IMPORTANCE:
Although venovenous extracorporeal membrane oxygenation (VV ECMO) has been used in case of COVID-19 induced acute respiratory distress syndrome (ARDS), outcomes and criteria for its application should be evaluated.
OBJECTIVES:
To describe patient characteristics and outcomes in patients receiving VV ECMO due to COVID-19–induced ARDS and to assess the possible impact of COVID-19 on mortality.
DESIGN, SETTING AND PARTICIPANTS:
Multicenter retrospective study in 15 ICUs worldwide. All adult patients (> 18 yr) were included if they received VV ECMO with ARDS as main indication. Two groups were created: a COVID-19 cohort from March 2020 to December 2020 and a “control” non-COVID ARDS cohort from January 2018 to July 2019.
MAIN OUTCOMES AND MEASURES:
Collected data consisted of patient demographics, baseline variables, ECMO characteristics, and patient outcomes. The primary outcome was 60-day mortality. Secondary outcomes included patient characteristics, COVID-19–related therapies before and during ECMO and complication rate. To assess the influence of COVID-19 on mortality, inverse probability weighted (IPW) analyses were used to correct for predefined confounding variables.
RESULTS:
A total of 193 patients with COVID-19 received VV ECMO. The main indication for VV ECMO consisted of refractory hypoxemia, either isolated or combined with refractory hypercapnia. Complications with the highest occurrence rate included hemorrhage, an additional infectious event or acute kidney injury. Mortality was 35% and 45% at 28 and 60 days, respectively. Those mortality rates did not differ between the first and second waves of COVID-19 in 2020. Furthermore, 60-day mortality was equal between patients with COVID-19 and non-COVID-19–associated ARDS receiving VV ECMO (hazard ratio 60-d mortality, 1.27; 95% CI, 0.82–1.98; p = 0.30).
CONCLUSIONS AND RELEVANCE:
Mortality for patients with COVID-19 who received VV ECMO was similar to that reported in other COVID-19 cohorts, although no differences were found between the first and second waves regarding mortality. In addition, after IPW, mortality was independent of the etiology of ARDS.
Keywords: acute respiratory distress syndrome, COVID-19, extracorporeal membrane oxygenation, mortality, venovenous extracorporeal membrane oxygenation
KEY POINTS
Questions: To describe patient characteristics and outcomes in VV ECMO for COVID-19 associated ARDS in 2020, and to evaluate whether COVID-19 influences mortality in patients receiving VV ECMO for ARDS.
Findings: In this retrospective observational study, 60-day mortality in patients receiving VV ECMO for COVID-19 ARDS was 45%. Moreover, 60-day mortality was equal between patients with COVID-19 and non-COVID-19 associated ARDS receiving VV ECMO.
Meaning: Mortality for patients with COVID-19 who received VV ECMO was similar to that reported in other COVID-19 cohorts, although no differences were found between the first and second wave regarding mortality neither for ARDS etiology.
The past decades, acute respiratory distress syndrome (ARDS) has been one of the first and primary indications for venovenous extracorporeal membrane oxygenation (VV ECMO) (1). By taking over oxygenation and carbon dioxide clearance in the extracorporeal circuit, VV ECMO functions as a supportive method in reversible pulmonary failure, refractory to other conventional therapies. VV ECMO can be beneficial by reducing the intensity of ventilatory support and thus the occurrence of ventilator-induced lung injury (1, 2). Post hoc Bayesian analyses of the ECMO to Rescue Lung Injury in Severe ARDS trial, which focused on the efficacy of VV ECMO in severe ARDS, suggested a high probability of mortality benefit of ECMO in severe ARDS, resulting in a further increased use of VV ECMO (3–5).
However, since the rise of COVID-19, the discussion on the role of ECMO in severe respiratory failure was reignited (6, 7). Despite the alarming initial experiences from China in COVID-19 patients receiving ECMO, this was followed by larger cohorts from Europe and the Extracorporeal Life Support Organization (ELSO) showing better survival rates of up to 60% (8, 9). In early 2022, over 10,000 patients had received ECMO for COVID-19–related indications and even dedicated guidelines exist for ECMO in COVID-19 (10, 11). However, some aspects still have to be taken into account. First, it has been widely described that substantial heterogeneity exists in ARDS, resulting in different optimal treatment per phenotype (12). These differences may even be more important in the critically ill receiving VV ECMO. Although previous studies did not find differences in survival between patients receiving ECMO for COVID and non-COVID-ARDS, those studies are often small and correction for confounders is limited (13–15). Second, a change in patient outcomes over time in 2020 was found by different study groups, showing an increase in mortality in the second wave (16, 17). This raises the question which criteria should be used for VV ECMO support in patients with COVID-19 ARDS.
The aim of this study is two-fold: first, to describe patient characteristics and outcomes in VV ECMO for COVID-19–associated ARDS in 2020; and second, to evaluate whether COVID-19 influences mortality in patients receiving VV ECMO for ARDS. We hypothesized that there is no significant difference in mortality in patients receiving VV ECMO for either COVID-19– or non-COVID-19–associated ARDS.
MATERIALS AND METHODS
Population
This international retrospective observational study was performed in 15 ICUs in Belgium, Germany, The Netherlands, Spain, and Sweden. The study was approved by the institutional review board of the Amsterdam University Medical Centers, location Academic Medical Centers W20_199#20.230, and, if indicated by national law, thereafter by local committees. This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was registered at the Netherlands Trial Registry on September 6, 2020 (NL8706).
This retrospective study consisted of two cohorts: a COVID-19 cohort and a non-COVID-19 cohort. Patients were included if they were 18 years old or older and received VV ECMO during ICU admission. In the COVID-19 cohort, patients were included if they were admitted to one of the participating ICUs between March 2020 and December 2020 and had a polymerase chain reaction-proven severe acute respiratory syndrome coronavirus 2 infection. The non-COVID-19 cohort was selected from a larger database, which contains data from ECMO patients between January 2018 and July 2019. Patients receiving other modes, including extracorporeal carbon dioxide removal, venoarterial ECMO, and veno-venoarterial ECMO, were excluded. In the non-COVID-cohort, additional exclusion criteria were: 1) if the main indication for ECMO was different than ARDS and 2) if the patient was admitted to other centers than available in the COVID-19 cohort to prevent between-center variance in protocols to influence patient outcomes.
Data Collection
In both cohorts, data were derived retrospectively from electronic patient files and entered in the electronic Case Registry Form. Data consisted of patient demographics (e.g., age, body mass index, medical history including cardiovascular and pulmonary diseases), baseline variables (e.g., Sequential Organ Failure Assessment score, 0–24 points and worst Pao2/Fio2 ratio at the day of ECMO initiation), and patient outcomes (successful weaning, survival rate, and complication rate). Also, ECMO-related variables such as date of initiation, day of decannulation, and the need for a second run were collected.
In the COVID-19 cohort, additional data collection was performed on before, during, and after ECMO variables. Before variables included worst arterial blood gas and mechanical ventilation (MV) parameters within 6 hours prior to ECMO initiation, rescue maneuvers applied (i.e., prone positioning, neuromuscular blockers), and the presence of complications (pulmonary embolism, venothrombotic event, acute kidney injury [AKI]) prior to ECMO initiation. During ECMO, variables included the application of rescue maneuvers to improve respiratory support and COVID-19 drugs (i.e., corticosteroids, complement inhibitors, antiviral agents) during ECMO. All definitions can be found in the supplementary materials (Appendix I, http://links.lww.com/CCX/B65). The observational study STrengthening the Reporting of OBservational studies in Epidemiology reporting guidelines were followed in the process of this article.
Outcome (Definition)
The primary outcome was 60-day mortality after initiation of ECMO. Secondary outcomes included patient characteristics, COVID-19–related therapies before and during ECMO, and complication rate.
Statistical Analysis
All analyses were performed using R statistics in the R Studio interface (Version 4.0.3 RStudio Team, Boston, MA) (18). Normality of data was determined using histograms and Q-Q plots. Normally distributed continuous variables were presented as mean (sd); non-normally distributed continuous variables as median (1st quartile–3rd quartile), and categorical variables as frequency and percentage. As a secondary analysis, all outcomes in the first and second waves were compared using chi-square or Mann-Whitney U tests in the COVID-19 cohort. The first wave was defined as ICU admission between March 1, 2020, and June 1, 2020; the second wave between June 1, 2020, and December 31, 2020. No correction for multiplicity took place; therefore, those results should be interpreted as hypothesis-generating. Survival in total, the first and second waves was depicted using Kaplan-Meier curves using the survival package.
Percentages of missing data per variable were determined using the mice package (19); all variables with over 25% missing data were deleted. In both cohorts, separately and combined, overall percentage of missing data was calculated and used to determine the number of multiple imputations applied in numerical variables, with a minimum of 5. Thus, as the overall percentage of missing data within all variables was less than 5%, still five imputed datasets were created. Primary and secondary outcome data regarding survival and complication rates were not imputed. An overview of missing data per variable can be found in Appendix II (http://links.lww.com/CCX/B65).
To compare 60-day mortality in the COVID-19 and non-COVID-19 cohort, inverse probability weighting (IPW) was used to correct for confounding variables. Confounding variables were identified based on the directed acyclic graph that was created prior to data analysis (Appendix III, http://links.lww.com/CCX/B65) using the R package “daggity” (20). A propensity score for the presence of COVID-19 was calculated based on the identified confounder variables. Inverse weighting was applied on this propensity score, thereby creating a pseudo-population in which the weighted averages reflect averages in the true population. By taking the mean IPW of all five imputed datasets, IPW was stabilized. Cases were excluded for further analysis if their inverse propensity score was greater than 10 and would therefore have a disproportionate influence on the results: one case was deleted. To evaluate covariate balance, and thus whether in this pseudo-population confounding was successfully removed, standardized mean differences (SMDs) of confounding variables were calculated prior and after weighting; a SMD of 0–0.1 is considered in balance (Appendix IV, http://links.lww.com/CCX/B65) (21). To assess the primary outcome, a chi-square test and Cox regression were used in the weight-adjusted and -unadjusted dataset. Last, a sensitivity analysis was performed using all confounding variables as independent variables in a multivariable logistic regression (Appendix V, http://links.lww.com/CCX/B65). In all analyses, a p value of less than 0.05 was considered significant.
RESULTS
COVID-19: Patient and ECMO Characteristics
Of the total of 222 patients, 29 were excluded for further analysis: 17 received ECMO outside of the predefined timeframe and 12 received other modes, resulting in 193 patients receiving ECMO due to COVID-19 in 2020 (Appendix VI, http://links.lww.com/CCX/B65). A complete overview of patient demographics can be found in Table 1. The majority of the patients were male (n = 150, 78%) and overweight (body mass index, 29.4 kg/m2; interquartile range [IQR], 26.3–32.2 kg/m2); 110 patients (57%) suffered from one or more comorbidities, mainly hypertension (n = 68, 35%) and diabetes (n = 49, 25%). Over two thirds were transferred from another referring hospital (n = 139, 72%). Median Pao2/Fio2 ratio prior to ECMO initiation was 69 mm Hg (55–94 mm Hg), and the majority was ventilated using controlled MV modes. Patients were admitted to a hospital 7 days (5–9 d) after their first symptoms, followed by ICU admission after 1 day (0–4 d; Table 2). ECMO was initiated at 7 days (5–14 d) after ICU admission. Indications for ECMO consisted mainly of refractory hypoxemia, followed by refractory hypoxemia combined with refractory hypercapnia. The duration of ECMO support was 15 days (9–24 d); only eight patients (4%) received a second run.
TABLE 1.
Patient Demographics
| Variable | COVID-19, n = 193 | First Wave, n = 97 | Second wave, n = 96 | p |
|---|---|---|---|---|
| Patient demographics | ||||
| Age, yr | 53 (48–60) | 53 (47–58) | 54 (48–62) | 0.14 |
| Body mass index, kg/m2 | 29.4 (26.3–32.2) | 28.7 (26.1–31.8) | 30.1 (27.2–33.8) | 0.04 |
| Male gender | 150 (78%) | 73 (75%) | 77 (80%) | 0.51 |
| Medical history | ||||
| Hypertension | 68 (35%) | 30 (31%) | 38 (40%) | 1.00 |
| Myocardial infarction | 11 (6%) | 2 (2%) | 9 (9%) | 0.13 |
| Diabetes mellitus | 49 (25%) | 13 (13%) | 36 (38%) | 0.001 |
| Asthma | 16 (8%) | 9 (9%) | 7 (7%) | 0.74 |
| Chronic obstructive pulmonary disease | 11 (6%) | 7 (7%) | 4 (4%) | 1.00 |
| Pulmonary hypertension | 3 (2%) | 3 (3%) | 0 (0%) | 0.42 |
| Chronic kidney failure | 10 (5%) | 5 (5%) | 5 (5%) | 1.00 |
| Liver cirrhosis | 3 (2%) | 1 (1%) | 2 (2%) | 0.99 |
| Malignancy | 4 (2%) | 2 (2%) | 2 (2%) | 1.00 |
| Immunocompromised state | 12 (6%) | 8 (8%) | 4 (4%) | 0.37 |
| Route of admission | 0.65 | |||
| Admission from emergency department | 24 (12%) | 10 (10%) | 14 (15%) | |
| Admission from general ward | 30 (16%) | 16 (17%) | 14 (15%) | |
| Admission from referring hospital | 139 (72%) | 71 (73%) | 68 (71%) | |
| Values prior to extracorporeal membrane oxygenation | ||||
| Lactate, mmol/L | 1.6 (1.2–2.5) | 1.6 (1.2–2.5) | 1.5 (1.2–2.6) | 0.71 |
| C-reactive protein, mg/L | 221 (97–313) | 265 (146–350) | 175 (77–267) | 0.001 |
| Sequential Organ Failure Assessment score | 9 (8–12) | 9 (7–11) | 10 (8–12) | 0.049 |
| Pao2/Fio2 ratio, mm Hg | 69 (55–94) | 64 (53–100) | 73 (58–93) | 0.45 |
| Ventilatory parameters | ||||
| Ventilatory mode | 0.08 | |||
| Pressure support ventilation | 14 (8%) | 4 (4%) | 10 (11%) | |
| Volume—CMV | 77 (42%) | 33 (36%) | 44 (48%) | |
| Pressure—CMV | 86 (47%) | 52 (56%) | 34 (37%) | |
| Volume—SIMV | 1 (1%) | 1 (1%) | 0 (0%) | |
| Pressure—SIMV | 1 (1%) | 0 (0%) | 1 (1%) | |
| Other | 6 (3%) | 3 (3%) | 3 (3%) | |
| Fio2, % | 100 (75–100) | 100 (70–100) | 100 (80–100) | 0.54 |
| Peak pressure, cm H2O | 32 (28–38) | 32 (30–38) | 30 (28–37) | 0.08 |
| Positive end-expiratory pressure, cm H2O | 12 (± 4) | 13 (± 4) | 12 (± 4) | 0.08 |
| Prone positioning prior | 157 (83%) | 81 (84%) | 76 (83%) | 1.00 |
| Neuromuscular blockersprior | 141 (78%) | 72 (78%) | 69 (77%) | 0.94 |
CMV = continuous mandatory ventilation, SIMV = synchronized intermittent mandatory ventilation.
Variables are stated as n (%) for categorical variables, mean ± sd for parametric, and median (1st quartile–3rd quartile) for nonparametric numeric data.
First wave is defined as ICU admission from March 1, 2020, to June 1, 2020; second wave is defined as ICU admission from June 1, 2020, to December 31, 2020.
All significant (p < 0.05) values are given in bold.
TABLE 2.
ECMO Characteristics and Patient
| Outcomes | COVID-19 Population, n = 193 | First Wave, n = 97 | Second Wave, n = 96 | p |
|---|---|---|---|---|
| Duration hospitalization—start ECMO, d | 10.5 (6–18) | 9 (6–15) | 13 (8–20) | 0.04 |
| Duration ICU admission—start ECMO, d | 7 (5–14) | 7 (5–12) | 9 (5–15) | 0.33 |
| Duration onset symptoms—start ECMO, d | 18 (14–26) | 17 (13–24) | 19 (14–26) | 0.44 |
| Transferred on ECMO to participating center | 63 (33%) | 32 (33%) | 31 (32%) | 1.00 |
| ECMO duration, d | 15 (9–24) | 14 (8–20) | 18 (10–30) | < 0.01 |
| Second run | 8 (4%) | 4 (4%) | 4 (4%) | 1.00 |
| Indication for ECMO | 0.49 | |||
| Refractory hypoxemia | 95 (49%) | 48 (50%) | 47 (49%) | |
| Refractory hypercapnia | 10 (5%) | 4 (4%) | 6 (6%) | |
| Combined hypercapnia and hypoxemia | 84 (44%) | 43 (44%) | 41 (43%) | |
| Other, i.e., pulmonary embolism | 4 (2%) | 2 (2%) | 2 (2%) | |
| Complications prior | ||||
| Pulmonary embolism | 18 (10%) | 6 (6%) | 12 (13%) | 0.21 |
| Venothrombotic event | 9 (5%) | 4 (4%) | 5 (5%) | 1.00 |
| Acute kidney injury | 34 (18%) | 19 (20%) | 15 (16%) | 0.57 |
| Renal replacement therapy | 18 (10%) | 11 (12%) | 7 (7%) | 0.46 |
| Complications during ECMO | ||||
| Hemorrhagic complication | 107 (56%) | 48 (50%) | 59 (62%) | 0.15 |
| Cannula-related | 23 (12%) | 11 (11%) | 12 (13%) | 0.98 |
| Hemorrhagic stroke | 17 (9%) | 8 (8%) | 9 (9%) | 0.98 |
| Gastrointestinal bleed | 16 (8%) | 5 (5%) | 11 (12%) | 0.19 |
| Arterial thrombotic complication | 6 (3%) | 4 (4%) | 2 (2%) | 0.68 |
| Venous thrombotic complication | 20 (10%) | 11 (12%) | 9 (9%) | 0.81 |
| Lower extremity | 6 (3%) | 3 (3%) | 3 (3%) | 1.00 |
| Upper extremity | 9 (5%) | 6 (6%) | 3 (3%) | 0.51 |
| Mechanical thrombotic complication | 26 (14%) | 10 (10%) | 16 (17%) | 0.29 |
| Cannula-related | 5 (3%) | 0 (0%) | 5 (5%) | 0.07 |
| Oxygenator | 24 (12%) | 10 (10%) | 14 (15%) | 0.50 |
| Pump | 2 (1%) | 0 (0%) | 2 (2%) | 0.47 |
| Pulmonary embolism | 13 (7%) | 6 (6%) | 7 (7%) | 1.00 |
| Superinfection | 114 (59%) | 57 (59%) | 57 (59%) | 1.00 |
| Acute kidney injury | 104 (54%) | 56 (58%) | 48 (50%) | 0.35 |
| Renal replacement therapy | 97 (50%) | 55 (57%) | 42 (44%) | 0.10 |
| Survival | ||||
| 28-d mortality | 67 (35%) | 38 (39%) | 29 (30%) | 0.25 |
| 60-d mortality | 87 (45%) | 43 (44%) | 44 (46%) | 0.95 |
ECMO = extracorporeal membrane oxygenation.
Variables are stated as n (%) for categorical variables, mean ± sd for parametric, and median (1st quartile–third quartile) for nonparametric numeric data.
First wave is defined as ICU admission from March 1, 2020, to June 1, 2020; second wave is defined as ICU admission from June 1, 2020, to December 31, 2020.
All significant (p < 0.05) values are given in bold.
COVID-19: Therapies
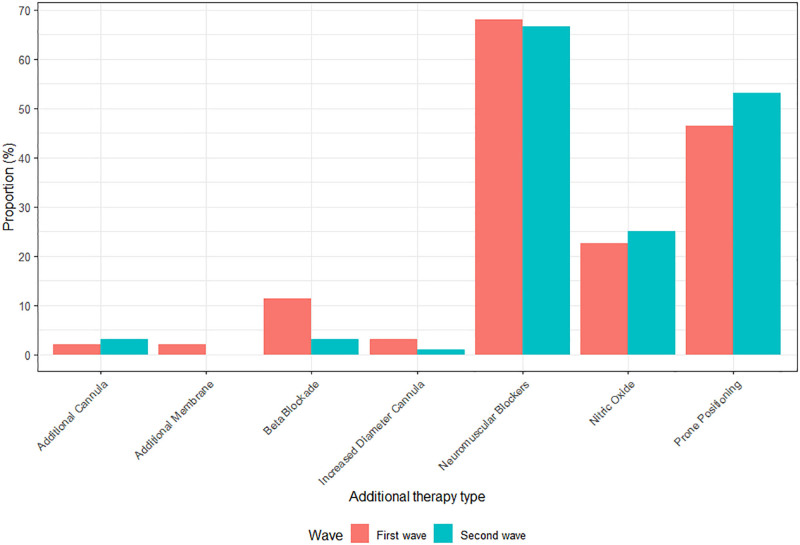
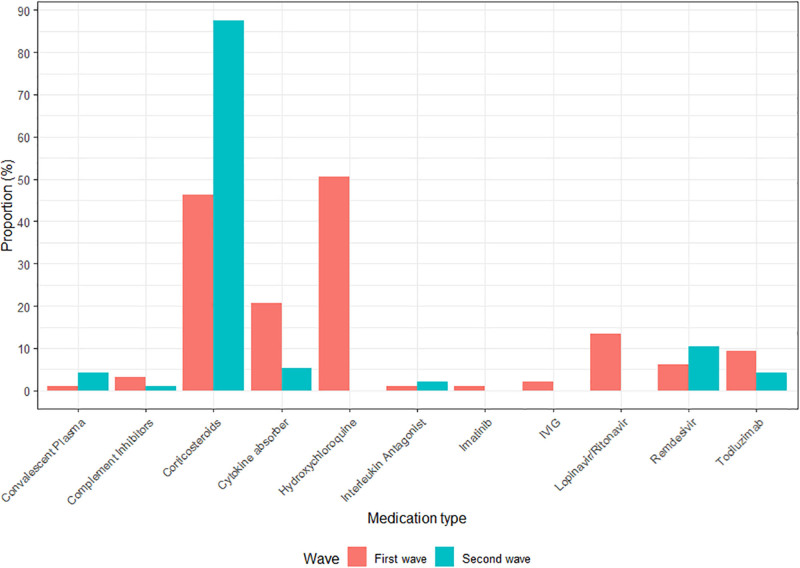
All COVID-19–related therapies during ECMO are shown as frequencies in Figures 1 and 2, and absolute count in Appendix VII (http://links.lww.com/CCX/B65). During ECMO, 96 patients (50%) received prone positioning: most of them had also received prone positioning before ECMO initiation (86/92, four missing). The same accounted for neuromuscular blockers, in which 111 out of 130 patients received neuromuscular blockers both before ECMO and during ECMO. Mostly applied COVID-19–related drugs consisted of corticosteroids, hydroxychloroquine, and cytokine absorber (n = 129, n = 49, and n = 25, respectively).
Figure 1.
Additional therapies: oxygenation-improving maneuvers during extracorporeal membrane oxygenation (ECMO). Red first wave (ICU admission from March 1, 2020, to June 1, 2020), blue second wave in 2020 (ICU admission from June 1, 2020, to December 31, 2020).
Figure 2.
Additional therapies: medication during extracorporeal membrane oxygenation (ECMO). Red first wave (ICU admission from March 1, 2020, to June 1, 2020), blue second wave in 2020 (ICU admission from June 1, 2020, to December 31, 2020). IVIG = IV immunoglobulin therapy.
COVID-19: Complications and Mortality
Overall, 75% of all patients (n = 145) suffered from one or more complications, mostly hemorrhage (n = 107, 56%), an additional infectious event (n = 114, 59%), and AKI (n = 104, 54%), as shown in Table 2. At 28 days after ECMO initiation, 35% of all patients had died, of which almost nine out of 10 died from reaching a state of irreversible respiratory failure during ECMO resulting in palliation (n = 59). The mortality rate further increased to 45% at day 60 after ECMO initiation.
COVID-19: First and Second Waves
Compared with the first wave, VV ECMO rates were similar in the second wave (96 vs 97 patients in total). Differences in patient demographics between the waves can be found in Tables 1 and 2. ECMO duration was longer in the second wave (first wave 14 d [IQR, 8–20 d] vs second wave 18 d [10–30 d]), as stated in Table 2. Although there were no differences in additional therapies during ECMO such as prone positioning and neuromuscular blockers (Fig. 1), differences were found in COVID-19–related drugs. Figure 2 shows that opposite to the first wave, when hydroxychloroquine and lopinavir/ritonavir were used in 51% and 13%, respectively, no patients received those drugs in the second wave (p < 0.001 and p = 0.001). On the other hand, the use of corticosteroids drastically increased in the second wave from 46% to 88% (p < 0.001; Appendix VII, http://links.lww.com/CCX/B65). Last, no differences were found in complication rates, nor in 28- or 60-day mortality between the first and second waves.
COVID-19 Versus Non-COVID-19: Complications and Mortality
A total of 116 patients received VV ECMO for non-COVID-19–associated ARDS. Mortality at 28- and 60-day between COVID-19 and non-COVID-19 patients was not significantly different (COVID-19 vs non-COVID-19: 28-d mortality [35% vs 34%; p = 0.25] and 60-d mortality [47% vs 41%; p = 0.37]; Appendix VIII, http://links.lww.com/CCX/B65). After IPW, covariate balance was found for all confounders but institute (Appendix IX, http://links.lww.com/CCX/B65). Patient demographics in the pseudo-population resulting from IPW can be found in Table 3. Both 28- and 60-day mortality remained nonsignificant between the groups. The weighted hazard ratio for 60-day survival was 1.27 (95% CI, 0.82–1.98; p = 0.30) comparing COVID-19– with non-COVID-19–associated ARDS. No differences were found in complication rate with the exception of a new infectious event, which had a higher occurrence in COVID-19–associated ARDS (COVID-19 vs non-COVID-19: n = 47.4 [42.8%] vs n = 108.7 [57.6%]; p = 0.05).
TABLE 3.
COVID and Non-COVID Acute Respiratory Distress Syndrome After Inverse Probability Weighting
| Variable | Overall, n =299.47 | Non-COVID, n = 110.75 | COVID, n = 188.72 | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr | 53 (47–60) | 55 (47–61) | 52 (47–60) | 0.52 |
| Body mass index, kg/m2 | 29.0 (25.8–32.7) | 27.8 (24.7–35.8) | 29.4 (26.5–32.0) | 0.82 |
| Male gender, n (%) | 219.2 (73.2%) | 82.2 (74.3%) | 137 (72.6%) | 0.78 |
| Medical history | ||||
| Mean comorbidity count | 0.74 (0.94) | 0.76 (0.87) | 0.73 (0.98) | 0.82 |
| Hypertension | 97.2 (32.4%) | 37.8 (34.1%) | 59.4 (31.5%) | 0.73 |
| Myocardial infarction | 19.9 (6.6%) | 8.3 (7.5%) | 11.6 (6.1%) | 0.70 |
| Diabetes mellitus | 60.8 (20.3%) | 24.3 (22.0%) | 36.5 (19.4%) | 0.75 |
| Chronic obstructive pulmonary disease | 18.5 (6.2%) | 7.5 (6.8%) | 11.0 (5.8%) | 0.75 |
| Pulmonary hypertension | 3.8 (1.3%) | 1.3 (1.1%) | 2.6 (1.4%) | 0.84 |
| Chronic kidney disease | 15.3 (5.1%) | 5.7 (5.1%) | 9.6 (5.1%) | 0.99 |
| Liver cirrhosis | 4.7 (1.6%) | 1.7 (1.6%) | 3.0 (1.6%) | 0.99 |
| Malignancy | 14.1 (4.7%) | 10.0 (9.0%) | 4.2 (2.2%) | 0.01 |
| Values prior to ECMO | ||||
| Lactate, mmol/L | 1.7 (1.2–2.8) | 1.9 (1.3–3.7) | 1.6 (1.2–2.3) | 0.06 |
| Sequential Organ Failure Assessment | 10.1 (± 3.5) | 11.4 (± 3.9) | 9.4 (±3.0) | < 0.01 |
| Pao2/Fio2 ratio, mm Hg | 67 (52–94) | 60 (40–98) | 68 (55–93) | 0.17 |
| ECMO characteristics | ||||
| Duration ICU admission—start ECMO, d | 4.74 (0.90–9.77) | 0.29 (0.01–2.79) | 6.87 (3.88–12.02) | < 0.001 |
| ECMO duration, d | 13.7 (8.0–22.0) | 13.0 (6.8–21.1) | 13.7 (8.6–22.6) | 0.30 |
| Second run | 12.2 (4.1%) | 5.3 (4.8%) | 7.0 (3.7%) | 0.63 |
| Complications | ||||
| Complication count | 0.61 | |||
| 0 | 52.0 (17.4%) | 18.0 (16.2%) | 34.0 (18.0%) | |
| 1 to 2 | 220.4 (73.6%) | 80.3 (72.4%) | 140.1 (74.3%) | |
| 3 or more | 27.0 (9.0%) | 12.5 (11.3%) | 14.6 (7.7%) | |
| Hemorrhagic event | 165.3 (55.4%) | 60.5 (54.6%) | 104.9 (55.8%) | 0.87 |
| Mechanical thrombotic event | 43.4 (14.5%) | 19.9 (17.9%) | 23.5 (12.5%) | 0.27 |
| Venous thrombotic event | 26.6 (8.9%) | 7.2 (6.5%) | 19.4 (10.3%) | 0.26 |
| Acute kidney injury | 155.3 (51.8%) | 59.0 (53.3%) | 96.3 (51.0%) | 0.77 |
| Infectious event | 156.2 (52.1%) | 47.4 (42.8%) | 108.7 (57.6%) | 0.05 |
| Renal replacement therapy | 151.1 (50.5%) | 61.5 (55.5%) | 89.6 (47.5%) | 0.31 |
| Outcomes | ||||
| Successful weaning | 171.4 (57.5%) | 71.3 (64.7%) | 100.1 (53.2%) | 0.13 |
| 28-d mortality | 104.67 (35.0%) | 33.75 (30.4%) | 73.52 (39.0%) | 0.37 |
| 60-d mortality | 127.78 (42.7%) | 41.7 (37.6%) | 91.3 (48.4%) | 0.33 |
| Weighted pooled hazard ratio (60-d mortality) | 1.27 (95% CI, 0.82–1.98) | 0.30 | ||
ECMO = extracorporeal membrane oxygenation.
Variables are stated as n (%) for categorical variables, mean ± sd for parametric, and median (1st quartile–third quartile) for nonparametric numeric data.
All significant (p < 0.05) values are given in bold.
DISCUSSION
This multicenter international study evaluated patient outcomes of a large patient cohort with COVID-19 ARDS receiving VV ECMO and demonstrated the following: first, mortality at 28 and 60 days was 35% and 45%, respectively. Second, these mortality rates did not differ between the first and second waves in 2020. Third, no differences were found in day 28 and day 60 mortality between COVID-19 and non-COVID-19 ARDS patients receiving VV ECMO, neither after correction for confounders using IPW.
The mortality rates of our cohort are in line with previously published data in patients receiving VV ECMO for COVID-19 ARDS (22, 23). After initial improved survival few months into the pandemic (8, 9, 15), survival seemed to decrease both in Europe as worldwide in the second half of 2020 (17, 24). Possible causes described included center experience, patient selection, treatment, and the final disposition of patients (24). We found equal survival in the first and second waves at both 28 and 60 days. This discrepancy may be explained by the following reasons. First, it has been demonstrated that both early application of ECMO throughout the pandemic as center experience influences mortality (24). In our study, all centers have extensive experience with ECMO and have been continuing its use throughout the pandemic. Second, concerning patient selection, we did not find differences in patient and ECMO characteristics, such as longer duration between ICU admission and initiation of ECMO. On the contrary, previous studies that do show a higher mortality in the second wave also find a longer ICU admission—ECMO initiation interval in the second wave as a possible explanation (16, 17). This could imply that no large changes in patient selection have been applied in our cohort during the year. Third, different definitions have been used in both survival status and cutoff dates for the waves. However, when changing the wave cutoff date to either May 1, 2020 (Barbaro et al [24]) or June 30, 2020 (Riera et al [17]), 28-day and 60-day mortality between the waves remained the same in our cohort. Last, our complication rate remained unchanged, on the contrary to previous studies describing an increased occurrence rate of, that is, new-onset pneumonia and vascular thrombosis.
Since the start of the pandemic, many studies have been performed regarding optimal care to patients with COVID-19, including different maneuvers and drugs. We found a high application rate of both neuromuscular blockers and prone positioning before and during ECMO. This is in line with previous studies and follows current ELSO recommendations (11, 22). Concerning drugs, an increase in the use of corticosteroids, and full decrease of the use of hydroxychloroquine took place between the first and second waves. This can be explained by the publication of several studies reporting their value in the treatment of COVID-19 (25, 26). Last, we found a lower C-reactive protein value in the second wave compared with the first wave, possibly explained by the use of tocilizumab and, to a lesser extent, corticosteroids, which use results in a reduced C-reactive protein level (27).
Early 2020, it was questioned whether ECMO could play the same role for COVID-19 ARDS as it had been in ARDS previously. In other pandemics, including the Middle East respiratory syndrome outbreak, an association of improved outcomes in patients with ARDS on ECMO had been demonstrated (28). In our study, no differences were found in 28- and 60-day mortality between COVID-19–associated ARDS and non-COVID-19 ARDS, both unadjusted as adjusted for confounders. This is supported by previous studies showing equal survival at different time points (13, 14, 23, 29). A recent study by Fanelli et al (23) found, when comparing patients receiving ECMO for either Influenza A H1N1 or COVID-19 ARDS, that 60-day mortality was only in the disadvantage of COVID-19 when unadjusted for confounders. Also, we find a similar complication rate in both COVID-19–associated ARDS and non-COVID-19 ARDS, with the exception of a higher infectious event rate in COVID-19, which may be caused by different COVID-19 targeted therapies.
In the future, it may thus be questioned whether distinctions should be made regarding the cause of ARDS and the use of VV ECMO. In the latest ELSO guidelines for management of adult patients supported with VV ECMO, no such distinctions are made concerning the indication ARDS for VV ECMO (30). Nevertheless, some differences in patient characteristics do exist between the different causes of ARDS. Current guidelines state MV for over 7 days with high plateau pressures and Fio2 levels should be considered a relative contraindication for initiating VV ECMO (11, 30). It has been reported that in COVID-19, both ventilation duration before ECMO and the ventilation duration during ECMO are relatively long compared with other etiologies (23). However, based on current studies, no final conclusions can be drawn regarding whether this advised “7 day limit” also applies to COVID-19–associated ARDS, as results appear to be contradictory to previous studies. Several studies failed to show an association between the duration of MV prior to ECMO and survival (31–33). Taking into account the improvements in lung protective ventilation and the use of noninvasive ventilation methods, future studies and guidelines should rather focus on other patient characteristics such as ARDS phenotype than one absolute cutoff for the initiation of ECMO.
This study has several strengths and limitations. One major strength of our study is the relatively large sample size in both the COVID-19 and the non-COVID-19 cohort. By using IPW, we were able to reduce the influence of confounders and use the majority of our study population. However, some limitations should also be recognized. First, due to the difference in sample size between the hospitals, we were not able to fully correct for the confounder “institute” in our IPW population. Although previous studies have described that center experience could influence patient outcomes (24, 34), all centers in our cohort have extensive experience in the application of ECMO of over several years. Second, several parameters were not available for the non-COVID-19 cohort, including ventilatory parameters, before the initiation of ECMO. Third, we did not collect longitudinal data during ECMO, which may have shown differences in the patient’s development during ECMO support.
CONCLUSIONS
In conclusion, mortality for patients with COVID-19 who received VV ECMO was similar to that reported in other COVID-19 cohorts, although no differences were found between the first and second waves regarding mortality. In addition, after IPW, mortality was independent of the etiology of ARDS.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Raasveld and Vlaar had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Raasveld and Vlaar were involved in concept and design. Drs. Raasveld, van Baarle, and Vlaar involved in statistical analysis. Dr. Vlaar were involved in supervision. All authors were involved in acquisition, analysis, or interpretation of data; drafting of the article; and critical revision of the article for important intellectual content.
Dr. Broman is a member of the Medical Advisory Boards of Eurosets Srl., Medolla, Italy, and Xenios AG, Heilbronn, Germany. Dr. Taccone is a member of the Medical Advisory Boards of Eurosets Srl., Medolla, Italy, and Xenios AG, Heilbronn, Germany. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review board of the Amsterdam University Medical Centers (W20_199#20.230), and, if applicable by national law, thereafter, from local Ethics Committees.
Due to the retrospective nature of the analysis, in accordance with article 9 paragraph 2 sub j GDPR jo. article 24 Dutch GDPR Implementation Act, and/or any additional data protection laws in its country, informed consent of data subjects is not required. It has been determined that requesting explicit permission from patients for this data processing is impossible or requires a disproportionate effort. Patients were not actively contacted for follow-up on survival status, other than stated in the electronic patient record.
After publication, encrypted data can be requested by contacting the corresponding author. Reasonable data request will be taken in consideration.
Trial registration: Netherlands trial registry, NL8706, date of registration: June 9, 2020.
REFERENCES
- 1.Brodie D, Slutsky AS, Combes A: Extracorporeal life support for adults with respiratory failure and related indications: A review. JAMA 2019; 322:557–568 [DOI] [PubMed] [Google Scholar]
- 2.Slutsky AS, Ranieri VM: Ventilator-induced lung injury. N Engl J Med 2013; 369:2126–2136 [DOI] [PubMed] [Google Scholar]
- 3.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet: Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018; 378:1965–1975 [DOI] [PubMed] [Google Scholar]
- 4.Goligher EC, Tomlinson G, Hajage D, et al. : Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA 2018; 320:2251–2259 [DOI] [PubMed] [Google Scholar]
- 5.Extracorporeal Life Support Organization: ELSO: International Report October 2021. 2021, p 1 Available at: https://www.elso.org/Portals/0/Files/Reports/2021_October/International%20Report%20October_page1.pdf. Accessed December 30, 2021
- 6.Henry BM: COVID-19, ECMO, and lymphopenia: A word of caution. Lancet Respir Med 2020; 8:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrams D, Lorusso R, Vincent JL, et al. : ECMO during the COVID-19 pandemic: When is it unjustified? Crit Care 2020; 24:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Hajage D, Lebreton G, et al. : Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir Med 2020; 2600:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbaro RP, MacLaren G, Boonstra PS, et al. ; Extracorporeal Life Support Organization: Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020; 396:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Extracorporeal Life Support Organization: ELSO.org - Full COVID-19 Registry Dashboard. 2021. Available at: https://www.elso.org/Registry/FullCOVID-19RegistryDashboard.aspx. Accessed April 14, 2022
- 11.Badulak J, Antonini MV, Stead CM, et al. ; ELSO COVID-19 Working Group Members: Extracorporeal membrane oxygenation for Covid-19: Updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J 2021; 67:485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson JG, Calfee CS: ARDS subphenotypes: Understanding a heterogeneous syndrome. Crit Care 2020; 24:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurihara C, Manerikar A, Gao CA, et al. : Outcomes after extracorporeal membrane oxygenation support in COVID-19 and non-COVID-19 patients. Artif Organs 2022; 46:688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roedl K, Kahn A, Jarczak D, et al. : Clinical characteristics, complications and outcomes of patients with severe acute respiratory distress syndrome related to COVID-19 or influenza requiring extracorporeal membrane oxygenation-a retrospective cohort study. J Clin Med 2021; 10:5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raasveld SJ, Delnoij TSR, Broman LM, et al. ; ETALON Study Group: Extracorporeal membrane oxygenation in patients with COVID-19: An international multicenter cohort study. J Intensive Care Med 2021; 36:910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M, Langouet E, Hajage D, et al. ; GRC RESPIRE Sorbonne Université: Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID-19 ARDS in Sorbonne hospitals, Paris. Crit Care 2021; 25:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riera J, Roncon-Albuquerque R, Jr, Fuset MP, et al. ; ECMOVIBER Study Group: Increased mortality in patients with COVID-19 receiving extracorporeal respiratory support during the second wave of the pandemic. Intensive Care Med 2021; 47:1490–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: A languate and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available at: https://www.R-project.org. Accessed September 28, 2022
- 19.van Buuren S, Groothuis-Oudshoorn K: mice: Multivariate imputation by chained equations in R. J Stat Softw 2011; 45:1–67 [Google Scholar]
- 20.Textor J, van der Zander B, Gilthorpe MS, et al. : Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int J Epidemiol 2016; 45:1887–1894 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC; Institute for Clinical Eveluative Sciences, Ontario C: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramanathan K, Shekar K, Ling RR, et al. : Extracorporeal membrane oxygenation for COVID-19: A systematic review and meta-analysis. Crit Care 2021; 25:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanelli V, Giani M, Grasselli G, et al. : Extracorporeal membrane oxygenation for COVID-19 and influenza H1N1 associated acute respiratory distress syndrome: A multicenter retrospective cohort study. Crit Care 2022; 26:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbaro RP, MacLaren G, Boonstra PS, et al. ; Extracorporeal Life Support Organization: Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021; 398:1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horby P, Landray M: No clinical benefit from use of hydroxychloroquine in hospitalized patients with COVID-19: statement from the chief investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) trial on hydroxychloroquine, 2020. Available at: https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19. Accessed December 30, 2021
- 27.Kooistra EJ, van Berkel M, van Kempen NF, et al. : Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. Crit Care 2021; 25:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alshahrani MS, Sindi A, Alshamsi F, et al. : Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care 2018; 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Autschbach T, Hatam N, Durak K, et al. : Outcomes of extracorporeal membrane oxygenation for acute respiratory distress syndrome in COVID-19 patients: A propensity-matched analysis. J Clin Med 2021; 10:2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonna JE, Abrams D, Brodie D, et al. : Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J 2021; 67:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermann M, Laxar D, Krall C, et al. : Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann Intensive Care 2022; 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivier PY, Ottavy G, Hoff J, et al. : Prolonged time from intubation to cannulation in VV-ECMO for COVID-19: Does it really matter? Crit Care 2021; 25:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt M, Bailey M, Sheldrake J, et al. : Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014; 189:1374–1382 [DOI] [PubMed] [Google Scholar]
- 34.Whebell S, Zhang J, Lewis R, et al. : Survival benefit of extracorporeal membrane oxygenation in severe COVID-19: A multi-centre-matched cohort study. Intensive Care Med 2022; 48:467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.