PURPOSE
Blood or marrow transplantation (BMT) is an integral part of consolidation and/or salvage therapy for patients with acute myeloid leukemia (AML). With the growing population of AML survivors, there is a need to understand the quality of their survival.
MATERIALS AND METHODS
This multisite study included 1,369 2-year survivors who underwent BMT for AML between 1974 and 2014 at age ≥ 21 years and 1,310 siblings. Using Common Terminology Criteria for Adverse Events, severe/life-threatening and fatal chronic health conditions were identified. Multivariable regression analysis was used to compare the risk of severe/life-threatening conditions and health status between survivors and siblings, and to identify risk factors for health conditions among BMT survivors.
RESULTS
The prevalence of severe/life-threatening conditions was 54.9% in BMT survivors compared with 28.5% in siblings (P < .001), yielding 3.8-fold higher odds of severe/life-threatening conditions (95% CI, 3.1 to 4.7) among the BMT survivors. The most prevalent conditions included subsequent neoplasms, diabetes, cataracts, venous thromboembolism, and joint replacement. Survivors were more likely to report poor general health (odds ratio [OR], 3.8; 95% CI, 2.8 to 5.1), activity limitation (OR, 3.7; 95% CI, 3.0 to 4.5), and functional impairment (OR, 2.9; 95% CI, 2.3 to 3.6). Among BMT recipients, the 20-year cumulative incidence of severe/life-threatening/fatal conditions was 68%. History of chronic graft-versus-host disease was associated with a higher risk of pulmonary disease (hazard ratio [HR], 3.1; 95% CI, 1.0 to 9.3), cataract (HR, 2.6; 95% CI, 1.4 to 3.8), and venous thromboembolism (HR, 2.3; 95% CI, 1.3 to 4.7). Relapse-related mortality (RRM) plateaued at 30%, whereas non-RRM increased to 50% at 30 years.
CONCLUSION
The burden of severe/life-threatening conditions is substantially higher in BMT recipients when compared with an unaffected comparison group, contributing to an increasing incidence of non-RRM over time. Chronic graft-versus-host disease was an important risk factor for severe/life-threatening/fatal conditions among BMT recipients, informing the need for close monitoring to anticipate and manage morbidity.
INTRODUCTION
Blood or marrow transplantation (BMT) is an established curative treatment option for patients with acute myeloid leukemia (AML).1,2 Improvement in transplantation strategies, supportive care, and increased donor source availability have led to an increase in the number of patients with AML who are treated with BMT, with notable increases in older recipients.1-3 In fact, AML is the most common indication for allogeneic BMT, with an estimated 3,500 patients undergoing BMT for AML annually in United States.2,3 Survival rates after BMT have improved steadily over the past 5 decades, resulting in a growing number of long-term survivors.1,4 However, these survivors are at high risk of developing severe or life-threatening chronic health conditions and premature death, attributed to pre-BMT therapeutic exposures, BMT-related conditioning, and persistent post-BMT complications such as chronic graft-versus-host disease (cGVHD).5-9
CONTEXT
Key Objective
To determine the burden of late morbidity and mortality in survivors of acute myeloid leukemia after blood or marrow transplantation (BMT), compare the burden of morbidity with that experienced by a noncancer comparison group, and identify subgroups with increased risk.
Knowledge Generated
The burden of chronic health conditions was consistently greater in BMT survivors compared with the noncancer comparison group across nearly all health outcomes, contributing to an increasing incidence of non–relapse-related mortality with time. Chronic graft-versus-host disease was an important risk factor for severe/life-threatening/fatal conditions among BMT recipients.
Relevance
These findings may inform evidence-based health screening recommendations that account for the evolving pattern of morbidity and mortality over time after BMT. Our findings support a long-term need for multidisciplinary management of patients with a history of chronic graft-versus-host disease, setting the stage for screening, early detection, and interventions to mitigate risk of adverse health outcomes.
There is a paucity of information regarding the burden of morbidity carried by AML survivors treated with BMT. Previous studies determining morbidity after BMT have been limited to patients who underwent BMT in childhood (< 21 years at BMT),10-13 or have focused on health-related quality of life and not the burden of morbidity,14,15 and have not included a non-BMT comparison cohort.8,10,12,16 We addressed these gaps by using the resources offered by the BMT Survivor Study (BMTSS). Specifically, we examined the burden of morbidity for severe or life-threatening chronic health conditions in adult survivors of AML treated with BMT, compared this burden of morbidity with that experienced by a noncancer comparison group, examined the association between BMT-related risk factors and chronic health conditions among BMT recipients, and described the overall and cause-specific late-mortality conditional on surviving varying lengths of time after BMT.
MATERIALS AND METHODS
BMTSS is a collaboration between the University of Alabama at Birmingham, City of Hope, and University of Minnesota, and examines the long-term outcomes of individuals who have survived ≥ 2 years after BMT performed at the participating institutions between 1974 and 2014. University of Alabama at Birmingham serves as the single institutional review board of record, and institutional review board approval was obtained at all three participating institutions; participants provided written informed consent according to the Declaration of Helsinki. For the current report, eligibility included a single BMT for AML between 1974 and 2014, survival for ≥ 2 years after BMT, and age at BMT ≥ 21 years. Vital status (alive or deceased) was ascertained as of December 31, 2020, using the following resources: National Death Index Plus, medical records, and institutional long-term follow-up efforts. Information on cause of death was obtained from the National Death Index Plus program and/or medical records. Of the 1,757 eligible patients, 529 had died after surviving ≥ 2 years after BMT. Of the 1,228 alive patients, 388 (22.1%) were lost to follow-up or refused study participation, yielding 840 (68.4%) participants followed for a mean of 8.6 years (± 5.7) after transplant. Compared with nonparticipants, participants were older at BMT, more likely to be non-Hispanic White, to have bone marrow as the stem-cell source, and to have undergone an unrelated allogeneic transplant, but less likely to have undergone BMT in recent years (Data Supplement, online only).
The noncancer comparison group comprised 1,310 nearest-age siblings of the larger cohort of BMT survivors17; selected siblings were therefore not all directly related to the BMT survivors included in the current study. The 840 alive survivors of AML and the 1,310 siblings completed a one-time 255-item BMTSS survey that included questions regarding sociodemographic characteristics (race/ethnicity, educational level, marital status, employment, household income, and insurance status), diagnosis by a health care provider of specific chronic health conditions (Data Supplement) with age at onset, and diagnosis of cGVHD. The BMTSS survey also asked about the participant's health status (poor general health, functional impairment, activity limitation, and anxiety or fear; Data Supplement).18,19 The reliability and accuracy of the BMTSS questionnaire have been previously established.20 We used Common Terminology Criteria for Adverse Events version 5.0 to assign a level of severity to each chronic health condition (grades: 1 = mild, 2 = moderate, 3 = severe, 4 = life-threatening/disabling, 5 = fatal; Data Supplement).7,21 Details regarding donor source (autologous or allogeneic), relapse risk at BMT (high or standard risk), stem-cell source (peripheral blood [PBSC], bone marrow, or cord blood), conditioning therapy (chemotherapy agent [yes/no] and total-body irradiation [TBI; yes/no]), and conditioning intensity (myeloablative, reduced intensity, or nonmyeloablative) were obtained from institutional BMT databases.
Statistical Analyses
BMT survivors compared with siblings.
We compared clinical and demographic characteristics of BMT survivors and siblings who completed a questionnaire. We compared the prevalence of any or multiple (≥ 2) grade 3 (severe) or 4 (life-threatening) chronic health conditions, as well as the domains of health status between BMT survivors and siblings. For multiple conditions, time to event was defined using the second condition. Missing values were handled as a separate category in the analyses. Multivariable logistic regression was used to adjust for potential confounders between the two groups, and to describe the odds (expressed as odds ratio [OR]) of developing a severe/life-threatening chronic health conditions or poor health status in survivors compared with siblings, with associated 95% CIs. For each multivariable model, we performed stepwise backward variable selection. Variables with P values < .1 were retained in the final models and are presented in the Data Supplement. For the analyses evaluating odds of reporting poor health status among BMT survivors versus siblings, we created two models, one with and one without chronic health conditions.
BMT survivors.
Cumulative incidence rates for grade 3-5 chronic health conditions and for the more prevalent conditions (> 5%) were calculated for the 1,369 BMTSS participants who completed a questionnaire (n = 840) or were deceased after surviving ≥ 2 years (n = 529), treating death due to nonchronic health conditions as a competing risk.22 Fine-Gray proportional subdistributional hazard models were developed to examine demographic and treatment-related risk factors for individual chronic health conditions; hazard ratios (HRs) with associated 95% CI were used to describe the magnitude of risk. Variables included in the models were selected as above and are provided in the Data Supplement. Recognizing the need to consider the changing practice of BMT over time, we created two multivariable models, one that included treatment era and one that did not. Next, we examined the burden of grades 3-5 conditions among the 1,120 allogeneic BMT recipients (81.8% of overall cohort), stratified by history of cGVHD. Survival estimates were calculated using the Kaplan-Meier method, conditional on increasing lengths of survival (2, 5, 10, 15, and 20 years) after BMT.23,24 The cumulative incidence of relapse-related (RRM) and non–relapse-related (NRM) mortality were computed by treating non–relapse-related and relapse-related deaths as competing risk, respectively.
Data were analyzed using SAS statistical software (version 9.4, SAS Institute, Inc, Cary, NC). All statistical tests were two-sided, and P values < .05 were considered statistically significant.
RESULTS
Demographic and Clinical Characteristics of Study Participants
The demographic characteristics of the 840 BMT survivors and 1,310 siblings are presented in Table 1. The mean age at study participation (55.9 years v 56.2 years) was comparable. Siblings were more likely to be female (60.3% v 52.1%, P < .001), non-Hispanic White (87.6% v 75.0%, P < .001), college graduates (55.0% v 46.7%, P < .001), and have an annual household income > $20,000 US dollars (84.6% v 77.0%, P < .001).
TABLE 1.
Demographic and Clinical Characteristics of the Study Participants
Disease and transplantation characteristics of the 1,369 BMT recipients are presented in Table 1. The mean age (± standard deviation) at BMT was 44.6 years (± 12.8), and the mean interval between BMT and questionnaire completion or death was 8.2 years (± 6.3). The majority (86.1%) of the cohort had received allogeneic BMT (50.9% related and 49.1% unrelated). Overall, 52.4% of the survivors underwent nonmyeloablative or reduced-intensity conditioning, and the most frequently used regimen was melphalan-/fludarabine-based (51.0%), followed by a TBI-based regimen (38.7%). PBSCs were used in 66.3% of survivors, and 62.7% were considered at low risk of relapse at BMT. Two thirds (67.6%) of the allogeneic BMT recipients carried a history of cGVHD.
Burden of Morbidity and Poor Health Status in BMT Survivors Versus Siblings
As shown in the Data Supplement, the prevalence of having any or multiple (≥ 2) grade 3 or 4 chronic health conditions was significantly greater in survivors compared with siblings (any condition: 54.9% v 28.5%, P < .001; multiple conditions: 23.9% v 7.8%, P < .001). BMT survivors had higher odds of developing a grade 3 or 4 chronic health condition (OR, 3.8; 95% CI, 3.1 to 4.7), multiple grade 3 or 4 conditions (OR, 4.3; 95% CI, 3.3 to 5.6), subsequent malignant neoplasm (SMN; OR, 16.9; 95% CI, 11.5 to 24.8), diabetes (OR, 4.6; 95% CI, 2.8 to 7.5), venous thromboembolism (OR, 3.5; 95% CI, 2.3 to 5.2), cataracts (OR, 2.8; 95% CI, 2.1 to 3.8), or undergo joint replacements (OR, 1.5; 95% CI, 1.1 to 2.1) compared with siblings. Furthermore, survivors were significantly more likely to report poor general health (OR, 3.8; 95% CI, 2.8 to 5.1), functional impairment (OR, 2.9; 95% CI, 2.3 to 3.6), activity limitation (OR, 3.7; 95% CI, 3.0 to 4.5), pain (OR, 2.2; 95% CI, 1.7 to 2.7), and anxiety or fears (OR, 2.4; 95% CI, 1.8 to 3.1; Table 2). These differences were attenuated but remained statistically significant when the model was adjusted for chronic health conditions (Table 2).
TABLE 2.
Prevalence and Risk of Poor Health Status Among Blood or Marrow Transplantation Survivors and Sibling Controls
Morbidity in BMT Recipients
The 10-year cumulative incidence of severe, life-threatening, or fatal (grades 3-5) chronic health conditions was 55% after BMT (Fig 1). The 10-year cumulative incidence was highest for SMNs (29.5%), followed by cataracts (15.6%), joint replacement (10.1%), venous thromboembolism (8.3%), and diabetes (6.8%; Fig 2). The most frequent SMN types included melanoma or squamous cell carcinoma of the skin (68.9%), breast cancer (6.7%), and colon cancer (1.8%). The latency for chronic health conditions varied, with longer latencies for SMN and cardiovascular diseases (Data Supplement). Compared with bone marrow recipients, PBSC or cord blood recipients were more likely to have a grade 3-5 chronic health condition, multiple conditions, cataracts, diabetes, or venous thromboembolism; individuals at high risk of relapse at BMT were also more likely to report cataracts (Data Supplement). However, these associations were no longer significant when the multivariable model was adjusted for treatment era (Data Supplement). Allogeneic BMT recipients were at increased risk of developing nearly all health outcomes, compared with autologous transplant recipients (Data Supplement), and this association remained significant for any grade 3-5 chronic health condition, multiple conditions, joint replacement, and cardiovascular disease, irrespective of adjustment for treatment era in the model (Data Supplement).
FIG 1.
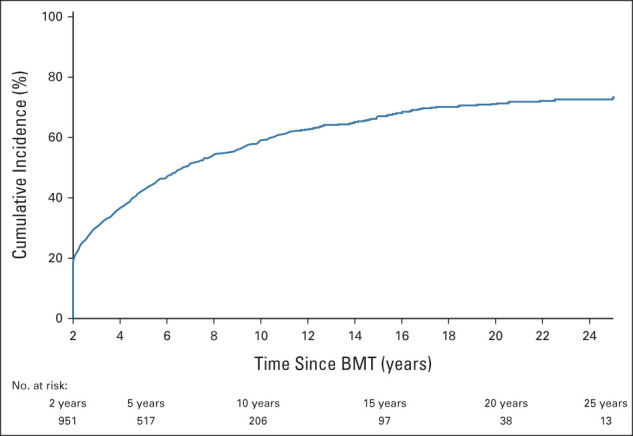
Cumulative incidence of grade 3-5 conditions among BMT survivors. BMT, blood or marrow transplantation.
FIG 2.
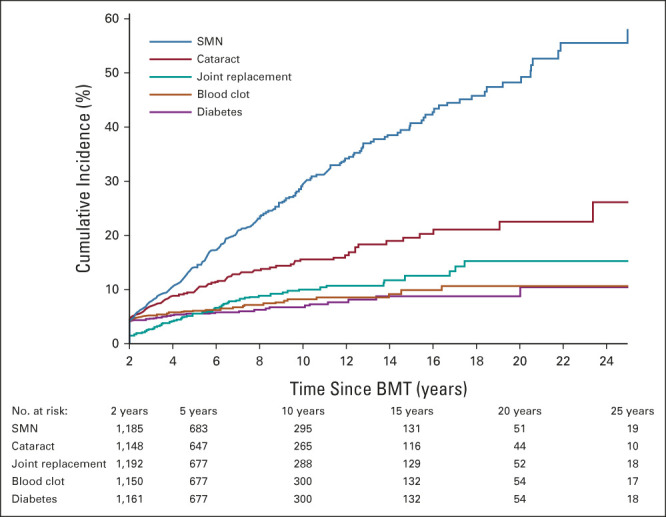
Cumulative incidence of select grade 3-5 conditions among BMT survivors. BMT, blood or marrow transplantation; SMN, subsequent malignant neoplasm.
Among allogeneic BMT recipients, the 10-year incidence of grade 3-5 conditions was significantly higher among those with a history of cGVHD (63.8% v 43.8%, P < .001, Data Supplement). Multivariable analyses revealed that history of cGVHD was associated with increased risk of grade 3-5 condition (HR, 1.3; 95% CI, 1.0 to 1.6), multiple grade 3-5 conditions (HR, 2.4; 95% CI, 1.6 to 3.5), pulmonary disease (HR, 3.1; 95% CI, 1.0 to 9.3), cataracts (HR, 2.6; 95% CI, 1.4 to 3.8), and venous thromboembolism (HR, 2.3; 95% CI, 1.3 to 4.7; Data Supplement). Patients with cGVHD were more likely to report poor general health (HR, 2.4; 95% CI, 1.5 to 4.1), activity limitation (HR, 2.5; 95% CI, 1.7 to 3.6), pain (OR, 2.0; 95% CI, 1.3 to 3.1), and anxiety or fears (OR, 1.7; 95% CI, 1.0 to 2.9), compared with those without cGVHD.
Late Mortality in BMT Recipients
Conditional on having surviving 2 years, the 5-year overall survival for this cohort was 76.4% (Fig 3). There was an improvement in 5-year survival rates conditional on having survived 5 years and 10 years (80.9% and 88.2%, respectively; Fig 3). As seen in Figure 4, the incidence of RRM plateaued at 30%, whereas the incidence of NRM continued to increase, approaching 50% at 30 years and crossing over the RRM rate at 12 years after BMT. Of note, the incidence of late relapse largely mirrored the incidence of RRM in this cohort (Data Supplement). Among 2-year survivors, the most common causes of death were primary disease (43.8%) and infection (21.3%), whereas the most common causes of death among 15-year and 20-year survivors were SMN (16.5% [15 years] and 21.4% [20 years]) and cardiovascular disease (16.5% [15 years] and 16.7% [20 years]).
FIG 3.
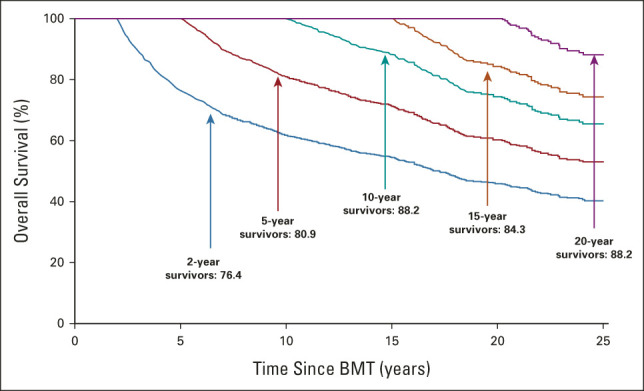
Five-year survival probability stratified on time survived since BMT (≥ 2, ≥ 5, ≥ 10, ≥ 15, and ≥ 20 years) among BMT survivors. BMT, blood or marrow transplantation.
FIG 4.
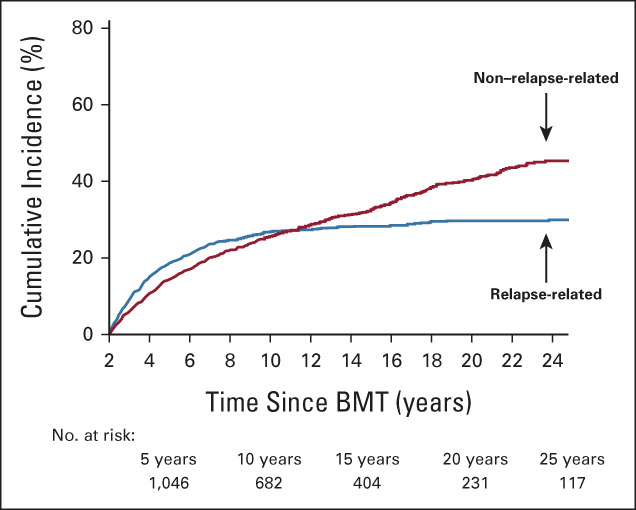
Cumulative incidence of cause-specific mortality among BMT survivors. BMT, blood or marrow transplantation.
DISCUSSION
Patients diagnosed with AML have benefited greatly from therapeutic advances over the past 4 decades, resulting in greater utilization of BMT and an overall improvement in survival rates.1,2 However, it is increasingly recognized that these treatment advances have come at a cost.5-9,25 We previously reported on the health outcomes of 401 survivors of acute lymphoblastic leukemia (n = 120) or myeloid leukemia (n = 281) who underwent BMT with mostly TBI-based myeloablative conditioning (acute lymphoblastic leukemia: 100%; AML: 86%) between 1974 and 1998 and a sibling comparison group.26 The current study builds on our previous report, and includes a larger (N = 1,369) and expanded cohort of patients with AML who underwent BMT during a more recent era (71% underwent BMT after 2000), with contemporary approaches (eg, non–TBI-based and/or nonmyeloablative conditioning, PBSC, or cord blood stem-cell source), were older at BMT, and had a longer follow-up after BMT.
This study represents the most comprehensive assessment of health outcomes in the largest cohort of long-term adult survivors of AML treated with BMT. The burden of chronic health conditions remained consistently greater in BMT survivors compared with siblings across nearly all outcomes, and this burden increased with longer follow-up after BMT. The magnitude of risk varied across health outcomes, ranging from 17.0-fold for SMN to 1.5-fold for joint replacement. Importantly, the latency for certain chronic health conditions such as SMN and cardiovascular disease was long (median 5 years after BMT [range, 2-30 years]), with no plateau in incidence over time, emphasizing the need for lifelong vigilance and for consideration of more aggressive screening strategies than what is currently recommended for this population.27 Nearly one in four BMT survivors rated their health as poor, and half reported significant activity limitations. Although health status was impaired across all domains, the magnitude of impairment was highest for activity limitation, and this was independent of the severity of the chronic health conditions. These findings highlight the increasingly recognized need for specialized, multidisciplinary, long-term follow-up care for AML survivors that incorporates physical function assessments in addition to surveillance for specific chronic health conditions.5
By 10 years after BMT, nearly 30% of survivors developed an SMN and 16% developed cataracts. By contrast, in a recent study of 1-year adolescent and young adult (15-39 years at diagnosis) survivors of AML, the 10-year incidence of SMN and cataracts was 4% and 10%, respectively.10 The difference in the incidence rates between the two studies may be attributable, in part, to the older demographic of our study population and differences in ascertainment of health outcomes. The findings from the current study may provide much-needed insight into the anticipated burden of morbidity in AML survivors, given the steady increase in average age at transplantation for AML over the past 4 decades.2,3,28
Allogeneic BMT recipients had a nearly two-fold higher risk of developing a grade 3-5 chronic health conditions, and a three-fold risk of developing multiple conditions, compared with autologous BMT recipients. However, use of autologous BMT, once considered a viable treatment strategy for AML, has steadily declined over time in favor of allogeneic BMT.1,3 Acknowledging this change in clinical practice, we performed subanalyses that were limited to survivors of allogeneic BMT, with a focus on cGVHD. cGVHD was associated with a 1.3-fold risk of having at least one grade 3-5 conditions and a 2.4-fold risk of having multiple such conditions, likely contributing to worse health status across nearly all domains examined. cGVHD was associated with increased risk of pulmonary disease, cataracts, and venous thromboembolism. These findings are in line with previous studies that have documented the association between cGVHD and/or its treatment and these health outcomes (ie, corticosteroid-associated cataracts,28,29 inflammation, and/or immobility leading to venous thromboembolism10,19,21), and underscore the importance of efforts to reduce the risk of GVHD at the time of BMT (eg, T-cell depletion and use of bone marrow stem-cell source) and continued efforts devoted toward developing treatments to reduce the severity of cGVHD after BMT.
The overall 5-year survival rate conditional on having survived 2 or more years was 76.4%, and there was a steady increase in survival, conditional on having lived at least 5 and 10 years. There was a modest decline in the 5-year survival rate conditional on surviving 15 years, largely because of the competing risk of NRM, including deaths due to SMN and cardiovascular disease. This is not surprising, given that cancer and cardiovascular diseases are leading causes of aging-related mortality in the United States. Nevertheless, studies examining cause-specific mortality in BMT survivors have shown that the relative risk of death due to these aging-related diseases remains greater than in the general population, even after adjusting for sociodemographic confounders.23,24,30 We also found a corresponding decline in RRM over time. Similar trends in cause-specific mortality with time from cancer diagnosis have been reported in childhood and adult cancer survivors and support the need to dynamically evaluate mortality risk after BMT.23,24,30-32
The findings from this study must be considered in the context of its limitations. We acknowledge differential participation rates by age, race/ethnicity, and certain transplant-related characteristics, which may have affected the generalizability of our findings. BMTSS relies on self-report for measuring chronic health conditions. Thus, this study summarizes the prevalence of and risk factors for chronic health conditions diagnosed by the health care system and communicated to cancer survivors and the noncancer comparison group. The reporting of health conditions is, therefore, a reflection of study participants' access to care, awareness among the health care providers of the risk of long-term complications, and communication of these outcomes to their patients. We have previously shown excellent correlation between medical records and self-reported outcomes, including cGVHD,20 and are confident that self-report can be used effectively to describe post-treatment complications that are diagnosed as part of routine health care delivery. Importantly, for the current study, we limited our outcomes of interest to those considered severe or life-threatening, which typically require medical or surgical intervention, thus minimizing the risk of recall bias.
In conclusion, this study provides a global assessment of the burden of morbidity in survivors of AML treated with BMT and finds that, compared with unaffected individuals, BMT survivors are at increased risk for severe/life-threatening health-related complications across nearly all domains. The overall burden of chronic conditions increases sharply with longer follow-up, contributing to a rising incidence of nonrelapse mortality. These findings may inform evidence-based health screening recommendations that account for the evolving pattern of morbidity and mortality over time for BMT survivors. Among allogeneic BMT recipients, those with a history of cGVHD were at highest risk of chronic health conditions and for reporting worse health status. These findings support a long-term need for multidisciplinary management of patients with a history of cGVHD, setting the stage for screening, early detection, and interventions to mitigate the risk of adverse health outcomes. The growing population of acute leukemia survivors treated with BMT (estimated > 40,000 in the United States alone by 2030)4 makes the development of such strategies imperative, to ensure these survivors live long and healthy lives well after their BMT.
Yanjun Chen
Employment: Edwards Lifesciences, Amgen
Wendy Landier
Research Funding: Merck Sharp & Dohme (Inst)
Daniel J. Weisdorf
Consulting or Advisory Role: Incyte, Fate Therapeutics
Research Funding: Incyte
Stephen J. Forman
Stock and Other Ownership Interests: MustangBio, Lixte Biotechnology
Consulting or Advisory Role: Alimera Sciences, Lixte Biotechnology, MustangBio
Research Funding: MustangBio
Patents, Royalties, Other Intellectual Property: MustangBio
Mukta Arora
Consulting or Advisory Role: Fate Therapeutics
Research Funding: Syndax (Inst), Kadmon (Inst), Pharmacyclics (Inst)
Smita Bhatia
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 3235
SUPPORT
Supported in part by grants from the National Cancer Institute (R01 CA078938, U01 CA213140), and the Leukemia Lymphoma Society (R6502-16).
AUTHOR CONTRIBUTIONS
Conception and design: Saro H. Armenian, Smita Bhatia
Financial support: Smita Bhatia
Administrative support: Jessica Wu, Elizabeth Schlichting, Stephen J. Forman, Smita Bhatia
Provision of study materials or patients: Saro H. Armenian, Elizabeth Schlichting, Ravi Bhatia, Smita Bhatia
Collection and assembly of data: Saro H. Armenian, Lindsay Hageman, Jessica Wu, Alysia Bosworth, Liton Francisco, Elizabeth Schlichting, Smita Bhatia
Data analysis and interpretation: Saro H. Armenian, Yanjun Chen, Jessica Wu, Wendy Landier, Ravi Bhatia, Donna Salzman, F. Lennie Wong, Daniel J. Weisdorf, Stephen J. Forman, Mukta Arora, Smita Bhatia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Burden of Long-Term Morbidity Borne by Survivors of Acute Myeloid Leukemia Treated With Blood or Marrow Transplantation: The Results of the BMT Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yanjun Chen
Employment: Edwards Lifesciences, Amgen
Wendy Landier
Research Funding: Merck Sharp & Dohme (Inst)
Daniel J. Weisdorf
Consulting or Advisory Role: Incyte, Fate Therapeutics
Research Funding: Incyte
Stephen J. Forman
Stock and Other Ownership Interests: MustangBio, Lixte Biotechnology
Consulting or Advisory Role: Alimera Sciences, Lixte Biotechnology, MustangBio
Research Funding: MustangBio
Patents, Royalties, Other Intellectual Property: MustangBio
Mukta Arora
Consulting or Advisory Role: Fate Therapeutics
Research Funding: Syndax (Inst), Kadmon (Inst), Pharmacyclics (Inst)
Smita Bhatia
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dholaria B, Savani BN, Hamilton BK, et al. : Hematopoietic cell transplantation in the treatment of newly diagnosed adult acute myeloid leukemia: An evidence-based review from the American Society of Transplantation and Cellular Therapy. Transplant Cell Ther 27:6-20, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Sekeres MA, Guyatt G, Abel G, et al. : American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv 4:3528-3549, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phelan R, Arora M, Chen M: Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. 2020. http://www.cibmtr.org
- 4.Majhail NS, Tao L, Bredeson C, et al. : Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant 19:1498-1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, Armenian SH, Landier W: How I monitor long-term and late effects after blood or marrow transplantation. Blood 130:1302-1314, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Arora M, Sun CL, Ness KK, et al. : Physiologic frailty in nonelderly hematopoietic cell transplantation patients: Results from the bone marrow transplant survivor study. JAMA Oncol 2:1277-1286, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun CL, Kersey JH, Francisco L, et al. : Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: Report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant 19:1073-1080, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Major CK, Kantarjian H, Sasaki K, et al. : Survivorship in AML—A landmark analysis on the outcomes of acute myelogenous leukemia patients after maintaining complete remission for at least 3 years. Leuk Lymphoma 61:3120-3127, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng MJ, Hourigan CS, Smith TJ: Adult acute myeloid leukemia long-term survivors. J Leuk (Los Angel) 2:26855, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CJ, Kim S, Tecca HR, et al. : Late effects after ablative allogeneic stem cell transplantation for adolescent and young adult acute myeloid leukemia. Blood Adv 4:983-992, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelmsson M, Glosli H, Ifversen M, et al. : Long-term health outcomes in survivors of childhood AML treated with allogeneic HSCT: A NOPHO-AML study. Bone Marrow Transplant 54:726-736, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Bernard F, Auquier P, Herrmann I, et al. : Health status of childhood leukemia survivors who received hematopoietic cell transplantation after BU or TBI: An LEA study. Bone Marrow Transplant 49:709-716, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Schultz KA, Chen L, Chen Z, et al. : Health conditions and quality of life in survivors of childhood acute myeloid leukemia comparing post remission chemotherapy to BMT: A report from the Children's Oncology Group. Pediatr Blood Cancer 61:729-736, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leunis A, Redekop WK, Uyl-de Groot CA, et al. : Impaired health-related quality of life in acute myeloid leukemia survivors: A single-center study. Eur J Haematol 93:198-206, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Efficace F, Breccia M, Avvisati G, et al. : Health-related quality of life, symptom burden, and comorbidity in long-term survivors of acute promyelocytic leukemia. Leukemia 33:1598-1607, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Duncan CN, Majhail NS, Brazauskas R, et al. : Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant 21:151-158, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia S, Dai C, Landier W, et al. : Trends in late mortality and life expectancy after allogeneic blood or marrow transplantation over 4 decades: A blood or marrow transplant survivor study report. JAMA Oncol 7:1626-1634, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armenian SH, Sun CL, Kawashima T, et al. : Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: A report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 118:1413-1420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser CJ, Bhatia S, Ness K, et al. : Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: A report from the Bone Marrow Transplant Survivor Study. Blood 108:2867-2873, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie AD, Robison LL, Bogue M, et al. : Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant 25:1191-1196, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Sun CL, Francisco L, Kawashima T, et al. : Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: A report from the Bone Marrow Transplant Survivor Study. Blood 116:3129-3139, 2010; quiz 3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray RJ: A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 23.Vanderwalde AM, Sun CL, Laddaran L, et al. : Conditional survival and cause-specific mortality after autologous hematopoietic cell transplantation for hematological malignancies. Leukemia 27:1139-1145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong FL, Teh JB, Atencio L, et al. : Conditional survival, cause-specific mortality, and risk factors of late mortality after allogeneic hematopoietic cell transplantation. J Natl Cancer Inst 112:1153-1161, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572-1582, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Baker KS, Ness KK, Weisdorf D, et al. : Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: A report from the Bone Marrow Transplant Survivor Study. Leukemia 24:2039-2047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majhail NS, Rizzo JD, Lee SJ, et al. : Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 18:348-371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathak M, Diep PP, Lai X, et al. : Ocular findings and ocular graft-versus-host disease after allogeneic stem cell transplantation without total body irradiation. Bone Marrow Transplant 53:863-872, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurney JG, Ness KK, Rosenthal J, et al. : Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood: Results from the Bone Marrow Transplant Survivor Study. Cancer 106:1402-1408, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Wingard JR, Majhail NS, Brazauskas R, et al. : Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29:2230-2239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong GT, Chen Y, Yasui Y, et al. : Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 374:833-842, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fidler-Benaoudia MM, Oeffinger KC, Yasui Y, et al. : A comparison of late mortality among survivors of childhood cancer in the United States and United Kingdom. J Natl Cancer Inst 113:562-571, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]