PURPOSE
Patients with pretreated estrogen receptor (ER)–positive/human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer have poor prognosis. Elacestrant is a novel, oral selective ER degrader that demonstrated activity in early studies.
METHODS
This randomized, open-label, phase III trial enrolled patients with ER-positive/HER2-negative advanced breast cancer who had one-two lines of endocrine therapy, required pretreatment with a cyclin-dependent kinase 4/6 inhibitor, and ≤ 1 chemotherapy. Patients were randomly assigned to elacestrant 400 mg orally once daily or standard-of-care (SOC) endocrine monotherapy. Primary end points were progression-free survival (PFS) by blinded independent central review in all patients and patients with detectable ESR1 mutations.
RESULTS
Patients were randomly assigned to elacestrant (n = 239) or SOC (n = 238). ESR1 mutation was detected in 47.8% of patients, and 43.4% received two prior endocrine therapies. PFS was prolonged in all patients (hazard ratio = 0.70; 95% CI, 0.55 to 0.88; P = .002) and patients with ESR1 mutation (hazard ratio = 0.55; 95% CI, 0.39 to 0.77; P = .0005). Treatment-related grade 3/4 adverse events occurred in 7.2% receiving elacestrant and 3.1% receiving SOC. Treatment-related adverse events leading to treatment discontinuations were 3.4% in the elacestrant arm versus 0.9% in SOC. Nausea of any grade occurred in 35.0% receiving elacestrant and 18.8% receiving SOC (grade 3/4, 2.5% and 0.9%, respectively).
CONCLUSION
Elacestrant is the first oral selective ER degrader demonstrating a significant PFS improvement versus SOC both in the overall population and in patients with ESR1 mutations with manageable safety in a phase III trial for patients with ER-positive/HER2-negative advanced breast cancer.
INTRODUCTION
Endocrine therapy, with either aromatase inhibitors (AI) or fulvestrant, plus a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor is the recommended first-line standard of care (SOC) for locally advanced or metastatic estrogen receptor (ER)–positive/human epidermal growth factor receptor 2 (HER2)–negative breast cancer.1-3 Subsequent progression is associated with endocrine resistance, which includes development of acquired mutations in a variety of genes such as erb-b2 receptor tyrosine kinase 2 (ERBB2), neurofibromin 1 (NF1), and estrogen receptor 1 (ESR1).4-6 Mutations in ESR1 result in estrogen-independent ER activation and, consequently, resistance to AIs but not ER inhibitors (eg, selective ER degraders [SERDs] and selective ER modulators).4,7 Current treatment guidelines recommend sequential endocrine therapy in the absence of visceral crisis or until all endocrine therapy options have been exhausted; tamoxifen with or without everolimus is another option as later-line therapy.1-3,8 However, the clinical activity of endocrine monotherapy in patients who have received prior CDK4/6 or mammalian target of rapamycin inhibition is limited, with a median progression-free survival (PFS) of approximately 2 months, highlighting a major unmet clinical need in the field.9-12
CONTEXT
Key Objective
What is the efficacy and safety of the novel oral selective estrogen degrader, elacestrant, in women with previously treated, estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer compared with standard-of-care (SOC) endocrine monotherapy?
Knowledge Generated
Among these patients, 43% of whom had two prior lines of endocrine therapy, elacestrant significantly reduced the risk of progression or death compared with SOC by 30% in the overall cohort (P = .002) and by 45% in patients with ESR1 mutation (P = .0005). The most common adverse event was nausea, which occurred in 35% of patients receiving elacestrant and 19% of patients receiving SOC. Elacestrant was discontinued for an adverse event in 6% of patients, and SOC was discontinued in 4% of patients.
Relevance
These data represent an opportunity to potentially offer a new oral endocrine therapy option to patients with previously treated metastatic hormone receptor–positive breast cancer, including ESR1-mutant breast cancer.
Elacestrant is a novel, nonsteroidal, oral SERD that degrades the ER alpha in a dose-dependent manner and inhibits estradiol-dependent ER-directed gene transcription and tumor growth in in vitro and in vivo preclinical models, including those harboring ESR1 mutations associated with endocrine resistance.13-16 Elacestrant demonstrated antitumor activity and tolerability in a phase I trial of heavily pretreated patients with advanced ER-positive/HER2-negative breast cancer, including patients with ESR1-mutated tumors.17 On the basis of the results, a phase III global trial (EMERALD) was conducted to compare the efficacy and safety of elacestrant compared with SOC endocrine therapy in patients with ER-positive/HER2-negative advanced or metastatic breast cancer who had progression after first- or second-line treatment with the combination of endocrine therapy and a CDK4/6 inhibitor and to compare efficacy between arms in patients with detectable ESR1 mutation.
METHODS
Study Design and Patients
EMERALD is an international, multicenter, randomized, open-label, phase III clinical study (Data Supplement, online only).18 Eligible patients were postmenopausal women or men age 18 years or older with histologically or cytologically proven ER-positive/HER2-negative breast adenocarcinoma and either locoregionally recurrent or metastatic disease. Disease progression must have occurred during or within 28 days after treatment with one or two prior lines of endocrine therapy for advanced/metastatic disease. Progression during or within 12 months of adjuvant endocrine therapy was included as a line of endocrine therapy for advanced/metastatic disease. Progression on previous CDK4/6 inhibitor treatment in combination with fulvestrant or an AI was required. One chemotherapy regimen in the advanced/metastatic setting was permitted. Additional eligibility requirements included Eastern Cooperative Oncology Group performance status 0 or 1 and measurable disease per RECIST version 1.119 or evaluable bone-only disease with at least one lytic or mixed lytic-blastic bone lesion (blastic-only metastases were not allowed).
ER and HER2 testing were performed by local laboratory. ER positivity was defined as ≥ 1% staining by immunohistochemistry,20 with or without progesterone receptor positivity. HER2 negativity was defined according to current guidelines.21
Key exclusion criteria included symptomatic metastatic visceral disease and any of the following cardiovascular events within 6 months of enrollment: severe/unstable angina, myocardial infarction, coronary/peripheral artery bypass graft, prolonged corrected QT interval grade ≥ 2, uncontrolled atrial fibrillation, ongoing grade ≥ 2 cardiac dysrhythmias, New York Heart Association Class II or greater heart failure, coagulopathy (thrombosis), and cerebrovascular accident.
Study Procedures
Patients were randomly assigned 1:1 to elacestrant or SOC therapy. Random assignment was stratified according to ESR1 mutational status, presence of visceral metastases, and previous treatment with fulvestrant. Elacestrant was dosed 400 mg orally once daily, with reductions to 300 mg or 200 mg daily permitted for toxicity. SOC treatment was per investigator's choice of fulvestrant, anastrozole, letrozole, or exemestane monotherapy and dosed according to the labeling. This guidance recommended use of a different endocrine therapy than the patient had received previously. Specifically, fulvestrant was recommended for patients who had not previously received fulvestrant and selection of AI on the basis of prior AI therapy. Detailed guidance for choice of SOC agent is provided in the Protocol (online only), as detailed in the Data Supplement.
Screening assessments included physical examination with 12-lead electrocardiogram, hematology, chemistry, and coagulation parameters. ESR1 mutational status was evaluated in cell-free circulating DNA at a central laboratory; blood samples were analyzed using the Guardant360 CDx (Guardant Health, Redwood City, CA). ESR1 mutations were defined as any missense mutation in codons 310-547. ESR1 mutation status was not provided to study sites during treatment. Tumor assessments were conducted with computed tomography/magnetic resonance imaging (CT/MRI), unless performed within 28 days from random assignment, and radionuclide bone scan or whole-body MRI, unless performed within the prior 12 weeks.
During treatment, electrocardiogram and laboratory parameters were performed predose on day 1 and 15 of cycle 1, day 1 of each subsequent cycle, and at the end of treatment. Tumor assessments with CT/MRI were performed every 8 weeks. Radionuclide bone scan or whole-body MRI was performed every 24 weeks in patients who had bone metastases at baseline. Abnormal bone lesions were confirmed with CT scan with bone windows or MRI. Complete responses (CRs) or partial responses had to be confirmed at least 4 weeks after first response. Adverse events (AEs) were collected until 30 days after the last study-drug dose.
End Points
The primary end points were PFS in all patients and in patients with detectable ESR1 mutation, each assessed by blinded independent central review (BICR) using standard RECIST v1.1 criteria. Key secondary end points were overall survival (OS) in all patients and in patients with ESR1 mutation. Other secondary end points included BICR-assessed PFS and OS in patients without detectable ESR1 mutation; PFS assessed by the investigator; objective response rate, duration of response, and clinical benefit rate ([CBR] the proportion of patients who experienced either a confirmed CR or partial response, or stable disease at ≥ 24 weeks from random assignment), assessed by BICR and the investigator; and safety and tolerability.
Trial Oversight
The trial was designed by a steering committee of independent investigators (Data Supplement) and the sponsor, Radius Health, Inc. The trial met regulatory requirements and was performed in accordance with ethical principles consistent with the Declaration of Helsinki and International Council of Harmonisation/Good Clinical Practice. An external independent data monitoring committee regularly reviewed the safety and efficacy data, including an interim futility analysis. The study protocol and relevant supporting information were approved by the institutional review board at each participating site. Each participant provided written informed consent. All authors were involved in the writing or critical review and editing of the manuscript and vouch for the fidelity of the trial to the protocol and for the accuracy and completeness of the data reported. Members of the steering committee guided the initial manuscript draft after an agreement to publish with all coauthors, with editorial assistance from professional medical writers funded by the sponsor.
Statistical Analysis
In this event-driven study, approximately 340 PFS events were required in the all-patient population to provide 92% power to detect a hazard ratio (HR) of 0.667 at the two-sided alpha level of .025. Approximately 160 PFS events were required in the ESR1-mutant population to provide 80% power to detect a HR of 0.610 at the two-sided alpha level of .025. The planned sample sizes were 466 patients in total and 220 patients with ESR1 mutation. The prespecified interim OS analysis occurred at the time of the PFS analysis with an allocated two-sided alpha level of .0001 according to the Haybittle-Peto rule.22,23 The final OS analysis will occur when approximately 50% of patients have died.
PFS and OS analyses were performed using standard Kaplan-Meier methods on the basis of the intention-to-treat populations for all patients and patients with ESR1 mutation. HR and 95% confidence interval (CI) for the difference between treatment groups in PFS and OS were estimated using the stratified Cox regression model, including treatment as a variable, and analyzed using the stratified log-rank test. To ensure that the family-wide error rate did not exceed 5%, multiplicity adjustments accounted for the analyses of two primary end points, two key secondary end points, and the key secondary end points at two time points. A truncated Hochberg procedure was used to test the primary end points. Given that both primary end points were met, an alpha of .05 was passed to OS. A Haybittle-Peto rule was used to adjust the alpha for analyses of OS at two time points. Other efficacy end points were analyzed without adjustment for P values at the two-sided alpha level of .05.
RESULTS
Patients and Treatment
Of 694 patients screened, 477 patients were randomly assigned to receive elacestrant (239 patients) or SOC therapy (238 patients) between February 2019 and October 2020 at 228 sites in 17 countries. The median age was 63 years (range, 24-89), and 228 patients (47.8%) had detectable ESR1 mutation. In the advanced/metastatic setting, 207 patients (43.4%) received two prior lines of endocrine therapy, and 106 patients (22.2%) received one prior chemotherapy regimen. Baseline characteristics were well-balanced between elacestrant and SOC therapy (Table 1). Most patients had visceral metastasis in both arms (163 patients [68.2%], elacestrant and 169 patients [71%], SOC therapy). Of the randomly assigned patients, 466 (97.7%) started treatment and 442 patients had discontinued study treatment at the data cutoff of September 6, 2021 (Data Supplement). The median duration of follow-up was 15.1 months.
TABLE 1.
Baseline Characteristics
Efficacy
PFS assessed by BICR was statistically significantly prolonged in the elacestrant arm versus the SOC arm in all patients (HR = 0.70; 95% CI, 0.55 to 0.88; P = .002) and in patients with ESR1 mutation (HR = 0.55; 95% CI, 0.39 to 0.77 P = .0005; Figs 1A and 1B). A closer look at the Kaplan-Meier curves revealed an initial drop in both arms, highlighting possible endocrine resistance in the second- /third-line setting, but then clear separation of the curves in the endocrine-sensitive setting. Since median PFS alone can be misleading in such a scenario (Data Supplement), landmark analysis at 6 and 12 months were conducted. Accordingly, 6-month PFS rates were 34.3% (95% CI, 27.2 to 41.5) versus 20.4% (95% CI, 14.1 to 26.7) for the elacestrant versus SOC arms, respectively, in all patients and 40.8% (95% CI, 30.1 to 51.4) versus 19.1% (95% CI, 10.5 to 27.8), respectively, in patients with ESR1 mutation. Similarly, 12-month PFS rates were 22.3% (95% CI, 15.2 to 29.4) versus 9.4% (95% CI, 4.0 to 14.8), respectively, in all patients versus 26.8% (95% CI, 16.2 to 37.4) and 8.2% (95% CI, 1.3 to 15.1), respectively, in patients with ESR1 mutation.
FIG 1.
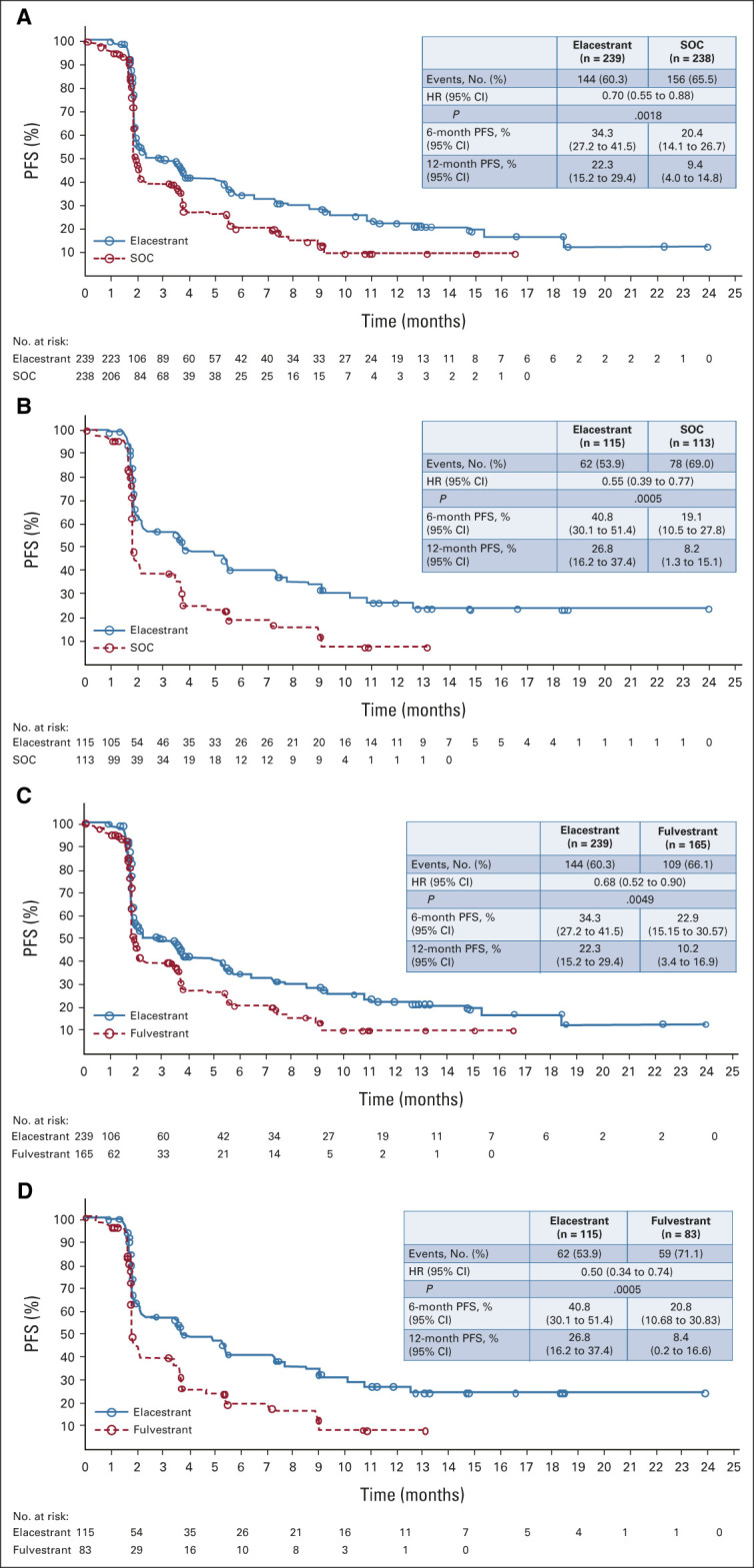
Kaplan-Meier estimates of PFS assessed by blinded independent central review are shown for (A) elacestrant versus SOC in all patients, (B) elacestrant versus SOC in patients with detectable ESR1 mutation, (C) elacestrant versus fulvestrant in all patients, and (D) elacestrant versus fulvestrant in patients with detectable ESR1 mutation. Analyses were performed on the intention-to-treat population. HR, hazard ratio; PFS, progression-free survival; SOC, standard of care.
The efficacy of elacestrant was maintained when compared with the fulvestrant subgroup in secondary analysis (Figs 1C and 1D). In analysis excluding the six patients who had received prior fulvestrant and received fulvestrant during the trial, results remained significant in favor of elacestrant, both in the overall population or ESR1 mutation cohort, in terms of statistical significance (P = .0019; .0006) estimates of median PFS (2.8 months v 1.9 months; 3.8 months v 1.9 months), 6-month PFS rate (34.3% v 20.6%; 40.8% v 19.3%), 12-month PFS rate (22.3% v 9.5%; 26.8% v 8.3%), or other efficacy outcomes (Data Supplement). The subgroup analysis of elacestrant versus AI also demonstrated a similar trend in the primary analyses (Data Supplement).
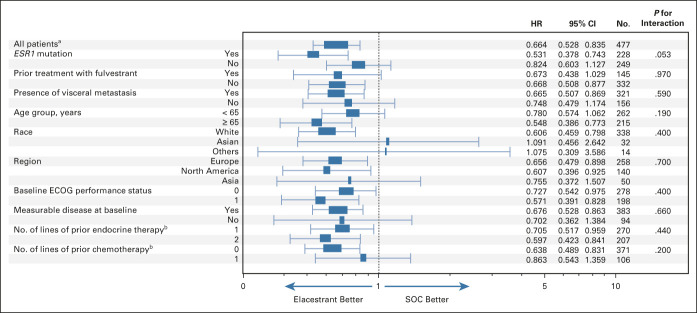
HRs for PFS numerically favored elacestrant across prespecified subgroups (Fig 2). In patients without ESR1 mutation detected, a similar pattern of curve separation was observed for PFS assessed by BICR (Data Supplement). PFS by local investigator and tumor response were consistent with these results (Data Supplement).
FIG 2.
Subgroup analysis of PFS in all patients. PFS, as assessed by blinded independent central review, in clinically relevant subgroups of patients with ER-positive/HER2-negative advanced breast cancer. Interaction P values were all nonsignificant indicating that elacestrant benefit on PFS is independent of subgroup. aNonstratified analysis. bIn the advanced setting. ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; PFS, progression-free survival; SOC, standard of care.
At the interim analysis of OS, 149 events had occurred in all patients with a HR of 0.75 (95% CI, 0.54 to 1.04; P = .08; Fig 3). In patients with ESR1 mutation, 68 events had occurred with a HR of 0.59 (95% CI, 0.36 to 0.96; P = .03, nonsignificant). In patients without ESR1 mutation, 81 events had occurred with a HR of 0.92 (95% CI, 0.59 to 1.42; P = .69; Data Supplement).
FIG 3.

Interim analysis of OS. Kaplan-Meier estimates of overall survival at the interim analysis are (A) shown for all patients and (B) patients with detectable ESR1 mutation. The differences in overall survival in this interim analysis were not statistically significant on the basis of the allocated two-sided alpha level of .0001. Analysis was performed on the intention-to-treat population. HR, hazard ratio; NS, nonsignificant; OS, overall survival; SOC, standard of care.
Safety
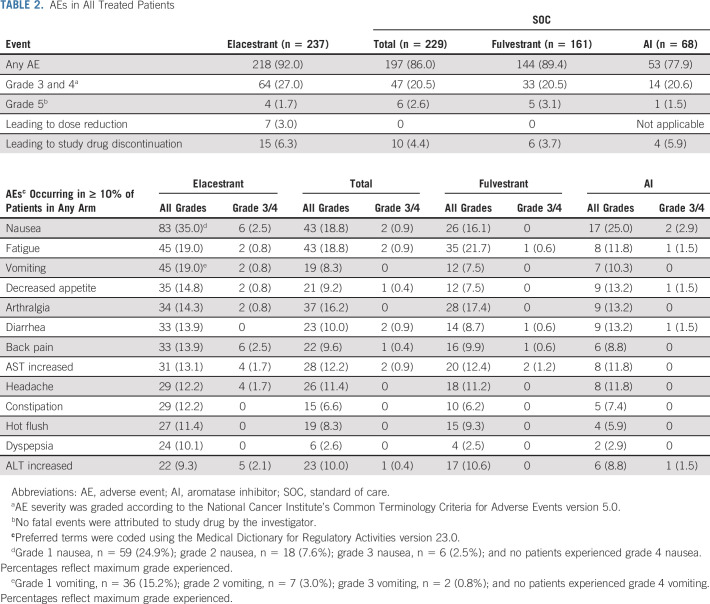
AEs occurred in 218 of 237 patients (92.0%) receiving elacestrant and 197 of 229 patients (86.0%) receiving SOC therapy (Table 2). The most common AEs observed with elacestrant versus SOC therapy, respectively, included nausea (35.0% v 18.8%), fatigue (19.0% v 18.8%), vomiting (19.0% v 8.3%), decreased appetite (14.8% v 9.2%), and arthralgia (14.3% v 16.2%). Grade 3/4 AEs occurred in 64 patients (27.0%) receiving elacestrant and 47 patients (20.5%) receiving SOC therapy. The most common grade 3/4 AEs were nausea (six patients, 2.5%), back pain (six patients, 2.5%), and increased ALT (five patients, 2.1%) in the elacestrant arm and nausea, fatigue, diarrhea, and increased AST (each occurring in two patients [0.9%]) in the SOC arm. AEs led to treatment discontinuation in 15 patients (6.3%) in the elacestrant arm and 10 patients (4.4%) in the SOC arm. Events deemed treatment-related by the investigator occurred in 150 patients (63.3%) receiving elacestrant and 100 patients (43.7%) receiving SOC therapy, with those of grade 3/4 severity occurring in 17 (7.2%) and seven (3.1%) patients, respectively (Data Supplement). No deaths assessed as treatment-related were reported in either arm.
TABLE 2.
AEs in All Treated Patients
DISCUSSION
This randomized phase III clinical trial demonstrated that elacestrant was associated with a statistically significantly prolonged PFS compared with SOC endocrine therapy in patients with advanced/metastatic ER-positive/HER2-negative breast cancer who had progressed on prior endocrine and CDK4/6 inhibitor therapy. The benefit was observed in the full cohort and in patients with detectable ESR1 mutations. The interim OS analysis demonstrated HRs of 0.59 in the ESR1 mutation population and 0.75 in the overall population. The final OS results will be provided in the future when data are mature. Elacestrant exhibited manageable toxicity with most AEs of grade 1 or 2 severity. The most frequent AE was nausea and was of grade 3 severity in 2.5% of patients. No cardiac or ocular toxicity, reported with other SERDs,24-26 was observed.
Elacestrant is the first oral SERD to demonstrate improved efficacy compared with SOC endocrine therapy in patients with advanced breast cancer. Nearly two decades have passed since the last endocrine therapy, fulvestrant, was approved in 2002 for patients with ER-positive metastatic breast cancer.27 In EMERALD, patients receiving elacestrant had superior PFS compared with those receiving fulvestrant. In addition to improved efficacy, elacestrant offers an oral option to intramuscular fulvestrant injection. Because of the initial drop in PFS in both arms, median PFS may not be a sufficient measure of efficacy in the overall population (2.8 months v 1.9 months) or ESR1 mutation cohort (3.8 months v 1.9 months). Rather, it is important to evaluate efficacy over longer periods of time using the HR and landmark analyses at 6 and 12 months in this population. The HRs reflect a 30% relative reduction in progression or death in the overall cohort and a 45% relative reduction in the ESR1-mutant cohort. The landmark analyses at 6 and 12 months demonstrated substantial improvements in PFS at these later time points with elacestrant. We consider these differences to be clinically meaningful in patients with limited treatment options. The magnitude of PFS improvement was lower in patients without detectable ESR1 mutation, possibly reflecting a second- /third-line post-CDK 4/6 inhibitor setting in which tumors are likely more dependent on alternate growth factor pathways and less dependent on the ER pathway, thus limiting the benefit of endocrine monotherapy.28 Note, the PFS results in this subset should be interpreted with caution given that this analysis was not the primary end point.
When placed in context of modern later-line endocrine therapy in the post-CDK 4/6 inhibitor setting and patients with visceral metastasis, the SOC arm's performance was generally consistent with available results in clinical trials. In a recent phase II trial, fulvestrant was associated with a median PFS of 1.9 months and a CBR of 13.7%.9 SOC therapy also performed as expected from ESR1 mutation clinical data. In plasmaMATCH, high-dose fulvestrant was associated with a median PFS of 2.2 months and a CBR of 16% among patients with detectable ESR1 mutation.10
EMERALD demonstrated that elacestrant as a single agent reduces the risk of progression or death, as compared with current SOC single-agent endocrine therapies. Therefore, when single-agent endocrine therapy is appropriate at a later line, our findings are applicable and demonstrate that elacestrant is a more effective option than fulvestrant or an AI. In this trial, tamoxifen was excluded from the SOC arm on the basis of current guidelines for endocrine therapy, which prioritize AIs and fulvestrant before tamoxifen,1,2 clinical trial results demonstrating superiority of AIs to tamoxifen,29,30 and a desire to limit heterogeneity in the SOC arm. There is no indication from the literature that tamoxifen would have led to prolonged PFS in the control arm because of its inferiority to AIs and fulvestrant.
In this study, endocrine therapy was administered as second-line single-agent therapy to approximately 57% of all patients. We recognize that in certain regions, particularly the United States and Europe, combination therapy with fulvestrant is increasingly being used as the second-line SOC treatment, particularly for patients with PIK3CA-mutant breast cancer on the basis of results from recent clinical trials (SOLAR-1 and BYlieve).31,32 However, the goal of this study, like other ongoing studies with oral SERDs in the second- or third-line setting, was to compare a novel endocrine therapy versus currently available endocrine therapies, rather than evaluate combination regimens. The benefit of elacestrant over fulvestrant and AIs in our monotherapy trial also suggests that incorporating elacestrant as the preferred endocrine therapy backbone in future earlier-line combination studies is a promising strategy. Accordingly, the role of elacestrant/everolimus compared with exemestane/everolimus combination and elacestrant/alpelisib compared with fulvestrant/alpelisib combination requires further research. Notably, these historical combinations (exemestane/everolimus and fulvestrant/alpelisib) exhibited an approximate 20% discontinuation rate for AEs in clinical trials.31,33
A strength of our study was the requirement that all patients had previously received a CDK4/6 inhibitor, consistent with current practice guidelines.8 It should be noted that the study used open-label design; as in our opinion, administering placebo intramuscularly was unethical. Accordingly, the primary end point was based on BICR. The central results were consistent with local investigator results providing additional assurance regarding therapeutic efficacy.
In conclusion, elacestrant is the first oral SERD that demonstrated a significant improvement in PFS versus SOC therapy in a randomized phase III study in patients with ER-positive/HER2-negative advanced or metastatic breast cancer in the second- or third-line setting. Elacestrant showed a predictable and manageable safety profile consistent with other endocrine therapies. These data represent a long-awaited opportunity to potentially offer second- or third-line, including heavily pretreated, patients with breast cancer a new effective option and further advance toward precision medicine in the ER-positive/HER2-negative subtype.
ACKNOWLEDGMENT
We thank Mark Phillips, PharmD, and Laura Evans, PharmD, of Phillips Gilmore Oncology Communications Inc, for professional assistance with manuscript preparation. Financial support for writing and editorial services was provided by Radius Health, Inc. and Berlin Chemie/Menarini Group.
Francois-Clement Bidard
Consulting or Advisory Role: Pfizer, AstraZeneca, Lilly, Novartis, Radius Health, Menarini, Sanofi (Inst)
Speakers' Bureau: Pfizer, Novartis, AstraZeneca, Roche, Lilly, Rain Therapeutics
Research Funding: Novartis (Inst), Pfizer (Inst), Menarini Silicon Biosystems (Inst), Prolynx (Inst)
Patents, Royalties, Other Intellectual Property: ESR1 & MSI detection techniques (patents; Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, AstraZeneca, Novartis
Virginia G. Kaklamani
Honoraria: Genentech, Novartis, Pfizer, Genomic Health, Puma Biotechnology, AstraZeneca, Seattle Genetics, daichi, Gilead Sciences
Consulting or Advisory Role: Amgen, Eisai, Puma Biotechnology, Celldex, AstraZeneca, Athenex, bioTheranostics
Speakers' Bureau: Genentech, Novartis, Genomic Health, Puma Biotechnology, Pfizer, AstraZeneca/Daiichi Sankyo
Research Funding: Eisai
Patrick Neven
Consulting or Advisory Role: Lilly (Inst), Pfizer (Inst), Novartis (Inst), Roche (Inst), Roche (Inst), Radius Health (Inst), Radius Health (Inst)
Travel, Accommodations, Expenses: Lilly (Inst), Pfizer (Inst), Roche (Inst)
Alberto J. Montero
Honoraria: Celgene, AstraZeneca, OncoSec
Consulting or Advisory Role: New Century Health, Welwaze
Uncompensated Relationships: Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/618396
Frédéric Forget
Travel, Accommodations, Expenses: Ipsen, Teva
Joo Hyuk Sohn
Research Funding: MSD (Inst), Roche (Inst), Novartis (Inst), Lilly (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst)
Other Relationship: Roche
Donatienne Taylor
Honoraria: AstraZeneca
Consulting or Advisory Role: Lilly, AstraZeneca, Roche/Genentech, Novartis, Agendia, MSD, Seattle Genetics
Travel, Accommodations, Expenses: MSD, AstraZeneca, Pfizer
Kathleen K. Harnden
Honoraria: E-Health Now
Speakers' Bureau: Eisai, Daiichi Sankyo/Astra Zeneca, Seattle Genetics, Merck
Hung Khong
Stock and Other Ownership Interests: MEI Pharma, TG Therapeutics, Lipocine, MustangBio, Tiziana Life Sciences, Vaxart, agenus
Consulting or Advisory Role: Celcuity
Research Funding: AstraZeneca
Patrick M. Dillon
Research Funding: Novartis (Inst), Pfizer (Inst), AbbVie (Inst), Newlink Genetics (Inst), Tesaro (Inst), Merck (Inst), Tolero Pharmaceuticals (Inst), Radius Health (Inst)
Sunil Babu
Employment: Fort Wayne Medical Oncology & Hematology
Stock and Other Ownership Interests: Fort Wayne Medical Oncology & Hematology, Lutheran hospital
Honoraria: Bristol Myers Squibb, Alexion Pharmaceuticals, Lilly, Bayer, AstraZeneca, Castle Biosciences, Pharmacosmos, Beigene, Kite, a Gilead company
Consulting or Advisory Role: Bristol Myers Squibb, Alexion Pharmaceuticals, AstraZeneca, argenx, Boehringer Ingelheim, Bayer, Kite, a Gilead company, Janssen Oncology, Amgen
Speakers' Bureau: Alexion Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Genentech/Roche (Inst), AstraZeneca/MedImmune (Inst), Janssen Oncology (Inst), Amgen (Inst), TG Therapeutics (Inst), AbbVie (Inst), Lilly (Inst), Alexion Pharmaceuticals (Inst), Merck (Inst), Novartis (Inst), Syndax (Inst), Nektar (Inst), Sanofi (Inst), argenx (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Alexion Pharmaceuticals, Lilly, Janssen Oncology, Genentech/Roche
Simon Waters
Honoraria: Novartis Pharmaceuticals UK Ltd
Consulting or Advisory Role: Novartis Pharmaceuticals UK Ltd, Sanofi/Aventis, Daiichi Sankyo/Astra Zeneca
Ines Deleu
Consulting or Advisory Role: BMS, Novartis, Pierre Fabre
Travel, Accommodations, Expenses: Sanofi, Pierre Fabre
José A. García Sáenz
Consulting or Advisory Role: Novartis, AstraZeneca, Lilly, AstraZeneca, Daiichi Sankyo/Astra Zeneca, Gilead Sciences, Seattle Genetics
Speakers' Bureau: Novartis
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Roche, Novartis
Emilio Bria
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Pfizer, Lilly, Bristol Myers Squibb, Roche
Research Funding: AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD
Marina Cazzaniga
Honoraria: Pierre Fabre, Roche, Pfizer
Consulting or Advisory Role: Pierre Fabre, Roche, Novartis
Research Funding: Lilly (Inst), Eisai Europe (Inst)
Janice Lu
Honoraria: Pfizer, Lilly, Sanofi/Aventis, AstraZeneca/Daiichi Sankyo, Seattle Genetics
Consulting or Advisory Role: Pfizer, Novartis, Lilly, Sanofi/Aventis, AstraZeneca/Daiichi Sankyo
Research Funding: Novartis (Inst), Lilly (Inst), Ambrx (Inst), Genentech (Inst), Radius Health (Inst)
Philippe Aftimos
Honoraria: Synthon, Roche, Gilead Sciences
Consulting or Advisory Role: Macrogenics, Boehringer Ingelheim, Novartis, Amcure, Roche, Novartis, Amgen, Servier, G1 Therapeutics, Radius Health, Deloitte, Menarini, Gilead Sciences
Travel, Accommodations, Expenses: Amgen, MSD Oncology, Roche Belgium, Pfizer
Javier Cortés
Stock and Other Ownership Interests: MedSIR, Nektar, Leuko
Honoraria: Novartis, Eisai, Celgene, Pfizer, Roche, Samsung, Lilly, Merck Sharp & Dohme, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Cellestia Biotech, AstraZeneca, Roche, Seattle Genetics, Daiichi Sankyo, ERYTECH Pharma, Polyphor, Athenex, Lilly, Servier, Merck Sharp & Dohme, GlaxoSmithKline, Leuko, Clovis Oncology, Bioasis, Boehringer Ingelheim, Ellipses Pharma, HiberCell, Bioinvent, GEMoaB, Gilead Sciences, Menarini, Zymeworks, Reveal Genomics
Research Funding: ARIAD (Inst), Astrazeneca (Inst), Baxalta (Inst), Bayer (Inst), Eisai (Inst), Guardant Health (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), Puma Biotechnology (Inst), Queen Mary university of London (Inst), Roche (Inst), Piqur (Inst)
Patents, Royalties, Other Intellectual Property: Pharmaceutical Combinations of A Pi3k Inhibitor And A Microtubule Destabilizing Agent. Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A, Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/ 0338368 A1
Travel, Accommodations, Expenses: Roche, Pfizer, Eisai, Novartis, Daiichi Sankyo, Gilead Sciences
Dirk Laurent
Employment: Berlin-Chemie, Merck KGaA
Stock and Other Ownership Interests: Bayer
Nassir Habboubi
Employment: Stemline Therapeutics
Leadership: Stemline Therapeutics
Maureen G. Conlan
Employment: Radius Health, Elevar Therapeutics
Stock and Other Ownership Interests: Radius Health
Aditya Bardia
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Spectrum Pharmaceuticals, bioTheranostics, Merck, Radius Health, Immunomedics (Inst), Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Radius Health (Inst), Innocrin Pharma (Inst), Immunomedics, Sanofi, Puma Biotechnology, Daiichi Sankyo/Astra Zeneca, Foundation Medicine, Philips Healthcare
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), AstraZeneca/Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/523675
No other potential conflicts of interest were reported.
See accompanying editorial on page 3227
PRIOR PRESENTATION
Presented in part at the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, December 7-10, 2021.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data that underlie the results reported in a published article may be requested for products and the relevant indications that have been authorized by the regulatory authorities in Europe/the United States (or, if not, two years have elapsed since the study completion). Stemline, a member of the Menarini Group, will review requests individually to determine whether (1) the requests are legitimate and relevant and meet sound scientific research principles, (2) are within the scope of the participants' informed consent, and (3) the request is compliant with any applicable law and regulation and with any contractual relationship that Stemline, its affiliates, and partners have in place with respect to the study and/or the relevant product. Before making data available, requestors will be required to agree in writing to certain obligations, including without limitation, compliance with applicable privacy, and other laws and regulations. Proposals should be directed to medicalinfo@stemline.com.
AUTHOR CONTRIBUTIONS
Conception and design: Virginia G. Kaklamani, Emilio Bria, Philippe Aftimos, Javier Cortés, Maureen G. Conlan, Aditya Bardia
Provision of study materials or patients: Francois-Clement Bidard, Virginia G. Kaklamani, Patrick Neven, Guillermo Streich, Marie-Ange Mouret-Reynier, Joo Hyuk Sohn, Donatienne Taylor, Judit Kocsis, Florence Dalenc, Patrick M. Dillon, Sunil Babu, Simon Waters, Ines Deleu, Emilio Bria, Marina Cazzaniga, Janice Lu, Javier Cortés, Giulia Tonini, Aditya Bardia
Collection and assembly of data: Francois-Clement Bidard, Virginia G. Kaklamani, Patrick Neven, Frédéric Forget, Marie-Ange Mouret-Reynier, Joo Hyuk Sohn, Kathleen K. Harnden, Hung Khong, Judit Kocsis, Patrick M. Dillon, Sunil Babu, Ines Deleu, José A. García Sáenz, Emilio Bria, Marina Cazzaniga, Dirk Laurent, Maureen G. Conlan, Aditya Bardia
Data analysis and interpretation: Francois-Clement Bidard, Virginia G. Kaklamani, Patrick Neven, Guillermo Streich, Alberto J. Montero, Frédéric Forget, Donatienne Taylor, Kathleen K. Harnden, Florence Dalenc, Patrick M. Dillon, Sunil Babu, Simon Waters, José A. García Sáenz, Emilio Bria, Janice Lu, Philippe Aftimos, Shubin Liu, Giulia Tonini, Dirk Laurent, Nassir Habboubi, Maureen G. Conlan, Aditya Bardia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Francois-Clement Bidard
Consulting or Advisory Role: Pfizer, AstraZeneca, Lilly, Novartis, Radius Health, Menarini, Sanofi (Inst)
Speakers' Bureau: Pfizer, Novartis, AstraZeneca, Roche, Lilly, Rain Therapeutics
Research Funding: Novartis (Inst), Pfizer (Inst), Menarini Silicon Biosystems (Inst), Prolynx (Inst)
Patents, Royalties, Other Intellectual Property: ESR1 & MSI detection techniques (patents; Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, AstraZeneca, Novartis
Virginia G. Kaklamani
Honoraria: Genentech, Novartis, Pfizer, Genomic Health, Puma Biotechnology, AstraZeneca, Seattle Genetics, daichi, Gilead Sciences
Consulting or Advisory Role: Amgen, Eisai, Puma Biotechnology, Celldex, AstraZeneca, Athenex, bioTheranostics
Speakers' Bureau: Genentech, Novartis, Genomic Health, Puma Biotechnology, Pfizer, AstraZeneca/Daiichi Sankyo
Research Funding: Eisai
Patrick Neven
Consulting or Advisory Role: Lilly (Inst), Pfizer (Inst), Novartis (Inst), Roche (Inst), Roche (Inst), Radius Health (Inst), Radius Health (Inst)
Travel, Accommodations, Expenses: Lilly (Inst), Pfizer (Inst), Roche (Inst)
Alberto J. Montero
Honoraria: Celgene, AstraZeneca, OncoSec
Consulting or Advisory Role: New Century Health, Welwaze
Uncompensated Relationships: Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/618396
Frédéric Forget
Travel, Accommodations, Expenses: Ipsen, Teva
Joo Hyuk Sohn
Research Funding: MSD (Inst), Roche (Inst), Novartis (Inst), Lilly (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst)
Other Relationship: Roche
Donatienne Taylor
Honoraria: AstraZeneca
Consulting or Advisory Role: Lilly, AstraZeneca, Roche/Genentech, Novartis, Agendia, MSD, Seattle Genetics
Travel, Accommodations, Expenses: MSD, AstraZeneca, Pfizer
Kathleen K. Harnden
Honoraria: E-Health Now
Speakers' Bureau: Eisai, Daiichi Sankyo/Astra Zeneca, Seattle Genetics, Merck
Hung Khong
Stock and Other Ownership Interests: MEI Pharma, TG Therapeutics, Lipocine, MustangBio, Tiziana Life Sciences, Vaxart, agenus
Consulting or Advisory Role: Celcuity
Research Funding: AstraZeneca
Patrick M. Dillon
Research Funding: Novartis (Inst), Pfizer (Inst), AbbVie (Inst), Newlink Genetics (Inst), Tesaro (Inst), Merck (Inst), Tolero Pharmaceuticals (Inst), Radius Health (Inst)
Sunil Babu
Employment: Fort Wayne Medical Oncology & Hematology
Stock and Other Ownership Interests: Fort Wayne Medical Oncology & Hematology, Lutheran hospital
Honoraria: Bristol Myers Squibb, Alexion Pharmaceuticals, Lilly, Bayer, AstraZeneca, Castle Biosciences, Pharmacosmos, Beigene, Kite, a Gilead company
Consulting or Advisory Role: Bristol Myers Squibb, Alexion Pharmaceuticals, AstraZeneca, argenx, Boehringer Ingelheim, Bayer, Kite, a Gilead company, Janssen Oncology, Amgen
Speakers' Bureau: Alexion Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Genentech/Roche (Inst), AstraZeneca/MedImmune (Inst), Janssen Oncology (Inst), Amgen (Inst), TG Therapeutics (Inst), AbbVie (Inst), Lilly (Inst), Alexion Pharmaceuticals (Inst), Merck (Inst), Novartis (Inst), Syndax (Inst), Nektar (Inst), Sanofi (Inst), argenx (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Alexion Pharmaceuticals, Lilly, Janssen Oncology, Genentech/Roche
Simon Waters
Honoraria: Novartis Pharmaceuticals UK Ltd
Consulting or Advisory Role: Novartis Pharmaceuticals UK Ltd, Sanofi/Aventis, Daiichi Sankyo/Astra Zeneca
Ines Deleu
Consulting or Advisory Role: BMS, Novartis, Pierre Fabre
Travel, Accommodations, Expenses: Sanofi, Pierre Fabre
José A. García Sáenz
Consulting or Advisory Role: Novartis, AstraZeneca, Lilly, AstraZeneca, Daiichi Sankyo/Astra Zeneca, Gilead Sciences, Seattle Genetics
Speakers' Bureau: Novartis
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Roche, Novartis
Emilio Bria
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Pfizer, Lilly, Bristol Myers Squibb, Roche
Research Funding: AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD
Marina Cazzaniga
Honoraria: Pierre Fabre, Roche, Pfizer
Consulting or Advisory Role: Pierre Fabre, Roche, Novartis
Research Funding: Lilly (Inst), Eisai Europe (Inst)
Janice Lu
Honoraria: Pfizer, Lilly, Sanofi/Aventis, AstraZeneca/Daiichi Sankyo, Seattle Genetics
Consulting or Advisory Role: Pfizer, Novartis, Lilly, Sanofi/Aventis, AstraZeneca/Daiichi Sankyo
Research Funding: Novartis (Inst), Lilly (Inst), Ambrx (Inst), Genentech (Inst), Radius Health (Inst)
Philippe Aftimos
Honoraria: Synthon, Roche, Gilead Sciences
Consulting or Advisory Role: Macrogenics, Boehringer Ingelheim, Novartis, Amcure, Roche, Novartis, Amgen, Servier, G1 Therapeutics, Radius Health, Deloitte, Menarini, Gilead Sciences
Travel, Accommodations, Expenses: Amgen, MSD Oncology, Roche Belgium, Pfizer
Javier Cortés
Stock and Other Ownership Interests: MedSIR, Nektar, Leuko
Honoraria: Novartis, Eisai, Celgene, Pfizer, Roche, Samsung, Lilly, Merck Sharp & Dohme, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Cellestia Biotech, AstraZeneca, Roche, Seattle Genetics, Daiichi Sankyo, ERYTECH Pharma, Polyphor, Athenex, Lilly, Servier, Merck Sharp & Dohme, GlaxoSmithKline, Leuko, Clovis Oncology, Bioasis, Boehringer Ingelheim, Ellipses Pharma, HiberCell, Bioinvent, GEMoaB, Gilead Sciences, Menarini, Zymeworks, Reveal Genomics
Research Funding: ARIAD (Inst), Astrazeneca (Inst), Baxalta (Inst), Bayer (Inst), Eisai (Inst), Guardant Health (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), Puma Biotechnology (Inst), Queen Mary university of London (Inst), Roche (Inst), Piqur (Inst)
Patents, Royalties, Other Intellectual Property: Pharmaceutical Combinations of A Pi3k Inhibitor And A Microtubule Destabilizing Agent. Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A, Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/ 0338368 A1
Travel, Accommodations, Expenses: Roche, Pfizer, Eisai, Novartis, Daiichi Sankyo, Gilead Sciences
Dirk Laurent
Employment: Berlin-Chemie, Merck KGaA
Stock and Other Ownership Interests: Bayer
Nassir Habboubi
Employment: Stemline Therapeutics
Leadership: Stemline Therapeutics
Maureen G. Conlan
Employment: Radius Health, Elevar Therapeutics
Stock and Other Ownership Interests: Radius Health
Aditya Bardia
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Spectrum Pharmaceuticals, bioTheranostics, Merck, Radius Health, Immunomedics (Inst), Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Radius Health (Inst), Innocrin Pharma (Inst), Immunomedics, Sanofi, Puma Biotechnology, Daiichi Sankyo/Astra Zeneca, Foundation Medicine, Philips Healthcare
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), AstraZeneca/Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/523675
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 8.2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [DOI] [PubMed] [Google Scholar]
- 2.Gennari A, André F, Barrios CH, et al. : ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 32:1475-1495, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Burstein HJ, Somerfield MR, Barton DL, et al. : Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol 39:3959-3977, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeselsohn R, Yelensky R, Buchwalter G, et al. : Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res 20:1757-1767, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Razavi P, Chang MT, Xu G, et al. : The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34:427-438, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein HJ: Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N Engl J Med 383:2557-2570, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Toy W, Shen Y, Won H, et al. : ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 45:1439-1445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moy B, Rumble RB, Come SE, et al. : Chemotherapy and targeted therapy for patients with human epidermal growth factor receptor 2-negative metastatic breast cancer that is either endocrine-pretreated or hormone receptor-negative: ASCO guideline update. J Clin Oncol 39:3938-3958, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Lindeman GJ, Bowen R, Jerzak JK, et al. : Results from VERONICA: A randomized, phase II study of second-/third-line venetoclax + fulvestrant vs. fulvestrant alone in ER-positive, HER2-negative, locally advanced, or metastatic breast cancer. J Clin Oncol 39, 2021. (suppl 15; abstr 1004) [Google Scholar]
- 10.Turner NC, Kingston B, Kilburn LS, et al. : Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol 21:1296-1308, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Leo A, Johnston S, Lee KS, et al. : Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19:87-100, 2018 [DOI] [PubMed] [Google Scholar]
- 12.André F, Ciruelos E, Rubovszky G, et al. : Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 38020:1929-1940, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Bihani T, Patel HK, Arlt H, et al. : Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER+ breast cancer patient-derived xenograft models. Clin Cancer Res 23:4793-4804, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Wardell SE, Nelson ER, Chao CA, et al. : Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr Relat Cancer 22:713-724, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garner F, Shomali M, Paquin D, et al. : RAD1901: A novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs 26:948-956, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel HK, Tao N, Lee KM, et al. : Elacestrant (RAD1901) exhibits anti-tumor activity in multiple ER+ breast cancer models resistant to CDK4/6 inhibitors. Breast Cancer Res 21:146, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardia A, Kaklamani V, Wilks S, et al. : Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-Positive, HER2-negative advanced breast cancer. J Clin Oncol 39:1360-1370, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardia A, Aftimos P, Bihani T, et al. : EMERALD: Phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol 15:3209-3218, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Hammond ME, Hayes DF, Dowsett M, et al. : American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784-2795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond MEH, Allison KH, et al. : HER2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update summary. JCO Oncol Pract 14:437-441, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Haybittle JL: Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 44:793-797, 1971 [DOI] [PubMed] [Google Scholar]
- 23.Peto R, Pike MC, Armitage P, et al. : Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 34:585-612, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton EP, Dees EC, Wang JS-Z, et al. : Phase I dose escalation of H3B-6545, a first-in-class highly selective ERα covalent antagonist (SERCA), in women with ER-positive, HER2-negative breast cancer (HR+ BC). J Clin Oncol 37, 2019. (suppl; abstr 1059) [Google Scholar]
- 25.Hamilton EP, Oliveira M, Banerji U, et al. : A phase I dose escalation and expansion study of the next generation oral SERD AZD9833 in women with ER-positive, HER2-negative advanced breast cancer. J Clin Oncol 38, 2020. (suppl; abstr 1024) [DOI] [PubMed] [Google Scholar]
- 26.Lim E, Jhaveri KL, Perez-Fidalgo JA, et al. : A phase Ib study to evaluate the oral selective estrogen receptor degrader GDC-9545 alone or combined with palbociclib in metastatic ER-positive HER2-negative breast cancer. J Clin Oncol 38, 2020. (suppl; abstr 1023) [Google Scholar]
- 27.Bross PF, Cohen MH, Williams GA, et al. : FDA drug approval summaries: Fulvestrant. Oncologist 7:477-480, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Griffiths JI, Chen J, Cosgrove PA, et al. : Serial single-cell genomics reveals convergent subclonal evolution of resistance as early-stage breast cancer patients progress on endocrine plus CDK4/6 therapy. Nat Cancer 2:658-671, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouridsen H, Gershanovich M, Sun Y, et al. : Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21:2101-2109, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Milla-Santos A, Milla L, Portella J, et al. : Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: A prospective, randomized, phase III study. Am J Clin Oncol 26:317-322, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Rugo HS, Lerebours F, Ciruelos E, et al. : Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol 22:489-498, 2021 [DOI] [PubMed] [Google Scholar]
- 32.André F, Ciruelos EM, Juric D, et al. : Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann Oncol 32:208-217, 2021 [DOI] [PubMed] [Google Scholar]
- 33.Baselga J, Campone M, Piccart M, et al. : Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520-529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that underlie the results reported in a published article may be requested for products and the relevant indications that have been authorized by the regulatory authorities in Europe/the United States (or, if not, two years have elapsed since the study completion). Stemline, a member of the Menarini Group, will review requests individually to determine whether (1) the requests are legitimate and relevant and meet sound scientific research principles, (2) are within the scope of the participants' informed consent, and (3) the request is compliant with any applicable law and regulation and with any contractual relationship that Stemline, its affiliates, and partners have in place with respect to the study and/or the relevant product. Before making data available, requestors will be required to agree in writing to certain obligations, including without limitation, compliance with applicable privacy, and other laws and regulations. Proposals should be directed to medicalinfo@stemline.com.