Abstract
Objective:
To compare outcomes of obese and non-obese pediatric patients with acute promyelocytic leukemia from the Cancer and Leukemia Group B trial CALGB 9710 and the Children’s Oncology Group trial AAML0631.
Methods:
Data including demographics, adverse events, overall and event free survival was analyzed.
Results:
The prevalence of obesity was 34% on C9710 and 35% on AAML0631. There was significantly lower overall and event free survival in the obese population on multivariable analysis on AAML0631 but not on CALGB 9710. Eleven patients died during therapy or in follow up.
Conclusion:
The prevalence of obesity is higher in pediatric patients with APL compared to the general population. The decreased EFS and OS in obese patients on AAML0631 suggests the presence of obesity can influence outcomes using the most current treatment. These findings support the need for further research on the potential role of obesity in pediatric APL leukemogenesis.
Keywords: Obesity, Pediatric, Acute Promyelocytic Leukemia
Introduction
Obesity continues to be a significant public health issue in the United States in both children1 and adults.2 In a recent study which reviewed obesity incidence from 1999–2016, it was reported that 18.5% of children aged 2–19 years were obese (class I) and 7.9% were extremely obese (class II and III).1,3 The effect obesity has on prognosis in children with acute myeloid leukemia (AML) is unclear, as some studies have reported a significant adverse effect on prognosis4–8 while other studies have not.9–12 There have been several analyses over the past decade which have shown an association between obesity and the AML sub-type acute promyelocytic leukemia (APL) in adults.14,15 Several clinical trials suggest that obesity may adversely affect outcome in APL. Breccia et al reported that increased body mass index (BMI) at diagnosis was an independent predictor of relapse and was associated with higher risk for differentiation syndrome in 144 adults and children (range 1‒76 years) with acute promyelocytic leukemia (APL) treated using the GIMEMA protocols AIDA-0493 and AIDA-2000.13 A pooled analysis from Cancer and Leukemia Group B (CALGB) also showed that adult obese patients (≥18 years of age) with APL had significantly worse overall survival (OS) and disease-free survival (DFS) compared to non-obese patients, but there was no difference in outcomes between obese and non-obese patients with non-APL acute myeloid leukemia (AML).8 CALGB is now part of the Alliance for Clinical Trials in Oncology.
The prevalence of obesity and its significance in outcomes in pediatric APL has not been previously reported, except for one abstract.16 We analyzed obesity and outcomes in pediatric patients with APL with data from the North American Leukemia Intergroup CALGB 9710 study and the Children’s Oncology Group AAML0631 study. The timing of deaths and other adverse events was reviewed to determine the significance of early versus late treatment risks for obese patients, as well as the potential influence of ethnicity in the development of APL.3
Methods
Patient Population and Treatment Protocols
Data was abstracted from pediatric patients diagnosed with APL who were enrolled on CALGB 9710 (Clinicaltrials.gov identifier NCT00003934) led by the Cancer and Leukemia Group B (Alliance) or enrolled on AAML0631, a phase III study for newly diagnosed childhood APL (NCT00866918). For both studies, the diagnosis of APL was initially based on morphology and was confirmed with detection of the PML-RARA gene translocation. Patients were dosed based on their observed weight. Each study was approved individually by the institutional review boards at participating centers.
The details of the study design and protocol for CALGB 9710 were described by Powell et al.17 and for AAML0631 by Kutny et al.18 There were differences between protocols in terms of use of all-trans retinoic acid (ATRA), arsenic trioxide (ATO), anthracycline agent and dose, cytosine arabinoside dose, and use of oral mercaptopurine and methotrexate. To highlight a few, on C9710 during induction, patients received ATRA on days 1–90 and daunorubicin (200 mg/m2). Patients > 15 years old were randomized to receive or not receive two cycles of ATO as part of consolidation, while patients < 15 years old did not receive ATO. On AAML0631 during induction, patients received ATRA on days 1–30 and idarubicin (36 mg/m2). Consolidation consisted of three (standard risk; SR) or four phases (high risk; HR); the first two phases were five-week cycles of ATRA and ATO, followed by ATRA with an anthracycline and cytosine arabinoside.
In terms of supportive care, per Powell et.al.17, on CALGB 9710, management of coagulopathy, transfusions, and antibiotics were at the discretion of the treating physician. Study requirements included coagulation tests at least 3 times per week until normal. Heparin use was discouraged. Treatment of suspected APL differentiation syndrome included dexamethasone 10 mg twice daily for 3 days. Per AAML0631, specific supportive care recommendations included avoiding leukapheresis, maintaining platelet count above 50,000/µL and fibrinogen above 100 mg/dL during the first 10 days of Induction or until the coagulopathy resolved, avoiding use of heparin or anti-fibrinolytics, azole antifungal use during times of ATRA and routine use of growth factors.
Body Mass Index (BMI)
BMI at diagnosis was determined by dividing patient weight (kilograms) by the square of the height (meters). Patients ≥ 20 years of age were divided into weight categories according to criteria from the World Health Organization (WHO)19: underweight (BMI <18.5 kg/m2), normal weight (BMI ≥18.5 to <25 kg/m2), overweight (BMI ≥25 to <30 kg/m2), or obese (BMI ≥30 kg/m2). Children and adolescents (≥2 years of age and <20 years of age, respectively) were divided into weight categories according to normative data by age and gender from the Centers for Disease Control and Prevention: underweight (<5th percentile, normal weight (≥5th to <85th percentile), overweight (≥85th to <95th percentile), and obese (≥95th percentile).20 For the patients younger than 2 years of age, the WHO growth chart was used to classify their weight category.21 Obese patients < 22 years old who died while enrolled on either clinical trial were further classified into degrees of obesity based on recently published classification systems. Class I obesity is BMI > 95th percentile, Class II is > 120% of the 95th percentile, and Class III is > 140% of the 95th percentile or > 40 kg/m2 (whichever is greater). 3,22,23
Statistical Methods
For both studies, EFS and OS were analyzed using Kaplan-Meier curves (Fig. 1 and 2).24 EFS was defined as the time from the study entry to first event, where an event was defined as failure to achieve a complete response (CR), relapse after achieving a CR, or death. Differences between obese and non-obese groups were assessed using the log-rank test and with Cox regression with white blood cell (WBC) count (dichotomized as <10 × 109/L vs ≥10 × 109/L) as a covariate. Hazard ratios (obese to non-obese) and 95% confidence intervals are reported and P values <0.05 were considered statistically significant. Patients lost to follow-up were censored at their last known date of contact.
Figure 1. C9710 Kaplan-Meier Event Free and Overall Survival.
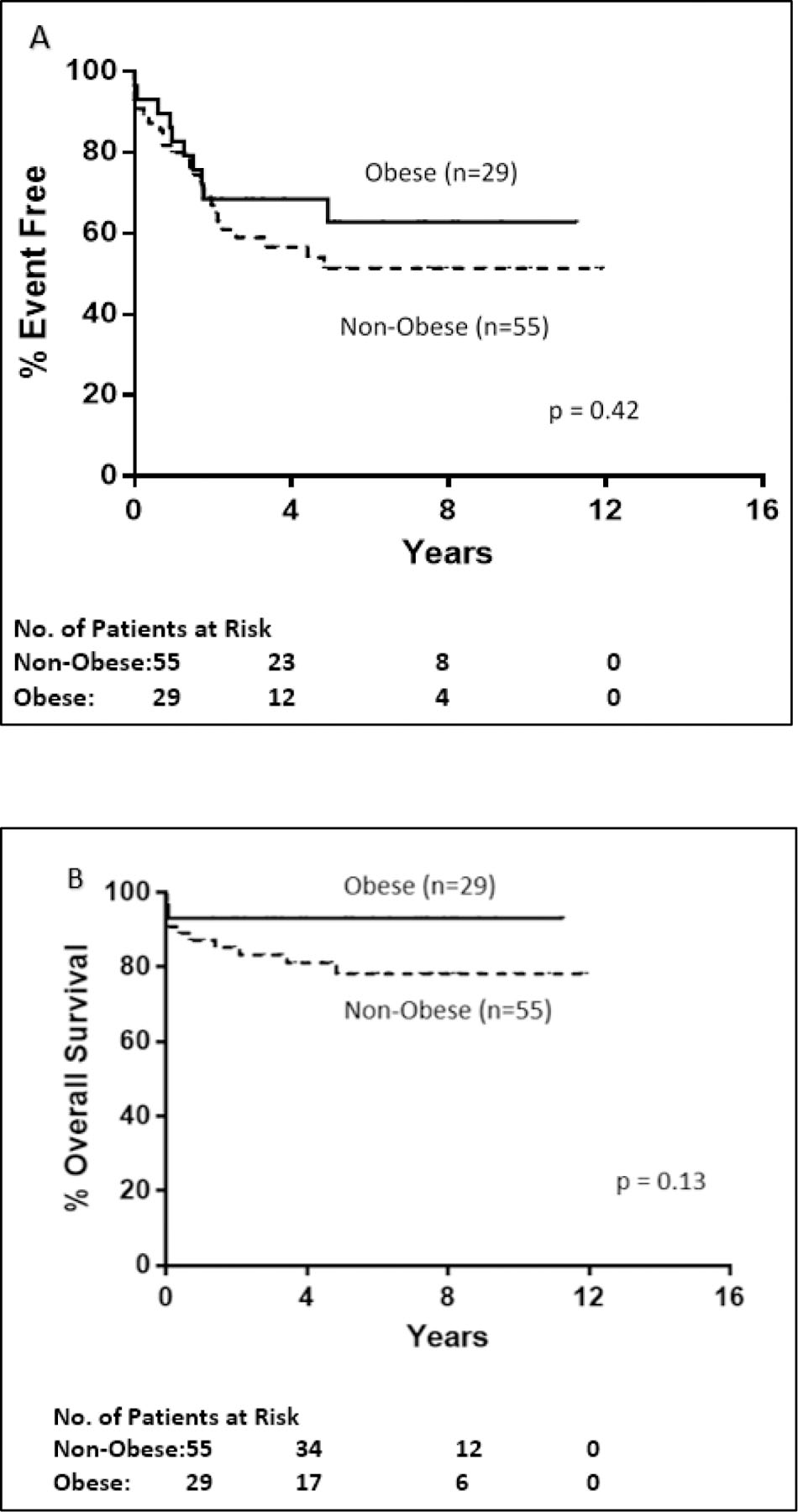
A. Event free survival for obese (n=29) versus non-obese (n=55) patients < 18 years old enrolled on C9710 was not statistically significant (p=0.42). Obese patients had a 5 year EFS of 63% versus 51% for non-obese pediatric patients. B. Overall survival for obese versus non-obese patients < 18 years old was not statistically significant. The 5-year OS for obese patients was 93% and non-obese pediatric patients 78%
Figure 2. AAML0631 Kaplan-Meier Event Free and Overall Survival.
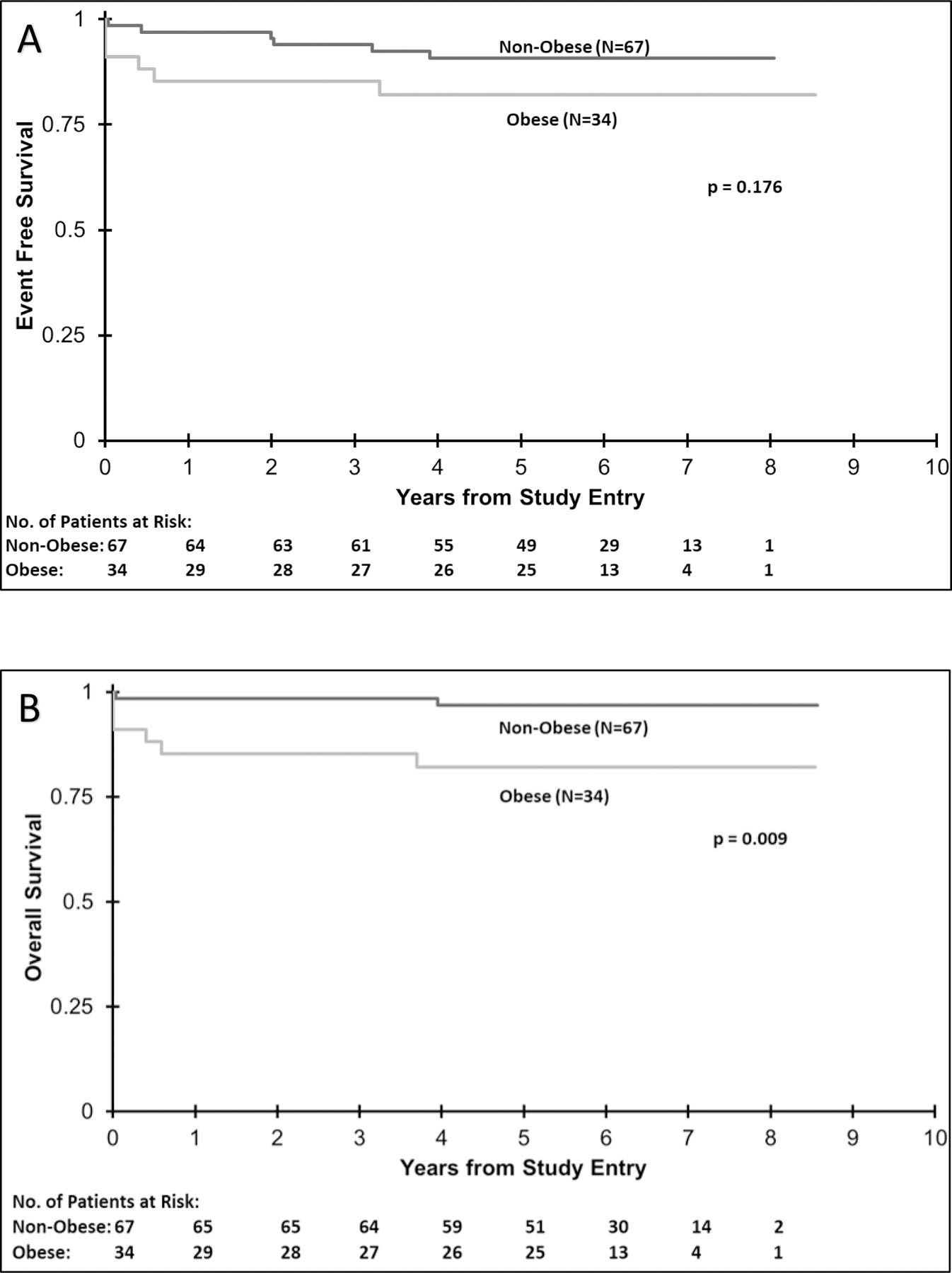
A. Event free survival for obese (n= 34) versus non-obese (n=67) patients 2 to < 22 years old on AAML0631 was not statistically significant (p=0.176). Obese patients had a 3 year EFS of 85% versus 94% for non-obese patients.
B. There was a statistically significant difference in 3-year overall survival in obese vs. non-obese patients (p=0.009). Obese patients had an OS of 85% vs. 99% for non-obese patients.
On CALGB 9710, categorical variables were compared between groups defined by age or weight using Fisher’s exact test, while the Wilcoxon rank sum test was used for continuous variables. Proportional hazards were tested in EFS and OS univariate analyses for each covariate. For EFS, white blood count (WBC) showed non proportional hazards while for OS, both WBC and age categories showed non proportional hazards. Therefore, in the multivariate analyses of Table 3, the models for CALGB 9710 OS and EFS were re-run adjusting for non-proportional hazards.
TABLE 3.
Multivariable Analysis of Overall and Event Free Survival
| C9710 | |||||||
|---|---|---|---|---|---|---|---|
| OS from study entry | EFS from study entry | ||||||
| Variables | HR | 95% CI | p | HR | 95% CI | p | |
| Weight | Obese vs Non-Obese | 0.36 | 0.08 – 1.67 | 0.19 | 0.77 | 0.36 – 1.64 | 0.49 |
| WBC Count at Diagnosis | <10×109/L vs ≥ 10 ×109/L | 0.16 | 0.05 – 0.57 | 0.004* | 0.51 | 0.25 – 1.06 | 0.07 |
| Age | Per 5 year interval | 1.004 | 0.96 – 1.05 | 0.86 | 0.90 | 0.66 – 1.28 | 0.56 |
| Sex | Female vs Male | 0.77 | 0.24 – 2.46 | 0.66 | 0.81 | 0.40 – 1.61 | 0.54 |
| Ethnicity | Hispanic vs Non Hispanic | 0.29 | 0.04 – 2.27 | 0.24 | 0.31 | 0.09 – 1.01 | 0.052 |
| Hispanic vs Unknown | 0.11 | 0.006 – 2.14 | 0.15 | 0.26 | 0.04 – 1.67 | 0.15 | |
| Non-Hispanic vs Unknown | 0.39 | 0.04 – 3.69 | 0.41 | 0.84 | 0.18 – 3.83 | 0.82 | |
| AAML0631 | |||||||
| OS from Study Entry | EFS from study entry | ||||||
| N | HR | 95% CI | p | HR | 95% | p | |
| Weight | |||||||
| Non-Obese | 67 | 1 | 1 | ||||
| Obese | 34 | 12.993 | 2.49–67.75 | 0.002* | 3.661 | 1.1 – 12.17 | 0.034* |
| WBC Count At Diagnosis | |||||||
| WBC <10×109/L | 66 | 1 | 1 | ||||
| WBC ≥ 10 ×109/L | 35 | 14.209 | 2.68–75.46 | 0.002* | 6.535 | 1.81 – 23.61 | 0.004* |
| Age | |||||||
| ≥ 10 years | 77 | 1 | 1 | ||||
| < 10 years | 24 | 0.323 | 0.04 – 2.72 | 0.298 | 0.525 | 0.11 – 2.44 | 0.411 |
OS = Overall Survival; EFS = Event Free Survival; HR = Hazard Risk; WBC = White Blood Cell
On AAML0631, disease free survival was defined as time from end of consolidation until relapse or death. Cumulative incidence methods were used to calculate relapse rate (RR), which was defined as time from end of consolidation for patients in CR to relapse or death, and deaths without relapse were considered competing events.
The above analyses included all patients < 18 years old on CALGB 9710 and all patients on AAML0631, which enrolled those 2 to < 22 years old. BMI data was missing for 9 patients on C9710. Additionally, FLT3 status was an optional datapoint and not available from this data set. All patients on AAML0631 had available BMI data and all other necessary endpoints for this study. Additional data for patients < 22 years old who experienced a death is described but no further statistical analysis was done for this population due to small sample size.
Results
Patient Characteristics
Patient characteristics for the CALGB 9710 pediatric population and the AAML0631 trial population are summarized in table 1. On C9710, 84 of the 529 total patients were ≤ 18 years old (range 1.3 – 18.0 years). Median follow-up time was 61.2 months (range 0.03–143.1 months). Obese patients made up 34.5% of the cohort; 16.7% were overweight and 3.6% were underweight. Of the 29 obese patients, 16 were class I, 9 were class II, and 4 were class III. Data in regard to FLT3 status was not available in relation to obesity status. Nineteen of the 84 patients < 18 years old received ATO; 14 were non-obese (73%) and 5 were obese (26%) (Supp. Table 1). Differentiation syndrome data specific to obese patients was not available.
TABLE 1.
Population Characteristics for C9710 and AAML0631
| C9710 | AAML0631 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obese (N =29) | Non-Obese (n=55) | Obese vs. Non-obese | Obese (N =34) | Non-Obese (n=67) | Obese vs. Non-obese | |||||||||||
| N | % | N | % | p | N | % | N | % | p | |||||||
| Sex | ||||||||||||||||
| Male | 18 | 62% | 27 | 49% | 0.36 | 15 | 44% | 29 | 43% | 0.936 | ||||||
| Female | 11 | 38% | 28 | 51% | 19 | 56% | 38 | 57% | ||||||||
| Ethnicity | ||||||||||||||||
| Non-Hispanic | 23 | 79% | 41 | 75% | 0.92 | 26 | 81% | 53 | 80% | 0.912 | ||||||
| Hispanic | 5 | 17% | 10 | 18% | 6 | 19% | 13 | 20% | ||||||||
| Unknown | 1 | 3% | 4 | 7% | 2 | 1 | ||||||||||
| Race | ||||||||||||||||
| White | 23 | 79% | 37 | 70% | 0.94 | 27 | 90% | 51 | 81% | 0.371 | ||||||
| Black | 3 | 10% | 7 | 13% | 1 | 3% | 9 | 14% | 0.159 | |||||||
| Asian | 1 | 3% | 3 | 6% | 0 | 0% | 3 | 5% | 0.549 | |||||||
| Other | 1 | 3% | 4 | 8% | Native American | 2 | 7% | 0 | 0% | 0.102 | ||||||
| Unknown | 1 | 3% | 2 | 4% | 4 | 4 | ||||||||||
| Peripheral blood WBC Count | ||||||||||||||||
| WBC <10 ×109/L | 22 | 76% | 38 | 69% | 0.62 | 26 | 76% | 40 | 60% | 0.094 | ||||||
| WBC >10 ×109/L | 7 | 24% | 17 | 31% | 8 | 24% | 27 | 40% | ||||||||
| FLT3 mutation | ||||||||||||||||
| - | - | No | 19 | 58% | 35 | 57% | 0.531 | |||||||||
| - | - | Yes | 14 | 42% | 26 | 43% | ||||||||||
| - | - | Unknown | 1 | 6 | ||||||||||||
WBC = White Blood Cell; FLT3 = FMS-Like Tyrosine Kinase 3
On AAML0631, the 101 patients ranged in age from 2.01 – 21.34 years. The median follow-up time for AAML0631 patients not experiencing an EFS event was 5.97 years. Obese patients made up 33.6% of the cohort, overweight patients 17.8%, and underweight patients 3.9%. There was no significant difference in ethnicity between obese and non-obese patients. Obese patients had a lower peripheral blast percentage compared to non-obese patients (p=0.049). There were no significant differences in FLT3 status, WBC count at diagnosis, bone marrow blast percentage, or platelet or hemoglobin values at diagnosis. Of the 34 obese patients, 18 were class I, 11 were class II, and 5 were class III obesity. There were no differences among these patients in terms of demographics Twenty patients were diagnosed with differentiation syndrome during induction. Causes of death during induction included coagulopathy (2) and multiorgan dysfunction (2), and differentiation syndrome was thought to have contributed to these deaths. Three out of those four deaths were obese patients.
BMI is not associated with adverse events in AAML0631 or CALGB 9710
Adverse event reports from both CALGB 9710 and AAML0631 showed no significant difference in toxicities observed between obese versus non-obese patients (Table 2). On AAML0631, there were several adverse events which trended up in obese patients. For example, vascular disorders occurred in 13% of non-obese patients versus 29% of obese patients (p=0.052) and respiratory, thoracic, and mediastinal adverse events occurred in 27% of non-obese patients versus 44% of obese patients (p=0.081) (Supp. Table 2)
TABLE 2.
Adverse Events For All Courses
| C9710 | AAML0631 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Event | Non-Obese (n=55) |
Obese (n=29) |
p-value | Non-Obese (n=67) | Obese (n=34) | p-value | |||||
| N | % | N | % | N | % | N | % | ||||
| Blood/bone marrow | 48 | 87.0% | 25 | 86.2% | 0.99 | 42 | 63% | 19 | 56% | 0.509 | |
| Infection | 39 | 70.9% | 24 | 82.8% | 0.29 | 47 | 70% | 24 | 71% | 0.964 | |
| Metabolic | 16 | 29.1% | 13 | 44.8% | 0.16 | 26 | 39% | 11 | 32% | 0.525 | |
| Pulmonary | 6 | 10.9% | 3 | 10.3% | 0.99 | 18 | 27% | 15 | 44% | 0.081 | |
| Pain | 16 | 29.1% | 10 | 34.5% | 0.63 | - | - | - | - | - | |
Association between Obesity, OS, and EFS on CALGB 9710 and AAML0631
On CALGB 9710, there was no significant difference in EFS or OS between obese and non-obese patients. The 5-year EFS was 62.8% (95% CI: 43.8% – 81.8%) for obese pediatric patients and 51.4% (95% CI: 37.3% – 65.5%) for non-obese pediatric patients (P=0.42; Fig. 1A). The 5-year OS for obese and non-obese pediatric patients was 93.1% (95% CI: 83.9% – 100.0%) and 78.2% (95% CI: 66.6% – 89.9%), respectively (P=0.13; Fig. 1B). EFS and OS was examined by univariate analyses for WBC (≥10 × 109/L or <10 × 109/L), age (in 5-year intervals), sex, and ethnicity (Hispanic vs non-Hispanic). None of these factors were significantly associated with EFS in univariate analyses. (Supp. Table 3) In multivariable analysis, only WBC count at diagnosis was associated with lower OS in C9710 (p = 0.004) (Table 3). In dividing obese patients into classes, three-year EFS was 81% for Class I (n=16), 56% for Class II (n=9) and 50% for Class III (n=4) (p=0.37). There was one death at 24 days in a Class II obese patient and one at 4 days in Class III obese patient.
On AAML0631, the 3-year EFS for obese patients was 85% and for non-obese patients was 94% (p=0.176) (Fig. 2A). There was a statistically significant difference in 3-year OS in obese vs. non-obese patients (85% vs. 99%; p=0.009) (Fig. 2B). Six out of 34 (17.6%) obese patients died, compared to 2 out of 67 (3.0%) non-obese patients. Dividing obese patients into classes, three year EFS was 89% for Class I, 82% for Class II, and 80% for Class III (p = 0.552). Treatment related mortality (TRM) was significantly different between groups; obese patients had 3-year TRM of 4% (95% CI: 2–6%) and non-obese patients had a TRM of 0% (p=0.044) (Supp. Table 4). OS and EFS in this cohort were examined by univariate and multivariable analyses based on weight group, WBC count, age and sex. In univariate analysis, weight and WBC count were significantly associated with OS, but only WBC count was significantly associated with EFS. (Supp. Table 3) However, in multivariable analyses, obesity was strongly significantly associated with OS and EFS (p=0.002 and p=0.034 respectively). (Table 3)
Deaths of pediatric patients on CALGB 9710 and AAML0631 during induction
On CALGB 9710, three of the five obese patient deaths in patients < 22 years old occurred during induction (Table 4) on days 2, 4, and 24. Causes of death were related to APL rather than treatment. Two deaths were associated with strokes; ischemic in one case and hemorrhagic in the other. BMI values for these patients were 30.6 (class I), 42.5 (class III), and 30.8 (class III) kg/m2. They were all ≥ 10 years old and all high risk by diagnostic WBC: 12.5, 82.7, and 61.4 ×10e9/L respectively. These two males and one female were all non-Hispanic ethnicity.
TABLE 4.
Patient Characteristics of Pediatric Obese Deaths
| C9710 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | Gender | Race | Ethnicity | BMI (kg/m2) | Obesity Class | WBC at Dx | Days until Death | Primary Cause of Death | Other Causes |
| 19.7 | Male | White | Non-Hispanic | 30.6 | Class I | 12.5 | 2 | Protocol Disease Related | Cardiac Respiratory Arrest |
| 15.8 | Female | Black/African American | Non-Hispanic | 42.5 | Class III | 82.7 | 4 | Protocol Disease Related | Ischemic Stroke/Brain Infarcts |
| 10.9 | Male | White | Non-Hispanic | 30.8 | Class III | 61.4 | 24 | Protocol Disease Related | Basilar Artery Hemorrhagic Stroke |
| 19.7 | Male | American | Non-Hispanic | 31.6 | Class I | 4.9 | 1257 | Protocol Disease Related | Disease |
| 18.9 | Male | Multiple Races | Unknown | 41.6 | Class III | 5.6 | 1326 | Not related to protocol treatment or disease | Massive Pulmonary Embolism |
| AAML0631 | |||||||||
| Age at diagnosis (years) | Gender | Race | Ethnicity | BMI (kg/m2) | Obesity Class | Stratum | Days Until Death | Primary Cause of Death | Other Causes |
| 17.7 | Male | White | Hispanic | 32.7 | Class I | HR | 1 | Due to this disease | None |
| 15.0 | Female | White | Non-Hispanic | 34.375 | Class II | HR | 1 | Multi-organ failure | Due to this disease |
| 15.5 | Male | White | Non-Hispanic | 35.8 | Class II | HR | 2 | Due to other cause | Respiratory failure, anemia, coagulopathy, electrolyte abnormalities, elevated WBC, thrombocytopenia, ARDS |
| 15.7 | Female | Unknown/Other | Non-Hispanic | 44.7 | Class III | HR | 147 | Multi-organ Failure | Infection (Serratia Marcescens) |
| 18.8 | Female | White | Non-Hispanic | 32.5 | Class I | SR | 215 | Due to other cause | Drug Toxicity of Amitriptyline and Zolpidem |
| 9.9 | Female | White | Non-Hispanic | 28.7 | Class II | HR | 1205 | Multi-organ failure | Hemorrhage, CNS; hepatotoxicity, portal venous thrombosis |
BMI = Body Mass Index; HR = High Risk; SR = Standard Risk; WBC = White Blood Cell; ARDS = Acute Respiratory Distress Syndrome; CNS = Central Nervous System
On AAML0631, three of the six obese patient deaths occurred during induction (Table 4) on days 1, 1, and 2. In these three cases, death was attributed to APL diagnosis, multi-organ failure with APL as a contributing factor, and a combination of factors, including respiratory failure from ARDS, leukocytosis, coagulopathy, and electrolyte abnormalities. The BMI of these patients was 32.7 (class I), 34.4 (class I), and 35.8 (class II) kg/m2. They were aged 17 years, 15 years, and 15 years. There were two males and one female, and one patient was Hispanic.
Deaths of pediatric obese patients on CALGB 9710 and AAML0631 After Induction
On CALGB 9710, there were two deaths which occurred after induction. One patient died at day 1257 after enrollment due to relapsed APL; this patient was non-Hispanic and was 19.7 years old at diagnosis with a BMI of 31.6 kg/m2 (class I). The second patient died at 1326 days after enrollment of a pulmonary embolism; he was 18.9 years old, ethnicity unknown, with a BMI of 41.6 kg/m2 (class III). On AAML0631, there were three deaths which occurred after induction. The causes of death included infection at day 147, a non-chemotherapy drug overdose at day 215, and CNS hemorrhage and portal venous thrombosis at 3.7 years from diagnosis. This last patient was assumed to have relapsed disease. These patients had BMIs of 44.7 (class III), 32.5 (class I), and 28.7 (class II) kg/m2. All were female and non-Hispanic ethnicity.
Discussion
APL in the pediatric population is a rare disease, thus cross sectional analysis of data obtained over many years of experience is critical to enhancing our understanding of the characteristics which effect outcomes in these patients. To our knowledge, this report constitutes the largest published analysis of obesity in pediatric APL and demonstrates the high prevalence of obesity at diagnosis in this patient population. The overall prevalence of obesity on these two studies was 34%, which is increased from the reported obesity rate of 19.5–24.8% in children and adolescents in the United States over the time period of these clinical trials.3 Our findings are consistent with prior reports in adults that increasing BMI is associated with a diagnosis of APL among patients with AML25,26,27. Additionally, increasing obesity class trended towards worse EFS in the obese populations on both CALGB 9710 and AAML0631, though small sample size on both trials precluded any significant conclusions to be made from this data alone.
The increased prevalence of obesity at diagnosis in pediatric APL suggests that obesity may predispose to the development of APL. In AAML0631, there was a statistically significant difference between peripheral blast percentage in obese versus non-obese patients, with obese patients having a lower peripheral blast count at diagnosis than non-obese patients. This could be a clue about the underlying mechanisms of obesity-driven APL and the differences in the pathogenesis of APL in the setting of obesity. There have been several potential proposed mechanisms in the leukemogenesis of APL, including the role of the leptin protein as a potential oncologic driver. Leptin regulates fat metabolism and is made by adipocytes, a key structural component in high concentration in the bone marrow. An isoform of the leptin receptor has been found on CD34+ hematopoietic stem cells and has been found in larger numbers on APL cells. Tabe et al. demonstrated that an upregulation of PML-RARα on leukemic cells is associated with the upregulation of particular isoforms of the leptin receptor.28 Additionally, analysis of AML transcriptomes by Mazzarella et al. found that APL was associated with an upregulation in pathways associated with long chain fatty acid metabolism, which the authors postulate might lead to several differences in signaling which could promote leukemogenesis.27 FLT3 mutations were also noted to be positively associated with obesity in both non-APL AML and in APL in this reference, however there was no difference in prevalence of FLT3 mutation in obese versus non-obese cohort on AAML0631. Collectively, this demonstrates the need for more focused prospective data collection, as establishing a relationship between APL, obesity, and adverse outcomes could impact prospective treatment decisions including adjusting dosing of chemotherapy on ideal versus actual body weight, especially in light of the significant association of obesity in treatment related mortality on AAML0631.
The difference in outcomes of obese patients on CALGB 9710 versus AAML0631 should be highlighted. AAML0631 demonstrated a statistically significant decrease in OS and EFS for obese patients in multivariable analysis, but CALGB 9710 demonstrated no such association. There are several possible explanations for this finding. There were a few notable differences in chemotherapy usage. AAML0631 included one or two cycles of high dose cytarabine in consolidation, depending on risk group, while C9710 included low dose cytarabine in induction only, which could have potentially contributed to the increase in post-induction treatment related mortality on AAML0631. The anthracycline agent differed between the two trials; daunorubicin was used on C9710 and idarubicin and mitoxantrone were used on AAML0631. The latter two are generally associated with more toxicity, including prolonged count suppression. All patients received ATO on AAML0631, while only patients > 15 years old were randomized to receive ATO in the C9710 study. It is difficult to know conclusively how the differential use of ATO impacted the observed deaths; ATO can improve survival for all APL patients through decreased RR but whether there is a unique balance of toxicity versus survival in obese patients is not clear from the data we have available on these trials. Despite the differences in findings between the two trials, the findings of decreased OS (HR 12.9) and EFS (HR 3.6) on multivariate analysis at a level of p=0.002 and p=0.034 respectively on AAML0631 in the obese patient population is felt to be significant by the authors, and deserves to be highlighted. AAML0631 is the most current trial with published data regarding treatment of APL in the pediatric population and its chemotherapy regimen and supportive care guidelines are considered standard of care. Thus, any future research and clinical treatment trials should be designed with this finding in mind in order to both explore the possible causation between obesity and APL in pediatric population as well as provide appropriate supportive care and medication adjustments for the obese populations.
Unlike other studies,4 which reported an association between obesity and adverse events, our analyses did not show the same effect, suggesting that the differences in survival outcomes were not related to adverse treatment effects. However, it should be noted that although not statistically significant, there was a trend towards an increase in certain types of adverse events on AAML0631, in particular bleeding and thrombosis events, which should be a focus in future analyses and treatment trials.
Our description of individual obese patients who died showed some interesting patterns. Nine of the 11 obese patients were > 15.0 years old and 4 of 6 who died during induction had severe obesity (Class II and III). There were no observable trends in sex, ethnicity, or race. The causes of induction deaths were all attributed to APL. Three patients on the AAML0631 trial had fatal CNS vascular events (ICH, ischemic stroke, hemorrhagic stroke). Deaths which occurred post-induction and after completion of chemotherapy were reported to be caused by thrombotic events (pulmonary and hepatic), drug toxicity (unrelated to APL or its treatment), and relapse. These identifiable patient characteristics of age and severe obesity could guide supportive care and management of patients at initial diagnosis and during induction in order to limit early mortality.
There are several limitations to this report including its design as a retrospective analysis. As with many pediatric studies, the small number of patients overall limits the power to observe statistically significant differences between groups and makes it challenging to draw definitive conclusions. There were inherent differences in the structures of the studies which complicated combining data from both trials and there are likely other unknown or unappreciated differences between the two patient cohorts. Data from CALGB 9710 was collected for patients 18 years and under while AAML0631 data included patients from ages 2 to 21.99 years. However, it must be remembered that even with these limitations, this represents the largest body of data on this topic and should encourage further studies about the role obesity plays in the development of APL as well as the aggressive supportive care measures needed for obese APL patients during their initial diagnosis and throughout treatment in order to improve their outcomes.
Supplementary Material
Acknowledgement:
COG AAML0631 was supported by NCTN Operations Center Grant U10CA180886, NCTN Statistics & Data Center Grant U10CA180899, and St. Baldrick’s Foundation.
CALGB 9710 was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology.
Abbreviations
- AML
Acute myeloid leukemia
- APL
Acute promyelocytic leukemia
- ATO
Arsenic trioxide
- ATRA
All-trans retinoic acid
- BMI
Body mass index
- CALGB
Cancer and Leukemia Group B
- CI
Confidence interval
- COG
Children’s Oncology Group
- CR
Complete response
- CTC
Common Toxicity Criteria
- DFS
Disease-free survival
- EFS
Event-free survival
- HR
Hazard ratio
- OS
Overall survival
- RR
Relapse risk
- TRM
Treatment Related Mortality
- WBC
White blood cell
- WHO
World Health Organization
Footnotes
Conflicts of Interest
PL is an employee of AbbVie.
JG is an employee of Novartis.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012:307(5):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012:307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 3.Skinner AC, Ravanbakht SN, Skelton JA, et al. Prevalence of obesity and severe obesity in US Children, 1999–2016. JAMA Pediatr 2018:141(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lange BJ, Gerbing RB, Feusner J, et al. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA 2005:293(2):203–211. [DOI] [PubMed] [Google Scholar]
- 5.Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcome in pediatric acute lymphoplastic leukemia. J Clin Oncol 2007:25(15):2063–9. [DOI] [PubMed] [Google Scholar]
- 6.Orgel E, Tucci J, Alhushki W, et al. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood 2014:124(26):3932–3938. [DOI] [PubMed] [Google Scholar]
- 7.Aplenc R, Zhang MJ, Sung L, et al. Effect of body mass in children with hematologic malignancies undergoing allogeneic bone marrow transplantation. Blood 2014:123(22):3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo JJ, Mulkey F, Geyer S, et al. Relationship between obesity and clinical outcomie in adults with acute myeloid leukemia: A pooled analysis from four CALGB (alliance) clinical trials. Am J Jematol 2016: 91(2):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hijiya N, Panetta JC, Zhou Y, et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood 2006:108(13):3997–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medeiros BC, Othus M, Estey EH, et al. Impact of body-mass index on the outcome of adult patients with acute myeloid leukemia. Haematologica 2012:97(9):1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Licht AS, Hyland AJ, et al. Is obesity a prognostic factor for acute myeloid leukemia outcome? Ann Hematol 2012:91(3):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzell CM, Gallagher EM, Earl M, et al. Outcomes in obese and overweight acute myeloid leukemia patients receiving chemotherapy dosed according to actual body weight. Am J Hematol 2013:88(10):906–909. [DOI] [PubMed] [Google Scholar]
- 13.Breccia M, Mazzarella L, Bagnardi V, et al. Increased BMI correlates with higher risk of disease relapse and differentiation syndrome in patients with acute promyelocytic leukemia treated with the AIDA protocols. Blood 2012:119(1):49–54. [DOI] [PubMed] [Google Scholar]
- 14.Tedesco J, Qualtieri J, Head D, et al. High Prevalance of Obesity of Acute Promeylocytic Leukemia (APL): Implications for Differentiating Agents in APL and Metabolic Dynrome. Ther Adv Hematol 2001; 2(3):141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong O, Harris F, Yiying W et al. A hospital based case control study of acute myeloid leukemia in Shanghai: analysis of personal characteristics, lifestyle and envionrmental risk factors by subtypes of the WHO classification. Regul Toxicol Pharmacol 2009; 55(3): 340–352. [DOI] [PubMed] [Google Scholar]
- 16.Feusner J, Kim H, Gregory J et al. Obesity in Newly Diagnosed Childhood Acute Promyelocytic Leukemia. Blood 2006: 108(11), 4494. [Google Scholar]
- 17.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 2010:116(19):3751–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutny MA, Alonzo TA, Gerbing R, et al. Arsenic Trioxide Consolidation Allows Anthracycline Dose Reduction for Pediatric Patients with Acute Promyelocytic Leukemia: Report From the Children’s Oncology Group Phase III Historically Controlled Trial AAML0631. Journal of Clinical Oncology 2017: 35:26, 3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, 2009. Percentile Data Files with LMS Values. U.S. Department of Health & Human Services. Accessed 15 May 2021. <https://www.cdc.gov/growthcharts/percentile_data_files.htm> [Google Scholar]
- 21.Centers for Disease Control and Prevention, 2019. A SAS Program for the WHO Growth Charts (ages 0 to <2 years). U.S. Department of Health & Human Services. Accessed 15 May 2021. <https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm> [Google Scholar]
- 22.Skinner AC, Perrin EM, Moss LA, et al. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med 2015: 373(14):1307–17. [DOI] [PubMed] [Google Scholar]
- 23.Freedman DS and Berenson GS. Tracking of BMI z Scores of Severe Obesity. JAMA Pediatr 2017: 140(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958:53:457–481. [Google Scholar]
- 25.Estey E, Thall P, Kantarjian H, et al. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia 1997:11(10):1661–1664. [DOI] [PubMed] [Google Scholar]
- 26.Tallman MS, Andersen JW, Schiffer CA et al. All-trans-retinoic-acid in acute promyelocytic leukemia. N Engl J Med 1997; 337(15): 1021–1028. [DOI] [PubMed] [Google Scholar]
- 27.Mazzarella L, Botteri E, Matthews A, et al. Obesity is a risk factor for acute promyelocytic leukemia: evidence from population and cross sectional studies and correlation with FLT3 mutations and polyunsaturationed fatty acid metabolism. Haematologica 2020;105(6):1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabe Y, Konopleva M, Munsell MF et al. PML-RARα is associated with leptin-receptor induction: the role of mesenchymal stem cell-derived adipocytes in APL cell survival. Blood 2004: 103(5), 1815–1822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


