) | Mitigates the severity of supine hypertension (not systematically assessed). Upright SBP increase: 11 mm Hg |
| Local passive heat [54] | peripheral vasodilation and blood redistribution | 38°C heating pad placed under the torso | Supine SBP decrease: 19–28 mm Hg. Upright SBP increase: 19 mmHg (in patients with ≥20 mmHg drop in SBP with heat) |
Whereas most therapies for OH have relied on increasing blood pressure by inducing arterial vasoconstriction, the primary hemodynamic mechanism of OH is gravity-induced venous pooling, mostly in the splanchnic circulation, that leads to a dramatic reduction in venous return and stroke volume. Compression garments, applied to the lower body, have been used to treat OH by improving venous return, but are difficult for patients to apply at a constant effective pressure. An alternative option recently developed is an automated abdominal binder that senses upright posture and inflates to a servo-controlled constant pressure (Table 1) [30]. Preliminary studies suggest that this device is as effective in improving upright BP as the alpha- agonist midodrine, arguably the most frequently used medication in the treatment of OH. The device is activated only on standing, so it has no effect on seated or supine BP.
Thus, several alternatives are now available to improve orthostatic hypotension while reducing the risk of worsening sHTN.
3. Risks Associated with OH and with sHTN, and the Dilemma of Which to Treat
The incidence of OH and hypertension increases with aging, so it is not surprising that they often coexist. Indeed, hypertension is the most common comorbidity in cohorts of individuals with OH [31]. There are known long-term risks associated with either OH and hypertension. OH is an independent risk factor of overall mortality, even after considering traditional risk factors including hypertension [6]. OH is not only associated with increased cardiovascular mortality, but also with non-cardiovascular mortality; it is not clear if this represents a causal relation or if OH is a marker of frailty or other risk factors. In the general population, hypertension is also associated with well-known risks, including end-organ damage (e.g., heart, kidney, and brain) and increased mortality. Thus, there are compelling reasons to treat both conditions, but until recently it has not been clear if treating hypertension will worsen OH. This potential treatment dilemma appears to be resolved in the general population. Hypertension is not only associated with OH, but the incidence of OH and falls is greatest in patients with uncontrolled hypertension than in those with controlled hypertension or normotension [32]. Observational studies have shown that there is a relationship between OH and hypertension, but not with the use of antihypertensive medications, suggesting that treatment of hypertension does not have a negative impact on OH [33]. A more recent meta-analysis of large randomized clinical trials for the treatment of essential hypertension has shown that intense BP-lowering treatment actually decreased the risk for OH [34].
On the other hand, in patients with autonomic failure, there is still disagreement whether or not to treat sHTN in part because of uncertainties about the risk/benefit analysis. Cross-sectional studies have shown that sHTN is associated with cardiac hypertrophy [35, 36] and impaired renal function [37], indicating that impaired sympathetic function does not confer protection from end-organ damage. Based on data from the general population, one can infer that this end-organ damage would lead to cardiovascular and renal disease, stroke, and death, but large epidemiological studies are not available and unlikely to be undertaken. In can be argued that the decision to treat sHTN with the goal of preventing long-term adverse events should be individualized considering the patient underlying diagnosis and prognosis. For example, patients with MSA have shorter life expectancy because of the progression of neurodegeneration [38] and may not benefit from aggressive treatment of sHTN [39]. On the other hand, patients with PAF have a better prognosis and generally survive longer and may reap a greater long-term benefit of treating sHTN compared to MSA.
Because of these uncertainties, many have advocated that treatment of OH should be prioritized over management of sHTN. The main argument is that the short-term risks associated with OH are greater than the potential long-term risks of sHTN. Of particular concern is the risk of falls known to be increased in OH patients [13]. The risk of falls is particularly concerning in patients with movement disorders, and a common cause of hospitalizations in PD. The cause of falls is likely multifactorial in PD, but OH is believed to be a contributing factor. Another concern is the association between OH and cognitive impairment and dementia (see [13] for review of the evidence). Thus, it has been suggested that management of OH should be prioritized over treatment of sHTN [13].
It is not known, however, if treatment of sHTN will increase or lower the risk of falls or the development of dementia. Furthermore, the presence of sHTN is also associated with an increased risk of dementia. For example, the presence of sHTN is associated with brain white matter lesions [11, 40] and cognitive dysfunction [11] in patients with MSA and PD. Also, reverse dipping (i.e., blood pressure paradoxically increasing during sleep), often seen in autonomic failure patients, is associated with small vessel cerebrovascular disease and subsequent cognitive impairment in the general elderly population [41].
4. Nocturnal sHTN begets Daytime OH
Even if the long-term negative consequences of sHTN are not completely known, sHTN can have an immediate negative impact to OH because of nocturnal pressure diuresis, which was discussed shortly in the introduction. Not only high blood pressure itself affects pressure diuresis, but also chronic hypertensive status may worsen arterial blood pressure, and thereby aggravate pressure diuresis through a vicious cycle (i.e., progressive renal damage resulting in an additional increase in arterial pressure and glomerular hydrostatic pressure, leading to worsening of renal functionn) (Figure 1) [42]. Autonomic failure patients have a reversal of the normal diurnal pattern of natriuresis and lose approximately twice more urinary sodium (on average 4 g), and volume (on average 1.3 L) during the night compared to the day. This explains why patients often experience worsening of their OH symptoms early in the morning. This nocturia not only impairs sleep and quality of life but may also increase the risk of syncope and falls during the night.
Figure 1. Supine nocturnal hypertension begets daytime OH because of pressure diuresis.
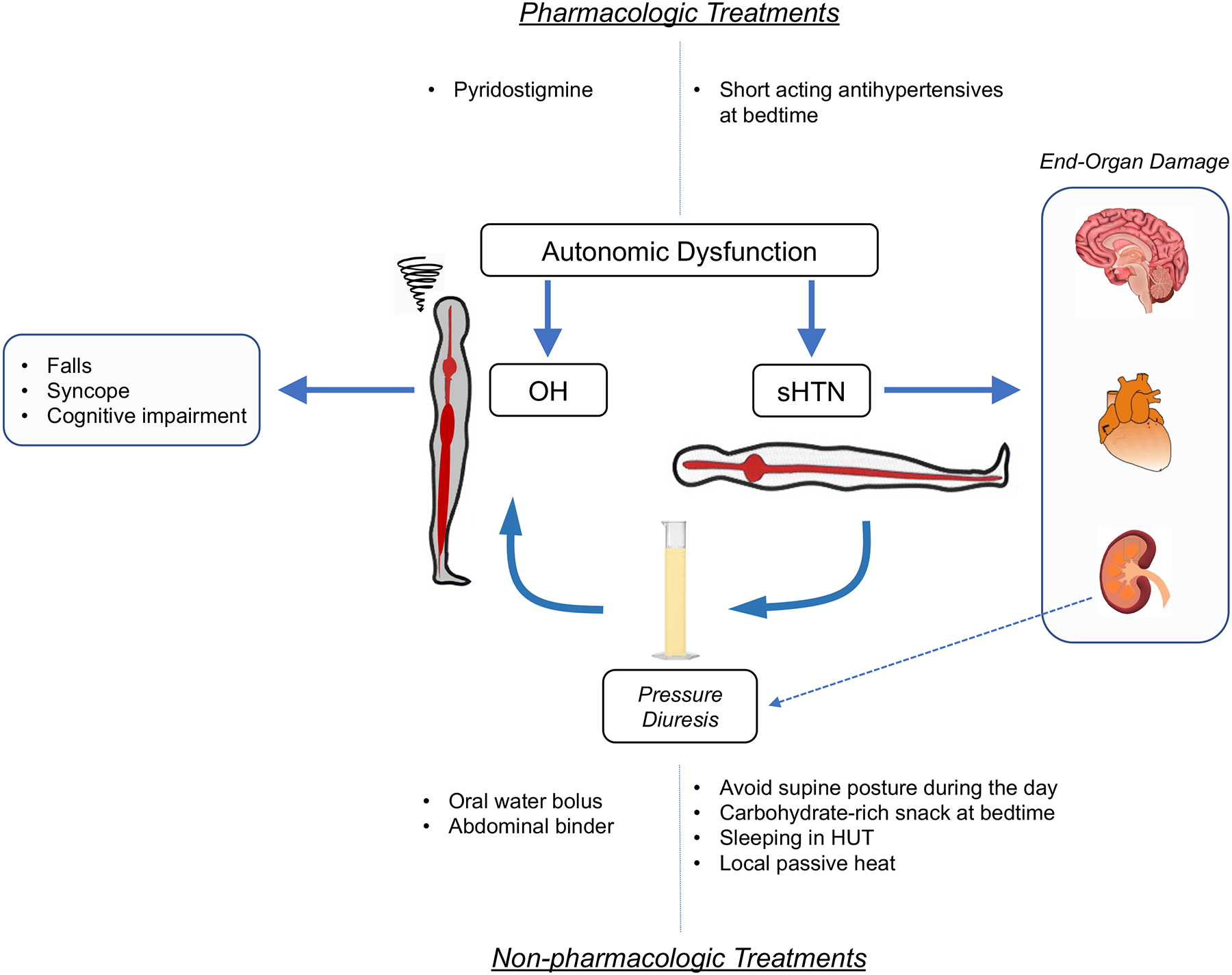
Impaired autonomic cardiovascular regulation leads to symptomatic OH which increases the risk of falls and syncope. It also worsens preexisting essential hypertension or causes de novo sHTN that is associated not only with known long-term end organ damage, but can also contribute to daytime OH by inducing nocturnal pressure diuresis. Pharmacologic and non-pharmacologic approaches can be used to treat OH while reducing the risk of worsening sHTN, and to treat sHTN while reducing the risk of worsening OH, as discussed in the text.
Thus, there is an argument to be made that treating sHTN, in ways that reduce the risk of worsening OH, would be beneficial to these patients. Novel therapeutic approaches, reviewed below, also raise the possibility that it may be possible to treat sHTN in a way that would improve OH.
5. Treating sHTN While Reducing the Risk of Worsening OH
Most patients with OH and sHTN have normal seated BPs. Thus, during the day, the most effective way to prevent hypertension without worsening OH is simply to avoid the supine position, particularly after taking pressor drugs to treat OH, or while wearing compression stockings or abdominal binders. If rest is needed during the day, patients should sit in a reclining chair with their feet on the floor. A few patients, however, can still be hypertensive while seated, which greatly complicates their management. Short-acting antihypertensives that are less likely to worsen OH such as angiotensin II receptor blockers (e.g., losartan) and calcium channel blockers (e.g., nifedipine) might be considered (Table 1). Alpha blockers and diuretics can exacerbate OH and should be avoided.
While sHTN can be effectively controlled by avoiding recumbency during the daytime, it remains a management problem during nighttime in most patients. However, about one-third of patients with sHTN retain the normal BP dipping pattern, with elevated levels early in the night that then dip to normal values, with a nadir around 4 AM [43]. A 24-hr ambulatory BP monitor, time-programmable monitor, or manual BP measurements at 4 AM can be useful to detect this pattern. Antihypertensive medications may not be necessary, and these patients may only require ingestion of a carbohydrate-rich snack at bedtime to induce postprandial hypotension and transiently lower BP [44].
In patients with sustained sHTN throughout the night (i.e., nocturnal sHTN), treatment has been focused on the use of short-acting antihypertensive drugs given at bedtime [7, 12]. The appropriate choice of medication is based on small, single-dose clinical trials showing short-term decreases in nighttime BP.
Plasma renin activity is undetectable in most patients with OH, likely due to renal sympathetic denervation [45]. Paradoxically, angiotensin II levels are elevated, and aldosterone levels are normal, suggesting that these downstream products of the renin-angiotensin pathway may contribute to the sHTN of autonomic failure [20, 21]. Indeed, the AT1 blocker losartan (50 mg at bedtime) and the mineralocorticoid receptor antagonist eplerenone (50 mg at bedtime) decreased nighttime SBP by about 32 mmHg at 6 and 8 hours after administration, respectively (Figure 2) [20, 21]. In contrast, the angiotensin-converting enzyme inhibitor captopril had less of an effect on SBP [20]. Nighttime natriuresis and fluid loss were only decreased by losartan, and none of them improved morning OH [20, 21].
Figure 2. Comparison of therapies available for treatment of Nocturnal sHTN.
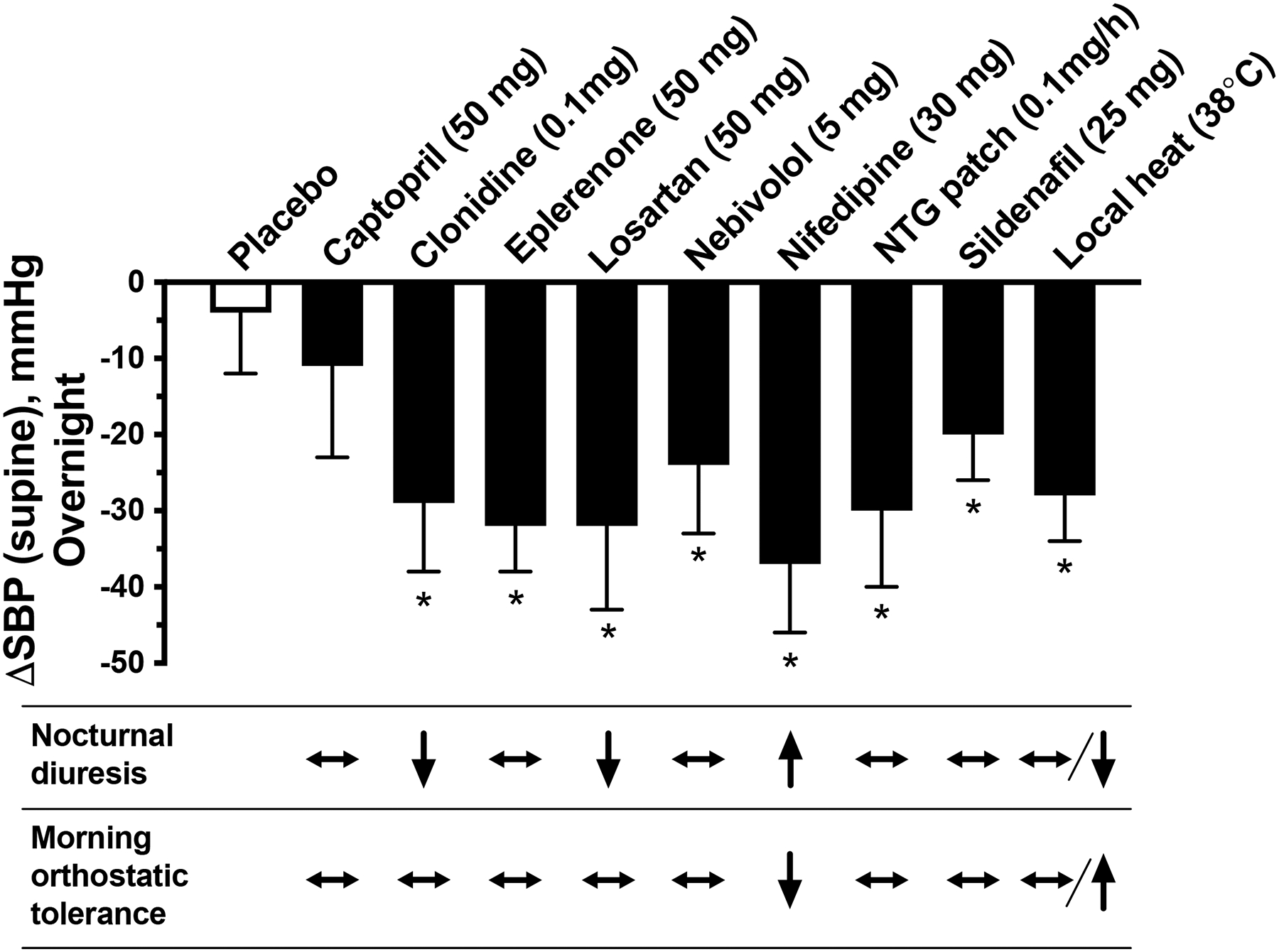
Bar-graph depicts the BP-lowering effects of different therapies shown as maximal reductions in nocturnal supine systolic BP from baseline (ΔSBP) after a single dose (PO) of the different medications given at 8 PM, or local heat (38°C) applied from 10 PM to 6 AM. *P<0.05 vs. placebo. Values are expressed as mean±SEM.
The table depicts the qualitative effect of these interventions on nocturnal diuresis (8 PM to 8 AM), and morning orthostatic tolerance (i.e., ability to endure orthostatic BP drop or hypotension) determined during a 10-minute standing test at 8 AM.
Patients with autonomic failure are exquisitely sensitive to nitric oxide donors. Transdermal nitroglycerin patches (0.1 mg/h) applied at bedtime and removed in the morning have been effective in reducing BP in these patients [25, 46]. SBP maximally decreased by 36 mmHg 4 hours after administration (Figure2). Increasing nitric oxide bioavailability with the phosphodiesterase 5 inhibitor sildenafil (25 mg at bedtime) also lowered nocturnal BP with a maximal SBP reduction of 24 mmHg at 8-hour post drug [19]. Similar antihypertensive effects were seen with nebivolol (5 mg at bedtime), a selective β1-receptor blocker with nitric oxide-mediated vasodilating properties [19]. This effect is not mediated through beta-blockade, because metoprolol had no BP-lowering effect [19]. None of the medications, however, had an effect on nocturnal urinary sodium excretion or orthostatic tolerance in the morning [19, 25, 46].
The short-acting calcium channel blocker nifedipine (30 mg at bedtime) induces sustained reductions in BP throughout the night with maximal reductions in SBP of 37 mmHg 4 hours after administration (Figure 2) [25]. Despite this effect, it induced nocturnal natriuresis and worsened orthostatic hypotension the next morning, which limits the utility of calcium channel blockade in the management of sHTN. If used, we recommend 30 mg extended-release at bedtime or 10 mg short-acting.
Central sympatholytic drugs such as the α-2 agonist clonidine (0.1 mg in the evening) have been effective in controlling sHTN in patients with residual sympathetic function, particularly in those with central autonomic dysfunction such as MSA patients [46]. Clonidine produced a maximal decrease in BP of 29 mmHg after 6 hours of administration and reduced nighttime natriuresis (Figure 2). Morning orthostatic tolerance, however, was not improved likely due to residual effects of the medication [46]. This agent should be administered in the afternoon rather than at bedtime, to reduce this residual hypotensive effect in the morning.
It is important to note that these studies were single-dose inpatient trials in which the possibility of worsening OH during the night was not systematically assessed. Although none of these studies reported increased nocturnal falls during treatment (e.g., when they stand up at nighttime to urinate), patients should still be advised about this potential risk, and the use of a urinal or bedside commode should be encouraged.
Thus, several antihypertensives are effective in lowering BP during the night. However, the goal of treating nocturnal sHTN is not only to lower BP to prevent end-organ damage and reduce cardiovascular risks, but also to decrease nighttime pressure diuresis and nocturia, and thereby improve daytime OH and quality of life. Unfortunately, no medication studied so far has met all the criteria for an ideal therapy for sHTN. Of the medications described above, only clonidine and losartan reduced nighttime diuresis, and none improved morning orthostatic tolerance (Figure 2). Thus, there is an unmet need to develop an alternative approach that would effectively treat nocturnal hypertension while improving nocturia and daytime OH.
6. Treating sHTN to improve OH
Sleeping in a head-up tilt (HUT) position has long been advocated in the management of sHTN. Small observational studies showed that this is one of the few interventions that both decreased nocturnal natriuresis and improved morning OH and symptoms, even in patients without sHTN [47, 48]. To be effective, however, this approach required sleeping with the entire bed head-up tilted by at least 12° (~16-inch elevation of the head of an average bed) [47, 48]. This degree of tilt limits the effectiveness of this approach because it is difficult for patients (and their caregivers) to tolerate [44, 49]. Lesser degrees of HUT are better tolerated but also less effective. In the only randomized clinical trial assessing the efficacy of HUT sleeping, a HUT of 5° (~6-inch elevation of the head of the bed) failed to improve morning OH [50].
A 10° of HUT (~13-inch elevation of the head of the bed) is the highest degree of tilt that patients can tolerate [44], and one that can be achieved by standard electric beds or by placing a wedge under the mattress or blocks under the legs of the bed’s headboard. However, the efficacy and tolerability of this degree of tilt have not been assessed systematically. In our experience, this degree of HUT produces only modest decreases in BP in most patients. Finally, patients should be advised of the potential risk of falls when getting out of a tilted bed. Alternatively, sleeping with only the head of the bed tilted up by 30° (i.e., semi-recumbent position) has been suggested, especially for patients not tolerating tilting of the whole bed [44]. The efficacy of this approach, however, has yet to be tested.
A common complain of patients with OH is that exposure to ambient heat significantly worsens their OH symptoms (i.e., heat intolerance) [51]. Ambient heat induces nitric oxide-dependent skin vasodilation and normal compensatory autonomic responses prevent BP from falling. Heat-induced skin vasodilation is preserved in these autonomic failure patients [52, 53], and because these patients have increased sensitivity to the vasodilatory effects of nitric oxide, we tested whether heat had an acute BP-lowering effect and whether this effect can be used to treat their nocturnal sHTN [54]. We found that low levels of local passive heat (40–42°C applied with a heating pad placed over the abdomen for 2 hours) had an acute and profound BP-lowering effect (maximal decrease in SBP of 19 mmHg), with a rapid onset of action and recovery (<30 minutes). We then conducted a proof-of-concept study with overnight local passive heat therapy (38°C applied with a water-perfused heating pad placed under the torso from 10 PM to 6 AM). We found that it effectively lowered nocturnal BP, resulting in a maximal SBP reduction of 28 mmHg, an effect comparable to that of pharmacologic antihypertensive treatments we have previously studied (Figure 2) [19–21, 25, 46]. Nocturnal urinary loss and morning OH improved in patients with greater BP responses to overnight heat. Of note, overnight heat therapy was well-tolerated, and more importantly, nocturnal body temperature was not significantly affected. Our findings suggested that overnight heat therapy may offer a novel non-pharmacologic approach to treat nocturnal sHTN, one of the few that meets the criteria for an ideal treatment of sHTN. However, future studies are still needed to assess the long-term efficacy and safety of this approach.
6. Conclusions
Impairment of the autonomic nervous system causes disabling orthostatic hypotension. The loss of cardiovascular regulation also causes supine hypertension, which is seen in a majority of patients. In patients with preserved efferent noradrenergic fibers (e.g., MSA), sHTN is driven by residual but unregulated sympathetic tone and can be reversed with central sympatholytics or ganglionic blockers. In patients with peripheral forms of autonomic denervation (e.g, pure autonomic failure and Parkinson’s disease) the sHTN is driven by increased vascular resistance of unclear etiology. The presence of sHTN complicates the treatment of OH and vice versa. Whenever possible, treatment of OH that worsens sHTN (e.g., fludrocortisone) should be avoided in favor of approaches that have little (pyridostigmine) or no effect (abdominal binders) on sHTN.
Supine hypertension is associated with end-organ damage (cardiac hypertrophy and renal impairment), but there has been a reluctance to treat sHTN for fear of worsening OH. However, in most patients, it is possible to treat sHTN while decreasing the risk of aggravating OH. During the day, simply avoiding the supine position is all that is needed in most patients. About one-third of patients with sHTN have a preserved nighttime BP dipping pattern and may only require a high-carbohydrate snack at bedtime to transiently lower BP.
Patients with sustained nocturnal sHTN can be treated with short-acting antihypertensives and several have been shown to effectively control nocturnal sHTN. However, the ideal management approach should also reduce nocturia and natriuresis, to improve quality of life and daytime OH. None of the medications tested so far meet these goals, but the novel development of non-pharmacological approaches like the application of passive heat show promise. If validated, local passive heat, and other non-pharmacological therapies to be developed, would be transformative, solving the controversy whether to treat, or not, sHTN. They offer the promise to treat nocturnal sHTN with the goal of improving daytime OH.
Funding
This work was supported by the National Institutes of Health (NIH) grants R01 HL144568, HL149386, U54 NS065736, R01 HL122847 and UL1 TR002243, and by the Overton and Jeannette Smith Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the NIH.
Conflicts of interest/Competing interests
Italo Biaggioni has received consultant fees and research support from Lundbeck and Theravance Biopharma, Inc for the development of therapies for orthostatic hypotension. Italo Biaggioni and Luis Okamoto are patent holders for the use of an automated binder in the treatment of orthostatic hypotension. Jin-Woo Park declares no conflict of interest.
Footnotes
Publisher's Disclaimer: This version of the article has been accepted for publication, after peer review (when applicable) and is subject to Springer Nature’s AM terms of use, but is not the Version of Record and does not reflect post-acceptance improvements, or any corrections. The Version of Record is available online at: https://doi.org/10.1007/s12350-021-02811-7
Consent for publication
All authors have approved the final version of the manuscript and provided consent for publication.
References
- 1.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. [DOI] [PubMed] [Google Scholar]
- 2.Farrell MC, Shibao CA. Morbidity and mortality in orthostatic hypotension. Auton Neurosci. 2020;229:102717. [DOI] [PubMed] [Google Scholar]
- 3.Aung AK, Corcoran SJ, Nagalingam V, Paul E, Newnham HH. Prevalence, associations, and risk factors for orthostatic hypotension in medical, surgical, and trauma inpatients: an observational cohort study. Ochsner J. 2012;12(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Kim N, Park J, Hong H, Kong ID, Kang H. Orthostatic hypotension and health-related quality of life among community-living older people in Korea. Qual Life Res. 2020;29(1):303–12. [DOI] [PubMed] [Google Scholar]
- 5.Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. The American journal of medicine. 2000;108(2):106–11. [DOI] [PubMed] [Google Scholar]
- 6.Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100(5):406–13. [DOI] [PubMed] [Google Scholar]
- 7. •.Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS) : Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res. 2018;28(4):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]; A Consensus statement, based mostly on expert opinion, about the definition and diagnosis of supine hypertension in the context of cardiovascular autonomic failure.
- 8.Biaggioni I Orthostatic Hypotension in the Hypertensive Patient. Am J Hypertens. 2018;31(12):1255–9. [DOI] [PubMed] [Google Scholar]
- 9.Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30(5):1062–7. [DOI] [PubMed] [Google Scholar]
- 10.Fanciulli A, Göbel G, Ndayisaba JP, Granata R, Duerr S, Strano S et al. Supine hypertension in Parkinson’s disease and multiple system atrophy. Clin Auton Res. 2016;26(2):97–105. [DOI] [PubMed] [Google Scholar]
- 11.Umehara T, Matsuno H, Toyoda C, Oka H. Clinical characteristics of supine hypertension in de novo Parkinson disease. Clin Auton Res. 2016;26(1):15–21. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. •.Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15(9):954–66. [DOI] [PubMed] [Google Scholar]; This review compares the risks associated with orthostatic hypotension and with supine hypertension, and argues that treatment for orthostatic hypotension should take priority over the management of supine hypertension because of the immediate risks of the former.
- 14.Kronenberg MW, Forman MB, Onrot J, Robertson D. Enhanced left ventricular contractility in autonomic failure: assessment using pressure-volume relations. J Am Coll Cardiol. 1990;15(6):1334–42. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DS, Polinsky RJ, Garty M, Robertson D, Brown RT, Biaggioni I et al. Patterns of plasma levels of catechols in neurogenic orthostatic hypotension. Annals of Neurology. 1989;26:558–63. [DOI] [PubMed] [Google Scholar]
- 16.Biaggioni I The Pharmacology of Autonomic Failure: From Hypotension to Hypertension. Pharmacol Rev. 2017;69(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D et al. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101(23):2710–5. [DOI] [PubMed] [Google Scholar]
- 18.Gamboa A, Shibao C, Diedrich A, Paranjape SY, Farley G, Christman B et al. Excessive nitric oxide function and blood pressure regulation in patients with autonomic failure. Hypertension. 2008;51(6):1531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto LE, Gamboa A, Shibao CA, Arnold AC, Choi L, Black BK et al. Nebivolol, but not metoprolol, lowers blood pressure in nitric oxide-sensitive human hypertension. Hypertension. 2014;64(6):1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D et al. Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension. 2013;61(3):701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. •.Arnold AC, Okamoto LE, Gamboa A, Black BK, Raj SR, Elijovich F et al. Mineralocorticoid Receptor Activation Contributes to the Supine Hypertension of Autonomic Failure. Hypertension. 2016;67(2):424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; A single dose of eplerenone acutely reduced nocturnal supine hypertension by ~30 mm Hg in patients with severe autonomic failure. These findings suggest that mineralocorticoid receptor activation contributes to supine hypertension in autonomic failure; the acute effects of eplerenone suggest that non-canonical mineralocorticoid actions.
- 22. •.Grijalva CG, Biaggioni I, Griffin MR, Shibao CA. Fludrocortisone Is Associated With a Higher Risk of All-Cause Hospitalizations Compared With Midodrine in Patients With Orthostatic Hypotension. J Am Heart Assoc. 2017;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]; Fludrocortisone is one of the earliest treatments for orthostatic hypotension. Despite its wide use, there is little evidence about its safety and efficacy. This pharmacoepidemiologic study found that, compared to midodrine, users of fludrocortisone had higher rates of all-cause hospitalizations, especially in patients with congestive heart failure.
- 23.Ramirez CE, Okamoto LE, Arnold AC, Gamboa A, Diedrich A, Choi L et al. Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2014;64(6):1235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biaggioni I, Arthur Hewitt L, Rowse GJ, Kaufmann H. Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol. 2017;17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water. Lancet. 1999;353(9154):723. [DOI] [PubMed] [Google Scholar]
- 26.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. JNeurolNeurosurgPsychiatry. 2003;74(9):1294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, Hines S et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Archives of Neurology. 2006;63(4):513–8. [DOI] [PubMed] [Google Scholar]
- 28.Park JW, Okamoto LE, Shibao CA, Biaggioni I. Pharmacologic treatment of orthostatic hypotension. Auton Neurosci. 2020;229:102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibao C, Okamoto LE, Gamboa A, Yu C, Diedrich A, Raj SR et al. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2010;56(5):847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ••.Okamoto LE, Diedrich A, Baudenbacher FJ, Harder R, Whitfield JS, Iqbal F et al. Efficacy of Servo-Controlled Splanchnic Venous Compression in the Treatment of Orthostatic Hypotension: A Randomized Comparison With Midodrine. Hypertension. 2016;68(2):418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]; The primary hemodynamic mechanism of orthostatic hypotension is splanchnic venous pooling leading to a reduction in venous return. This study described a device that detects upright posture and automatically inflates an abdominal binder to a servo-controlled constant pressure. This automated abdominal binder was found to be as effective as midodrine in improving upright blood pressure and reducing symptoms.
- 31.Shibao C, Grijalva CG, Lipsitz LA, Biaggioni I, Griffin MR. Early discontinuation of treatment in patients with orthostatic hypotension. Auton Neurosci. 2013;177(2):291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59(3):383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ooi WL, Barrett S, Hossain M, Kelley-Gagnon M, Lipsitz LA. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. JAMA : the journal of the American Medical Association. 1997;277(16):1299–304. [PubMed] [Google Scholar]
- 34. ••.Juraschek SP, Hu JR, Cluett JL, Ishak A, Mita C, Lipsitz LA et al. Effects of Intensive Blood Pressure Treatment on Orthostatic Hypotension : A Systematic Review and Individual Participant-based Meta-analysis. Ann Intern Med. 2020;174(1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]; Treatment of hypertension is often relaxed in orthostatic hypotensive patients for fear of worsening their condition. In this metanalysis of 18,466 individuals participating in nine large randomized controlled antihypertensive trials. The investigators found, contrary to widespread concerns, that intensive blood pressure treatment reduces the risk of orthostatic hypotension.
- 35.Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet. 2000;355(9205):725–6. [DOI] [PubMed] [Google Scholar]
- 36.Maule S, Milan A, Grosso T, Veglio F. Left ventricular hypertrophy in patients with autonomic failure. Am J Hypertens. 2006;19(10):1049–54. [DOI] [PubMed] [Google Scholar]
- 37.Garland EM, Gamboa A, Okamoto L, Raj SR, Black BK, Davis TL et al. Renal impairment of pure autonomic failure. Hypertension. 2009;54(5):1057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverberg R, Naparstek Y, Lewis BS, Levy M. Angina pectoris with normal coronary arteries in Shy-Drager syndrome. J Neurol Neurosurg Psychiatry. 1979;42(10):910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biaggioni I, Robertson RM. Hypertension in orthostatic hypotension and autonomic dysfunction. Cardiol Clin. 2002;20(2):291–301, vii. [DOI] [PubMed] [Google Scholar]
- 40.Kim JS, Oh YS, Lee KS, Kim YI, Yang DW, Goldstein DS. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology. 2012;79(13):1323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Cui Y, Zhao Y, Dong Y, Wang J, Duan D et al. Association of Circadian Rhythm of Blood Pressure and Cerebral Small Vessel Disease in Community-Based Elderly Population. J Gerontol A Biol Sci Med Sci. 2019;74(8):1322–30. [DOI] [PubMed] [Google Scholar]
- 42.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension. 1990;15(6 Pt 1):547–59. [DOI] [PubMed] [Google Scholar]
- 43.Okamoto LE, Gamboa A, Shibao C, Black BK, Diedrich A, Raj SR et al. Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension. 2009;53(2):363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan J, Fanciulli A, Tank J, Calandra-Buonaura G, Cheshire WP, Cortelli P et al. Management of supine hypertension in patients with neurogenic orthostatic hypotension: scientific statement of the American Autonomic Society, European Federation of Autonomic Societies, and the European Society of Hypertension. J Hypertens. 2019;37(8):1541–6. [DOI] [PubMed] [Google Scholar]
- 45.Biaggioni I, Garcia F, Inagami T, Haile V. Hyporeninemic normoaldosteronism in severe autonomic failure. J Clin Endocrinol Metab. 1993;76(3):580–6. [DOI] [PubMed] [Google Scholar]
- 46.Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B et al. Clonidine for the treatment of supine hypertension and pressure natriuresis in autonomic failure. Hypertension. 2006;47(3):522–6. [DOI] [PubMed] [Google Scholar]
- 47.Maclean AR, Allen EV. Orthostatic hypotension and orthostatic tachycardia: Treatment with the “Head-up” bed. Journal of the American Medical Association. 1940;115(25):2162–7. [Google Scholar]
- 48.Ten Harkel AD, Van Lieshout JJ, Wieling W. Treatment of orthostatic hypotension with sleeping in the head-up tilt position, alone and in combination with fludrocortisone. J Intern Med. 1992;232(2):139–45. [DOI] [PubMed] [Google Scholar]
- 49.Wieling W, Van Lieshout JJ, Hainsworth R. Extracellular fluid volume expansion in patients with posturally related syncope. Clin Auton Res. 2002;12(4):242–9. [DOI] [PubMed] [Google Scholar]
- 50.Fan CW, Walsh C, Cunningham CJ. The effect of sleeping with the head of the bed elevated six inches on elderly patients with orthostatic hypotension: an open randomised controlled trial. Age Ageing. 2011;40(2):187–92. [DOI] [PubMed] [Google Scholar]
- 51.Pathak A, Lapeyre-Mestre M, Montastruc JL, Senard JM. Heat-related morbidity in patients with orthostatic hypotension and primary autonomic failure. Mov Disord. 2005;20(9):1213–9. [DOI] [PubMed] [Google Scholar]
- 52.Charkoudian N, Eisenach JH, Atkinson JL, Fealey RD, Joyner MJ. Effects of chronic sympathectomy on locally mediated cutaneous vasodilation in humans. J Appl Physiol (1985). 2002;92(2):685–90. [DOI] [PubMed] [Google Scholar]
- 53.Yamanaka Y, Asahina M, Mathias CJ, Akaogi Y, Koyama Y, Hattori T. Skin vasodilator response to local heating in multiple system atrophy. Mov Disord. 2007;22(16):2405–8. [DOI] [PubMed] [Google Scholar]
- 54. ••.Okamoto LE, Celedonio JE, Smith EC, Gamboa A, Shibao CA, Diedrich A et al. Local Passive Heat for the Treatment of Hypertension in Autonomic Failure. Journal of the American Heart Association. 2021;10(7):e018979. [DOI] [PMC free article] [PubMed] [Google Scholar]; Following the clinical observation that patients with autonomic failure are intolerant to heat exposure, investigators found that local passive heat acutely decreased blood pressure ~20 mmHg with a rapid onset of action and recovery. Overnight application of a water-perfused heating pad applied under the torso produced a maximal decrease of BP of ~30 mm Hg. The decrease in nocturnal BP correlated with a decrease in urinary volume and an improvement in morning orthostatic hypotension. This proof-of-concept study supports the notion that treatment of nocturnal hypertension can improve daytime orthostaic hypotension.
- 55.Low PA, Singer W. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008;7(5):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan J, Biaggioni I. Diagnosis and treatment of supine hypertension in autonomic failure patients with orthostatic hypotension. J Clin Hypertens (Greenwich). 2002;4(2):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Lieshout JJ, ten Harkel AD, Wieling W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin Auton Res. 2000;10(1):35–42. [DOI] [PubMed] [Google Scholar]


