Abstract
This study describes the isolation and characterization of a unique class of TolC mutants that, under steady-state growth conditions, secreted normal levels of largely inactive alpha-hemolysin. Unlike the reduced activity in the culture supernatants, the cell-associated hemolytic activity in these mutants was identical to that in the parental strain, thus reflecting a normal intracellular toxin activation event. Treatment of the secreted toxin with guanidine hydrochloride significantly restored cytolytic activity, suggesting that the diminished activity may have been due to the aggregation or misfolding of the toxin molecules. Consistent with this notion, sedimentation and filtration analyses showed that alpha-hemolysin secreted from the mutant strain has a mass greater than that secreted from the parental strain. Experiments designed to monitor the time course of alpha-hemolysin release showed delayed appearance of toxin in the culture supernatant of the mutant strain, thus indicating a possible defect in alpha-hemolysin translocation or release. Eight different TolC substitutions displaying this toxin secretion defect were scattered throughout the protein, of which six localized in the periplasmically exposed α-helical domain, while the remaining two mapped within the outer membrane-embedded β-barrel domain of TolC. A plausible model for the secretion of inactive alpha-hemolysin in these TolC mutants is discussed in the context of the recently determined three-dimensional structure of TolC.
Uropathogenic strains of Escherichia coli secrete a pore-forming cytotoxin called alpha-hemolysin, also known as HlyA (for reviews, see references 4 and 40). The hlyCABD genes mediate synthesis, activation, and secretion of alpha-hemolysin (20). hlyA encodes for a 110,000-molecular-weight inactive protoxin, which is posttranslationally modified into an active form by HlyC-mediated acylation at two lysine residues (15, 18, 34). While acylation is obligatory for HlyA's cytolytic activity, it is not essential for secretion into the medium (26) or for its ability to form pores in synthetic membranes (18). HlyA is exported through a type I secretory pathway in which proteins are exported across the bacterial envelope by an N-terminal signal sequence-independent manner (20, 30). Instead, HlyA's export signal lies at its C-terminal end (27).
HlyB and HlyD constitute the inner membrane transport complex for HlyA secretion. HlyB is a member of the ATP-binding cassette transport protein superfamily (12). Its large cytoplasmic C-terminal domain binds ATP, whose hydrolysis provides the primary energy source for HlyA secretion (37). HlyD has a short N-terminal cytoplasmic region, a single transmembrane domain and a large periplasmic domain (33). It is a member of the membrane fusion protein class of proteins that are found as a part of the type I secretory pathway in gram-negative bacteria (7). These proteins are proposed to bridge the inner and outer membranes to facilitate the transport of various substrates into the medium.
Wandersman and Delepelaire (38) first showed the requirement for TolC in alpha-hemolysin secretion. TolC is also involved in the secretion of unrelated toxins (14, 42). TolC is an outer membrane protein (23) that has been implicated in many diverse cellular functions. tolC null mutants display a pleiotropic phenotype, including colicin tolerance (25), hypersensitivity to detergents (8, 24, 41), chromosomal partitioning defect (13), and reduced OmpF expression (22, 24, 31). In vitro reconstitution experiments showed that TolC forms a peptide-specific channel (2).
The in vivo secretion of alpha-hemolysin is dependent on an intact lipopolysaccharide (LPS) core (1, 35). However, unlike HlyA, HlyB, and TolC mutants, which are defective in the secretion of toxin, the culture supernatant of these LPS mutants largely contain inactive alpha-hemolysin. It was proposed that an intact LPS core influences toxin's tertiary structure during or after its secretion into the medium. Mutations affecting LPS, and only indirectly TolC's biogenesis, have been shown to reduce alpha-hemolysin secretion (39).
A recent study carried out by Thanabalu et al. (37) showed that HlyB and HlyD form a translocase complex independent of HlyA or TolC. During secretion, HlyA interacts individually with HlyB, HlyD, and TolC; HlyA's interaction with TolC is observed only when the toxin molecule is properly engaged with the HlyBD translocase. HlyB's ATPase activity is not needed to form the translocation complex but for releasing toxin molecules from the complex into the medium. The recently solved crystal structure of TolC shows that it has a large α-helical domain that extends 100 Å into the periplasmic space (17). The proximal end of TolC must interact with the accessory proteins to form a continuous conduit from the cytoplasm to the external surface for the transport of alpha-hemolysin into the medium.
In this study, we have taken a genetic approach to elucidate the role of TolC in alpha-hemolysin secretion. Since tolC null mutations confer a pleiotropic phenotype, missense mutations were sought that primarily affected the secretion of alpha-hemolysin without influencing other TolC-associated functions. A unique class of tolC mutants was obtained in which the steady-state secretion of alpha-hemolysin was unaffected but secreted toxin had significantly reduced cytolytic activity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
All bacterial strains and plasmids used in this study are listed in Table 1. The rich medium (Luria-Bertani [LB]) was prepared as previously described (34). The medium was supplemented with 25 μg of ampicillin/ml, 12.5 μg of chloramphenicol/ml, and 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) when needed. Blood agar plates were prepared by supplementing LB agar with 5% defibrinated sheep erythrocytes (Remel, Lenexa, Kans.).
TABLE 1.
Bacterial strains, bacteriophage, and plasmids
| Strain, bacteriophage, or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| MC4100 | F−araD139 Δ(argF-lac)U139 rpsL150 flbB5301 ptsF25 deoC1 thi-1 rbsR relA | Casadaban (3) |
| P2731 | W1485F−tolC::Tn10–48 | Morona and Reeves (23) |
| RAM958 | MC4100 tolC::Tn10–48 | This study |
| RAM959 | RAM958/pTrc99A | This study |
| RAM960 | RAM958/pTrc99A-tolC+ | This study |
| RAM961 | RAM958/pTrc-tolC(TolCL42P) | This study |
| RAM962 | RAM958/pTrc-tolC(TolCS257P) | This study |
| RAM963 | RAM958/pTrc-tolC(TolCA343T) | This study |
| RAM964 | RAM958/pTrc-tolC(TolCA360V) | This study |
| RAM965 | RAM958/pTrc-tolC(TolCA360T) | This study |
| RAM966 | RAM958/pTrc-tolC(TolCG147D) | This study |
| RAM967 | RAM958/pTrc-tolC(TolCT434M) | This study |
| RAM968 | RAM958/pTrc-tolC(TolCT140A) | This study |
| RAM969 | MC4100/pSF4000 (hlyCABD+) | This study |
| RAM970 | RAM958/pSF4000 | This study |
| RAM971 | RAM959/pSF4000 | This study |
| RAM972 | RAM960/pSF4000 | This study |
| RAM973 | RAM961/pSF4000 | This study |
| RAM974 | RAM962/pSF4000 | This study |
| RAM975 | RAM963/pSF4000 | This study |
| RAM976 | RAM964/pSF4000 | This study |
| RAM977 | RAM965/pSF4000 | This study |
| RAM978 | RAM966/pSF4000 | This study |
| RAM979 | RAM967/pSF4000 | This study |
| RAM980 | RAM968/pSF4000 | This study |
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB) (F′ traD36 proAB lacIqZΔM15) | Stratagene |
| XL1-Red | endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac mutD5 mutS mutT::Tn10 | Stratagene |
| K53 | Produces colicin E1 | Davies and Reeves (6) |
| Bacteriophage | TLS | Carl Schnaitman |
| Plasmids | ||
| pTrc99A | Apr; expression vector | Pharmacia |
| pACYC184 | Cmr Tcr | Chang and Cohen (5) |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Tcr, tetracycline resistance.
Plasmid construction and mutagenesis.
The tolC gene was cloned under the control of an IPTG-inducible promoter of pTrc99A (Pharmacia). For this two mutagenic primers were utilized: the forward primer (5′-GAATGCCCATGGGGAAATTGCTCCCCATTC-3′) created a unique NcoI site (underlined), and the reverse primer (5′-GCGGCAGATAACCCGAAGCTTTACGGTTGCC-3′) created a unique HindIII site (underlined). In creating the NcoI site, the second codon of the TolC signal sequence was changed from AAG (lysine) to GGG (glycine). tolC DNA was amplified from the chromosome by PCR, digested with NcoI and HindIII, and ligated into appropriately restricted pTrc99A. Under noninducing growth conditions (without IPTG), the level of plasmid-encoded TolC was extremely low, as determined by Western blot analysis, but in the presence of 0.4 mM IPTG its level was similar (85%) to that produced from the chromosomal gene.
pTrc-tolC+ was used to transform strain XL1-Red (Table 1) according to the manufacturer's instructions (Stratagene). Transformants were selected on LBA-ampicillin plates. Plasmid DNA obtained from the pool of Apr colonies was transformed into a tolC::Tn10 strain and transformants were screened for the desired tolC phenotype.
SDS-PAGE and Western blot analyses.
The French press lysis method was used to isolate cell envelopes from whole cells (21). Membrane proteins were subsequently analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (19). For Western blot analysis, protein samples were analyzed on mini (7 by 8 cm) SDS-polyacrylamide gels and transferred onto Immuno-Lite membranes (Bio-Rad) by using a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad). Blots were incubated with primary antibodies raised against HlyA or TolC (1:5,000 dilution) for 2 h and then with the secondary antibody (goat-α-rabbit immunoglobulin G) for 1 h. Detection was carried according to the manufacturer's protocol (Bio-Rad).
Sensitivities to colicin E1, bacteriophage, and antibiotics.
Strain K53 was grown to stationary phase and exposed to UV radiation for 90 s to induce colicin production. The UV-irradiated cells were diluted 1:50 and grown overnight. Cells were treated with chloroform and centrifuged at 2,000 × g for 10 min at room temperature in a GH-3.7 horizontal rotor (Beckman). Twofold dilutions were prepared from the supernatant, and 10 μl of each dilution was spotted over a lawn of bacterial strain that was to be tested for colicin E1 sensitivity. Sensitivity to bacteriophage was also done by a serial dilution method described. For antibiotic sensitivity, presoaked antibiotic disks (Difco) were placed on bacterial lawns prepared on LBA. The zones of inhibition (in millimeters) were measured after incubation of the plates for 18 h at 37°C.
Hemolysin enzyme assay.
Hemolysin enzymatic assays were carried out as previously described (10). Cells were harvested at an optical density at 600 nm (OD600) of 0.8 by centrifugation. The supernatant was filtered through 0.45-μm (pore-size) syringe filters (Acrodisc; Pall Corp.). To obtain hemolytic activity within a linear range, the filtered supernatant was diluted fivefold in an enzyme assay buffer composed of 20 mM CaCl2, 10 mM Tris-HCl (pH 7.5), and 160 mM NaCl. Sheep erythrocytes were prepared for the assay by washing them three times in 0.9% NaCl. The enzyme assay reaction mixture (for each time point) contained 900 μl of enzyme assay buffer, 20 μl of washed erythrocytes, and 80 μl of the diluted culture supernatant. Enzymatic reactions were carried out at 37°C in a shaking water bath. Aliquots (800 μl) withdrawn at various time points were chilled immediately by placing them on ice. Unlysed erythrocytes were removed by centrifugation (5 min, 12,000 × g at 4°C), and 500 μl of the supernatant was used to measure the absorbance at OD530 (as an indication of the amount of released hemoglobin). The hemolytic activity is defined as OD530/OD600/ml of culture supernatant (10). To test the effect of a denaturant on hemolytic activity, filtered culture supernatants were incubated with 6 M guanidine hydrochloride (GuHCl) for 15 min at room temperature. Enzyme assays on the GuHCl-treated supernatant was carried out as described above. The final concentration of GuHCl in enzymatic assays was 0.096 M.
Time course analysis of alpha-hemolysin secretion.
Cultures were grown in LB containing ampicillin and chloramphenicol without IPTG to an OD600 of ∼0.3, at which point TolC synthesis was induced with 0.4 mM IPTG. A 5-ml aliquot was removed every 10 min for up to 60 min. Cells were pelleted by centrifugation in a bench-top centrifuge for 15 min at room temperature. The filtered supernatant was used to examine extracellular HlyA levels; TolC levels were determined from envelopes prepared from the pellet. HlyA and TolC levels were analyzed by Western blots.
RESULTS
Isolation of tolC mutants.
Two genetic strategies were employed to isolate tolC mutants defective in alpha-hemolysin secretion. One strategy involved direct mutant selection in which a plasmid carrying the tolC gene was mutagenized to avoid mutations mapping in other genes that could also confer the desired phenotype. A mutator strain (mutD mutS mutT) was used for plasmid mutagenesis and a randomly mutagenized pool of tolC plasmids was transformed into a tolC::Tn10 strain containing an hly plasmid. Transformants were replica plated onto blood agar medium containing novobiocin and two additional antibiotics to select for the tolC and hly plasmids. The presence of novobiocin (10 μg/ml) selected against tolC null mutants, which are hypersensitive to this and other hydrophobic antibiotics. Mutants defective in alpha-hemolysin secretion produced smaller hemolytic zones compared to the tolC+ parental strain. Of the 18,000 transformants examined, 16 showed smaller hemolytic zones.
In the second strategy, missense tolC mutations were first enriched through an indirect selection that exploited two prominent characteristics of tolC null mutants, namely, hypersensitivity toward detergents (such as deoxycholate), and resistance (tolerance) against colicin E1. By demanding simultaneous resistance against these agents, we expected to enrich for isolates proficient in efflux activity and thus deoxycholate resistance (Docr), but defective in colicin transport (E1r). Mutagenized plasmids were transformed into a tolC::Tn10 strain (Docs E1r), and transformants were replica plated onto a medium containing deoxycholate and colicin E1. Plasmids isolated from Docr E1r colonies were then introduced into a tolC::Tn10 strain harboring an hly plasmid. Hemolytic zones were examined on blood agar medium containing appropriate antibiotics to select for both the tolC and the hly plasmids. Of the 23,000 transformants screened, 57 displayed an E1r Docr phenotype. Plasmids obtained from eight such isolates conferred an apparent alpha-hemolysin secretion defect since they produced colonies with smaller opaque hemolytic zones. In all, the two strategies yielded 24 desired isolates out of 41,000 transformants.
Protein and nucleotide sequence analyses.
TolC was analyzed to see whether reduced protein levels resulted in the observed phenotype. Envelope proteins were transferred onto a membrane and hybridized with polyclonal antibodies raised against TolC and LamB (as an internal control). The data showed that only 10 of the 24 mutants produced a detectable level of TolC (Table 2). These 10 mutants were analyzed further. Nucleotide sequence of the entire tolC gene from 10 mutants was determined through employing primers complementary to the tolC sequence. This analysis revealed that 9 of the 10 isolates contained a single-base-pair substitution within a region of tolC encoding for the mature portion of the protein (Table 2). One mutant with an alteration affecting the last residue of the signal sequence was not analyzed further. Two mutants had an identical substitution (A343T), while two other isolates had different substitutions at the same site (A360T and A360V).
TABLE 2.
Summary of tolC mutants
| Strain | Mutation | Substitutiona | Mean TolC levelb ± SD | Sensitivity to inhibitors
|
||
|---|---|---|---|---|---|---|
| E1c | Novd | TLSe | ||||
| RAM660 | None | TolC-wt | 100 | 1 | 9 | 1 |
| RAM959 | tolC::Tn10 | TolC− | 0 | 2−5 | 23 | <10−10 |
| RAM961 | T125C | L42P | 31 ± 9 | 2−1 | 10 | 1 |
| RAM968 | A418G | T140A | 95 ± 5 | 1 | 9 | 1 |
| RAM966 | G440A | G147D | 26 ± 4 | 1 | 9 | 1 |
| RAM962 | T769C | S257P | 90 ± 10 | <2−5 | 10 | 10−10 |
| RAM963 | G1027A | A343T | 79 ± 3 | 1 | 9 | 1 |
| RAM965 | G1078A | A360T | 100 ± 12 | 1 | 9 | 1 |
| RAM964 | C1079T | A360V | 98 ± 5 | 1 | 9 | 1 |
| RAM967 | C1241T | T434M | 46 ± 11 | 1 | 12 | 1 |
Substitutions represent mature TolC residues.
TolC levels relative to the parental protein. Averages from three independent experiments are shown.
Sensitivities relative to the parental strain are shown. E1 sensitivity was determined by spotting twofold serial dilutions of colicin.
Zones of inhibition in millimeters; Nov, novobiocin (30 μg). The diameter the of presoaked antibiotic paper disks was 7 mm.
Sensitivity to the TLS bacteriophage was measured by determining the efficiency of plaquing.
Phenotypic characterization of tolC mutants.
None of the mutants showed hypersensitivity toward novobiocin (Table 2), which was expected since the isolation strategy demanded a proficient efflux activity. The slight increase in sensitivity observed in a mutant carrying a T434M substitution may have been due to the presence of reduced TolC levels in the envelope. The two mutants isolated through the colicin E1 selection method showed different degrees of sensitivity to colicin E1 in titration assays, with the S257P substitution resulting in complete insensitivity (Table 2). The other six mutants with substitutions in the mature portion of the TolC protein remained sensitive to colicin E1. tolC null mutants are known to be resistant to the bacteriophage TLS (9). All but one mutant, bearing a S257P substitution, were sensitive to TLS. Lastly, unlike the strain carrying a null tolC mutation, which drastically reduces OmpF levels (22, 24), all mutants isolated in this study showed normal OmpF expression (data not shown). These analyses showed that five mutants phenotypically resembled a strain expressing the parental TolC protein. The remaining three mutants bearing a L42P, S257P, or T434M substitution showed some degree of defect in at least one of the phenotypes associated with the tolC null mutation.
Secretion of alpha-hemolysin in tolC mutants.
Mutants and control strains were tested for their abilities to secrete alpha-hemolysin by quantifying the amount of toxin secreted in the supernatant of cultures grown to late log phase. Bacterial cells were removed by centrifugation, supernatants were filtered through 0.45-μm (pore-size) cellulose acetate filters, diluted, and spotted onto a polyvinylidene difluoride membrane for dot blot analysis. Filters were hybridized with polyclonal antibodies raised against alpha-hemolysin, and hybridized spots were quantified (Table 3). The data showed that, with the exception of a mutant carrying a L42P substitution, all other mutants secreted alpha-hemolysin in amounts similar to that secreted by a strain expressing the parental TolC protein. Identical results were obtained when alpha-hemolysin precipitated by trichloroacetic acid from the culture supernatant was analyzed by Western blots (data not shown).
TABLE 3.
Hemolysin levels and hemolytic activities in tolC mutants
| Strain | TolC | Alpha-hemolysin
|
||
|---|---|---|---|---|
| Zonea | Levelb | Activityb | ||
| RAM660 | TolC-wt | +++ | 100 | 100 |
| RAM959 | TolC− | − | 10 ± 10 | 2 ± 1 |
| RAM961 | L42P | + | 69 ± 11 | 4 ± 1 |
| RAM968 | T140A | + | 88 ± 2 | 10 ± 4 |
| RAM966 | G147D | ++ | 95 ± 5 | 28 ± 2 |
| RAM962 | S257P | ++c | 100 ± 14 | 9 ± 6 |
| RAM963 | A343T | + | 100 ± 8 | 46 ± 10 |
| RAM965 | A360T | ++ | 93 ± 2 | 51 ± 1 |
| RAM964 | A360V | ++ | 100 ± 1 | 47 ± 4 |
| RAM967 | T434M | ++ | 99 ± 1 | 49 ± 13 |
Qualitative measurements of hemolytic (halo) zones on blood agar plates are displayed.
Hemolysin activities and levels relative to the TolC+ strain. Averages from three independent experiments are shown.
Opaque hemolytic zone.
The results presented above were somewhat unexpected because mutants were identified as those producing smaller hemolytic zones on blood agar medium and hence were expected to have reduced secreted alpha-hemolysin. As mentioned above, in the case of the mutant expressing TolCL42P, the reduced level of secreted alpha-hemolysin could partly be attributed to the reduced TolC level. Interestingly, a mutant expressing TolCG147D produced even less TolC than that by a strain expressing TolCL42P and yet secreted alpha-hemolysin levels in the former strain that were similar to that in the parental strain. This showed that reduced TolC levels did not always correlate with reduced levels of secreted alpha-hemolysin.
Hemolytic activities of tolC mutants.
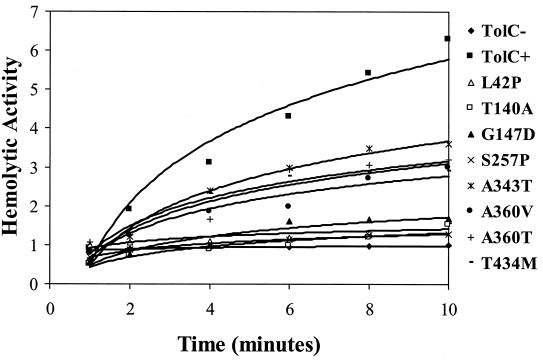
The cytolytic activity of secreted alpha-hemolysin from mutants and control strains was quantified in an attempt to resolve the above anomaly. The kinetics of blood lysis by culture supernatants were determined (Fig. 1), and relative values are presented in Table 3. The data revealed that the supernatants of all of the mutant cultures displayed significantly lower hemolytic activities than that observed in the parental strain. Based on their hemolytic activities, mutants can be divided into two groups. One group bearing a L42P, T140A, G147D, or S257P substitution showed dramatically lower activities ranging from 4 to 28% of the parental level. The second group, consisting of mutants with an A343T, A360T, A360V, or T434M substitution, had moderate activities ranging from 46 to 51% of the parental value. These results provided quantitative verification that the smaller hemolytic zones formed around colonies of mutant tolC strains were not due to reduced secreted alpha-hemolysin levels per se but rather to reduced hemolytic activities.
FIG. 1.
Cytolytic activities of secreted α-hemolysin from TolC+, TolC−, and TolC mutant strains. Graphs were obtained from three independent experiments in which the slope values did not vary more than 13% as shown in Table 3.
The reduced extracellular cytolytic activity of alpha-hemolysin may be due to its synthesis in an inactive form. To test this, the alpha-hemolysin level and activity were analyzed from whole-cell lysates prepared from extensively washed bacterial cells. TolC+ and TolC mutant strains had almost identical levels and activities of alpha-hemolysin (data not shown); thus, neither synthesis nor activation of alpha-hemolysin was affected in the mutant strains. Therefore, it appears that the defect leading to the presence of inactive alpha-hemolysin molecules in the supernatant involved their secretion through envelopes containing mutant TolC proteins.
Restoration of hemolytic activity by GuHCl.
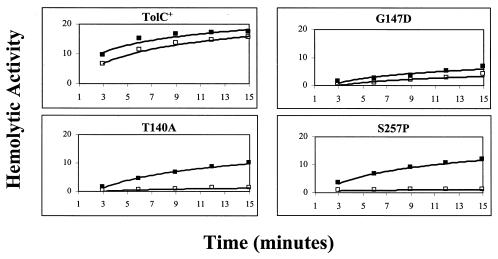
One possibility for the presence of inactive alpha-hemolysin in the supernatant involves its aggregation or misfolding during secretion or postsecretion. A precedent for this notion exists in LPS mutants, in which the secretion of alpha-hemolysin into the medium was normal but secreted alpha-hemolysin molecules were enzymatically inactive due to aggregation (35). If alpha-hemolysin in the tolC mutants is aggregated or misfolded, it may be possible to resolve aggregates or restore correct folding by treatments with chaotropic agents such as GuHCl and restore hemolytic activity. Consistent with this notion, we discovered that the addition of 6 M GuHCl and its subsequent dilution to 0.1 M stimulated the hemolytic activity of toxin secreted from all but one strain expressing the TolCG147D protein (Fig. 2). These results showed that the low hemolytic activities observed were likely due to the presence of misfolded or aggregated alpha-hemolysin in the medium.
FIG. 2.
Effect of GuHCl treatment on secreted alpha-hemolysin activity. Secreted alpha-hemolysin was treated with 6 M GuHCl (▪) or untreated (□) as described in Materials and Methods.
Conformational alterations of alpha-hemolysin secreted from the mutant strain.
We employed protease sensitivity assays, together with sedimentation and filtration analyses, to examine whether the inactivity of secreted alpha-hemolysin from the mutant was due to its aggregation or misfolding. Despite our repeated efforts with experiments involving limiting proteolysis with carboxypeptidase Y or trypsin, we failed to find any reproducible differences in the proteolytic pattern of alpha-hemolysin secreted from the mutant and parental strains.
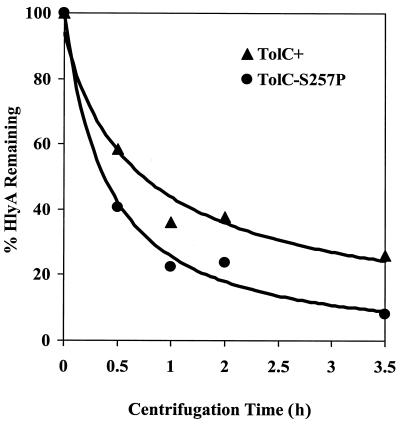
Subsequently, sedimentation and filtration analyses were carried out in an attempt to reveal a possible mass difference between alpha-hemolysin secreted from the mutant and parental strains. Cell-free culture supernatants from the strain (RAM974; TolCS257P) displaying the strongest hemolytic activity defect and the parental strain were centrifuged at 150,000 × g for up to 3.5 h. The levels of alpha-hemolysin from samples withdrawn after 0, 0.5, 1, 2, and 3.5 h of centrifugation were determined and plotted after normalizing them to the level obtained prior to centrifugation (Fig. 3). The results showed that alpha-hemolysin obtained from the mutant culture sedimented at a faster rate than that obtained from the parental strain. Consequently, three times more alpha-hemolysin remained in the supernatant obtained from the parental strain than the mutant strain after 3.5 h of centrifugation. The reduction in alpha-hemolysin levels with centrifugation time was not due to the degradation of toxin, since increasing amounts were present in the pellet (data not shown).
FIG. 3.
Sedimentation analysis of alpha-hemolysin secreted from strains expressing either wild-type TolC (▴) or TolCS257P (●). The amounts of alpha-hemolysin remaining in the supernatant were determined and plotted after normalization to values obtained prior to centrifugation. See Results for details.
Filtration of cell-free culture supernatants through membranes with a cutoff molecular-weight limit of 300,000 (Omega Macrosep 300K; Pall Gelman Science) showed that approximately four times more alpha-hemolysin was retained from the mutant culture supernatant than that from the parental culture supernatant. Taken together, these results indicated that alpha-hemolysin secreted from the mutant strain had a larger mass than that obtained from the parental strain.
Time course analysis of alpha-hemolysin secretion.
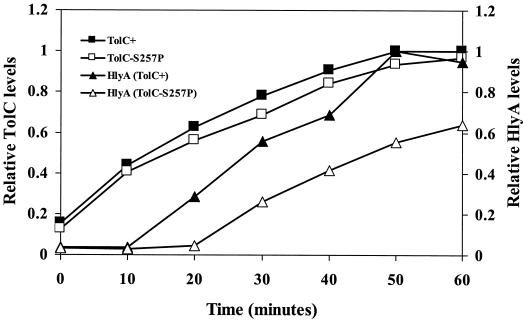
It is conceivable that an unproductive interaction between alpha-hemolysin and the mutant TolC protein impedes secretion, thus leading to misfolding and/or aggregation of the toxin molecules. To examine secretion, we exploited the dependency of alpha-hemolysin secretion on TolC whose synthesis from a plasmid was controlled by IPTG. In the absence of IPTG, only basal levels of TolC and alpha-hemolysin were detectable (Fig. 4). After the addition of IPTG, TolC levels rose rapidly; alpha-hemolysin was detected in the supernatant of a TolC+ culture at 10 min postinduction, and the levels rose steadily until 50 min postinduction.
FIG. 4.
Time course analysis of alpha-hemolysin secretion and TolC synthesis in strains expressing either wild-type TolC or TolCS257P proteins. The levels of alpha-hemolysin and TolC were determined after analyzing protein samples by use of Western blots. Further details of the experiment are provided in Materials and Methods.
We compared the parental secretion time course with a TolC mutant (TolCS257P) displaying the most severe alpha-hemolysin activity defect. In the mutant strain, TolC levels rose with a rate and amplitude similar to that observed for the TolC+ strain. However, the appearance of alpha-hemolysin in the medium was delayed by 10 min compared to the parental strain. Additionally, it appeared in the medium at a significantly slower rate than that observed in the parental strain. These results support the notion that impeded translocation or release of alpha-hemolysin into the medium may lead to its inactivation in the mutant strains.
DISCUSSION
A goal of this research has been to obtain missense tolC mutations that primarily affect alpha-hemolysin secretion without displaying the pleiotropic phenotype of a more frequent class of null mutations. This was achieved by selecting tolC mutants on medium containing inhibitors that must be removed by cells expressing TolC proficient in its efflux activity. The desired class of tolC mutants defective in alpha-hemolysin secretion was identified among colonies that formed relatively small hemolytic zones on blood agar medium.
A unique property of TolC mutants.
A novel class of tolC mutants emerged in which the steady-state level of secreted alpha-hemolysin was normal, but the secreted toxin was enzymatically less active. While the activity of extracellular alpha-hemolysin was reduced in the mutants, the cytoplasmic activity was identical to the parental strain, suggesting the toxin's inactivation during or after its secretion into the medium. Although all tolC mutants studied here showed reduced extracellular hemolytic activity, this defect was particularly pronounced in two mutants expressing TolCT140A or TolCS257P. In these mutants, TolC and secreted alpha-hemolysin levels were similar to that of the parental strain, but the extracellular hemolytic activity was 10-fold reduced.
Restoration of hemolytic activity.
In several instances, the treatment of inactive extracellular alpha-hemolysin with GuHCl substantially elevated its cytolytic activity. This increase in hemolytic activity likely reflected a decrease in aggregation and/or misfolding. Interestingly, a modest improvement of hemolytic activity was also observed in a strain expressing the wild-type TolC protein, indicating that a small population of secreted alpha-hemolysin molecules may exist as inactive aggregates. Indeed, the inactivation of secreted alpha-hemolysin due to aggregation in the aqueous environment is thought to be a normal phenomenon (11, 29). Since GuHCl restored the hemolytic activity in vitro, it appeared unlikely that the step of HlyC-mediated acylation, which is essential for the cytolytic activity (36), was affected in tolC mutants. Consistent with this notion, the cytoplasmic hemolytic activity in the mutant strains was found to be identical to that of the parental strain (data not shown).
tolC and rfa mutants display similar alpha-hemolysin activity defects.
Two other studies have shown the presence of predominantly inactive alpha-hemolysin molecules in the culture supernatant of mutant E. coli strains. In these cases, however, the genetic defect existed within the rfaC (1) and rfaP (35) genes whose products influence the composition of LPS inner core (32). rfaC and rfaP mutants display a pleiotropic phenotype, including hypersensitivity toward hydrophobic compounds and defective outer membrane protein biogenesis (28). Stanley et al. (35) demonstrated that aggregation of alpha-hemolysin in the culture supernatant resulted in the inactivation of its hemolytic activity. In the rfaC mutant, both the extracellular expression and activity of alpha-hemolysin was affected (1). These studies suggested that the intact LPS core interacts with alpha-hemolysin either during or after its secretion, and this interaction affords a conformational stability to the toxin molecule. Unlike the rfaC and rfaP mutants, the tolC mutants studied here do not display a severe membrane defect, as was evident from the lack of a hypersensitivity phenotype. Thus, it appears unlikely that the effect of mutant TolC proteins on the activity of alpha-hemolysin is indirect and produced through a gross LPS or membrane defect. Polyacrylamide gel analysis of LPS from envelopes of TolC mutants showed no qualitative or quantitative difference from LPS of the parental strain (data not shown), thus further supporting the notion that the reduced hemolytic activity in TolC mutants is not due to an effect on LPS.
Possible reasons for alpha-hemolysin inactivation.
Due to the relatively large size of alpha-hemolysin, it is likely to translocate through the TolC barrel in a partially unfolded state. Subsequent to its release into the medium or through its interaction with the target cell, the alpha-hemolysin molecule must then refold in order to become enzymatically active. In the TolC mutants studied here, this step of refolding might be defective. However, since not all substitutions map within the β-barrel domain of TolC, it is difficult to envisage how the terminal steps of alpha-hemolysin translocation, i.e., release and refolding, would be affected in the mutants. Time course studies involving alpha-hemolysin secretion in a mutant strain expressing TolCS257P showed (i) a delayed appearance of toxin in the supernatant and (ii) a slower rate of secretion compared to that observed in the parental strain. These observations suggest that the steps involving alpha-hemolysin translocation through the TolC barrel might be affected in the mutant strains. A block in translocation may lead to misfolding and/or aggregation of toxin molecules trapped within the TolC barrel.
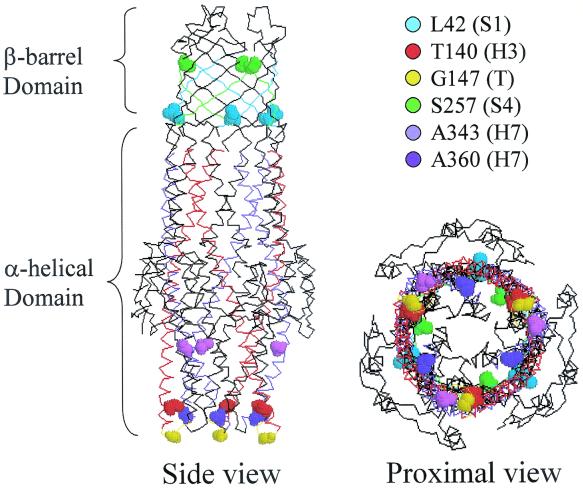
The crystal structure of TolC shows tapering of the proximal end due to the packing of H3/H4 (outer) and H7/H8 (inner) helices into coiled coils (17) (Fig. 5). It has been proposed that the opening of the proximal entrance during substrate translocation may involve uncoiling and realignment of the paired helices (17). It is conceivable that substitutions affecting residues T140, G147, A343, and A360, which are located at the proximal end, influence such helical movement and realignment and thus proper opening of the proximal end. It is also probable that the above substitutions affect TolC's interaction with the accessory proteins HlyB and/or HlyD. Since the TolC crystal structure lacked last 43 residues, the location of T434 in the folded protein is unknown.
FIG. 5.
Three-dimensional structure of TolC showing locations of various mutant substitutions that influence alpha-hemolysin secretion and activity. Views from the side and proximal end are shown. Colored circles reflect the location of various mutant sites (in space-filled representations) in α-helices (H), β-strands (S), or turns (T).
The above explanations, however, may not be applicable to the two substitutions affecting L42 and S257 because they are located within the exterior β-barrel domain of TolC (Fig. 5) and hence are less likely to influence the movement of α-helices or their interactions with HlyB and/or HlyD. A significant reduction in protein level suggests that L42P affects TolC's biogenesis. This is consistent with a study of OmpA showing that proline residues are not tolerated in the transmembrane β-strands (16). The presence of proline in the transmembrane β-strand may influence the barrel (channel) conformation and impede alpha-hemolysin translocation or release. In addition to the proposed conformational changes in the β-barrel, the lower TolC level is likely to contribute to the observed alpha-hemolysin secretion defect. Unlike L42P, S257P does not significantly affect the TolC level and hence biogenesis, yet its effect on alpha-hemolysin activity is quite severe. As S257 is located at the external tip of a β-strand (Fig. 5), it is conceivable its substitution by proline affects a later step of toxin translocation or release into the medium. In this context it is worth noting that the S257P substitution was also obtained among mutants affecting TLS bacteriophage binding (9), thus corroborating the notion of an altered surface structure.
Diploid analysis revealed that the expression of mutant TolC proteins other than TolCA343T in a tolC+ background resulted in a codominant phenotype with respect to secreted hemolytic activity (data not shown). This is presumably because alpha-hemolysin in merodiploid strains is released in the medium through both wild-type and mutant TolC pathways, thus resulting in the mixed accumulation of active and inactive toxin molecules. Interestingly, the TolCG147D protein produced the most prominent interfering effect. Since the level of TolCG147D in the membrane is significantly lower than in the parental protein, the observed dominance could in part be due to an affect on the biogenesis of wild-type protein. It is also worth noting that G147 is located in a turn region of the proximal α-helical domain (Fig. 5), which may interact with HlyB and/or HlyD during alpha-hemolysin translocation. The G147D substitution may result in an aberrant interaction between TolC and HlyB and/or HlyD, thus producing a strong dominant-negative phenotype.
The genetic study presented here points out that the TolC protein may not simply act as a passive conduit for the translocation of large alpha-hemolysin molecules. The various substitutions within TolC identified here may influence the dynamics of TolC's “chunnel” so as to hinder the ability of toxin to interact and translocate through it. Since the aggregation of alpha-hemolysin to some degree occurs naturally in the aqueous environment (11), this behavior may be accentuated if the toxin's translocation through TolC is hampered.
ACKNOWLEDGMENTS
We thank Rod Welch for providing the hemolysin-secreting E. coli strain and HlyA antibodies and Leanne Misra and Anne Marie Augustus for critically reading the manuscript.
This work was supported in part by a grant from the NIH (GM48167 to R.M.)
REFERENCES
- 1.Bauer M E, Welch R A. Pleiotropic effects of a mutation in rfaC on Escherichia coli hemolysin. Infect Immun. 1997;65:2218–2224. doi: 10.1128/iai.65.6.2218-2224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz R, Maier E, Gentschev I. TolC of Escherichia coli as an outer membrane channel. Zentbl Bakteriol. 1993;278:187–196. doi: 10.1016/s0934-8840(11)80836-4. [DOI] [PubMed] [Google Scholar]
- 3.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;141:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 4.Cavalieri S J, Bohach G A, Snyder I S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenecity. Microbiol Rev. 1984;48:326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies J K, Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975;123:102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinh T, Paulsen I T, Saier M H., Jr A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J Bacteriol. 1994;176:3225–3231. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fralick J A, Burns-Keliher L L. Additive effect of tolC and rfa mutations on the hydrophobic barrier of the outer membrane of Escherichia coli K-12. J Bacteriol. 1994;176:6404–6406. doi: 10.1128/jb.176.20.6404-6406.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.German G J, Misra R. The TolC protein of Escherichia coli serves as a cell-surface receptor for the newly characterized TLS bacteriophage. J Mol Biol. 2001;301:579–585. doi: 10.1006/jmbi.2001.4578. [DOI] [PubMed] [Google Scholar]
- 10.Goebel W, Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982;151:1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goñi F M, Ostolaza H. Escherichia coli α-hemolysin: a membrane-active protein toxin. Braz J Med Biol Res. 1998;31:1019–1034. doi: 10.1590/s0100-879x1998000800002. [DOI] [PubMed] [Google Scholar]
- 12.Higgins C F, Hiles I D, Salmond G P, Gill D R, Downie J A, Evans I J, Holland I B, Gray L, Buckel S, Bell A W, Hermondson M A. A family of related ATP binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986;323:448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- 13.Hiraga S, Niki H, Ogura T, Ichinose C, Mori H, Ezaki B, Jaffe A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989;171:1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang J, Zhong X, Tai P C. Interactions of dedicated export membrane proteins of colicin V secretion system: CvaV, a member of the protein fusion family, interacts with CvaB and TolC. J Bacteriol. 1997;179:6264–6270. doi: 10.1128/jb.179.20.6264-6270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Issartel J-P, Koronakis V, Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991;351:759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- 16.Koebnik R. Membrane assembly of the Escherichia coli outer membrane protein OmpA: exploring sequence constraints on transmembrane β-strands. J Mol Biol. 1999;285:1801–1810. doi: 10.1006/jmbi.1998.2405. [DOI] [PubMed] [Google Scholar]
- 17.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig A, Garcia F, Bauer S, Jarchau T, Benz R, Hoppe J, Goebel W. Analysis of in vivo activation of hemolysis (HlyA) from Escherichia coli. J Bacteriol. 1996;178:5422–5430. doi: 10.1128/jb.178.18.5422-5430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lugtenberg B, Meijers J, Peters R, van der Hoek P, van Alphen L. Electrophoretic resolution of the major outer membrane proteins of Escherichia coli K-12 into four bands. FEBS Lett. 1975;58:254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- 20.Mackman N, Nicaud J-M, Gray L, Holland I B. Secretion of hemolysin by Escherichia coli. Curr Top Microbiol Immunol. 1986;125:159–181. doi: 10.1007/978-3-642-71251-7_10. [DOI] [PubMed] [Google Scholar]
- 21.Misra R, Peterson A, Ferenci T, Silhavy T J. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli. J Biol Chem. 1991;266:13592–13597. [PubMed] [Google Scholar]
- 22.Misra R, Reeves P. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J Bacteriol. 1987;169:4722–4730. doi: 10.1128/jb.169.10.4722-4730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morona R, Reeves P. The tolC locus of Escherichia coli affects the expression of three major outer membrane proteins. J Bacteriol. 1982;150:1016–1023. doi: 10.1128/jb.150.3.1016-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morona R, Manning P A, Reeves P. Identification and characterization of the TolC protein, an outer membrane protein of Escherichia coli. J Bacteriol. 1983;153:693–699. doi: 10.1128/jb.153.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagel de Zwaig R, Luria S E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967;94:1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicaud J-M, Mackman N, Gray L, Holland I B. Characterization of HlyC and mechanism of activation and secretion of haemolysin from E. coli 2001. FEBS Lett. 1985;187:339–344. doi: 10.1016/0014-5793(85)81272-2. [DOI] [PubMed] [Google Scholar]
- 27.Nicaud J-M, Mackman N, Gray L, Holland I B. The C-terminal, 23-kD peptide of E. coli haemolysin 2001 contains all the information necessary for its secretion by the haemolysin (Hly) export machinery. FEBS Lett. 1986;204:331–335. doi: 10.1016/0014-5793(86)80838-9. [DOI] [PubMed] [Google Scholar]
- 28.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostolaza H, Bartolome B, Serra J L, de la Cruz F, Goñi F M. Alpha-haemolysin from E. coli: purification and self-aggregation properties. FEBS Lett. 1991;280:195–198. doi: 10.1016/0014-5793(91)80291-a. [DOI] [PubMed] [Google Scholar]
- 30.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolfe B, Onodera K. Demonstration of a missing membrane protein in colicin-tolerance mutants of E. coli K-12. Biochem Biophys Res Commun. 1971;44:767–773. doi: 10.1016/0006-291x(71)90776-5. [DOI] [PubMed] [Google Scholar]
- 32.Schnaitman C A, Klena J. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulein R, Gentschev I, Mollenkopf H J, Goebel W. A topological model for haemolysin translocator protein HlyD. Mol Gen Genet. 1992;234:155–163. doi: 10.1007/BF00272357. [DOI] [PubMed] [Google Scholar]
- 34.Silhavy T J, Berman M, Enquist L. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 35.Stanley P L, Diaz P, Bailey M J, Gygi D, Juarez A, Hughes C. Loss of activity in the secreted form of Escherichia coli haemolysis caused by an rfaP lesion in core lipopolysaccharide assembly. Mol Microbiol. 1993;10:781–787. doi: 10.1111/j.1365-2958.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 36.Stanley P L, Packman C, Koronakis V, Hughes C. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science. 1994;23:1992–1996. doi: 10.1126/science.7801126. [DOI] [PubMed] [Google Scholar]
- 37.Thanabalu T, Koronakis E, Hughes C, Koronakis V. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 1998;17:6487–6496. doi: 10.1093/emboj/17.22.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wandersman C, Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci USA. 1990;87:4746–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wandersman C, Létoffé S. Involvement of lipopolysaccharide in the secretion of Escherichia coli α-haemolysin and Erwinia chrysanthemi proteases. Mol Microbiol. 1993;7:141–150. doi: 10.1111/j.1365-2958.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 40.Welch R A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 41.Whitney E N. The tolC locus of Escherichia coli K12. Genetics. 1971;67:39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamanaka H, Nomura T, Fuji Y, Okamoto K. Need for TolC, an Escherichia coli outer membrane protein, in the secretion of heat-stable enterotoxin I across the outer membrane. Microb Pathog. 1998;25:111–120. doi: 10.1006/mpat.1998.0211. [DOI] [PubMed] [Google Scholar]