ABSTRACT
Objective
Simian immunodeficiency virus (SIV) infection of macaques recapitulates many aspects of HIV pathogenesis and is similarly affected by both genetic and environmental factors. Psychosocial stress is associated with immune system dysregulation and worse clinical outcomes in people with HIV. This study assessed the impact of single housing, as a model of psychosocial stress, on innate immune responses of pigtailed macaques (Macaca nemestrina) during acute SIV infection.
Methods
A retrospective analysis of acute SIV infection of 2- to si6-year-old male pigtailed macaques was performed to compare the innate immune responses of socially (n = 41) and singly (n = 35) housed animals. Measures included absolute monocyte count and subsets, and in a subset (n ≤ 18) platelet counts and activation data.
Results
SIV infection resulted in the expected innate immune parameter changes with a modulating effect from housing condition. Monocyte number increased after infection for both groups, driven by classical monocytes (CD14+CD16−), with a greater increase in socially housed animals (227%, p < .001, by day 14 compared with preinoculation time points). Platelet numbers recovered more quickly in the socially housed animals. Platelet activation (P-selectin) increased by 65% (p = .004) and major histocompatibility complex class I surface expression by 40% (p = .009) from preinoculation only in socially housed animals, whereas no change in these measures occurred in singly housed animals.
Conclusions
Chronic psychosocial stress produced by single housing may play an immunomodulatory role in the innate immune response to acute retroviral infection. Dysregulated innate immunity could be one of the pathways by which psychosocial stress contributes to immune suppression and increased disease severity in people with HIV.
Key words/Abbreviations: simian immunodeficiency virus, psychosocial stress, innate immunity, infectious disease, animal models, human immunodeficiency virus, ART = antiretroviral therapy, B = unstandardized β coefficient, CBC = complete blood count, CSF = cerebrospinal fluid, CNS = central nervous system, HIV = human immunodeficiency virus, PWH = people with HIV, SE = standard error, SIV = simian immunodeficiency virus
INTRODUCTION
Human immunodeficiency virus (HIV), the agent responsible for AIDS, affects an estimated 38 million people worldwide (1). Although about two-thirds of people with HIV (PWH) have access to antiretroviral therapy (ART), many remain untreated or experience interruption in access to medication, and approximately 690,000 deaths were attributed to AIDS-related illness in 2019 (1,2). In addition, clinical comorbidities still develop during ART. For example, central nervous system (CNS) complications remain highly prevalent in ART-treated individuals and are associated with lower quality of life and increased mortality (3). A major barrier to curing HIV infection is the establishment of latent viral reservoirs within resting cells and tissues inaccessible to ART (4,5). These reservoirs may play a role in predisposition to the comorbidities seen in PWH by serving as an ongoing inflammatory stimulus (6,7).
For these reasons, it is imperative that the immunomodulatory response to HIV infection is better understood. Clinical outcomes from HIV infection vary widely between individuals; these variations are modulated by both genetic and environmental factors that alter immune response (8–10). Among these, PWH are at greater risk of external stress from social factors, such as social isolation and loss of support due to the stigmatized nature of HIV infection. These psychosocial stressors (11) are associated with worse mental and physical quality of life in PWH compared with other chronic conditions and are associated with increased incidence of comorbidities such as cognitive impairment (12–17). Establishment of increased social support improves personal resilience and has been shown to mitigate the negative impacts of social stressors (14,17–19).
Chronic stressors and HIV infection independently induce persistent immune activation, and many studies have demonstrated the importance of immune dysregulation, rather than viral load, in the development of adverse outcomes (20,21). Monocytes in particular have been identified as a pivotal cell type in mediating damage in HIV infection secondary to inflammation. Activation of monocytes and macrophages, as indicated by plasma level of sCD14 and sCD163, is associated with a greater risk of cardiovascular disease, cognitive impairment, and other comorbidities in PWH (22,23). In particular, monocyte subsets expressing CD16 (CD14+CD16+ intermediate monocytes and CD14low CD16+ nonclassical monocytes) have been found to be permissive to HIV and simian immunodeficiency virus (SIV) infection. These monocytes may play an important role in transporting virus into the brain, contributing to the establishment of latent viral reservoirs in this sanctuary organ (23,24). Platelets also play an important role in modulating immune responses to HIV infection, demonstrated both in the formation of activated platelet-monocyte aggregates that drive the adoption of the CD16+ monocyte phenotype during acute infection and by the persistent platelet activation seen in PWH receiving ART (25–27).
Direct study of the impact of psychosocial stress in HIV infection is confounded by the complex nature of sociobehavioral factors and their potential to affect access to care (18,19). However, psychosocial stress can be recapitulated with animal models to allow for its investigation in a controlled setting (28–30). The pigtail macaque (Macaca nemestrina) model of SIV infection pathophysiologically resembles HIV infection in humans, and disease progression is similarly characterized by CD4 T-cell depletion and a persistent viral reservoir during ART (31,32). Previous work by this group demonstrates that the psychosocial stress associated with single housing results in elevated viral load and dysregulation of both CD4 and CD8 T-cell activation during acute SIV infection in this social species (33). Because these changes in viral dynamics and adaptive immunity occur acutely after infection, these findings raised the question of whether these differences may be driven by alterations in innate immune function. In this investigation, we seek to expand on previous findings by characterizing the innate immune response to inoculation with SIV in singly and socially housed macaques. A retrospective analysis of monocyte and platelet hematology and flow cytometry data was performed. We hypothesized that psychosocial stress would result in a less robust innate immune response during acute infection in the SIV-infected pigtail macaque model.
METHODS
Animals
A retrospective analysis of acute infection data from 76 two- to six-year-old male pigtailed macaques (M. nemestrina) dual-inoculated with a single stock of the neurovirulent clone SIV/17E-Fr and immunosuppressive swarm SIV/DeltaB670, as previously described (32,33), was performed. All were specific pathogen free for SIV, simian T-cell leukemia virus, and simian type D retrovirus before inoculation and negative for the Mane-A1*08401 major histocompatibility complex class I allele, which confers resistance to SIV (34). Three baseline time points of sample collection were performed before inoculation, each at least 2 weeks apart. Postinoculation samples were collected on days 7, 10, and 14. Macaques were sedated with ketamine (10 mg/kg intramuscular) at each time point for cerebrospinal fluid collection from the cisterna magna followed by collection of 13 ml of blood via femoral venipuncture. Samples were collected with a 21-gauge 1.5-inch needle directly into vacutainer tubes containing 3.2% sodium citrate at a 9:1 ratio of blood to anticoagulant for platelet samples or into syringes with acid citrate dextrose solution at a 5:1 ratio of blood to anticoagulant for monocyte samples. Socially housed macaques were always inoculated and sampled on the same day at the same time as the other animals in their pair or trio, and sedation order remained constant throughout the study. Most macaques initiated ART on day 12; those that did not were excluded from day 14 analysis (Supplemental Digital Content, Table S1, http://links.lww.com/PSYMED/A873).
All animals were housed in the same animal facility and had ad libitum food (Purina 5038) and water throughout the study, as well as daily enrichment from behavior staff. All macaques used before 2013 were kept in single housing (n = 35): animals were able to visualize and interact with conspecifics in the room, but had no direct, full contact. After 2013, incoming animals were grouped in compatible pairs or trios (n = 41) by an animal behaviorist and observed over time to ensure pair stability. Socially housed animals were all introduced at least 2 months before initiation of the study, and groupings remained stable with full contact for the duration of this study.
All procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee and were conducted in accordance with guidelines set forth in the Animal Welfare Regulations and the Guide for the Care and Use of Laboratory Animals. These studies were all conducted within a fully AAALAC-accredited facility.
Sample Processing
Complete blood counts (CBCs) were performed on citrated whole blood samples using either a Hemavet (Drew Scientific) hematology analyzer or a Procyte Dx Hematology Analyzer (IDEXX Laboratories; Supplemental Digital Content, Tables S1 and S2, http://links.lww.com/PSYMED/A873); both had been calibrated for use in pigtailed macaques. To assess monocyte phenotype, citrated whole blood was stained with antibodies for CD14 and CD16+/−TLR2 (Supplemental Digital Content, Table S3, http://links.lww.com/PSYMED/A873) for 20 minutes at room temperature, followed by a 10-minute fixation with BD FACS Lysing Solution (BD Biosciences) (35). To assess platelet activation, citrated whole blood was stained within 1 hour of collection with antibodies for CD42a, P-selectin, CD40L, and HLA-ABC (Supplemental Digital Content, Table S3, http://links.lww.com/PSYMED/A873) for 15 minutes at room temperature followed by fixation with 2% neutral-buffered formalin. The samples were analyzed by a single technician using a FACSCalibur (BD Biosciences) or a BD LSRFortessa (BD Biosciences) flow cytometer (Supplemental Digital Content, Tables S1 and S2, http://links.lww.com/PSYMED/A873).
Data Analysis
For this investigation, flow cytometry data files were reanalyzed in FlowJo (version 10) by a single blinded researcher. Results were compared between animals that were singly housed (2007–2012) versus those who were housed socially long term with a compatible conspecific (2013–2017). Because of the retrospective nature of this study, sample size for each parameter varies by the data available for each animal (Supplemental Digital Content, Table S4, http://links.lww.com/PSYMED/A873); day 14 samples were excluded from monkeys that did not initiate ART on day 12. Gating was performed as shown in Supplemental Digital Content, Figure S3, http://links.lww.com/PSYMED/A873.
To calculate absolute cell numbers for monocyte subtypes, the percent gated cells for each parameter were multiplied by the total monocyte number from the corresponding CBC for that animal and time point. A series of linear mixed-effects regression models were conducted to accommodate animals being nested in different studies. Post hoc analyses of hematology analyzer, animal age, and animal sedation order were conducted when possible and showed no effect on the outcomes under investigation. Flow cytometry machine used did show an effect on percentage of monocytes classified as CD14+CD16+ intermediate monocytes (post hoc data not shown). For this reason, only data from a single FACS machine (BD LSRFortessa) was used for subsequent analyses (n = 41 socially housed, n = 17 singly housed). Analysis assessed the influence of housing status from baseline (before infection) up to 14 days after inoculation and the relationship with previously published infection data (33). Statistical analyses were performed in SAS PROC MIXED (version 9.4) and GraphPad Prism (version 9.1.2), and graphs were generated using GraphPad Prism (version 9.1.2). Results with a p < .05 were considered significant after applying a false discovery rate correction to adjust for multiple comparisons.
RESULTS
Psychosocial Stress Associated With Transient Decline in Total Monocytes During Acute SIV Infection
Monocytes are innate immune effector cells that play a critical role in the initial response to viral challenge and thus are an important determinant in the outcome of acute HIV and SIV infection (36–38). To assess the changes in circulating monocyte numbers over the course of acute infection with SIV, CBCs were performed at three baseline time points and at 7, 10, and 14 days after inoculation (Figure 1A). Before inoculation, a single preinoculation time point showed a higher monocyte count in singly housed animals than in those that were socially housed (unstandardized β coefficient [B] = −0.13, standard error [SE] = 0.05, p = .012). Monocyte numbers increased over the first 2 weeks after infection with SIV in all animals, reflective of typical innate immune activation in response to viral inoculation (39–41). However, at day 7 after inoculation, the singly housed animals experienced a transient 40% decline in monocyte number that was not observed in socially housed animals (B = 0.29, 0.28, 0.29; SE = 0.06, 0.05, 0.06; p < .001 for all comparisons with preinoculation time points).
FIGURE 1.
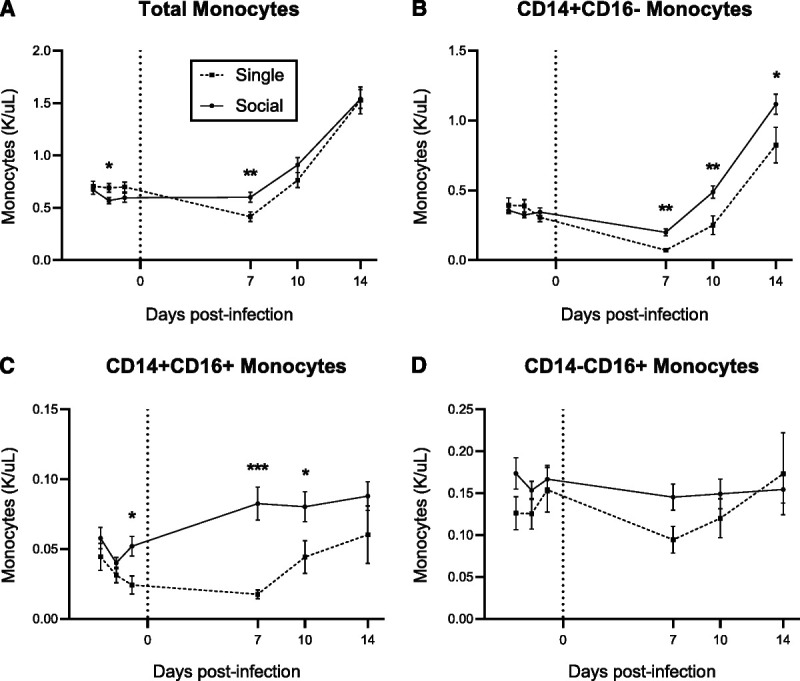
Monocyte response to acute SIV infection in singly compared with socially housed macaques. Housing groups were compared from baseline (three time points) to 7, 10, and 14 days after inoculation with SIV (for n for each time point, see Supplemental Digital Content, Table S4, http://links.lww.com/PSYMED/A873). ART was started on day 12 for all animals. A, White blood cell counts in whole blood CBCs were compared. Using a linear mixed-effects regression model, differences between housing groups over time were significant (F(5,406) = 4.27, p < .001). B–D, CD14 and CD16 monocytes expression profiles were compared using flow cytometry, and total monocyte count was used to calculate absolute numbers of each subtype. Error bars display the standard error of estimated least squares means generated by a linear mixed-effects regression model. * p < .05; ** p < .01; *** p < .001. SIV = simian immunodeficiency virus; ART = antiretroviral therapy; CBC = complete blood count.
Increase in Classical and Intermediate Monocytes During Acute Infection Delayed With Psychosocial Stress
Circulating monocytes can be classified by their transcriptional profiles into three major subtypes. Acute inflammatory processes stimulate release of CD14+ classical monocytes from the bone marrow, contributing to peripheral monocytosis during early infection (39–41). Monocytes expressing CD16 exhibit a more mature phenotype associated with patrolling the vasculature but have also been shown to be more susceptible to infection with HIV and SIV (23,42). Monocyte subsets were classified at each time point by their level of CD14 and CD16 expression. Classical monocytes were gated as monocytes negative for CD16 (CD14+CD16−), whereas intermediate (CD14+CD16+) and nonclassical (CD14−CD16+) monocytes were distinguished by the relative level of surface CD14 (Figures 1B–D). All macaques demonstrated the same pattern of change in classical (CD14+CD16−) monocyte number over acute infection (Figure 1B), consisting of transiently decreased classical monocytes at day 7 after inoculation followed by increased numbers by day 14. However, singly housed macaques experienced a greater decline in classical monocytes at day 7 (B = 0.32, 0.32, 0.23; SE = 0.05, 0.04, 0.05; p < .001 for all preinoculation time points) and numbers did not increase until day 14 (127% increase from baseline; B = −0.42, −0.42, −0.51; SE = 0.12 and p < .001 for all preinoculation time points), whereas socially housed macaques first demonstrated a 43% increase in classical monocytes on day 10 after inoculation (B = −0.13, −0.16, −0.15; SE = 0.04 and p ≤ .001 for all preinoculation time points), which increased to 227% by day 14 (B = −0.76, −0.79, −0.78; SE = 0.06 and p < .001 for all preinoculation time points). Thus, classical monocyte counts were lower in singly housed macaques at all acute infection time points, and the magnitude of this difference increased over time.
Before inoculation, all macaques had similar numbers of each monocyte subset, with the exception of intermediate monocytes at a single time point. In singly housed macaques, neither CD16+ monocyte subset count increased significantly after infection. Although classical monocytes represented the primary driver of change in total monocyte number in all macaques because of the large proportion of circulating monocytes with this phenotype, intermediate monocytes (CD14+CD16+; Figure 1C) also increased after SIV inoculation in socially housed macaques only (effect of housing: F(1,312) = 9.87, p = .002). There were no differences in nonclassical monocytes (CD14−CD16+; Figure 1D) between housing groups or over time.
Prior work has demonstrated differences in both CD4+ T-cell count and viral load in socially housed compared with singly housed macaques (33). To determine if there may be an association between the dynamics in circulating monocyte subsets and the progression of infection, previously published viral load and CD4 T-cell data (33) at day 7 after inoculation were compared with monocyte subsets in the context of housing groups (Figure 3). This time point demonstrated the largest differences in monocytes subsets between singly and socially housed animals and occurred before peak viral load. Numbers of the canonical target for SIV infection, the CD4+ T cell (Figure 3A), did not correlate with plasma or cerebrospinal fluid (CSF) viral load overall, or in the singly or socially housed subsets. Similarly, classical monocytes (CD14+CD16−; Figure 3B) showed no direct correlation with viral load in either the plasma or the CSF despite distinct patterns of association with viral load between socially and singly housed animals. Intermediate monocytes (CD14+CD16+; Figure 3C) also showed no correlation with viral load in the plasma for all macaques. However, CSF viral load showed an association with the number of intermediate monocytes (p = .03, Spearman r = 0.33) in socially housed macaques only, although no relationship was present in singly housed macaques. There was no relationship between either housing group or viral loads and nonclassical monocytes (Figure 3D).
FIGURE 3.
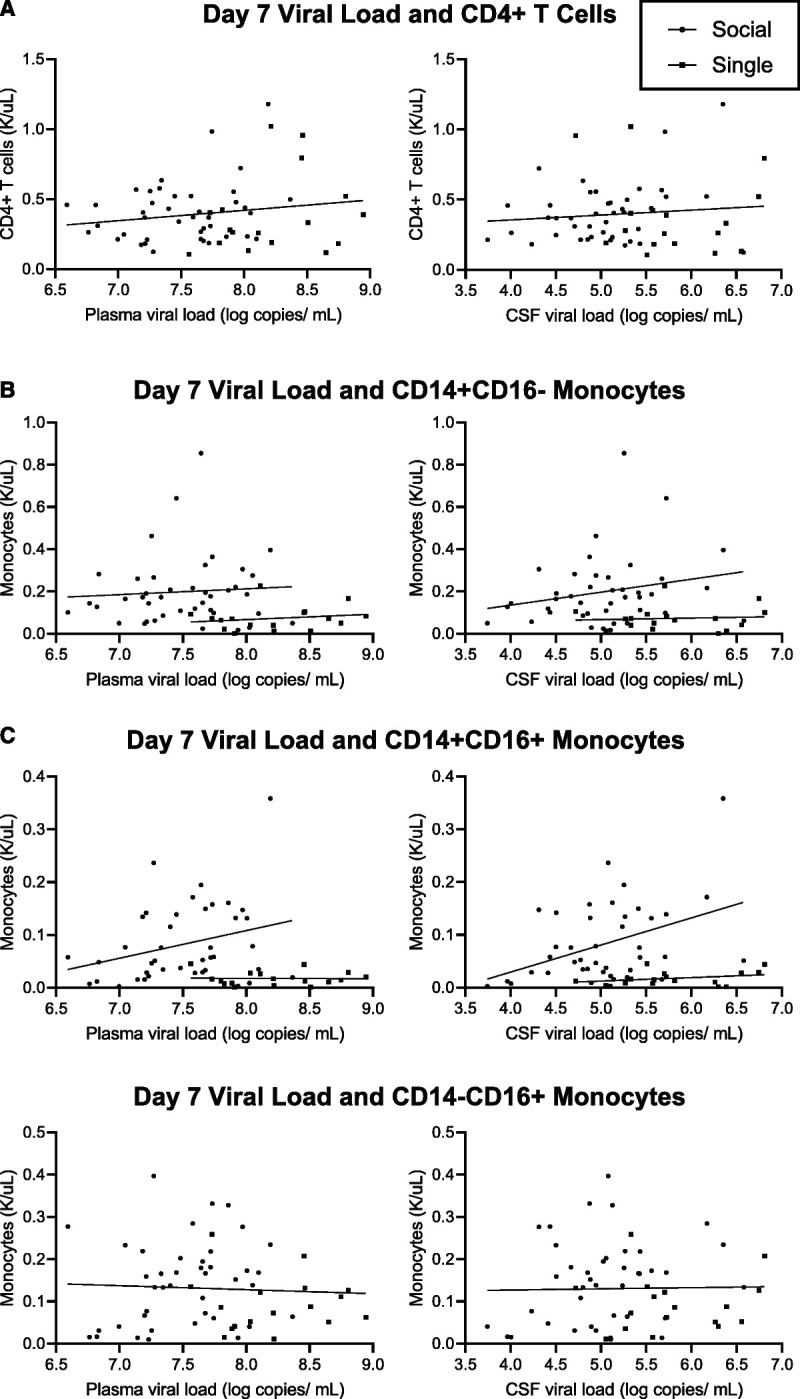
Correlation of housing status and immune parameters with viral load at day 7 after inoculation. Housing group and monocyte subsets at day 7 were correlated with previously reported data on CD4 T-cell count and plasma and CSF viral load (33). A least squares regression model was generated by using a sum-of-squares F test to determine if one line or individual lines best fit each data set. A, When directly compared, CD4 T-cell count did not correlate with plasma or CSF viral load overall, or in each housing subset. B, The line of best fit comparing CD14+CD16− monocytes and viral load was different between housing groups (p = .03 plasma, p = .004 CSF), but showed no correlation with viral load. C, The line of best fit for CD14+CD16+ monocytes was different between housing groups (p < .001), and socially housed animals only showed a positive correlation with CSF viral load (p = .03, Spearman r = 0.33). D, Plasma and CSF viral load showed no relationship with CD14−CD16+ monocytes. CSF = cerebrospinal fluid.
SIV-Associated Thrombocytopenia Prolonged With Psychosocial Stress
Transient thrombocytopenia is a typical manifestation of early retroviral infection, which resolves without observable clinical signs (21,25–27). Data from CBC also allowed for comparison of circulating platelet counts between singly and socially housed animals over the course of acute SIV infection (Figure 2A). Both groups developed thrombocytopenia after inoculation, consistent with previous reports of response to acute infection in SIV (25). Although the onset and magnitude of thrombocytopenia were similar between housing groups, platelet numbers in singly housed macaques did not return to baseline by day 14 after inoculation, leading to a divergence between housing groups at this time point (B = 130.46, SE = 29.79, p < .001). The nadir in platelet count occurred on day 10 for both housing groups, with a 58% decline from baseline in singly housed macaques and a 43% decline in socially housed macaques (Supplemental Digital Content, Figure S1A, http://links.lww.com/PSYMED/A873).
FIGURE 2.
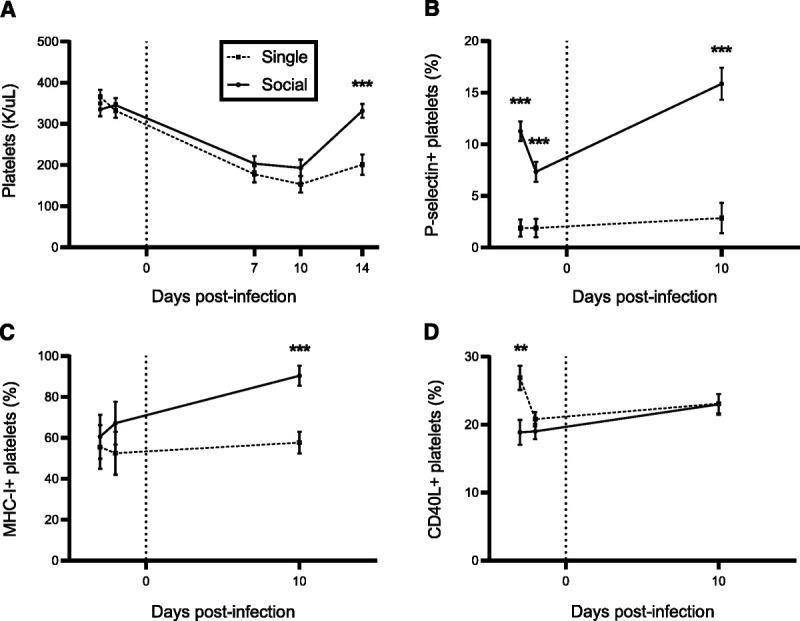
Platelet response to acute SIV infection in singly compared with socially housed macaques. Housing groups were compared at baseline (two time points) against day 10 after inoculation with SIV (for n for each time point, see Supplemental Digital Content, Table S4, http://links.lww.com/PSYMED/A873). ART was started on day 12 for all animals. A, Platelet counts in whole blood CBCs were compared. Singly housed animals showed a delayed recovery from thrombocytopenia during acute infection, failing to return to baseline by day 14. Differences between housing groups over time were significant (F(4,64) = 8.26, p < .001). B, Platelet P-selectin expression in singly housed macaques was less than in socially housed both at baseline and at day 10. C, The effects of housing (F(2,41) = 47.03, p < .001) and housing over time (F(2,41) = 53.29, p = .009) were significant. Platelet CD40L expression was not different between groups at day 10; however, a difference was present in a single baseline time point. In addition, the effect of housing over time was significant (F(2,41) = 10.03, p < .001). D, Platelet MHC-I expression was not as upregulated at day 10 in singly housed animals. In addition, the effect of housing over time was significant (F(2,39) = 4.18, p = .023). Error bars display the standard error of estimated least squares means generated by a linear mixed-effects regression model. * p < .05; ** p < .01; *** p < .001. SIV = simian immunodeficiency virus; ART = antiretroviral therapy; CBC = complete blood count.
Platelet Activation During Acute Infection Is Suppressed With Psychosocial Stress
Platelets are increasingly recognized for their role as immune effector cells upon activation, and both signal to other immune cells and directly interact with SIV and HIV in vivo (25,43–46). Platelet activation in whole blood samples was assessed using flow cytometry based on surface expression of P-selectin, CD40L, and MHC-I at two baseline time points and at day 10 after inoculation (Figures 2B–D; Supplemental Digital Content, Figures S1B–D, http://links.lww.com/PSYMED/A873). At day 10, singly housed macaques showed no change in expression of P-selectin and MHC-I from their baseline levels, although socially housed macaques exhibited an increase in platelet activation as shown by both increased P-selectin (65% increase from baseline, B = −4.59, −8.50; SE = 1.52, 0.92; p = .004, p < .001; Figure 2B) and MHC-I (40% increase, B = −29.76, −23.18; SE = 10.85, 11.16; p = .009, p = .044; Figure 2C) expression. P-selectin expression was 70% to 83% lower in singly housed than socially housed macaques before and after infection (B = 9.38, 5.46, 13.00; SE = 1.27, 1.32, 2.15; p < .001 at each time point), whereas MHC-I expression diverged only after infection (35% lower in singly housed animals, B = 32.72, SE = 7.28, p < .001). Conversely, no increase with infection or difference between groups was observed in CD40L expression (Figure 2D) other than a single elevated baseline time point in singly housed macaques (B = −8.02, SE = 2.55, p = .003), although the effect of housing over time was significant (F(2,41) = 10.03, p < .001).
DISCUSSION
The immune response of pigtail macaques to SIV infection was impacted by housing status, with singly housed macaques showing reduced expansion of classical and intermediate monocytes, prolonged thrombocytopenia, and a suppression of platelet activation during the first 2 weeks after inoculation. Circulating monocytes and platelets are two effector cells critical to a rapid response during immune challenge, and our findings indicate that both populations may exhibit a blunted antiviral response under a condition modeling psychosocial stress. These data reflect that psychosocial stress may induce clinically significant immunomodulatory effects in the innate immune system during acute SIV infection, and are consistent with previous work in this model showing alterations in T-cell phenotype before infection and higher viral loads after infection in singly housed macaques (33).
Psychosocial stressors, such as social adversity, disruption, or exclusion, can induce broad physiological changes through their effects on the hypothalamic-pituitary-adrenal axis and autonomic nervous system. In people, these effects are ubiquitously confounded with socioeconomic status, which is associated with health outcomes through additional factors such as access to health resources and risk behaviors. Because of the complexity of these relationships, it is difficult to demonstrate their direct influence on regulation of the immune system, especially in the context of HIV infection (47,48).
Macaques are naturally social animals that require interaction with conspecifics to maintain their psychological welfare (49,50). This trait makes them a natural model to recapitulate psychosocial stress in a controlled setting, and multiple experimental designs have been used to validate the physiological and behavioral changes that occur in macaques under different social conditions (29,51–53). Because this was a retrospective investigation, no stress biomarkers or behavioral assessments are available for the animals in this study. We acknowledge that this is a significant limitation of this study given that the complexity of social interactions in macaques can sometimes lead to individuals who experience greater stress from their social status than they would without contact with conspecifics (54–57). However, numerous publications have demonstrated the overall positive impact of social housing in comparison to single housing in diverse populations of macaques as assessed by both behavioral outcomes and immune function (53,58–60). One of these positive impacts is the phenomenon of social buffering. Social buffering, or the ability of a compatible social partner to improve coping, has been associated with fewer abnormal behaviors and reduced cortisol release in response to stressors (61). In particular, full contact housing with conspecifics has been shown to increase the amount of species-typical behavior, reduce maladaptive behaviors such as stereotypies, increase coping ability, and reduce the need for veterinary care (62). It is important to note that, although numerous studies report positive behavioral outcomes from social housing of macaques, which is currently the standard for assessment of animal welfare, measurement of cortisol as a biomarker of stress has yielded highly inconsistent results (60,63–66). There are a number of factors that may contribute to this variability, including method and timing of measurement as well as the numerous physiological factors that affect cortisol levels (67–69).
Previous studies investigating psychosocial stressors in SIV-infected macaques have demonstrated an adverse effect on disease outcomes, including altered glucocorticoid regulation, greater viral load, and decreased survival time (28,29). Although the binary system of psychosocial stress used by our study cannot fully recapitulate the complexity of psychosocial and socioeconomic effects experienced by PWH, this model demonstrates a relationship between reduced social contact and immunomodulation. The retrospective nature of this investigation limits the scope of longitudinal data because macaques were involved in different studies and, at the final time point, had received 2 days of ART intervention. In addition, the chronological separation between housing groups allows for the possibility that, despite rigorous efforts to control experimental procedures, unidentified variables may have contributed to the effects found in this investigation (Supplemental Digital Content, Table S5, http://links.lww.com/PSYMED/A873). Because of the limited availability of SIV infection data in female macaques, only males were included in this study. Future work will be needed to elucidate whether sex, age, diet, or the microbiota-immune axis also drive differences in these immune alterations. In addition, more investigation is needed to uncover potential mechanisms for how psychosocial stress may initiate these immunological changes.
Monocytes play a complex role in the response to and persistence of retroviral infections. As innate immune effector cells, they are important for mounting effective antiviral responses before the adaptive immune response develops. However, monocytes and tissue macrophages are also susceptible to infection with HIV or SIV and are a major contributor in the establishment of tissue reservoirs (22–24,36,42). In addition to being an integral component of the viral reservoir in the CNS, neuroinflammation leading to cognitive impairment is mediated by monocyte activation (7). Monocytes are similarly critical effectors in the systemic inflammatory response that persists during chronic HIV and SIV infection and contribute to the clinical symptoms seen in PWH, with tissue factor expression on persistently activated monocytes in PWH on ART specifically associated with coagulopathies (20,70). Inappropriate monocyte activation can also lead to excess production of proinflammatory cytokines, such as tumor necrosis factor, which can impair mucosal integrity and contribute to lipopolysaccharide and gut luminal microbe translocation (71).
Our findings indicate that the number of classical and intermediate monocytes is persistently lowered with psychosocial stress over the course of acute SIV infection. All macaques had a similar baseline before infection, but only singly housed animals showed a transient drop in classical monocytes on day 7, and although numbers increased in all animals, differences between housing groups persisted over time. Singly housed animals similarly did not show increased intermediate monocytes after infection; these differences were also present in the percentage of gated cells from immunophenotyping data (Supplemental Digital Content, Figure S2, http://links.lww.com/PSYMED/A873). Classical monocytes are the most numerous monocyte subset in circulation, representing cells that are newly released from bone marrow and predisposed to an inflammatory, antiviral phenotype (24,37,40). Classical monocytes are relatively resistant to infection and may play an important role in limiting the viral peak during acute infection through mechanisms involving increased interleukin 18 and inflammasome activation (37,38,41). Conversely, intermediate and nonclassical monocytes (CD14+CD16+ and CD14lowCD16+, respectively) are more permissive to HIV infection, and intermediate monocytes are associated with transport of virus across the blood-brain barrier and induction of CNS inflammation that persists with ART. These subsets of monocytes have been shown to increase in HIV and correlate with viral load in SIV infection (23,24). Despite the importance of nonclassical monocytes in retroviral pathogenesis, intermediate monocyte counts were lower in singly housed compared with socially housed animals. In addition, a transient decrease in total monocyte number was observed in singly housed animals on day 7 after infection. Peripheral monocyte counts are affected both by rate of monocyte extravasation into tissues and by the rate of release of new monocytes into circulation from hematopoietic bone marrow (40). Both of these factors can be impacted by stressors because they are directly affected by signaling from the sympathetic nervous system and hypothalamic-pituitary-adrenal axis (72,73). In addition to an effect on circulating cell numbers, models of psychosocial stress have demonstrated an altered transcriptome in classical monocytes that result in blunted antiviral responses and reduced glucocorticoid sensitivity (74). This monocytopenia in singly housed macaques could therefore be caused by increased migration into tissues, a lack of upregulation of monocyte release in response to infection, or a combination of these factors (39,41).
Although platelet activation has been associated with physiological stress in both people and animal models, it is not a reliable measure of stress because it is variable based on the chronicity of the stressor and is directly affected by multiple other parameters (75,76). Increased surface expression of P-selectin and MHC-I on platelets consistent with previous observations during acute infection (25) was observed in socially housed macaques, although no change in platelet activation parameters was seen in the singly housed animals. Platelets are another innate immune cell that play a role in responding to HIV infection and in symptoms of PWH. Because of the complexity of their functions, it remains unclear if platelets play a permissive or protective role during HIV infection (21,27,43). Platelets are able to uptake HIV virions and productively infect other cells in vitro (45,46). However, platelets also express TLRs, and stimulation with TLR7 upregulates P-selectin and CD154 as a direct antiviral response. Activated platelets contribute to antiviral responses through multiple mechanisms including interleukin 1β–mediated inflammasome activation and an array of immunomodulatory signals released by α-granules (43,77). For example, CXCL4 from α-granules has been demonstrated to inhibit host cell entry of HIV-1 (44). Release of α-granules also enhances antigen presentation by increasing surface MHC-I, and platelets have been demonstrated to increase T-cell activation in response to viral inoculation (78). Neutrophil (via P-selectin and CCL5) and monocyte (via P-selectin, CD40L, PGE2, and PF4) migration, activation, and survival are modulated both by direct interaction with activated platelets and by secreted signals (77). Persistent platelet activation, as indicated by increased CD40L and P-selectin, is one element of the chronic inflammatory state induced by HIV and has been reported to persist even during successful ART (27).
Psychosocial stress as modeled by single housing was associated with a failure to return to baseline platelet counts by 2 weeks after infection, although the magnitude of thrombocytopenia during acute infection was similar across all animals. Thrombocytopenia is a common finding in PWH and is associated with viral load and disease progression. Reduction in circulating platelet count can be caused by sequestration through activation and binding, increased destruction of platelets, or decreased production by megakaryocytes (79). Sequestration of platelets through the formation of platelet-monocyte aggregates has been associated with thrombocytopenia during acute SIV infection (25). However, thrombopoiesis may be affected by glucocorticoid resistance (80), and the concomitant lack of platelet activation seen with psychosocial stress suggests that persistence of thrombocytopenia may be related to a failure to appropriately upregulate platelet production rather than increased rate of sequestration. Additional investigation could clarify the mechanism driving thrombocytopenia in the context of psychosocial stress.
Activated platelets can signal to and directly bind with circulating immune cells (21,43), including monocytes (25), and the changes we observed in monocyte and platelet phenotype in psychosocial stress may be mechanistically connected. The formation of platelet-monocyte aggregates has been shown to increase monocyte adhesion to the endothelium and drive them toward a CD16+ phenotype (81,82), and both monocytosis and platelet activation during acute SIV infection may increase these interactions (25). Psychosocial stress was associated with reductions in intermediate monocytes, but no differences in the nadir of platelet count during acute infection in this study. Unfortunately, we did not have the opportunity to examine platelet-monocyte aggregates in this retrospective study. More work is needed to understand if decreased platelet-monocyte aggregation occurs during psychosocial stress and how this affects immune cell extravasation into tissues and susceptibility to retroviral infection.
The changes in monocyte and platelet phenotype associated with psychosocial stress in this study may reflect multiple functional deficits through which increased viral load can be established during acute SIV infection. Because these innate effector cells are critical components of the immune response before the development of adaptive immunity (37,43), lower monocyte and platelet numbers and activation in singly housed macaques may allow greater viral replication during early infection. Peak viral load during acute infection is associated with worse clinical outcomes in PWH (83) and is also associated with the extent the viral reservoir is established before the adaptive immune response develops (84). Thus, the loss of classical and intermediate monocytes and activated platelets with psychosocial stress may represent an immunosuppressive state that is permissive to SIV infection. These findings are consistent with previous work from our laboratory, which demonstrated higher viral loads in the blood and CSF of singly housed macaques during acute SIV infection. Activation of CD4 and CD8 T cells was greater in singly housed animals, which may represent compensation of the adaptive immune system to an inadequate innate response during acute infection (33). The interplay of different components of the immune system with virus and with each other is mechanistically complex and represents an area for future work. These findings emphasize the importance of mitigating psychosocial stressors in PWH, such as through provision of strong social support systems.
Of note, the associations between classical and intermediate monocyte numbers with plasma and CSF viral load differed significantly between the socially and singly housed animals. A positive correlation between intermediate monocyte number and CSF viral load was revealed only in animals that were socially housed. Intermediate monocytes have previously been implicated in the establishment of latent reservoirs in the brain and other tissues, and such an association could be interpreted as further support for such a hypothesis. Psychosocial stress may have masked detection of this relationship in singly housed animals and should be considered as a variable in future investigations.
Single housing, as a model of psychosocial stress, modulates the innate immune response to SIV infection as shown by reduced numbers of circulating classical and intermediate monocytes and platelets during acute infection. Platelet activation is similarly suppressed under conditions of limited social contact. These changes are suggestive of a reduced antiviral innate immune response and may represent an inadequate response to inoculation with SIV. This may allow for the establishment of a greater viral burden (33) and long-term clinical consequences of retroviral infection. These findings not only highlight the need for interventions to address chronic stressors such as social isolation in PWH but also illustrate the importance of cohabitation as the standard for housing of social species such as macaques. These data supply evidence that single housing can directly impact study outcomes in addition to compromising animal welfare. Robust animal models for infectious disease research must reflect a normal immunological state to best translate findings into successful treatments. Further work is needed to validate the translatability of these findings to PWH and identify the mechanisms by which psychosocial stress alters antiviral responses.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the critical contributions of Brandon Bullock and Ming Li to this investigation. Their work included technical assistance in completing animal studies and establishing techniques for sample preparation and assays. The authors also acknowledge the generous antiretroviral therapy donation from Abbvie (Abbott), Bristol-Myers Squibb, Gilead, Merck, Janssen Pharmaceuticals, Roche, and ViiV Healthcare.
Source of Funding and Conflicts of Interest: This study was supported by Grants for Laboratory Animal Science (GLAS), the National Institutes of Health (P30 AI094189, P01 AI131306, K01 OD018244, P30 MH075673, RR00116, P40 OD013117/U42 OD013117, R01 NS089482, NS097221, NS055651, MH61189, MH070306, NS36911, RR019995, and T32 OD011089), BSi, and Blaustein Pain Foundation. The authors declare no conflicts of interest.
Open Access publication for this article, which is part of a special themed issue of Psychosomatic Medicine, was funded by the National Institute of Mental Health.
Footnotes
Alternate corresponding author: Natalie Castell (e-mail: ncastel3@jhmi.edu).
Supplemental Digital Content
Contributor Information
Natalie Castell, Email: ncastel3@jhmi.edu.
Selena M. Guerrero-Martin, Email: sguerre3@jhmi.edu.
Leah H. Rubin, Email: lrubin@jhu.edu.
Erin N. Shirk, Email: eshirk1@jhmi.edu.
Jacqueline K. Brockhurst, Email: jbrockh1@jhmi.edu.
Claire E. Lyons, Email: celyons@mit.edu.
Kevin M. Najarro, Email: kevin.najarro@cuanschutz.edu.
Suzanne E. Queen, Email: squeena@jhmi.edu.
Bess W. Carlson, Email: besscarlson@outlook.com.
Robert J. Adams, Email: rjadams@jhmi.edu.
Craig N. Morrell, Email: craig_morrell@urmc.rochester.edu.
Lucio Gama, Email: lucio@jhmi.edu.
David R. Graham, Email: dgraham@jhmi.edu.
Christine Zink, Email: cz@jhmi.edu.
Joseph L. Mankowski, Email: jmankows@jhmi.edu.
Janice E. Clements, Email: jclements@jhmi.edu.
REFERENCES
- 1.Global HIV & AIDS Statistics—2020 Fact Sheet | UNAIDS. Available froat: https://www.unaids.org/en/resources/fact-sheet. Accessed March 23, 2021.
- 2.Global Statistics | HIV.gov. Available at: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics. Accessed March 23, 2021.
- 3.Anteraper SA Gopinath K Hoch MJ Waldrop-Valverde D Franklin D Letendre SL, et al. A comprehensive data-driven analysis framework for detecting impairments in brain function networks with resting state fMRI in HIV-infected individuals on cART. J Neurovirol 2021;27:239–48. [DOI] [PubMed] [Google Scholar]
- 4.Siliciano JD, Siliciano RF. Recent developments in the search for a cure for HIV-1 infection: targeting the latent reservoir for HIV-1. J Allergy Clin Immunol 2014;134:12–9. [DOI] [PubMed] [Google Scholar]
- 5.Dinoso JB Rabi SA Blankson JN Gama L Mankowski JL Siliciano RF, et al. A simian immunodeficiency virus–infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol 2009;83:9247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanal S, Schank M, El Gazzar M, Moorman JP, Yao ZQ. HIV-1 latency and viral reservoirs: existing reversal approaches and potential technologies, targets, and pathways involved in HIV latency studies. Cell 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams ME, Stein DJ, Joska JA, Naudé PJW. Cerebrospinal fluid immune markers and HIV-associated neurocognitive impairments: a systematic review. J Neuroimmunol 2021;358:577649. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien TR Blattner WA Waters D Eyster E Hilgartner MW Cohen AR, et al. Serum HIV-1 RNA levels and time to development of AIDS in the multicenter hemophilia cohort study. J Am Med Assoc 1996;276:105–10. [PubMed] [Google Scholar]
- 9.Fiebig EW Wright DJ Rawal BD Garrett PE Schumacher RT Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003;17:1871–9. [DOI] [PubMed] [Google Scholar]
- 10.Streeck H Lu R Beckwith N Milazzo M Liu M Routy J-P, et al. Emergence of individual HIV-specific CD8 T cell responses during primary HIV-1 infection can determine long-term disease outcome. J Virol 2014;88:12793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upton J. Psychosocial factors. In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. New York, NY: Springer New York; 2013:1580–1. [Google Scholar]
- 12.Leserman J Jackson ED Petitto JM Golden RN Silva SG Perkins DO, et al. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosom Med 1999;61:397–406. [DOI] [PubMed] [Google Scholar]
- 13.Hays RD Cunningham WE Sherbourne CD Wilson IB Wu AW Cleary PD, et al. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV Cost and Services Utilization Study. Am J Med 2000;108:714–22. [DOI] [PubMed] [Google Scholar]
- 14.Ashton E Vosvick M Chesney M Gore-Felton C Koopman C O’Shea K, et al. Social support and maladaptive coping as predictors of the change in physical health symptoms among persons living with HIV/AIDS. AIDS Patient Care STDS 2005;19:587–98. [DOI] [PubMed] [Google Scholar]
- 15.Greysen SR, Horwitz LI, Covinsky KE, Gordon K, Ohl ME, Justice AC. Does social isolation predict hospitalization and mortality among HIV+ and uninfected older veterans? J Am Geriatr Soc 2013;61:1456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marziali ME McLinden T Card KG Closson K Wang L Trigg J, et al. Social isolation and mortality among people living with HIV in British Columbia, Canada. AIDS Behav 2021;25:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis RJ Iudicello J Sun-Suslow N Grelotti D Cherner M Morgan E, et al. Social isolation is linked to inflammation in aging people with HIV and uninfected individuals. J Acquir Immune Defic Syndr 2021;86:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez IR Baño JR Ruz MAL del Arco Jimenez A Prados MC Liaño JP, et al. Health-related quality of life of patients with HIV: impact of sociodemographic, clinical and psychosocial factors. Qual Life Res 2005;14:1301–10. [DOI] [PubMed] [Google Scholar]
- 19.Engelhard EAN Smit C van Dijk PR Kuijper TM Wermeling PR Weel AE, et al. Health-related quality of life of people with HIV: an assessment of patient related factors and comparison with other chronic diseases. AIDS 2018;32:103–12. [DOI] [PubMed] [Google Scholar]
- 20.Temu TM Zifodya JS Polyak SJ Wagoner J Wanjalla CN Masyuko S, et al. Antiretroviral therapy reduces but does not normalize immune and vascular inflammatory markers in adults with chronic HIV infection in Kenya. AIDS 2021;35:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madzime M, Rossouw TM, Theron AJ, Anderson R, Steel HC. Interactions of HIV and antiretroviral therapy with neutrophils and platelets. Front Immunol 2021;12:634386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakritz JR, Robinson JA, Polydefkis MJ, Miller AD, Burdo TH. Loss of intraepidermal nerve fiber density during SIV peripheral neuropathy is mediated by monocyte activation and elevated monocyte chemotactic proteins. J Neuroinflammation 2015;12:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.León-Rivera R Veenstra M Donoso M Tell E Eugenin EA Morgello S, et al. Central nervous system (CNS) viral seeding by mature monocytes and potential therapies to reduce CNS viral reservoirs in the cART era. mBio 2021;12:e03633–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim W-K Sun Y Do H Autissier P Halpern EF Piatak M, et al. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol 2010;87:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metcalf Pate KA, Lyons CE, Dorsey JL, Shirk EN, Queen SE, Adams RJ. Platelet activation and platelet-monocyte aggregate formation contribute to decreased platelet count during acute simian immunodeficiency virus infection in pig-tailed macaques. J Infect Dis 2013;208:874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcantara S Reece J Amarasena T Rose RD Manitta J Amin J, et al. Thrombocytopenia is strongly associated with simian AIDS in pigtail macaques. J Acquir Immune Defic Syndr 2009;51:374–9. [DOI] [PubMed] [Google Scholar]
- 27.Nkambule BB Mxinwa V Mkandla Z Mutize T Mokgalaboni K Nyambuya TM, et al. Platelet activation in adult HIV-infected patients on antiretroviral therapy: a systematic review and meta-analysis. BMC Med 2020;18:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc Natl Acad Sci U S A 1998;95:4714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capitanio JP, Lerche NW. Social separation, housing relocation, and survival in simian AIDS: a retrospective analysis. Psychosom Med 1998;60:235–44. [DOI] [PubMed] [Google Scholar]
- 30.Cole SW, Mendoza SP, Capitanio JP. Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosom Med 2009;71:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deere JD, Schinazi RF, North TW. Simian immunodeficiency virus macaque models of HIV latency. Curr Opin HIV AIDS 2011;6:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clements JE, Mankowski JL, Gama L, Zink MC. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus–associated neurological disease: from mechanism to treatment. J Neurovirol 2008;14:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerrero-Martin SM Rubin LH McGee KM Shirk EN Queen SE Li M, et al. Psychosocial stress alters the immune response and results in higher viral load during acute SIV infection in a pigtailed macaque model of HIV. J Infect Dis 2021;224:2113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith MZ Fernandez CS Chung A Dale CJ De Rose R Lin J, et al. The pigtail macaque MHC class I allele Mane-A*10 presents an immundominant SIV Gag epitope: identification, tetramer development and implications of immune escape and reversion. J Med Primatol 2005;34:282–93. [DOI] [PubMed] [Google Scholar]
- 35.Shirk EN, Kral BG, Gama L. Toll-like receptor 2bright cells identify circulating monocytes in human and non-human primates. Cytometry A 2017;91:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabezanahary H Clain J Racine G Andreani G Benmadid-Laktout G Borde C, et al. Early antiretroviral therapy prevents viral infection of monocytes and inflammation in simian immunodeficiency virus–infected Rhesus macaques. J Virol 2020;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaccari M Fourati S Gordon SN Brown DR Bissa M Schifanella L, et al. HIV vaccine candidate activation of hypoxia and the inflammasome in CD14+ monocytes is associated with a decreased risk of SIVmac251 acquisition. Nat Med 2018;24:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorini G Fourati S Vaccari M Rahman MA Gordon SN Brown DR, et al. Engagement of monocytes, NK cells, and CD4+ Th1 cells by ALVAC-SIV vaccination results in a decreased risk of SIVmac251 vaginal acquisition. PLoS Pathog 2020;16:e1008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yáñez A, Goodridge HS, Gozalbo D, Gil ML. TLRs control hematopoiesis during infection. Eur J Immunol 2013;43:2526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu YP, Thomas GD, Hedrick CC. 2014 Jeffrey M. Hoeg award lecture: transcriptional control of monocyte development. Arterioscler Thromb Vasc Biol 2016;36:1722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arfi V Rivière L Jarrosson-Wuillème L Goujon C Rigal D Darlix J-L, et al. Characterization of the early steps of infection of primary blood monocytes by human immunodeficiency virus type 1. J Virol 2008;82:6557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowlin BT, Wang J, Schafer JL, Autissier P, Burdo TH, Williams KC. Monocyte subsets exhibit transcriptional plasticity and a shared response to interferon in SIV-infected rhesus macaques. J Leukoc Biol 2018;103:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribeiro LS, Migliari Branco L, Franklin BS. Regulation of innate immune responses by platelets. Front Immunol 2019;10:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon Tsegaye T Gnirß K Rahe-Meyer N Kiene M Krämer-Kühl A Behrens G, et al. Platelet activation suppresses HIV-1 infection of T cells. Retrovirology 2013;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson SR, Singh MV, Dewhurst S, Schifitto G, Maggirwar SB. Platelets function as an acute viral reservoir during HIV-1 infection by harboring virus and T-cell complex formation. Blood Adv 2020;4:4512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Real F Capron C Sennepin A Arrigucci R Zhu A Sannier G, et al. Platelets from HIV-infected individuals on antiretroviral drug therapy with poor CD4+ T cell recovery can harbor replication-competent HIV despite viral suppression. Sci Transl Med 2020;12. [DOI] [PubMed] [Google Scholar]
- 47.Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol 2009;60:501–24. [DOI] [PubMed] [Google Scholar]
- 48.Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta2-adrenergic receptor in children with asthma. Proc Natl Acad Sci U S A 2006;103:5496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder-Mackler N Sanz J Kohn JN Brinkworth JF Morrow S Shaver AO, et al. Social status alters immune regulation and response to infection in macaques. Science 2016;354:1041–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiVincenti L, Wyatt JD. Pair housing of macaques in research facilities: a science-based review of benefits and risks. J Am Assoc Lab Anim Sci 2011;50:856–63. [PMC free article] [PubMed] [Google Scholar]
- 51.Capitanio JP, Lerche NW. Psychosocial factors and disease progression in simian AIDS: a preliminary report. AIDS 1991;5:1103–6. [DOI] [PubMed] [Google Scholar]
- 52.Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol 2015;66:733–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pahar B Baker KC Jay AN Russell-Lodrigue KE Srivastav SK Aye PP, et al. Effects of social housing changes on immunity and vaccine-specific immune responses in adolescent male Rhesus macaques. Front Immunol 2020;11:565746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology 2012;37:1071–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proc Natl Acad Sci U S A 2012;109:9017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abbott DH Keverne EB Bercovitch FB Shively CA Mendoza SP Saltzman W, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav 2003;43:67–82. [DOI] [PubMed] [Google Scholar]
- 57.Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis). J Neuroendocrinol 2009;21:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, Capitanio J. Laboratory rhesus macaque social housing and social changes: implications for research. Am J Primatol 2017;79:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hennessy MB, Chun K, Capitanio JP. Depressive-like behavior, its sensitization, social buffering, and altered cytokine responses in rhesus macaques moved from outdoor social groups to indoor housing. Soc Neurosci 2017;12:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker KC Bloomsmith MA Oettinger B Neu K Griffis C Schoof V, et al. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Appl Anim Behav Sci 2012;137:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilbert MH, Baker KC. Social buffering in adult male rhesus macaques (Macaca mulatta): effects of stressful events in single vs. Pair housing. J Med Primatol 2011;40:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schapiro SJ, Bushong D. Effects of enrichment on veterinary treatment of laboratory Rhesus macaques (Macaca mulatta). Animal Welfare 1994;3:25–36. [Google Scholar]
- 63.Zijlmans DGM Meijer L Vernes MK Wubben JAM Hofman L Louwerse AL, et al. Effect of housing conditions on cortisol and body fat levels in female rhesus macaques. Biology 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hannibal DL Cassidy LC Vandeleest J Semple S Barnard A Chun K, et al. Intermittent pair-housing, pair relationship qualities, and HPA activity in adult female rhesus macaques. Am J Primatol 2018;80:e22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. Life at the top: rank and stress in wild male baboons. Science 2011;333:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cavigelli SA, Caruso MJ. Sex, social status and physiological stress in primates: the importance of social and glucocorticoid dynamics. Philos Trans R Soc Lond B Biol Sci 2015;370:20140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vandeleest JJ Capitanio JP Hamel A Meyer J Novak M Mendoza SP, et al. Social stability influences the association between adrenal responsiveness and hair cortisol concentrations in rhesus macaques. Psychoneuroendocrinology 2019;100:164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer JS, Novak MA. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 2012;153:4120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boulay E Troncy E Pugsley M St-Pierre J Downey A-M Smutova V, et al. Combined cardiopulmonary assessments using impedance and digital implants in conscious freely moving cynomolgus monkeys, beagle dogs, and göttingen minipigs: pharmacological characterization and social housing effects. Int J Toxicol 2021;40:530–41. [DOI] [PubMed] [Google Scholar]
- 70.Schechter ME Andrade BB He T Richter GH Tosh KW Policicchio BB, et al. Inflammatory monocytes expressing tissue factor drive SIV and HIV coagulopathy. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dutertre C-A Amraoui S DeRosa A Jourdain J-P Vimeux L Goguet M, et al. Pivotal role of M-DC8+ monocytes from viremic HIV-infected patients in TNFα overproduction in response to microbial products. Blood 2012;120:2259–68. [DOI] [PubMed] [Google Scholar]
- 72.Ramirez K, Fornaguera-Trías J, Sheridan JF. Stress-induced microglia activation and monocyte trafficking to the brain underlie the development of anxiety and depression. Curr Top Behav Neurosci 2017;31:155–72. [DOI] [PubMed] [Google Scholar]
- 73.van de Wouw M Sichetti M Long-Smith CM Ritz NL Moloney GM Cusack A-M, et al. Acute stress increases monocyte levels and modulates receptor expression in healthy females. Brain Behav Immun 2021;94:463–8. [DOI] [PubMed] [Google Scholar]
- 74.Cole SW, Capitanio JP, Chun K, Arevalo JMG, Ma J, Cacioppo JT. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci U S A 2015;112:15142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koudouovoh-Tripp P Hüfner K Egeter J Kandler C Giesinger JM Sopper S, et al. Stress enhances proinflammatory platelet activity: the impact of acute and chronic mental stress. J Neuroimmune Pharmacol 2021;16:500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandrini L, Ieraci A, Amadio P, Popoli M, Tremoli E, Barbieri SS. Apocynin prevents abnormal megakaryopoiesis and platelet activation induced by chronic stress. Oxid Med Cell Longev 2017;2017:9258937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y, Zhong H, Zhao Y, Luo X, Gao W. Role of platelet biomarkers in inflammatory response. Biomark Res 2020;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zufferey A, Schvartz D, Nolli S, Reny J-L, Sanchez J-C, Fontana P. Characterization of the platelet granule proteome: evidence of the presence of MHC1 in alpha-granules. J Proteomics 2014;101:130–40. [DOI] [PubMed] [Google Scholar]
- 79.Metcalf Pate KA, Mankowski JL. HIV and SIV associated thrombocytopenia: an expanding role for platelets in the pathogenesis of HIV. Drug Discov Today Dis Mech 2011;8:e25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Isidori AM, Minnetti M, Sbardella E, Graziadio C, Grossman AB. Mechanisms in endocrinology: the spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol 2015;173:R101–13. [DOI] [PubMed] [Google Scholar]
- 81.da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol 2006;79:499–507. [DOI] [PubMed] [Google Scholar]
- 82.Passacquale G, Vamadevan P, Pereira L, Hamid C, Corrigall V, Ferro A. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLos One 2011;6:e25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robb ML, Ananworanich J. Lessons from acute HIV infection. Curr Opin HIV AIDS 2016;11:555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krebs SJ, Ananworanich J. Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr Opin HIV AIDS 2016;11:163–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


