Abstract
Macular telangiectasia Type 2 (MacTel) is a gradually progressive disease that affects the quality of life by impairing both distant and near vision. It had previously been considered a vascular condition, but recent evidence suggests a neurodegenerative etiology, with primary involvement of Muller cells. Retinal pigment epithelium (RPE) hyperplasia and subretinal neovascularization (SNV) are responsible for most of the vision loss in advanced cases. Neurotrophic factors in the non-proliferative phase and intravitreal anti-Vascular Endothelial growth factor (VEGF) in the proliferative phase have shown to retard the progression of the disease. This review will discuss the pathophysiology, clinical features, important diagnostic imaging studies and available treatment options for MacTel.
Keywords: macular telangiectasia type 2, MacTel, CNTF, ciliary neurotrophic factor
Introduction
Macular telangiectasia type 2 (MacTel) is a slowly progressive disease of the macula. It had previously been considered a vascular condition, but recent evidence suggests a neurodegenerative etiology, with primary involvement of Muller cells. Retinal pigment epithelium (RPE) hyperplasia and subretinal neovascularization (SNV) are responsible for most of the vision loss in advanced cases.
This review will discuss the pathophysiology, clinical features, important diagnostic imaging studies, and available treatment options for MacTel.
Demography
MacTel is a bilateral asymmetric condition1 affecting patients over the age of 40 years,1,2 with a predilection for women.1,3 The Beaver Dam Eye Study reported a prevalence of 0.1% in 4790 individuals between the ages of 43 and 86 years,4 but the Melbourne Collaborative Cohort estimated a considerably lower prevalence of 0.0045% in 22,415 participants.2 MacTel is equally prevalent throughout the world with no racial predilection.5
Pathogenesis
Vascular Theory
Gass and Oyakawa proposed that stasis within the retinal veins due to arterial crossing impingement plays an eitiologic role.1 They believed that chronic exudation from the telangiectasia leads to outer retinal atrophy and RPE metaplasia.
Several inconsistencies plagued the widespread acceptance of the vascular theory. MacTel usually starts in the temporal parafovea though the reason for this predilection is unclear. Anastomoses between the superior and inferior temporal arcade vessels could be associated with vascular decompensation, though the reason behind this mechanism had not been elucidated.6 Retinal thickness is substantially reduced (not increased) despite dye leakage on fluorescein angiography (FA) and vision loss due to photoreceptor atrophy occurs without any evidence of cystoid macular edema.7 Optical coherence tomography (OCT) shows abnormalities inconsistent with vascular leakage and leakage on FA often predates the development of telangiectasia.
Neurodegenerative Theory
Muller cells are important to maintain the blood retinal barrier, and providing trophic factors to the surrounding neurons. Muller cell dysfunction is central to the neurodegenerative theory of MacTel.8
The Muller cells surround the neurons and provide them with nutrition. The Muller cell processes are in close contact with the retinal blood vessels in the outer plexus.8–10 Nutritional deprivation may be a major factor leading to the loss of retinal transparency commonly seen in MacTel.7 Muller cell dysfunction is associated with atrophy and disorganization of the outer retina, leading to hyporeflective spaces in the outer retinal layers seen on OCT.10 OCT and ERG changes in the form of inner lamellar holes and reduced cone responses manifested before the development of typical vascular changes.10 Xanthophyll, that is primarily stored in Muller cells, is diminished in early MacTel.
Vascular Proliferative Changes
Neurodegeneration leads to retinal thinning. As the disease progresses, associated capillary endothelial cell degeneration leads to hypoxia.11,12 The combination of VEGF secretion and Muller cell dysfunction leads to intraretinal edema, that when occurs with neuroretinal thinning may be associated with normal retinal thickness in the early stages.13–15
It has been suggested that the SNV probably originates from the retinal blood vessels and Indocyanine green angiography (ICGA) has shown intraretinal anastomosis.11,16,17
Genetics
Reports of familial clusters and affected monozygotic twin suggest that MacTel has a genetic component, although no hereditary pattern has yet been established.18–20 The MacTel project group suggested an autosomal dominant inheritance pattern with variable penetrance.21 Twin studies with asymmetric findings among twins suggest that epigenetics may be the cause for discordance among monozygotic twins.22 Secondary factors, such as smoking, diabetes mellitus (DM), coronary artery disease or hypertension, may increase the risk of clinical disease.4,5
Genome-wide linkage analysis has identified a single peak on chromosome 1 at 1q41-42 that may lead to the development of MacTel.21 Serine biosynthesis and lipid recycling in the retina occur primarily in the Muller cells and RPE. Defects in metabolism with accumulation of deoxysphingolipids result in a loss of photoreceptors in MacTel.23,24 Eade et al have found a missense mutation in the PHGDH gene involved in serine synthesis pathway in ~3.2% of affected individuals.25 The PHGDH mutation decreased serine biosynthesis with accumulation of deoxysphingolipids in RPE cells.26 Table 1 gives details of known genes associated with MacTel.
Table 1.
Genes Associated with Mactel
| Study | Genes Involved | Salient Features |
|---|---|---|
| Parmalee et al, 201221 | Single peak on chromosome 1 at 1q41-42 significant LOD score | |
| Eade et al, 202125 | Phosphoglycerate dehydrogenase deficiency (PHGDH) on 1p12 locus | Implicated in glycine and serine metabolism |
| Gantner et al, 202126 | SPTLC1/2 on chromosome 9q22.1-q22.3 | Associated with hereditary sensory and autonomic neuropathy type 1, may be associated with MacTel |
| Bonelli et al, 202127 | A single nucleotide polymorphism (SNP) at locus 5q14.3 | Associated with variation in retinal vascular diameter |
| Scerri, T. S. et al, 201728 | CPS 1 on 2q34 locus | Implicated in glycine and serine metabolism |
Ocular and Systemic Associations
Up to 45% of patients with MacTel have a systemic disorder most commonly DM, obesity, hypertension and cardio-vascular diseases.5,29 Though Gass and Blodi recognized that DM could predispose patients to develop MacTel the incidence of diabetic retinopathy was found to be significantly lesser in diabetic patients with MacTel.11,12 MacTel has also been found to be associated with celiac sprue,30 polycythemia vera, Alport disease, CREST syndrome31 and pseudoxanthoma elasticum.32 MacTel has also been associated with ocular conditions like soft confluent drusen,33 microhemangiomas of the pupillary margin,34 full thickness macular holes (FTMH)35 and epiretinal membranes.7
Clinical Features
Gass and Blodi categorized MacTel based on disease severity into five stages: Stage 1 – slight loss of the retinal transparency, usually in the temporal juxtafoveal area. Stage 2 – Loss of transparency all around the fovea with superficial crystals may be seen. Stage 3 - right-angled venules; stage 4 – hyperplastic RPE within the retina; and stage 5 – retinal neovascularization or exudation from SNV.11 Yannuzzi revised the classification into nonproliferative and proliferative disease.12 Nonproliferative MacTel refers to exudative telangiectasia and foveal atrophy and Proliferative disease refers to proliferative changes with SNV and fibrosis. The Modified Mactel classification described in 2013, divides MacTel into 5 stages.36 The first stage is nonproliferative deep capillary involvement characterised by inner retinal thickening and a few cysts. The second stage superficial capillary shows loss of transparency and few telangiectatic vessels temporally. Stage 3 is subinternal limiting membrane and foveal area involvement with distortion and constriction of FAZ. Stage 4 is proliferative stage showing presence of telangiectatic vessels in deep and superficial capillary plexus, with proliferation upto sub ILM and subretinal spaces. The stage 5 fibrovascular proliferation shows subretinal vascular complex perfused by descending arterioles and venules. Venkatesh et al proposed a preproliferative stage of MacTel that could help identify patients at risk of proliferative disease.37
Retinal graying usually begins temporally and then expands in an oval fashion, with the horizontal linear dimension being greater than the vertical dimension, usually within one disc diameter from the central fovea. The earliest clinical feature is a mild grayish discoloration of the temporal retina due to loss of retinal transparency (Figure 1). 11,38 Vascular changes are absent or rarely evident early in the disease and FA is often required to identify them.39 Ironically, leakage of fluorescein on FA is not associated with cystoid changes in the retina, and there is lack of lipid exudation or haemorrhages.39
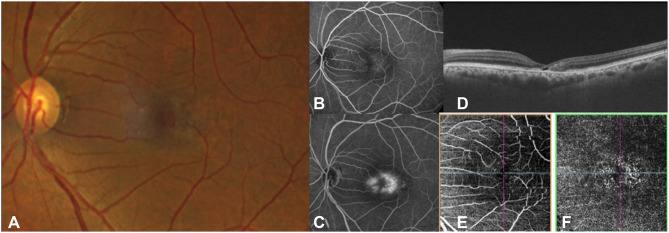
Figure 1.
Fundus photograph (A) and early (B) and late (C) fluorescein angiography, OCT (D) and OCTA (E and F) in a patient with MacTel. There is loss of retinal transparency temporal to fovea with angiographic leakage. (B and C) OCT (D) shows thinning of the parafoveal retina with hypo-reflective cavities in the outer retina. The OCTA (E and F) demonstrates dilation and telangiectasis of the deep capillary plexus temporal to fovea.
Blunted, slightly dilated venules are seen in later disease stages of the disease with pigment hyperplasia (Figure 2). The venules do not narrow as they approach the foveola and they appear to dive at right angles into the deeper retinal layers. The right angled venules originate from the deep capillary plexus and form a stellate radiating pattern because of tissue contraction in the temporal macula.40 They may appear dilated due to increased blood flow through the telangiectatic capillaries.23
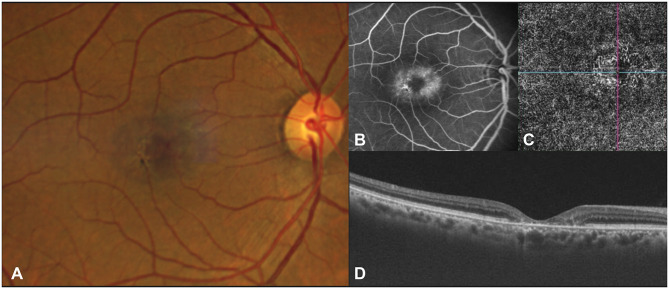
Figure 2.
Fundus photograph (A) showing blunted retinal venule and early pigmentation. The corresponding fluorescein angiogram (B) shows mild leakage in the late phase with hypofluorescence at areas of pigmentation. The OCTA (C) demonstrates dilation and telangiectasis of the deep capillary plexus temporal to fovea, and the OCT (D) shows foveal thinning with loss of the ellipsoid zone.
Eyes with MacTel have multiple tiny, yellow, crystalline deposits on the inner retinal surface adjacent to the telangiectasis (Figure 3). Nearly half of the patients exhibit crystalline deposits (60% bilaterally), but they do not correlate with the severity of disease.41,42 The source of the crystalline deposits remains unknown, though research has suggested that they are due to degenerating Muller cells or lipofuscin-containing pigmented cells.11,43 The cells containing brown pigment that surround abnormal blood vessels may represent hyperplasia of the RPE.12
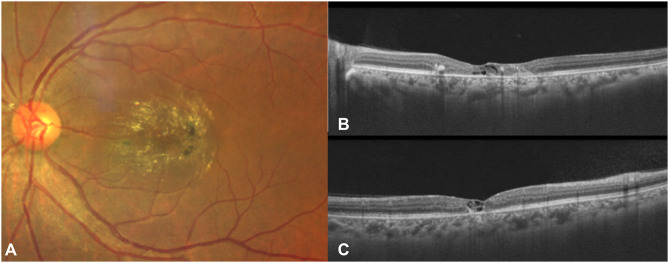
Figure 3.
Fundus photograph (A) showing pronounced crystalline deposits, blunted retinal venules, and pigment proliferation. OCT images (horizontal and vertical cross-sections represented as (B and C) respectively) show loss of outer-retinal layers with inner and outer retinal hypo-reflective cavities. Back shadowing beneath a hyper-reflective region corresponds to pigmentary changes on the fundus photo.
Yellow foveal lesions occur in up to 5% of cases of MacTel. Focal atrophy of the foveola may create a lamellar macular hole and FTMH may be due to atrophic foveal changes, vitreomacular traction, or progression from a lamellar macular hole.40,44,45 Eyes with FTMH secondary to MacTel are considered poor surgical candidates.39,45
Gass and Oyakawa observed that progressive loss of photoreceptors allows hyperplastic RPE cells to migrate along the right angled vessels to form stellate intraretinal hyperpigmented black plaques.1 Meleth et al used OCT to verify that pigment clumping is initiated in areas of outer retinal thinning and atrophy.46 Intraretinal pigments correlate with disease progression but the relation between plaques and retinal vascular proliferation is controversial.46,47
Advanced MacTel is characterized by outer retinal degeneration, atrophy, and scarring. SNV (Figure 4) develops in a minority of patients3 but it can result in significant loss of vision due to subretinal hemorrhage, cystoid macular edema, hard exudates, and disciform scarring.12 If the SNV enters the subretinal space, chorio-retinal shunts may develop and neovascular membranes that resemble markedly ectatic capillaries might represent retinal–retinal anastomoses.47 Patients with SNV in one eye have an increased risk for development in the fellow eye.48
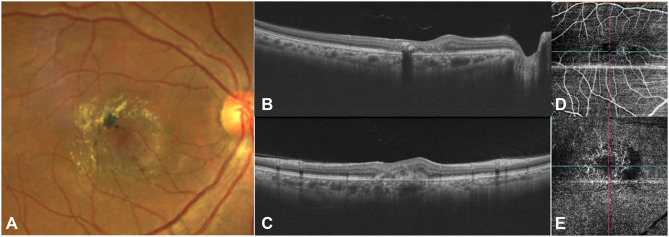
Figure 4.
Fundus photograph (A) depicts an active SNV with subretinal hemorrhage inferior to the fovea. Prominent crystalline deposits and pigment proliferation are seen temporal to fovea. The OCT images (horizontal and vertical cross-sections represented as (B and C) respectively) show sub-retinal hyper-reflective material with overlying retinal thickening and the OCTA (D and E) demonstrates a vascular network with a hypo-reflective flow void in the deep capillary plexus.
Visual Function
Patients with MacTel have significant more difficulty with near vision compared to distance, and often have central visual field defects. Patients with no or minimal decrease in binocular best corrected visual acuity (BCVA) often have significantly reduced reading ability because of central field defects. For efficient reading, patients need BCVA with preservation of at least four degrees of central field to process words and perform eye movements.49
MacTel patients often have profound paracentral scotomas that can be identified with microperitmetry. Visual acuity is generally stable during long-term follow-up studies.3,6,11 Watzke et al observed that 25% of their patients maintained a stable visual acuity over 17 years6 and Gass and Blodi reported that only 33% of their patients deteriorated to a visual acuity of less than 20/200.11 The natural history of MacTel has been described in Table 2.
Table 2.
Natural History of MacTel 2
| Study | Number of Patients And Duration of Disease | Visual Acuity Parameters | Salient Features |
|---|---|---|---|
| Marsonia et al, 20213 | 82 eyes of 47 patients, 4.5 years | Most patients maintained BCVA from logMAR 0.25 ± 0.25 to 0.46 ± 0.42 | Major cause of poor vision observed was SNV (active in 10.98% and scarred in 7.32%), foveal atrophy (10.98%) and central pigmented plaques (3.66%). The incidence of sight-threatening lesions like SNV (10.6%) |
| Clemons et al, 20105 | 310 patients, 3.2 years | BCVA was 20/20 or better in 16% of these eyes | More than half of the patients had ≥20/32 in their better eye over a 3.2 year follow up |
| Shukla et al, 201245 | 203 eyes of 103 patients, 2.5 years | Mean logMAR BCVA declined from 0.35 to 0.43 by 2.5 years (P < 0.0001) | 14% incidence of SNV. Final mean logMAR BCVA was 0.61 (20/80) in the eyes with SNV and 0.40 (20/50) in eyes without SNV |
| Peto et al, 201750 | 507 participants, 4.2 years | BCVA decreased 1.07 ± 0.05 letters per year. Of all eyes, 15% lost ≥15 letters after 5 years | The rate of BCVA loss was significantly higher in eyes with central EZ loss at baseline (−1.40 ± 0.14 letters, P < 0.001) |
Fundus Fluorescein Angiography
FA has been the gold standard for diagnosing MacTel, and leakage on FA may precede any other visible changes. Characteristically, FA shows dilated leaking capillaries temporal to the foveoa in the early phase, and diffuse hyperfluorescence in the late phase. These vascular changes in the deep capillary plexus, as shown by stereoscopic angiographic studies, are usually bilaterally symmetric and the amount of “staining” is similar in the late phase of the angiogram.14 The leakage in MacTel is not associated with retinal edema and thickening, suggesting that partial breakdown of the blood-retinal barrier allows passage of small molecules such as fluorescein but not the larger albumin.51
Occasionally, an area of intense focal hyperfluorescence within the area of diffuse hyperfluorescence can occur. This intense hyperfluorescence could be due to staining at the level of the outer retina or the development of intraretinal new vessels.37 SNV usually develops near migrating intraretinal pigment epithelium, appears to originate from the deep retinal circulation, and is characterized by early and late fluorescein leakage.52
Optical Coherence Tomography (OCT)
The earliest sign noted on OCT is temporal widening of the foveal pit, due to thinning of the temporal juxtafoveal retina.22 Most of the thinning occurs in the outer nuclear/Henle’s fiber layer. Charbel Issa et al showed that eyes with decreased central foveal thickness may actually have better light sensitivity than eyes with normal foveal thickness.53
The most common OCT findings are hyporeflective cavities in the inner and outer neurosensory retina and disruption of the external limiting membrane (ELM), photoreceptor inner segment-outer segment border and interdigitation zone.54 Cystoid spaces in eyes with MacTel have more boundary irregularities and lower reflectivity than those in eyes with MacTel 1, retinal vein occlusions and diabetic macular edema.55 These atrophic hyporeflective spaces are usually situated in the foveal pit with a predilection for the temporal slope and may resemble lamellar macular holes. The hyporeflective spaces in the outer retinal layers are due to photoreceptor loss.1 Microcavitations surround the area around increased reflectivity associated with right-angle veins.
Müller cell degeneration may lead to outer retinal atrophy, foveal thinning and the ILM “drape” sign. As the disease progresses, inner hypo-reflective cavities may change, possibly due to loss of supporting structures, surrounding atrophy and decreasing leakage. The hyperreflective cavities may disappear, only to reappear over time.
Eyes with disorganized inner retinal layers collapsed outer retinal layers and disrupted outer retinal hyperreflective bands have significantly worse visual acuity.56 The amount of ellipsoid zone loss on SD-OCT corresponds with deficits in microperimetry and may be used as a diagnostic feature and outcome measure. The extent of photoreceptor loss correlates with visual impairment, and limiting photoreceptor loss appears to be the most promising therapeutic approach to maintaining vision. Current clinical trials with ciliary neurotrophic factor (CNTF) implants use photoreceptor loss as a marker of efficacy.56,57
Increased reflectivity in various layers due to SNV or pigment hyperplasia may be seen. Macular holes, and foveal detachment occur less commonly. Subretinal deposits, if present, may represent debris from photoreceptor elements, which could cause increased foveal autofluorescence.58 Hyper-reflective intraretinal lesions with back shadowing on OCT usually correspond to pigment plaques. They first begin to appear in the outer retina before migrating into the inner retinal layers, but larger, flat lesions may start within the inner retina.59
Neovascular complexes may present as thick, hyper-reflective lesions on OCT, located in the outer retina. A thicker retina temporal to the fovea compared to the nasal fovea, without any retinal fluid, may indicate early SNV. In the presence of SNV, highly reflective dots can be seen in the inner and outer nuclear layers.60,61
Atrophy of the outer retina is the most common cause of vision loss. Choroidal thinning due to ischemia has been proposed as a cause but Chhablani et al found no difference in choroidal thickness between eyes with MacTel and age-matched healthy eyes.62 A recent study by Chandran et al has shown that fellow eyes of patients with MacTel showed telangiectasia and FAZ changes in the superficial and deep capillary plexuses prior to any neurodegenerative changes on SD-OCT.63
Optical Coherence Tomography- Angiography
OCT angiography is useful in classifying the severity of the disease and identifying neovascularisation activity in MacTel. The earliest vascular changes in MacTel occur temporal to the fovea in the deep capillary network, apparent on OCTA as reduced vascular density and telangiectatic vessels in the deep vascular plexus. Progressively, the entire capillary network around the fovea becomes involved, in both the superficial and deep plexus.64 The abnormalities in the deep vascular network may be identified on the OCTA, very early in the disease when the OCT is within normal limits. Abnormalities in retinal vascular density, length and diameter of vessels, and fractal dimension are seen.65 Foveal avascular zone (FAZ) irregularities and abnormal shape or dragging of vessels perifoveally appear in stage 3 and 4 of the disease.66 New vessel invasion of the outer and subretinal spaces occurs beneath areas with the greatest flow abnormalities.40
Autofluorescence
Increased blue light fundus autofluorescence (FAF) in the fovea that precedes clinical and fluorescein angiographic finding is one of the earliest changes in MacTel.67 This is likely due to macular pigment depletion from the fovea rather than increased lipofuscin accumulation in the RPE or displacement of the pigment.68 Normal eyes show central foveal masking on 488 nm FAF due to the central accumulation of macular pigment, but reduced macular pigment density or changes in its topographic distribution may affect this. Chhablani et al showed that 93.3% of eyes with MacTel had loss of foveal autofluorescence.69
The distribution of macular pigment optical density (MPOD) mirrors the amount of leakage on FA. There is an initial reduction in MPOD, presumably due to the loss of the xanthophyll pigments, temporally followed by the entire fovea and the appearance of a circular perifoveal ring. Disturbances in macular pigment occur with other ocular diseases but this pattern of MPOD is unique to MacTel.70,71
Microperimetry
Microperimetry sensitivity is reduced in the early stages of MacTel but visual acuity is unaffected, suggesting that microperimetry can pick up functional changes before vision becomes affected. In more advanced disease, paracentral sensitivity usually declines two degrees temporal to the foveal center though this depends on the presence and extent of photoreceptor atrophy.72
Multi-Focal Electro-Retinography
Narayanan et al found that in 28 eyes with proliferative MacTel both N1 and P1 amplitudes on the multifocal ERG were significantly lower compared to normal control.73 There was, however, no correlation with visual acuity and OCT thickness.
Treatment
Treatment of neovascular MacTel has improved with anti-vascular endothelial growth factor (anti-VEGF) drugs but treatment of non-proliferative MacTel remains controversial.
Non Neovascular Mactel
Focal and grid laser photocoagulation, photodynamic therapy (PDT), intravitreal triamcinolone acetonide (IVTA) and anti-VEGF drugs have been used to treat non-proliferative MacTel.
The visual acuity did not change with laser. Macular edema reduced in only 20% of eyes.74 Compared with untreated eyes, laser photocoagulation is associated with distortion of retinal vessels, more fibrovascular tissue, retinal pigment epithelial clumping, new draining venules, and retinal haemorrhages.6,49,75
PDT alone and in combination with intravitreal ranibizumab does not appear to be effective to improve visual acuity, or retinal atrophy.76,77 Similarly, IVTA has not shown to be beneficial to improve the visual acuity.78 Subthreshold micropulse laser with pulses of 15-ms duration have been used in 10 eyes of MacTel. A decrease in inner and outer retinal lacunae with preservation of retinal thickness and improvement in visual acuity by 10 letters was observed in these patients.79
Use of anti-VEGF drugs does not appear to alter the long-term outcomes in non-proliferative Mactel. There is a short-term benefit of reduction of leakage on FA, normalization of macular capillaries and decrease in retinal thickness. A few studies have reported gain in visual acuity,14,80 while other studies have not found substantial benefit.81,82 The reduction in retinal thickness is temporary and rebound increase in parafoveal leakage and central retinal thickness has been reported.11,83 Vascular changes may lead to deficiency of oxygen and nutrients to the neurosensory retina.14 Anti-VEGF agents may also cause photoreceptor degeneration. Hence, use of anti-VEGF drugs in the nonproliferative stage is still controversial.
Pars plana vitrectomy (PPV) combined with ILM peeling does not improve the anatomical or functional outcome in MacTel.84 PPV and ILM peeling is commonly performed in eyes with ERM or full-thickness macular holes, and it has been suggested that vitreous adhesion and traction may cause foveal changes in MacTel. PPV is not routinely recommended in MacTel.
Since the earliest finding in MacTel is redistribution of macular carotenoid pigment beginning in the temporal perifovea, supplementation with lutein, xeazanthin and meso-zeaxanthin has been studied. However, this failed to show any benefit.85,86 The role of carotenoid supplementation remains uncertain and needs further study.
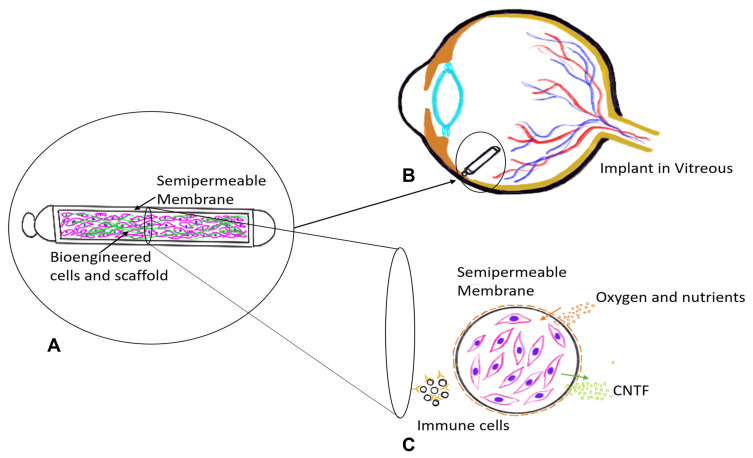
CNTF appears to reduce photoreceptor loss in animal models of outer retinal degeneration. CNTF supplementation with the Neurotech 501 cell implant (Neurotech Pharmaceuticals, Inc, Cumberland, RI) is being evaluated.87–89 This innovative, cell-based drug delivery system is surgically anchored at the pars plana with a titanium loop (Figure 5). Genetically modified human RPE cells are encapsulated in a semipermeable membrane and programmed to synthesize CNTF that is released into the vitreous.90
Figure 5.
Representative Illustration of Ciliary Neurotrophic factor (CNTF)-secreting implant (A) using Encapsulated cell technology. The implant consists of genetically bioengineered CNTF secreting cells enclosed in a semipermeable membrane. It is surgically anchored at the pars plana using a suture clip (B). The cross section image shows the membrane that allows nutrients to diffuse in and CNTF agent to diffuse out while not allowing immune cells to reach the CNTF secreting cells (C).
Phase 2 trials with 99 eyes demonstrated that CNTF significantly slowed the progression of retinal degeneration.91 Sham treatment showed greater progression of neurodegeneration, decrease in retinal sensitivity and reading speed compared to eyes treated with CNTF. There have been no safety concerns with the use of CNTF. Phase 3 trials are under way in the US, Europe, and Australia.
Neovascular Mactel
Active SNV is clinically suspected in the presence of subretinal hemorrhage, thickening of the retina and/or visible membrane at the macula. SNV is confirmed with FA and OCTA imaging.
Subretinal surgery to remove the neovascular membrane has been performed but its strong adherence to the overlying neurosensory retina leads to poor outcomes.92 Parafoveal scarring and impaired reading ability have limited the use of thermal laser photocoagulation93 and PDT has not been shown to improve visual acuity.94,95 Transpupillary thermotherapy (TTT) led to regression of the neovascular network in 11 of 13 eyes at 3 months.96
Anti-VEGF drugs are able to improve visual acuity and control the CNV.97–99 In a retrospective series of 16 patients followed over 12 months, Narayanan et al reported improvement in mean visual acuity from 20/120 to 20/70.48 They also found that the number of injections was significantly lower (1.9 injections per eye) than needed for other diseases such as age-related macular degeneration. In another study of 25 patients, the mean BCVA improved (20/91 to 20/62) and the central macular thickness decreased.100
Earlier diagnosis of SNV with high-resolution OCT may lead to better visual outcomes. Treatment outcomes of SNV have been described in Table 3.
Table 3.
Treatment Outcomes of SNV
| Study | Number of Eyes | Baseline BCVA | Final BCVA | Mean CMT | F/u Duration (Months) | Mean No. of Injections (Intravitreal Bevacizumab) |
|---|---|---|---|---|---|---|
| Narayanan, 201248 | 16 | 20/120 | 20/70 | CMT decreased from 235.57±108.65 µm to 174.91± 56.97 µm | 12 | 1.9 |
| Abdelaziz, 201697 | 22 | 20/200 | 20/100 | – | 18 | 3 |
| Baz, 201798 | 10 | 0. 62 ± 0.35 logMAR | 0.54 ± 0.35 logMAR | CMT decreased from 251 ± 25 µm to 239 ± 39 µm | 54.7±16.0 | 1.7 |
| Ozkaya, 201399 | 26 | 20/100 | 20/40 | CMT decreased from 318 µm to 198 µm | 26 | 6 |
| Toygar, 2016100 | 25 | 20/91 | 20/62 | CMT decreased from 254 µ to 205 µm | 42± 34 | 8.4 |
| Roller, 2011101 | 9 | 0.48±0.29 (decimal) | 0.77± 0.35 (decimal) | CMT decreased from 328±139 µm to 265±142 µm | 26±11 | 2.3 |
Conclusion
MacTel is a neurodegenerative disease that is found among people over the age of 40 years throughout the world. The neurodegenerative mechanism proposes that dysfunction of the parafoveal Müller cells leads to retinal thinning and subsequent vascular changes. However, the pathogenesis of the disease till remains elusive. MacTel causes limited vision loss and may not progress after a few years. Treatment with CNTF to slow disease progression holds promise and anti-VEGF therapy is often able to control SNV.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100(5):769–780. doi: 10.1001/archopht.1982.01030030773010 [DOI] [PubMed] [Google Scholar]
- 2.Aung KZ, Wickremasinghe SS, Makeyeva G, Robman L, Guymer RH. The prevalence estimates of macular telangiectasia type 2: the Melbourne collaborative cohort study. Retina. 2010;30(3):473–478. doi: 10.1097/IAE.0b013e3181bd2c71 [DOI] [PubMed] [Google Scholar]
- 3.Marsonia K, Kiran Chandra K, Ali MH, Chhablani J, Narayanan R. Long term follow-up of visual acuity and incidence of subretinal neovascularization in Mactel type 2 in 82 eyes. Semin Ophthalmol. 2022;37(2):136–141. doi: 10.1080/08820538.2021.1929347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Blodi BA, Meuer SM, Myers CE, Chew EY, Klein BE. The prevalence of macular telangiectasia type 2 in the Beaver Dam eye study. Am J Ophthalmol. 2010;150(1):55–62. doi: 10.1016/j.ajo.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemons TE, Gillies MC, Chew EY, et al. Baseline characteristics of participants in the natural history study of macular telangiectasia (MacTel) MacTel project report no 2. Ophthalmic Epidemiol. 2010;17(1):66–73. doi: 10.3109/09286580903450361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watzke RC, Klein ML, Folk JC, et al. Long-term juxtafoveal retinal telangiectasia. Retina. 2005;25(6):727–735. doi: 10.1097/00006982-200509000-00007 [DOI] [PubMed] [Google Scholar]
- 7.Cohen SM, Cohen ML, El-Jabali F, Pautler SE. Optical coherence tomography findings in nonproliferative group 2a idiopathic juxtafoveal retinal telangiectasis. Retina. 2007;27(1):59–66. doi: 10.1097/01.iae.0000256663.94734.e1 [DOI] [PubMed] [Google Scholar]
- 8.Newman E, Reichenbach A. The Muller cell: a functional element of the retina. Trends Neurosci. 1996;19(8):307–312. doi: 10.1016/0166-2236(96)10040-0 [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa S, Tamai M. Muller cells in the human foveal region. Curr Eye Res. 2001;22(1):34–41. doi: 10.1076/ceyr.22.1.34.6979 [DOI] [PubMed] [Google Scholar]
- 10.Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 11.Gass JD, Blodi BA. Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology. 1993;100(10):1536–1546. doi: 10.1016/S0161-6420(93)31447-8 [DOI] [PubMed] [Google Scholar]
- 12.Yannuzzi LA, Bardal AM, Freund KB, Chen KJ, Eandi CM, Blodi B. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124(4):450–460. doi: 10.1001/archopht.124.4.450 [DOI] [PubMed] [Google Scholar]
- 13.Charbel Issa P, Finger RP, Kruse K, Baumuller S, Scholl HP, Holz FG. Monthly ranibizumab for nonproliferative macular telangiectasia type 2: a 12-month prospective study. Am J Ophthalmol. 2011;151(5):876–886. doi: 10.1016/j.ajo.2010.11.019 [DOI] [PubMed] [Google Scholar]
- 14.Charbel Issa P, Finger RP, Holz FG, Scholl HP. Eighteen-month follow-up of intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Br J Ophthalmol. 2008;92(7):941–945. doi: 10.1136/bjo.2007.129627 [DOI] [PubMed] [Google Scholar]
- 15.Querques G, Delle Noci N. Juxtafoveal telangiectasias. Ophthalmology. 2008;115(9):1636. doi: 10.1016/j.ophtha.2008.03.029 [DOI] [PubMed] [Google Scholar]
- 16.Gass JD. Juxtafoveal telangiectasis-a name change? Retina. 2005;25(2):234–236. doi: 10.1097/00006982-200502000-00028 [DOI] [PubMed] [Google Scholar]
- 17.Soheilian M, Tavallali A, Peyman GA. Identification of intraretinal neovascularization by high-speed indocyanine green angiography in idiopathic perifoveal telangiectasia. Ophthalmic Surg Lasers Imaging. 2007;38(2):167–169. doi: 10.3928/15428877-20070301-16 [DOI] [PubMed] [Google Scholar]
- 18.Menchini U, Virgili G, Bandello F, Malara C, Rapizzi E, Lanzetta P. Bilateral juxtafoveolar telangiectasis in monozygotic twins. Am J Ophthalmol. 2000;129(3):401–403. doi: 10.1016/S0002-9394(99)00380-3 [DOI] [PubMed] [Google Scholar]
- 19.Putteman A, Toussaint D, Graff E, Verougstraete C. Idiopathic familial juxtafoveolar retinal telangiectasias. Bull Soc Belge Ophtalmol. 1984;209:81–90. [PubMed] [Google Scholar]
- 20.Siddiqui N, Fekrat S. Group 2A idiopathic juxtafoveolar retinal telangiectasia in monozygotic twins. Am J Ophthalmol. 2005;139(3):568–570. doi: 10.1016/j.ajo.2004.09.030 [DOI] [PubMed] [Google Scholar]
- 21.Parmalee NL, Schubert C, Figueroa M, et al.; MacTel Project. Identification of a potential susceptibility locus for macular telangiectasia type 2. PLoS One. 2012;7(8):e24268. doi: 10.1371/journal.pone.0024268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillies MC, Zhu M, Chew E, et al. Familial asymptomatic macular telangiectasia type 2. Ophthalmology. 2009;116(12):2422–2429. doi: 10.1016/j.ophtha.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charbel Issa P, Gillies MC, Chew EY, et al. Macular telangiectasia type 2. Prog Retin Eye Res. 2013;34:49–77. doi: 10.1016/j.preteyeres.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powner MB, Gillies MC, Zhu M, Vevis K, Hunyor AP, Fruttiger M. Loss of Müller’s cells and photoreceptors in macular telangiectasia type 2. Ophthalmology. 2013;120:2344–2352. doi: 10.1016/j.ophtha.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 25.Eade K, Gantnr M, Hostyk JA, et al. Serine biosynthesis defect due to haploinsufficiency of PHGDH causes retinal disease. Nat Metab. 2021;3:366–377. doi: 10.1038/s42255-021-00361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gantner ML, Eade K, Wallace M, et al. Serine and lipid metabolism in macular disease and peripheral neuropathy. N Engl J Med. 2019;381(15):1422–1433. doi: 10.1056/NEJMoa1815111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonelli R, Ansell BRE, Lotta L, et al. Genetic disruption of serine biosynthesis is a key driver of macular telangiectasia type 2 aetiology and progression. Genome Med. 2021;13(1):39. doi: 10.1186/s13073-021-00848-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scerri TS, Quaglieri A, Cai C, et al. Genome-wide analyses identify common variants associated with macular telangiectasia type 2. Nat Genet. 2017;49(4):559–567. doi: 10.1038/ng.3799 [DOI] [PubMed] [Google Scholar]
- 29.Clemons TE, Gillies MC, Chew EY, et al. Medical characteristics of patients with macular telangiectasia type 2 (MacTel Type 2) MacTel project report no. 3. Ophthalmic Epidemiol. 2013;20(2):109–113. doi: 10.3109/09286586.2013.766757 [DOI] [PubMed] [Google Scholar]
- 30.Lee HC, Liu M, Ho AC. Idiopathic juxtafoveal telangiectasis in association with celiac sprue. Arch Ophthalmol. 2004;122(3):411–413. doi: 10.1001/archopht.122.3.411 [DOI] [PubMed] [Google Scholar]
- 31.Huerva V, Sanchez MC. Juxtafoveolar telangiectasis associated with CREST syndrome. Ocul Immunol Inflamm. 2008;16(4):195–197. doi: 10.1080/09273940802217867 [DOI] [PubMed] [Google Scholar]
- 32.Parodi MB, Iacono P, Ravalico G. Subretinal neovascular membrane associated with type 2a idiopathic juxtafoveolar telangiectasis in pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol. 2007;245(4):612–615. doi: 10.1007/s00417-006-0402-7 [DOI] [PubMed] [Google Scholar]
- 33.Querques G, Coscas G, Soubrane G, Souied EH. Type II idiopathic macular telangiectasia and soft confluent drusen. Eur J Ophthalmol. 2010;20(2):466–468. doi: 10.1177/112067211002000234 [DOI] [PubMed] [Google Scholar]
- 34.Bakke EF, Drolsum L. Iris microhaemangiomas and idiopathic juxtafoveolar retinal telangiectasis. Acta Ophthalmol Scand. 2006;84(6):818–822. doi: 10.1111/j.1600-0420.2006.00708.x [DOI] [PubMed] [Google Scholar]
- 35.Charbel Issa P, Scholl HP, Gaudric A, et al. Macular full-thickness and lamellar holes in association with type 2 idiopathic macular telangiectasia. Eye. 2009;23(2):435–441. doi: 10.1038/sj.eye.6703003 [DOI] [PubMed] [Google Scholar]
- 36.Chin EK, Kim DY, Hunter AAIII, et al. Staging of macular telangiectasia: power-Doppler optical coherence tomography and macular pigment optical density. Invest Ophthalmol Vis Sci. 2013;54:4459–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatesh R, Reddy NG, Mishra P, et al. The preproliferative stage in type 2 macular telangiectasia (MacTel type 2). Graefes Arch Clin Exp Ophthalmol. 2022;260(1):121–132. doi: 10.1007/s00417-021-05371-1 [DOI] [PubMed] [Google Scholar]
- 38.Abujamra S, Bonanomi MT, Cresta FB, Machado CG, Pimentel SL, Caramelli CB. Idiopathic juxtafoveolar retinal telangiectasis: clinical pattern in 19 cases. Ophthalmologica. 2000;214(6):406–411. doi: 10.1159/000027534 [DOI] [PubMed] [Google Scholar]
- 39.Casswell AG, Chaine G, Rush P, Bird AC. Paramacular telangiectasis. Trans Ophthalmol Soc UK. 1986;10:683–692. [PubMed] [Google Scholar]
- 40.Spaide R, Suzuki M, Yannuzzi L, Matet A, Behar-Cohen F. Volume-rendered angiographic and structural optical coherence tomography angiography of macular telangiectasia type 2. Retina. 2017;37:424–435. doi: 10.1097/IAE.0000000000001344 [DOI] [PubMed] [Google Scholar]
- 41.Sallo FB, Leung I, Chung M, et al. Retinal crystals in type 2 idiopathic macular telangiectasia. Ophthalmology. 2011;118(12):2461–2467. doi: 10.1016/j.ophtha.2011.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moisseiev J, Lewis H, Bartov E, Fine SL, Murphy RP. Superficial retinal refractile deposits in juxtafoveal telangiectasis. Am J Ophthalmol. 1990;109(5):604–605. doi: 10.1016/S0002-9394(14)70699-3 [DOI] [PubMed] [Google Scholar]
- 43.Eliassi-Rad B, Green WR. Histopathologic study of presumed parafoveal telangiectasis. Retina. 1999;19(4):332–335. doi: 10.1097/00006982-199907000-00011 [DOI] [PubMed] [Google Scholar]
- 44.Olson JL, Mandava N. Macular hole formation associated with idiopathic parafoveal telangiectasia. Graefes Arch Clin Exp Ophthalmol. 2006;244(3):411–412. doi: 10.1007/s00417-005-0057-9 [DOI] [PubMed] [Google Scholar]
- 45.Shukla D. Evolution and management of macular hole secondary to type 2 idiopathic macular telangiectasia. Eye. 2011;25(4):532–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meleth AD, Toy BC, Nigam D, et al. Prevalence and progression of pigment clumping associated with idiopathic macular telangiectasia type 2. Retina. 2013;33(4):762–770. doi: 10.1097/IAE.0b013e3182695bb3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelbrecht NE, Aaberg TM Jr, Sung J, Lewis ML. Neovascular membranes associated with idiopathic juxtafoveolar telangiectasis. Arch Ophthalmol. 2002;120(3):320–324. doi: 10.1001/archopht.120.3.320 [DOI] [PubMed] [Google Scholar]
- 48.Narayanan R, Chhablani J, Sinha M, et al. Efficacy of anti-vascular endothelial growth factor therapy in subretinal neovascularization secondary to macular telangiectasia type 2. Retina. 2012;32(10):2001–2005. doi: 10.1097/IAE.0b013e3182625c1d [DOI] [PubMed] [Google Scholar]
- 49.Trauzettel-Klosinski S. Reading disorders due to visual field defects: a neuroophthalmological view. Neuro-Ophthalmology. 2002;27:79e90. doi: 10.1076/noph.27.1.79.14298 [DOI] [Google Scholar]
- 50.Peto T, Heeren T, Clemons T, et al.; The MacTel Research Group. Correlation of clinical and structural progression with visual acuity loss in macular telangiectasia type 2: MacTel project report no. 6. Retina. 2018;38:S8–S13. doi: 10.1097/IAE.0000000000001697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan G, Balaratnasingam C, Yu PK, et al. Quantitative morphometry of perifoveal capillary networks in the human retina. Invest Ophthalmol Vis Sci. 2012;53(9):5502–5514. doi: 10.1167/iovs.12-10265 [DOI] [PubMed] [Google Scholar]
- 52.Chhablani J, Mithal K, Rao H, Narayanan R. Diagnosis of subretinal neovascularization associated with idiopathic juxtafoveal retinal telangiectasia - fluorescein angiography versus spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):549–553. doi: 10.1007/s00417-013-2491-4 [DOI] [PubMed] [Google Scholar]
- 53.Charbel Issa P, Helb HM, Holz FG, Scholl HP. Correlation of macular function with retinal thickness in nonproliferative type 2 idiopathic macular telangiectasia. Am J Ophthalmol. 2008;145(1):169–175. doi: 10.1016/j.ajo.2007.08.028 [DOI] [PubMed] [Google Scholar]
- 54.Kim YH, Chung YR, Oh J, et al. Optical coherence tomographic features of macular telangiectasia type 2: Korean macular telangiectasia type 2 study-report no. 1. Sci Rep. 2020;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh JH, Oh J, Togloom A, Kim SW, Huh K. Characteristics of cystoid spaces in type 2 idiopathic macular telangiectasia on spectral domain optical coherence tomography images. Retina. 2014;34(6):1123–1131. doi: 10.1097/IAE.0000000000000038 [DOI] [PubMed] [Google Scholar]
- 56.Pauleikhoff D, Pauleikhoff L, Chew EY. Imaging endpoints for clinical trials in MacTel type 2. Eye. 2022;36(2):284–293. doi: 10.1038/s41433-021-01723-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heeren TFC, Kitka D, Florea D, et al. Longitudinal Correlation of ellipsoid zone loss and functional loss in macular telangiectasia type 2. Retina. 2018;38((Suppl1)):S20–S26. doi: 10.1097/IAE.0000000000001715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherepanoff S, Killingsworth MC, Zhu M, et al. Ultrastructural and clinical evidence of subretinal debris accumulation in type 2 macular telangiectasia. Br J Ophthalmol. 2012;96(11):1404e1409. doi: 10.1136/bjophthalmol-2011-301009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta V, Gupta A, Dogra MR, Agarwal A. Optical coherence tomography in group 2A idiopathic juxtafoveolar telangiectasis. Ophthalmic Surg Lasers Imaging. 2005;36(6):482–486. doi: 10.3928/1542-8877-20051101-08 [DOI] [PubMed] [Google Scholar]
- 60.Gaudric A, Ducos de Lahitte G, Cohen SY, Massin P, Haouchine B. Optical coherence tomography in group 2A idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 2006;124:1410–1419. doi: 10.1001/archopht.124.10.1410 [DOI] [PubMed] [Google Scholar]
- 61.Paunescu LA, Ko TH, Duker JS, et al. Idiopathic juxtafoveal retinal telangiectasis: new findings by ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113(1):48. doi: 10.1016/j.ophtha.2005.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chhablani J, Kozak I, Jonnadula GB, et al. Choroidal thickness in macular telangiectasia type 2. Retina. 2014;34(9):1819–1823. doi: 10.1097/IAE.0000000000000180 [DOI] [PubMed] [Google Scholar]
- 63.Chandran K, Giridhar A, Gopalakrishnan M, Sivaprasad S. Microvascular changes precede visible neurodegeneration in fellow eyes of patients with asymmetric type 2 macular telangiectasia. Eye. 2021;36:1623–1630. doi: 10.1038/s41433-021-01699-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeimer M, Gutfleisch M, Heimes B, Spital G, Lommatzsch A, Pauleikhoff D. Association between changes in macular vasculature in optical coherence tomography- and fluorescein-angiography and distribution of macular pigment in type 2 idiopathic macular teleangiectasia. Retina. 2015;35:2307–16Z. doi: 10.1097/IAE.0000000000000868 [DOI] [PubMed] [Google Scholar]
- 65.Pauleikhoff D, Gunnemann F, Book M, Rothaus K. Progression of vascular changes in macular telangiectasia type 2: comparison between SD-OCT and OCT angiography. Graefes Arch Clin Exp Ophthalmol. 2019;257:1381–1392. doi: 10.1007/s00417-019-04323-0 [DOI] [PubMed] [Google Scholar]
- 66.Nalcı H, Şermet F, Demirel S, Özmert E. Optical coherence tomography angiography findings in type-2 macular telangiectasia. Turk J Ophthalmol. 2017;47(5):279–284. doi: 10.4274/tjo.68335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong WT, Forooghian F, Majumdar Z, Bonner RF, Cunningham D, Chew EY. Fundus autofluorescence in type 2 idiopathic macular telangiectasia: correlation with optical coherence tomography and microperimetry. Am J Ophthalmol. 2009;148(4):573–583. doi: 10.1016/j.ajo.2009.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeimer MB, Kromer I, Spital G, Lommatzsch A, Pauleikhoff D. Macular telangiectasia: patterns of distribution of macular pigment and response to supplementation. Retina. 2010;30(8):1282–1293. doi: 10.1097/IAE.0b013e3181e096dd [DOI] [PubMed] [Google Scholar]
- 69.Chhablani JK, Narayanan R. Fundus autofluorescence patterns in type 2A idiopathic juxtafoveolar retinal telangiectasis. Eur J Ophthalmol. 2012;22(3):398–403. doi: 10.5301/ejo.5000008 [DOI] [PubMed] [Google Scholar]
- 70.Charbel Issa P, Berendschot TT, Staurenghi G, Holz FG, Scholl HP. Confocal blue reflectance imaging in type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2008;49(3):1172–1177. doi: 10.1167/iovs.07-0636 [DOI] [PubMed] [Google Scholar]
- 71.Charbel Issa P, van der Veen RL, Stijfs A, Holz FG, Scholl HP, Berendschot TT. Quantification of reduced macular pigment optical density in the central retina in macular telangiectasia type 2. Exp Eye Res. 2009;89(1):25–31. doi: 10.1016/j.exer.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 72.Charbel Issa P, Helb HM, Rohrschneider K, Holz FG, Scholl HPN. Microperimetric assessment of patients with type II macular telangiectasia. Invest Ophthalmol Vis Sci. 2007;48:3788e3795. [DOI] [PubMed] [Google Scholar]
- 73.Narayanan R, Dave V, Rani PK, et al. Multifocal electroretinography in type 2 idiopathic macular telangiectasia. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1311–1318. doi: 10.1007/s00417-012-2191-5 [DOI] [PubMed] [Google Scholar]
- 74.Park DW, Schatz H, McDonald HR, Johnson RN. Grid laser photocoagulation for macular edema in bilateral juxtafoveal telangiectasis. Ophthalmology. 1997;104:1838e1846. doi: 10.1016/S0161-6420(97)30019-0 [DOI] [PubMed] [Google Scholar]
- 75.Friedman SM, Mames RN, Stewart MW. Subretinal hemorrhage after grid laser photocoagulation for idiopathic juxtafoveolar retinal telangiectasis. Ophthalmic Surg. 1993;24:551e553. [PubMed] [Google Scholar]
- 76.De Lahitte GD, Cohen SY, Gaudric A. Lack of apparent short-term benefit of photodynamic therapy in bilateral, acquired, parafoveal telangiectasis without subretinal neovascularization. Am J Ophthalmol. 2004;138(5):892–894. doi: 10.1016/j.ajo.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 77.Zehetner C, Haas G, Treiblmayr B, Kieselbach GF, Kralinger MT. Reduced-fluence photodynamic therapy combined with ranibizumab for nonproliferative macular telangiectasia type 2. Ophthalmologica. 2013;229(4):195. doi: 10.1159/000350033 [DOI] [PubMed] [Google Scholar]
- 78.Wu L, Evans T, Arevalo JF, et al. Long-term effect of intravitreal triamcinolone in the nonproliferative stage of type II idiopathic parafoveal telangiectasia. Retina. 2008;28(2):314–319. doi: 10.1097/IAE.0b013e31814cf03e [DOI] [PubMed] [Google Scholar]
- 79.Lavinsky D, Wang J, Huie P, et al. Nondamaging retinal laser therapy: rationale and applications to the macula. Invest Ophthalmol Vis Sci. 2016;57(6):2488–2500. doi: 10.1167/iovs.15-18981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toy BC, Koo E, Cukras C, Meyerle CB, Chew EY, Wong WT. Treatment of nonneovascular idiopathic macular telangiectasia type 2 with intravitreal ranibizumab: results of a Phase II clinical trial. Retina. 2012;32(5):996–1006. doi: 10.1097/IAE.0b013e31824690a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kovach JL, Rosenfeld PJ. Bevacizumab (avastin) therapy for idiopathic macular telangiectasia type II. Retina. 2009;29(1):27–32. doi: 10.1097/IAE.0b013e31818ba9de [DOI] [PubMed] [Google Scholar]
- 82.Kupitz EH, Heeren TFC, Holz FG, Issa PC. Poor longterm outcome of anti-vascular endothelial growth factor therapy in nonproliferative macular Ophthalmol Ther (2019) 8: 155–175173 telangiectasia type 2. Retina. 2015;35:2619–2626. doi: 10.1097/IAE.0000000000000715 [DOI] [PubMed] [Google Scholar]
- 83.Matsumoto Y, Yuzawa M. Intravitreal bevacizumab therapy for idiopathic macular telangiectasia. Jpn J Ophthalmol. 2010;54:320–324. doi: 10.1007/s10384-010-0810-4 [DOI] [PubMed] [Google Scholar]
- 84.Sigler EJ, Randolph JC, Calzada JI, Charles S. Pars plana vitrectomy with internal limiting membrane removal in type 2 idiopathic macular telangiectasia. Retinal Cases Brief Rep. 2013;7(4):380–385. doi: 10.1097/ICB.0b013e318297f69a [DOI] [PubMed] [Google Scholar]
- 85.Tan ACS, Balaratnasingam C, Yannuzzi LA. Treatment of macular telangiectasia type 2 with carotenoid supplements containing meso-zeaxanthin: a pilot study. Ophthal Surg Lasers Imaging Retina. 2016;47:528–535. doi: 10.3928/23258160-20160601-04 [DOI] [PubMed] [Google Scholar]
- 86.Choi RY, Gorusupudi A, Wegner K, Sharifzadeh M, Gellermann W, Bernstein PS. Macular pigment distribution responses to high-dose zeaxanthin supplementation in patients with macular telangiectasia type 2. Retina. 2017;37(12):2238–2247. doi: 10.1097/IAE.0000000000001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dorrell MI, Aguilar E, Jacobson R, et al. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J Clin Invest. 2009;119:611e623. doi: 10.1172/JCI35977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012;31:136. doi: 10.1016/j.preteyeres.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen W, Fruttiger M, Zhu L, et al. Conditional Muller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci. 2012;32:15715e15727. doi: 10.1523/JNEUROSCI.2841-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chew EY, Clemons TE, Peto T, et al. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a Phase 1 safety trial. Am J Ophthalmol. 2015;159:659–666.e1. doi: 10.1016/j.ajo.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chew EY, Clemons TE, Jaffe GJ, et al. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2. Ophthalmology. 2018;126:S0161642018314271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berger AS, McCuen BW, Brown GC, Brownlow RL. Surgical removal of subfoveal neovascularization in idiopathic juxtafoveolar retinal telangiectasis. Retina. 1997;17(2):94–98. doi: 10.1097/00006982-199703000-00002 [DOI] [PubMed] [Google Scholar]
- 93.Vaze A, Gillies M. Salient features and management options of macular telangiectasia type 2: a review and update. Expert Rev Ophthalmol. 2016;11(6):429–441. doi: 10.1080/17469899.2016.1251311 [DOI] [Google Scholar]
- 94.Potter MJ, Szabo SM, Chan EY, Morris AH. Photodynamic therapy of a subretinal neovascular membrane in type 2A idiopathic juxtafoveolar retinal telangiectasis. Am J Ophthalmol. 2002;133(1):149–151. doi: 10.1016/S0002-9394(01)01205-3 [DOI] [PubMed] [Google Scholar]
- 95.Shanmugam MP, Agarwal M. RPE atrophy following photodynamic therapy in type 2A idiopathic parafoveal telangiectasis. Indian J Ophthalmol. 2005;53:61–63. doi: 10.4103/0301-4738.15289 [DOI] [PubMed] [Google Scholar]
- 96.Shukla D, Singh J, Kolluru CM, Kim R, Namperumalsamy P. Transpupillary thermotherapy for subfoveal neovascularization secondary to group 2A idiopathic juxtafoveolar telangiectasis. Am J Ophthalmol. 2004;138(1):147–149. doi: 10.1016/j.ajo.2004.01.047 [DOI] [PubMed] [Google Scholar]
- 97.Abdelaziz M, Rostamizadeh M, Schartman J, et al. The use of anti-vascular endothelial growth factor therapies in the macular telangiectasia associated with choroidal neovascularization. Invest Ophthalmol Vis Sci. 2016;57(12):21455962. [Google Scholar]
- 98.Baz O, Yilmaz I, Alagoz C, et al. Efficacy of intravitreal bevacizumab in treatment of proliferative type 2 idiopathic juxtafoveal telangiectasia. Turk J Ophthalmol. 2017;47(3):144–148. doi: 10.4274/tjo.04874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ozkaya A, Alkin Z, Karakucuk Y, Yazici AT, Demirok A. Long term result of intravitreal bevacizumab in a patient newly transformed to proliferative macular telangiectasia type 2. Middle East Afr J Ophthalmol. 2013;20(4):360–362. doi: 10.4103/0974-9233.120005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toygar O, Guess M, Youssef D, Miller D. Longterm outcomes of intravitreal bevacizumab therapy for subretinal neovascularization secondary to idiopathic macular telangiectasia type 2. Retina. 2016;36(11):2150–2157. doi: 10.1097/IAE.0000000000001035 [DOI] [PubMed] [Google Scholar]
- 101.Roller AB, Folk JC, Patel NM, et al. Intravitreal bevacizumab for treatment of proliferative and nonproliferative type 2 idiopathic macular telangiectasia. Retina. 2011;31(9):1848–1855. doi: 10.1097/IAE.0b013e31820d3feb [DOI] [PubMed] [Google Scholar]