PURPOSE
To report pancreas surveillance outcomes of high-risk individuals within the multicenter Cancer of Pancreas Screening-5 (CAPS5) study and to update outcomes of patients enrolled in prior CAPS studies.
METHODS
Individuals recommended for pancreas surveillance were prospectively enrolled into one of eight CAPS5 study centers between 2014 and 2021. The primary end point was the stage distribution of pancreatic ductal adenocarcinoma (PDAC) detected (stage I v higher-stage). Overall survival was determined using the Kaplan-Meier method.
RESULTS
Of 1,461 high-risk individuals enrolled into CAPS5, 48.5% had a pathogenic variant in a PDAC-susceptibility gene. Ten patients were diagnosed with PDAC, one of whom was diagnosed with metastatic PDAC 4 years after dropping out of surveillance. Of the remaining nine, seven (77.8%) had a stage I PDAC (by surgical pathology) detected during surveillance; one had stage II, and one had stage III disease. Seven of these nine patients with PDAC were alive after a median follow-up of 2.6 years. Eight additional patients underwent surgical resection for worrisome lesions; three had high-grade and five had low-grade dysplasia in their resected specimens. In the entire CAPS cohort (CAPS1-5 studies, 1,731 patients), 26 PDAC cases have been diagnosed, 19 within surveillance, 57.9% of whom had stage I and 5.2% had stage IV disease. By contrast, six of the seven PDACs (85.7%) detected outside surveillance were stage IV. Five-year survival to date of the patients with a screen-detected PDAC is 73.3%, and median overall survival is 9.8 years, compared with 1.5 years for patients diagnosed with PDAC outside surveillance (hazard ratio [95% CI]; 0.13 [0.03 to 0.50], P = .003).
CONCLUSION
Most pancreatic cancers diagnosed within the CAPS high-risk cohort in the recent years have had stage I disease with long-term survival.
INTRODUCTION
Pancreatic cancer is a deadly disease that is projected to become the second most common cause of cancer death in the United States by 2026,1 with a similar trend of increasing worldwide incidence.2 Pancreatic cancer survival has improved modestly in recent years; overall 5-year survival now approaches 11%, the poor survival largely attributed to the late stage at diagnosis for most patients.3,4 In particular, very few patients are diagnosed with stage I pancreatic ductal adenocarcinoma (PDAC).3 However, the percentage of patients diagnosed with stage I PDAC has been increasing in the United States in the past decade,3 which may be due to a number of factors, including improvements in diagnostic imaging, better access to care leading to earlier diagnosis of symptomatic disease (a stage I PDAC diagnosis associated with having better insurance coverage),3 and the enrollment of more at-risk individuals (with familial/genetic risk and/or incidentally detected pancreatic cysts) into pancreatic surveillance programs.
CONTEXT
Key Objective
To evaluate the stage at diagnosis and outcome of individuals diagnosed with pancreatic cancer and high-grade dysplasia while undergoing recommended, typically annual, pancreas imaging surveillance with magnetic resonance imaging and endoscopic ultrasound for their familial/inherited risk in the multicenter Cancer of Pancreas Screening-5 (CAPS5) study and in the Johns Hopkins CAPS study, initiated 20+ years ago.
Knowledge Generated
The majority of patients diagnosed with pancreatic ductal adenocarcinoma while under pancreas surveillance have stage I disease and can achieve long-term survival; the median survival of patients diagnosed with pancreatic ductal adenocarcinoma while under surveillance in the CAPS program is 9.8 years. The predominance of stage I disease is in marked contrast to the advanced stage at presentation for the majority of patients who present with symptomatic pancreatic cancer.
Relevance
Regular pancreatic imaging surveillance should be offered to patients who meet recommended pancreatic surveillance criteria.
Pancreas surveillance has been recommended for individuals estimated to be at high risk of developing PDAC (5% or higher estimated lifetime risk).5-8 The risk increases with the number of affected first-degree relatives with pancreatic cancer and in those who carry a pathogenic germline variant in a pancreatic cancer susceptibility gene.9-11 The goals of pancreas surveillance are to reduce pancreatic cancer mortality through early detection, at a stage when the disease is most curable (stage I PDAC), or when there is only a noninvasive neoplasm with high-grade dysplasia (HGD), and to do so with minimal harm.6 In 2019, the US Preventative Services Task Force recommended against pancreas screening of asymptomatic average-risk adults and those with a family history, but it did not address the role of surveillance in germline mutation carriers.12,13 Recent evidence indicates that pancreas surveillance can downstage pancreatic cancers diagnosed in high-risk individuals (HRIs), although this is based upon only a few studies,12,14-16 with relatively few PDACs diagnosed, and only a small percentage of which were diagnosed with stage I disease.17
The goal of this study was to describe the outcomes to date of the multicenter Cancer of Pancreas Screening-5 (CAPS5) study, which opened in 2014, in particular with a focus on the number of stage I PDACs diagnosed, and to provide updated survival outcomes in the 20+-year CAPS (1998-2021) program overall.
METHODS
Study Design and Participants
CAPS5 (ClinicalTrials.gov identifier: NCT02000089) is a multicenter, prospective cohort study involving eight academic medical centers in the United States that opened to enrollment in 2014; we report on all HRIs enrolled until June 2021. CAPS5 was approved by the Johns Hopkins Institutional Review Board and the institutional review boards at each of the CAPS sites with written informed consent provided by all participants. CAPS5 enrolled patients recommended by the study clinicians to undergo pancreatic surveillance because of their estimated elevated risk of developing PDAC (Fig 1) as per the CAPS international consensus guidelines.5,6 A small percentage of patients (described in the Data Supplement [online only]), were enrolled based upon other risk criteria, such as BRCA2 or ATM mutation carriers without a family history,18 or those with other family history criteria (eg, having three second-degree relatives with pancreatic cancer, without an affected first-degree relative). CAPS participants were enrolled on the basis of recommended age criteria.5,6
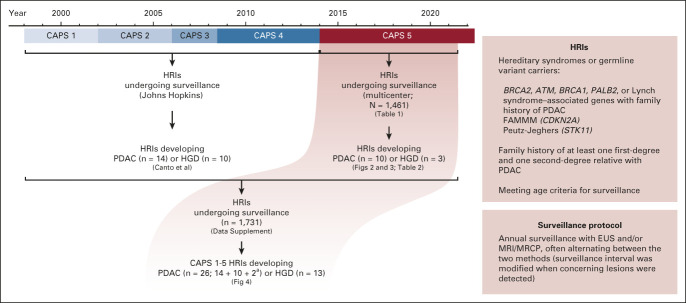
FIG 1.
Diagram of the CAPS1-5 enrollment periods from 1998 to 2021 summarizing the outcomes of individuals in the CAPS5 study and combined updated outcomes of the CAPS1-5 studies. A summary of the CAPS5 study criteria and surveillance protocol is provided in the boxes and described with more details in the methods section. aTwo HRIs from the CAPS1-4 cohort stopped surveillance and then developed PDAC after the last report of that cohort in the study by Canto et al.14 CAPS, Cancer of Pancreas Screening; EUS, endoscopic ultrasound; FAMMM, familial atypical multiple mole melanoma; HGD, high-grade dysplasia; HRI, high-risk individual; MRCP, magnetic resonance cholangiopancreatography; MRI, magnetic resonance imaging; PDAC, pancreatic ductal adenocarcinoma.
In this report, we also provide an updated analysis of the Johns Hopkins CAPS study single-center experience since 1998 (this CAPS1-4 study cohort was described previously14), combining it with the multicenter CAPS5 study cohort data (Fig 1). For patients enrolled in earlier CAPS studies, the follow-up time was calculated from the time of initial baseline screening until the date of last follow-up (see also the Data Supplement for additional methods).
Statistics
The primary outcome of this study was the early detection of stage I PDAC (PDACs were staged per the American Joint Committee on Cancer staging manual, eighth edition) or a noninvasive neoplasm with HGD among HRIs undergoing surveillance, which is reported descriptively with frequencies and percentages. Separate analyses are provided for the CAPS5 cohort and for those enrolled in all the CAPS programs (CAPS1-5). The latter analysis includes an update (since 2017) of the outcomes of patients who progressed to PDAC or a noninvasive neoplasm with HGD since the last CAPS report.14 The study's secondary outcome was overall survival after a diagnosis of PDAC or HGD for HRIs enrolled in all CAPS studies (CAPS1-5), which was estimated using the Kaplan-Meier method. Overall survival of individuals diagnosed with PDAC was compared between those in the CAPS program whose disease was or was not screen-detected (ie, diagnosed during routine surveillance as opposed to presenting with concerning symptoms outside of surveillance or between surveillance intervals) with Cox proportional hazards models. We report hazard ratios (HRs) both unadjusted and adjusted for age at diagnosis and whether patients were enrolled for family history or pathogenic variant in a PDAC susceptibility gene.
RESULTS
Study Population
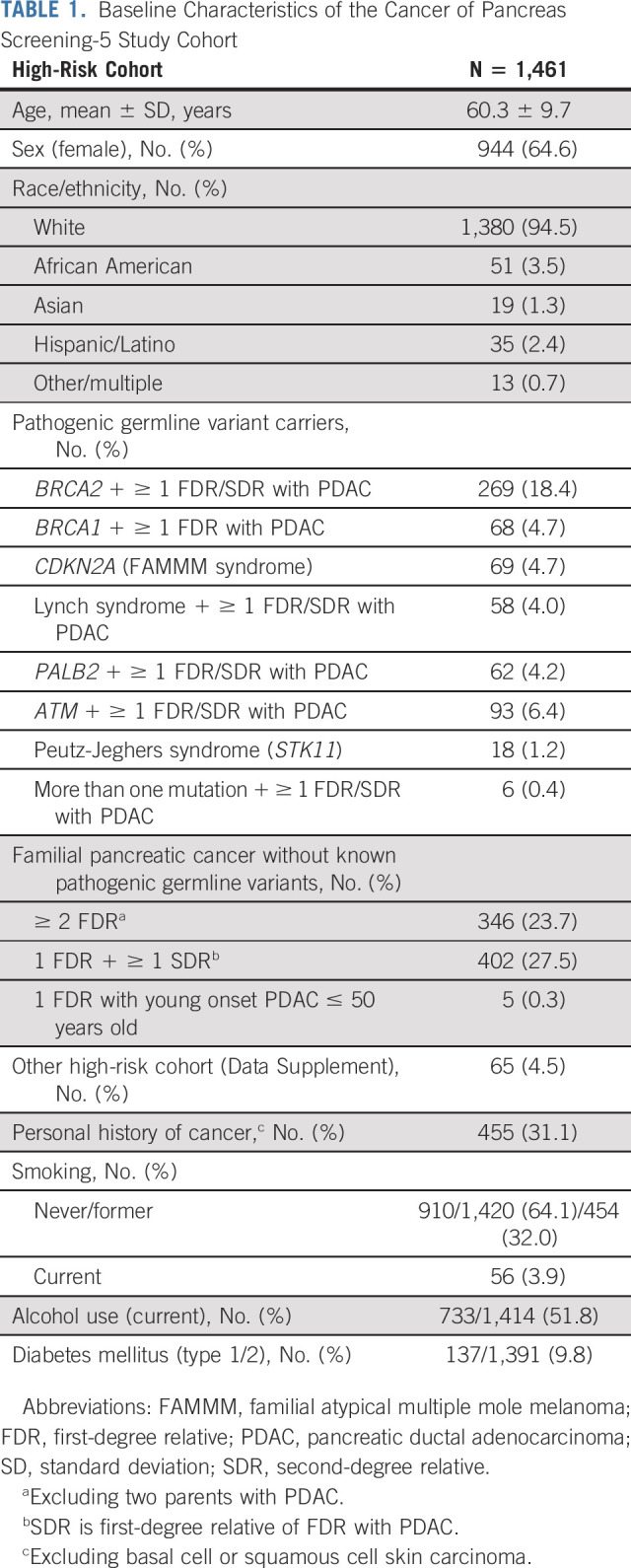
The baseline characteristics of the 1,461 subjects in the CAPS5 study cohort are presented in Table 1. 48.5% of participants had a pathogenic germline variant, including 18.4% with a BRCA2 and 6.4% with an ATM variant. Around one third of the cohort had a personal history of cancer, with breast cancer being the most commonly reported cancer (15.8% of the entire cohort). To date, 451 (30.9%) of the total CAPS5 cohort have undergone one baseline screening examination (due in part to COVID-19–related delays19). Of these 451 HRIs, 215 (47.7%) of the participants had their baseline screening visit less than a year from the date of data extraction, whereas the remaining 236 patients or 16.1% of the entire HRI cohort had a baseline screening visit (≥ 1 year from data extraction date) that has not yet been followed up with any subsequent surveillance. The remaining 1010 HRIs (69.1%) had a median follow-up duration of 4 years (interquartile range, 2.2-5.6 years) for a total of 4,462 person-years of surveillance.
TABLE 1.
Baseline Characteristics of the Cancer of Pancreas Screening-5 Study Cohort
Pancreatic Cancers and Other Resected Lesions
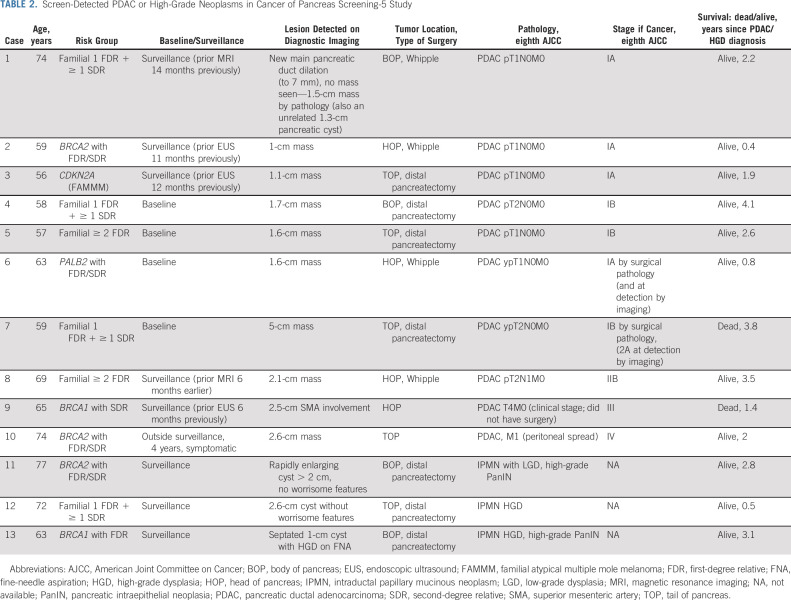
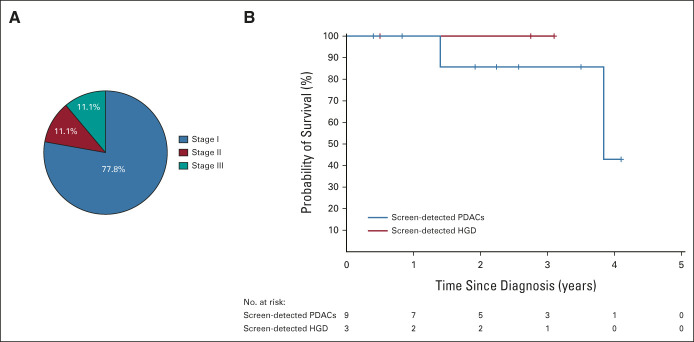
During the CAPS5 study surveillance period, nine patients were diagnosed with a screen-detected PDAC (either at baseline or at subsequent surveillance visits). One additional patient presented with symptomatic metastatic PDAC 4 years after their baseline and only surveillance (Table 2). The majority of pancreatic cancers detected during surveillance (7/9, 77.8%) were stage I by surgical pathology (n = 4 stage IA, n = 3 stage IB); one patient had stage IIB cancer (case 8), and one (case 9) had a stage III cancer (clinically staged) with superior mesenteric artery involvement (Fig 2A; case 9 did not undergo surgical resection). Overall, 8/9 (88.9%) of the screen-detected PDACs were resectable. Two of the stage I PDACs were surgically staged after neoadjuvant chemotherapy (their stages at diagnosis by imaging, before neoadjuvant therapy were stage IA and IIA, Table 2). The PDACs detected during surveillance were detected between 6 and 14 months after their previous imaging test (Table 2). Most of the PDACs were detected by imaging as small pancreatic masses; one case had concerning main pancreatic duct dilation without a detectable mass; none of the PDACs arose from an intraductal papillary mucinous neoplasm (IPMN). Comparing the proportion of stage I PDACs to the national average is highly significant; in 2016, 5.4% of 8,398 PDACs in the SEER database (National Cancer Institute-SEER) were stage I by surgical pathology (P < .00001, Fisher's exact test).3
TABLE 2.
Screen-Detected PDAC or High-Grade Neoplasms in Cancer of Pancreas Screening-5 Study
FIG 2.
PDAC stage and survival in the CAPS5 study cohort. (A) Distribution of stages (eighth edition American Joint Committee on Cancer) of screen-detected PDACs (n = 9) detected during surveillance. (B) Kaplan-Meier curve showing overall survival of all screen-detected PDACs and high-grade neoplasms in the CAPS5 study. CAPS, Cancer of Pancreas Screening; HGD, high-grade dysplasia; PDAC, pancreatic ductal adenocarcinoma.
Seven of the nine patients diagnosed with a screen-detected PDAC are alive, with median overall survival to date of 3.84 years. The Kaplan-Meier survival curves of the patients with PDAC and a noninvasive neoplasm with HGD are shown in Figure 2B. Seven patients are alive at last follow-up; one patient with stage IB PDAC (BRCA1) and another who had stage III PDAC (familial-risk) died after 3.84 and 1.4 years from diagnosis, respectively. One patient (stage IB) had a biopsy-proven lung recurrence 1.7 years after surgery, but is currently alive (4.1 years after surgery). The one patient (BRCA2) diagnosed with PDAC 4 years after dropping out of surveillance, who was also diagnosed with colorectal cancer around the same time, is still alive 2 years after diagnosis.
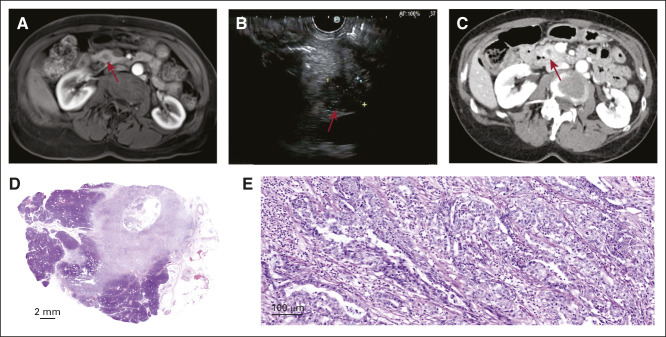
Figure 3 (case 2) shows an example of a stage IA PDAC detected by using magnetic resonance imaging (MRI) performed 1 year after a normal-appearing endoscopic ultrasound (EUS; Data Supplement). The Data Supplement shows another case of a stage IA PDAC detected by surveillance EUS as a 1.1-cm hypoechoic mass and confirmed with secretin-enhanced MRI/magnetic resonance cholangiopancreatography (case 3).
FIG 3.
Example of a screen-detected stage IA pancreatic cancer (case 2). (A) Surveillance magnetic resonance imaging showing a new 1-cm hypoenhancing lesion in the head of the pancreas (arrow pointing to mass). (B) Confirmatory EUS showing a 1.5-cm hypoechoic lesion in the head of the pancreas without invasion of nearby vessels with cytology (not shown) diagnostic of a moderately differentiated adenocarcinoma. (C) Confirmatory computed tomography of the abdomen showing s 1.5-cm pancreatic head mass without upstream dilation or atrophy. (D) Whole-slide scanned image of a resected 1.4-cm lesion showing at 5× (E) a moderately differentiated invasive ductal adenocarcinoma confined to the pancreas. EUS, endoscopic ultrasound.
In addition, eight patients had pancreatic resections for concerning cystic lesions detected during surveillance; three were found to have HGD (in high-grade pancreatic intraepithelial neoplasia or IPMN; summarized in Table 2). The remaining five patients who underwent surgical resection for worrisome lesions had only low-grade dysplasia (Data Supplement).20 There were no significant surgical complications.
Overall, 10 (56%) of the 18 patients identified with PDAC or who underwent surgical resection had pathologies that meet recommended goals of surveillance (seven stage I PDAC, and three with a precancerous neoplasm with HGD).5,6 During the CAPS5 study period, 73 patients under pancreas surveillance were diagnosed with other cancers, an incidence of 1.68 cancers per 100 patient-years of follow-up. The most common cancers diagnosed were breast (n = 17), prostate (n = 11), and melanoma (n = 7; Data Supplement).
CAPS1-5 Combined Cohort
The 1,731 HRIs (42.6% with germline mutations) enrolled into the CAPS1-5 studies to date have had a median follow-up of 2.8 years (interquartile range, 1.4-4.8 years) with a total of 5,041 person-years of surveillance (their baseline characteristics are described in the Data Supplement). Overall, 26 patients in the entire CAPS program have had a diagnosis of PDAC over the past 20+ years, corresponding to a detection rate (including baseline and surveillance) of 5.15 PDACs diagnosed per 1,000 person-years of surveillance; one individual diagnosed with PDAC per year for every 194 screened. Since the last CAPS report (which described outcomes of CAPS1-4 subjects up to 201714), two additional HRIs, one who had enrolled into the CAPS4 and one who had enrolled into CAPS3, developed PDAC outside of surveillance (both stage IV), at 4 and 7 years, respectively, after their last pancreas surveillance imaging.
Among the 26 patients with PDAC, 38.5% (10/26) had a known pathogenic germline variant (10 of the 16 without a known variant had undergone negative multigene panel testing). Since 2018, National Comprehensive Cancer Network guidelines have recommended individuals with PDAC and their first-degree relatives be offered susceptibility gene testing.21,22 Among the 26 PDACs, 19 were detected during surveillance, 57.9% of whom had stage I disease, 15.8% stage II, 21.1% stage III, and 5.2% stage IV (Fig 4A). In the CAPS1-5 cohort, six of the seven cases (85.7%) whose PDAC was detected outside surveillance were stage IV (Fig 4B). The median (standard deviation/range) age at diagnosis for all PDAC cases was 65.5 (9.5/45-81) years; for those with stage I PDAC, it was 65.3 (7.8/57-77) years; for those with stage IV, it was 69.1 (8.9/59-81) years (P = .37). Regarding management of IPMN, 10 patients had resections of IPMN with HGD, but none of the resected PDACs were associated with an IPMN by surgical pathology.
FIG 4.
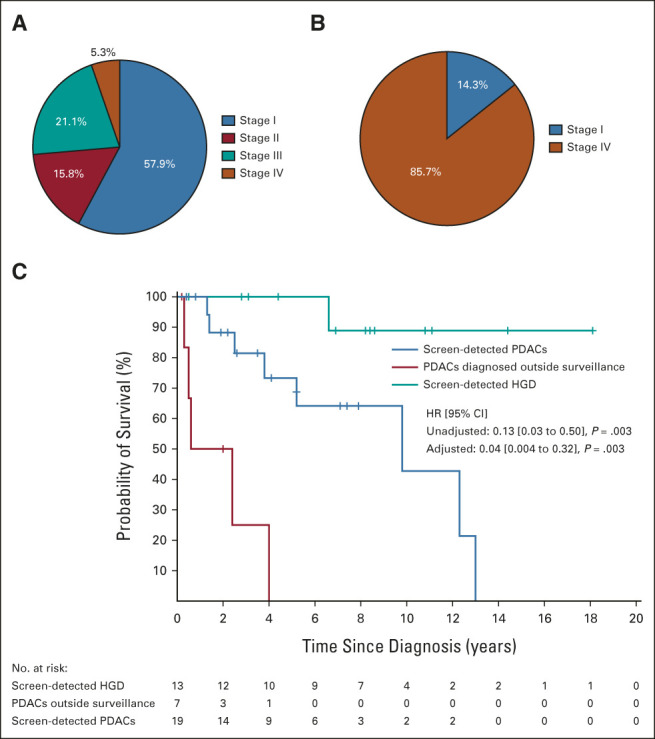
Screen-detected pancreatic cancers in the combined Cancer of Pancreas Screening 1-5 cohorts. (A) Graph showing eighth edition American Joint Committee on Cancer stage distribution of the screen-detected PDACs (n = 19) and (B) PDACs detected outside surveillance (n = 7). (C) Kaplan-Meier curves showing survival of all screen-detected PDACs, PDACs diagnosed outside surveillance, and screen-detected HGD. HGD, high-grade dysplasia; HR, hazard ratio; PDAC, pancreatic ductal adenocarcinoma.
Individuals in the CAPS1-5 cohort with screen-detected PDAC had a significantly longer survival than those whose PDACs were diagnosed outside surveillance (HR unadjusted: 0.13 [95% CI, 0.03 to 0.50], P = .003). The median survival for the patients with a screen-detected PDAC was 9.8 years (95% CI, 5.2 to ∼ years, ∼; small sample size precludes upper survival limit estimate), compared with 1.5 years (0.50 to ∼ years) for patients whose PDACs were diagnosed outside surveillance (Fig 4C), and the 5-year survival to date of the CAPS screen-detected PDAC cases is 73.3% (54 to 100).
Adjusting for factors besides stage that could affect survival, specifically, age at PDAC diagnosis and risk group (pathogenic variant carriers v familial risk), yielded a slightly larger effect size (HR 0.04 [95% CI, 0.004 to 0.32], P = .003). Overall, of the 13 patients with HGD in IPMN or pancreatic intraepithelial neoplasias who underwent resection, 12 (92%) were alive at last follow-up; one patient died 6.5 years after pancreatic resection of end-stage renal disease (after a kidney transplant for polycystic kidney disease).
DISCUSSION
Among HRIs undergoing surveillance in the CAPS5 study, PDAC was diagnosed in one of every 160 person-years and there was a predominance of stage I PDACs among screen-detected PDACs diagnosed, with 77.8% of pancreatic cancers staged at surgical pathology as stage I, 88.9% with resectable disease, and a median survival of 9.8 years. These results compare favorably with the stage at diagnosis of usual PDAC cases diagnosed outside surveillance (> 50% metastatic disease, < 20% localized and resectable, and < 5% surgically staged as stage I [SEER3,23]), but are consistent with the long-term survival among PDAC cases in the SEER database with stage I disease by surgical pathology.3
The CAPS5 study results differ in some respects from the recent report of the Dutch Familial Pancreatic Cancer Surveillance Study Group.16 This study reported a PDAC resectability rate of only 60%, which may be a function of the predominance of CDKN2A mutation carriers in their high-risk cohort, which have more aggressive disease than other hereditary syndromes and comprised 70% of their patients with an incident pancreatic cancer.24 As a result of this experience, consideration is being given to having CDKN2A carriers undergo more frequent surveillance (such as biannually). A 2016 study by Vasen et al reported stage I PDACs comprised six of the 15 (40%) PDACs detected during surveillance. Although the follow-up period of the screen-detected PDACs diagnosed in the multicenter CAPS5 study cohort to date is relatively short, there is evidence of improved survival, especially when compared with the 11% 5-year overall survival of patients with PDAC in the United States.4
The survival benefit associated with pancreas surveillance is clearly evident in the entire CAPS cohort (CAPS1-5; Fig 4) with long-term follow-up; the 5-year overall survival among screen-detected PDACs was 73.3% in this updated analysis. The patients who progressed to PDAC in our study included carriers with germline pathogenic variants and those who met familial-risk criteria only. This observation is consistent with previous reports that the PDAC risk in the familial group appears significant enough to warrant surveillance.9,10,14 Our results support current CAPS surveillance recommendations and argue against the notion of limiting pancreatic surveillance to those high-risk individuals with known pathogenic mutations.16,25,26 One guideline that deserves re-evaluation and further study in light of the benefits of pancreas surveillance is the recommendation that a family history of PDAC be required for BRCA2 and ATM mutation carriers.27 This recommendation, part of an effort to enroll only highest-risk individuals, was developed in the early years of pancreas surveillance in an effort to optimize outcomes. Further evidence of the value of regular surveillance and appropriate surgical intervention can be inferred from the proportion of cases of IPMN with HGD without an IPMN-associated invasive ductal adenocarcinoma (n = 10), to the number with an IPMN-associated PDAC (n = 0). This proportion is in marked contrast to what is typically observed in surgical series, where IPMN with HGD is commonly associated with an invasive PDAC.28
There were five pancreatic resections performed during the study period for worrisome-type findings (dilated main pancreatic duct and mass-like lesions), which turned out to harbor low-grade dysplasia only. These cases are discussed at a multidisciplinary conference, and the decision to resect is based on clinical judgment in accordance with expert consensus.6,7 By consensus, these patients would likely have been better off not having surgical resection, but it is notable that these five cases represent a smaller proportion of resections for low-grade lesions than has been reported in prior studies14,15,29,30 and likely results from better selection of patients for surgery. The cases that had resections for low-grade dysplasia highlight the limitations of current EUS and MRI imaging to characterize concerning lesions and the need to improve our diagnostic imaging tools to minimize having progress to advanced-stage PDAC, for example, with artificial intelligence–based methods,31,32 the use of accurate circulating blood-based biomarkers (circulating tumor DNA for multicancer detection,33 genotype-stratified cancer antigen 19-934,35), or other approaches.36-38 A blood test for pancreatic cancer early detection would be attractive, but such a test should approach or complement the diagnostic performance of EUS/MRI for stage I PDAC. And the low incidence of PDAC, even in a high-risk cohort such as CAPS, necessitates using sensitive, high-specificity (> 98%) tests, as even modest reductions in diagnostic test specificity yield increasing numbers of false-positive tests, especially when multiple testing occurs over many years.
The improvement in surveillance outcomes reported here compared with previous reports is likely the combined effect of multiple factors, including our case mix (CDKN2A carriers appear to have more aggressive disease), the diagnostic performance of our study team, and the cumulative experience gained from undertaking pancreatic screening studies over more than 20 years. Pancreas surveillance of HRIs and the management of screen-detected lesions is best done at expert centers by multidisciplinary teams. At the same time, the low diagnostic yield of PDACs in our cohort highlights the need to develop more refined estimates of pancreatic cancer risk. Few studies have evaluated the cost-effectiveness of pancreas surveillance.39,40 Better estimates of risk would help determine who should undergo surveillance, and at what age surveillance should begin. Commencing surveillance closer to the age range when most PDACs are diagnosed is one potential way diagnostic yield and cost-effectiveness could be improved.
Some limitations to our study include our relatively short follow-up of the CAPS5 study cohort included in this study; however, we do have long-term follow-up of cases from our earlier CAPS studies. Long-term survival outcomes are needed to minimize confounding from lead-time bias. Comparing outcomes of those diagnosed with a screen-detected PDAC to those whose PDAC was diagnosed after dropping out of surveillance is worthwhile because these groups have similar demographic/risk characteristics, and for whom data on pancreatic cancer outcomes is limited,41-43 but the number of such cases was small. Few PDACs have been diagnosed-to-date in the CAPS program; a larger cohort with longer follow-up would generate better precision estimates for the proportion of stage I PDACs diagnosable with surveillance.
In conclusion, pancreatic surveillance of HRIs can dramatically downstage PDACs diagnosed; most of the patients with PDACs diagnosed in the multicenter CAPS5 study to date had stage I disease. The long-term survival among patients in the CAPS cohort is excellent (currently 73.3% 5-year overall survival). Longer follow-up is needed to better define the benefits of EUS and MRI-based surveillance in high-risk cohorts, along with long-term studies designed to evaluate the role of emerging biomarker tests.
ACKNOWLEDGMENT
The authors wish to thank all patients for their participation in the CAPS studies. In addition, the authors thank all study coordinators who have assisted in patient enrollment and data collection at their respective centers: Alyson Caruso, Barbara Clerkin, Christine DeCapite, Eve Karloski, Ildiko Fulip, Jordan Heiman, Chinedu Iheyinwa Ukaegbu, Lisa Vasquez, Marietta Kocher, Nancy Furey, Sara Booz, Sarah Volk, Scott Merenda, and Hilary Cosby, who is also the lead study coordinator of the multicenter CAPS5 study.
See accompanying editorial on page 3230
SUPPORT
Supported by NIH Grant Nos. (CA210170; M.G., CA176828; M.G., CA62924; A.P.K., P30CA013696; A.K.R.). This work was also supported by the Pancreatic Cancer Action Network (M.G.), the V Foundation (M.G.), Susan Wojcicki and Dennis Troper (M.G.), the Lustgarten Foundation (M.G.), Smith Family Research Fund (B.W.K.), the Bowen-Chapman Fund (S.S.), and by a Stand Up To Cancer-Lustgarten Foundation Pancreatic Cancer Interception Translational Cancer Research Grant (Grant No.: SU2C-AACR-DT25-17; M.G.). Stand Up To Cancer is a program of the Entertainment Industry Foundation. SU2C research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. M.G. is the Sol Goldman Professor of Pancreatic Cancer Research. The funders had no role in study design or implement or in manuscript preparation.
AUTHOR CONTRIBUTIONS
Conception and design: Mohamad Dbouk, Sapna Syngal, Anil K. Rustgi, Ihab Kamel, Ralph H. Hruban, Marcia Irene Canto, Michael Goggins
Financial support: Sapna Syngal, Marcia Irene Canto, Michael Goggins
Administrative support: Michael Goggins
Provision of study materials or patients: Bryson W. Katona, Amitabh Chak, Sapna Syngal, James J. Farrell, Fay Kastrinos, Elena M. Stoffel, Anil K. Rustgi, Beth Dudley, Ankit Chhoda, Gregory G. Ginsberg, Alison P. Klein, Jin He, Eun Ji Shin, Anne Marie Lennon, Marcia Irene Canto, Michael Goggins
Collection and assembly of data: Mohamad Dbouk, Bryson W. Katona, Randall E. Brand, Amitabh Chak, Sapna Syngal, James J. Farrell, Fay Kastrinos, Elena M. Stoffel, Anil K. Rustgi, Beth Dudley, Linda S. Lee, Gregory G. Ginsberg, Alison P. Klein, Ihab Kamel, Jin He, Eun Ji Shin, Marcia Irene Canto, Michael Goggins
Data analysis and interpretation: Mohamad Dbouk, Bryson W. Katona, Amitabh Chak, Sapna Syngal, James J. Farrell, Elena M. Stoffel, Amanda L. Blackford, Anil K. Rustgi, Ankit Chhoda, Richard Kwon, Alison P. Klein, Ihab Kamel, Anne Marie Lennon, Marcia Irene Canto, Michael Goggins
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The Multicenter Cancer of Pancreas Screening Study: Impact on Stage and Survival
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Bryson W. Katona
Other Relationship: Janssen, Immunovia, Epigenomics, Guardant, Freenome, Universal diagnostics
Uncompensated Relationships: Invitae, Ambry, GeneDx
Randall E. Brand
Consulting or Advisory Role: Immunovia, Freenome (Inst)
Research Funding: Immunovia (Inst), Freenome (Inst)
Amitabh Chak
Stock and Other Ownership Interests: Lucid Diagnostics
Honoraria: CDx Diagnostics
Consulting or Advisory Role: US Endoscopy, Microtek
Research Funding: STERIS
Patents, Royalties, Other Intellectual Property: Methods and compositions for detecting gastrointestinal and other cancers US 8415100 B2. Sanford D. Markowitz, Joseph Willis, Amitabh Chak, Rom Leidner Pending: 2012272697 (Australia) EP 12802150.8 (Europe) 2,840,324 (Canada) Device for Collecting a Biological Sample: Amitabh Chak, Rebecca Blice, Sanford D. Markowitz, Dean Secrest, Dennis Siedlak, Jeffrey Taggart, Joseph E. Willis Pending: PCT/US14/070060 Methylated Markers of Esophageal Neoplasia. Amitabh Chak, Omar de la Cruz, Thomas LaFramboise, Sanford D. Markowitz, Helen Moinova, Joseph E. Willis Pending: 62/099,021 (Inst)
Sapna Syngal
Patents, Royalties, Other Intellectual Property: Dana-Farber Cancer Institute has a registered service mark for the PREMM5 model and holds copyrights for the PREMM questionnaires (Inst); Myriad Genetics (through Dana-Farber Cancer Institute) paid an inventor share of the IP (license issue fee)
James J. Farrell
Honoraria: Immunovia
Consulting or Advisory Role: Cook Medical
Research Funding: Immunovia
Fay Kastrinos
Consulting or Advisory Role: Ambry Genetics/Konica Minolta, Immunovia, Iterative Scopes
Research Funding: Immunovia, Janssen
Elena M. Stoffel
Research Funding: Cancer Prevention Pharmaceuticals (Inst)
Linda S. Lee
Consulting or Advisory Role: Boston Scientific, Fujifilm
Research Funding: Fujifilm
Gregory G. Ginsberg
Consulting or Advisory Role: Olympus Medical Systems
Research Funding: Fractyl
Expert Testimony: Cook Medical
Alison P. Klein
Consulting or Advisory Role: OptumInsight, Merck
Research Funding: OptumLabs
Ihab Kamel
Research Funding: Siemens Healthineers
Ralph H. Hruban
Research Funding: Applied Materials Inc
Patents, Royalties, Other Intellectual Property: Dr Hruban has the potential to receive royalty payments from Thrive Earlier Detection Corp for the TERT in bladder cancers and GNAS inventions in an arrangement reviewed and approved by the Johns Hopkins University
Eun Ji Shin
Consulting or Advisory Role: Boston Scientific
Anne Marie Lennon
Patents, Royalties, Other Intellectual Property: Patent for CancerSEEK
Marcia Irene Canto
Consulting or Advisory Role: Castle Biosciences, Bluestar Genomics
Research Funding: Pentax Medical Corporation (Inst), Endogastric Solutions (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from UpToDate, online
Michael Goggins
Patents, Royalties, Other Intellectual Property: Royalty related to licensing as a codiscoverer of PALB2 as a pancreatic cancer susceptibility gene to Myriad Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rahib L, Wehner MR, Matrisian LM, et al. : Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open 4:e214708, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Lok V, Ngai CH, et al. : Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology 160:744-754, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Blackford AL, Canto MI, Klein AP, et al. : Recent trends in the incidence and survival of stage 1A pancreatic cancer: A Surveillance, Epidemiology, and End Results analysis. J Natl Cancer Inst 112:1162-1169, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA Cancer J Clin 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 5.Canto MI, Harinck F, Hruban RH, et al. : International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 62:339-347, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goggins M, Overbeek KA, Brand R, et al. : Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) consortium. Gut 69:7-17, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslanian HR, Lee JH, Canto MI: AGA clinical practice update on pancreas cancer screening in high-risk individuals: Expert review. Gastroenterology 159:358-362, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Syngal S, Brand RE, Church JM, et al. : ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110:223-262, 2015; quiz 263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe T, Blackford AL, Tamura K, et al. : Deleterious germline mutations are a risk factor for neoplastic progression among high-risk individuals undergoing pancreatic surveillance. J Clin Oncol 37:1070-1080, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein AP, Brune KA, Petersen GM, et al. : Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 64:2634-2638, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Hu C, Hart SN, Polley EC, et al. : Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 319:2401-2409, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrikson NB, Aiello Bowles EJ, Blasi PR, et al. : Screening for pancreatic cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 322:445-454, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Lucas AL, Kastrinos F: Screening for pancreatic cancer. JAMA 322:407-408, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Canto MI, Almario JA, Schulick RD, et al. : Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 155:740-751.e2, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corral JE, Mareth KF, Riegert-Johnson DL, et al. : Diagnostic yield from screening asymptomatic individuals at high risk for pancreatic cancer: A meta-analysis of cohort studies. Clin Gastroenterol Hepatol 17:41-53, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Overbeek KA, Levink IJM, Koopmann BDM, et al. : Long-term yield of pancreatic cancer surveillance in high-risk individuals. Gut 71:1152-1160, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overbeek K, Goggins MG, Dbouk M, et al. : Timeline of development of pancreatic cancer and implications for successful early detection in high-risk individuals. Gastroenterology 162:772-785.e4, 2022 [DOI] [PubMed] [Google Scholar]
- 18.Katona BW, Long JM, Ahmad NA, et al. : EUS-based pancreatic cancer surveillance in BRCA1/BRCA2/PALB2/ATM carriers without a family history of pancreatic cancer. Cancer Prev Res (Phila) 14:1033-1040, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katona B, Mahmud N, Dbouk M, et al. : COVID-19 related pancreatic cancer surveillance disruptions amongst high-risk individuals. Pancreatology 21:1048-1051, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brune K, Abe T, Canto M, et al. : Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol 30:1067-1076, 2006 [PMC free article] [PubMed] [Google Scholar]
- 21.Daly MB, Pal T, Berry MP, et al. : Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:77-102, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Tempero MA, Malafa MP, Al-Hawary M, et al. : Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:439-457, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Khalaf N, El-Serag HB, Abrams HR, et al. : Burden of pancreatic cancer: From epidemiology to practice. Clin Gastroenterol Hepatol 19:876-884, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasen HF, Wasser M, van Mil A, et al. : Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 140:850-856, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Vasen H, Ibrahim I, Ponce CG, et al. : Benefit of surveillance for pancreatic cancer in high-risk individuals: Outcome of long-term prospective follow-up studies from three European expert centers. J Clin Oncol 34:2010-2019, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Potjer TP: Pancreatic cancer surveillance and its ongoing challenges: Is it time to refine our eligibility criteria? Gut 71:1047-1049, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Calderwood AH, Sawhney MS, Thosani NC, et al. : American Society for Gastrointestinal Endoscopy guideline on screening for pancreatic cancer in individuals with genetic susceptibility: Methodology and review of evidence. Gastrointest Endosc 95:827-854.e3, 2022 [DOI] [PubMed] [Google Scholar]
- 28.Khoury RE, Kabir C, Maker VK, et al. : What is the incidence of malignancy in resected intraductal papillary mucinous neoplasms? An analysis of over 100 US institutions in a single year. Ann Surg Oncol 25:1746-1751, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Dbouk M, Brewer Gutierrez OI, Lennon AM, et al. : Guidelines on management of pancreatic cysts detected in high-risk individuals: An evaluation of the 2017 Fukuoka guidelines and the 2020 International Cancer of the Pancreas Screening (CAPS) consortium statements. Pancreatology 21:613-621, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Canto MI, Kerdsirichairat T, Yeo CJ, et al. : Surgical outcomes after pancreatic resection of screening-detected lesions in individuals at high risk for developing pancreatic cancer. J Gastrointest Surg 115:1-9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenner B, Chari ST, Kelsen D, et al. : Artificial intelligence and early detection of pancreatic cancer: 2020 summative review. Pancreas 50:251-279, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisberg EM, Chu LC, Park S, et al. : Deep lessons learned: Radiology, oncology, pathology, and computer science experts unite around artificial intelligence to strive for earlier pancreatic cancer diagnosis. Diagn Interv Imaging 101:111-115, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Lennon AM, Buchanan AH, Kinde I, et al. : Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 369:eabb9601, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe T, Koi C, Kohi S, et al. : Gene variants that affect levels of circulating tumor markers increase identification of patients with pancreatic cancer. Clin Gastroenterol Hepatol 18:1161-1169.e5, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka H, Tamura K, Abe T, et al. : Serum carboxypeptidase activity and genotype-stratified CA19-9 to detect early-stage pancreatic cancer. Clin Gastroenterol Hepatol 10.1016/j.cgh.2021.10.008 [epub ahead of print on October 11, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levink IJM, Nesteruk K, Visser DI, et al. : Optimization of pancreatic juice collection: A first step toward biomarker discovery and early detection of pancreatic cancer. Am J Gastroenterol 115:2103-2108, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Suenaga M, Yu J, Shindo K, et al. : Pancreatic juice mutation concentrations can help predict the grade of dysplasia in patients undergoing pancreatic surveillance. Clin Cancer Res 24:2963-2974, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu J, Sadakari Y, Shindo K, et al. : Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut 66:1677-1687, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Saumoy M, Oh A, et al. : Threshold analysis of the cost-effectiveness of endoscopic ultrasound in patients at high risk for pancreatic ductal adenocarcinoma. Pancreas 50:807-814, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corral JE, Das A, Bruno MJ, et al. : Cost-effectiveness of pancreatic cancer surveillance in high-risk individuals: An economic analysis. Pancreas 48:526-536, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Momtaz P, O'Connor CA, Chou JF, et al. : Pancreas cancer and BRCA: A critical subset of patients with improving therapeutic outcomes. Cancer 127:4393-4402, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji J, Forsti A, Sundquist J, et al. : Survival in familial pancreatic cancer. Pancreatology 8:252-256, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Yurgelun MB, Chittenden AB, Morales-Oyarvide V, et al. : Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med 21:213-223, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]