PURPOSE
The status of p53 in a tumor can be inferred by next-generation sequencing (NGS) or by immunohistochemistry (IHC). We examined the association between p53 IHC and sequence and whether p53 IHC alone, or integrated with TP53 NGS, predicts the outcome.
METHODS
From GOG-86P, a randomized phase II study of chemotherapy combined with either bevacizumab or temsirolimus in advanced endometrial cancer, 213 cases had p53 protein expression data measured by IHC and TP53 NGS data. An analysis was designed to integrate p53 expression by IHC with the presence or absence of a TP53 mutation. These variables were further correlated with progression-free survival (PFS) and overall survival (OS) in the chemotherapy plus bevacizumab arms versus the chemotherapy plus temsirolimus arm.
RESULTS
In the analysis of p53 IHC, the most striking treatment effect favoring bevacizumab was in cases where p53 was overexpressed (PFS hazard ratio [HR]: 0.46, 95% CI, 0.26 to 0.88; OS HR: 0.31, 95% CI, 0.16 to 0.62). On integrated analysis, patients with TP53 missense mutations and p53 protein overexpression had a similar treatment effect on PFS (HR: 0.41, 95% CI, 0.22 to 0.83) and OS (HR: 0.28, 95% CI, 0.14 to 0.59) favoring bevacizumab plus chemotherapy relative to temsirolimus plus chemotherapy. Concordance between TP53 NGS and p53 IHC was 88%. Concordance was 92% when cases with TP53 mutations and POLE mutations or mismatch repair deficiency were removed.
CONCLUSION
IHC for p53 alone or when integrated with sequencing for TP53 identifies a specific, high-risk tumor genotype/phenotype for which bevacizumab is particularly beneficial in improving outcomes when combined with chemotherapy.
INTRODUCTION
Cancer of the uterine corpus, or endometrial cancer, is the most common gynecologic malignancy in the United States.1 Furthermore, endometrial cancer incidence and mortality are on the rise worldwide.1-3 Nearly 66,000 cases are expected in 2022, and 13,000 lives will be lost to this pervasive and understudied disease.4,5 In response to these alarming statistics, cooperative groups such as NRG Oncology and its antecedent organization, the Gynecologic Oncology Group (GOG), have sought to improve outcomes for women with advanced and recurrent diseases.
CONTEXT
Key Objective
Clinical management of advanced endometrial cancer is transitioning from histologic subtype to molecular stratification. The most aggressive molecular subtype is abnormal p53; however, a major debate is how to appropriately determine p53 status. We performed a retrospective analysis of p53 status by immunohistochemistry (IHC) and next-generation sequencing (NGS) using specimens from a completed clinical trial, GOG-86P.
Knowledge Generated
p53 expression by IHC strongly correlated with mutational status in > 90% of cases. p53 overexpression by IHC identified patients with improved outcomes when bevacizumab is added to the chemotherapy backbone, with an overall survival hazard ratio of 0.31.
Relevance
Determining p53 status by IHC is a fast, cost-effective, and reliable method that can be easily integrated into clinical management of advanced endometrial cancer. The integration of sequencing with IHC may allow better stratification of cases in which the p53 immunostaining reveals an unusual pattern, such as cytoplasmic or highly heterogeneous p53 expression.
GOG-86P was one of the first studies to combine molecular inhibitors such as bevacizumab or temsirolimus with chemotherapy in patients with advanced endometrial cancer.6 GOG-86P was a three-arm, randomized phase II study of paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab as the initial therapy for measurable advanced or recurrent endometrial cancer.6 The conclusion of that study was that the primary end point, grouped progression-free survival (PFS), was not significantly increased in any experimental arm compared with historical controls from GOG-209 who were treated with chemotherapy alone.7 However, a recently published follow-up exploratory analysis assessed outcomes on the basis of mutations in the tumor suppressor TP53,8 the most commonly mutated gene in advanced/recurrent endometrial cancer.9 When the overall impact of TP53 mutations determined by next-generation sequencing (NGS) was assessed on all experimental arms of the study combined, those patients whose tumors harbored mutated TP53 did worse, both by PFS and by overall survival (OS), compared with patients whose tumors had wild-type (WT) TP53.8 This is not surprising given the association between mutant p53 and aggressive histology10-14 and highlights the acute need to develop more effective therapies for patients with mutated TP53 tumors.15,16
However, when cases with TP53 mutations were evaluated and compared on the individual experimental arms of GOG-86P, a picture of differential sensitivity emerged. The conclusion of the original GOG-86P report showed no PFS benefit compared with historical controls treated with chemotherapy alone. However, this more recent exploratory analysis indicates that bevacizumab combined with chemotherapy is superior to temsirolimus plus chemotherapy, specifically in patients with TP53-mutated tumors as determined by NGS,8 although a chemotherapy-only reference arm was not included in the GOG-86P trial design.6 Indeed, the risks of progression and/or death for those patients were significantly decreased in the bevacizumab arms (PFS hazard ratio [HR]: 0.48, 95% CI, 0.31 to 0.75; OS HR: 0.61, 95% CI, 0.38 to 0.98). On the other hand, patients with WT TP53 did not experience improved outcomes on either arm. Since the original GOG-86P included a third arm of bevacizumab in combination with a different microtubule spindle disruptor (ixabepilone) in place of paclitaxel, this retrospective study combined the two bevacizumab-containing arms into a single group to increase power to detect differences. However, the improvement in PFS and OS with p53-mutated tumors persisted when the bevacizumab-containing arms were analyzed independently.8
Given the identification of mutated TP53 as a relevant biomarker for improved outcomes in the bevacizumab plus chemotherapy arms of GOG-86P, we wished to determine how best to assess tissues for p53 status. Fortunately, translational end points for 213 patients on GOG-86P included both immunohistochemistry (IHC) for p53 and NGS of TP53, enabling the current analyses. Herein, we performed a comparison between IHC and NGS and determined the association of both variables with histology and clinical outcomes. As with the previous exploratory study of GOG-86P,8 we combined data from the two bevacizumab-containing arms into a single group and compared outcomes with those of patients in the temsirolimus plus chemotherapy group.
METHODS
Detailed methods are provided in the Data Supplement (online only).
Study Cohort
GOG-86P (ClinicalTrials.gov identifier: NCT00977574) was a three-arm, randomized phase II study of paclitaxel/carboplatin/bevacizumab (NSC#704865, IND#7921), paclitaxel/carboplatin/temsirolimus (NSC#683864, IND#61010), or ixabepilone/carboplatin/bevacizumab (NSC#710428, IND#59699) as initial therapy for measurable stage III or IVA, stage IVB, or recurrent endometrial cancer. Biospecimens were collected from patients who consented to participate in the translational research component of the study. The collection of archival formalin-fixed paraffin-embedded tissue and tumor DNA was coordinated by the NRG Oncology Biospecimen Bank. From these cases, 213 tumors were analyzed using IHC for p53 and NGS for TP53. NGS methodology has been previously reported in detail.8 The distribution of p53 expression (IHC) and mutational status (TP53 NGS) by race and ethnicity are provided in the Data Supplement.
TP53 Mutation Classification Criteria
TP53 mutations by NGS were grouped into four categories: (1) WT: mutations that did not result in an amino acid substitution; (2) truncating: frameshift or splice site mutations that result in a truncated protein; (3) gain of function (GOF): missense mutations that are canonical gain-of-function mutations (P151S, Y163C, R175H, L194R, Y220C, R248Q, R248W, R273C, R273H, R273L, and R282W11-13); and (4) unclassified missense mutations or indels, referred to herein as somatic variants of unknown function (sVUFs).
p53 IHC
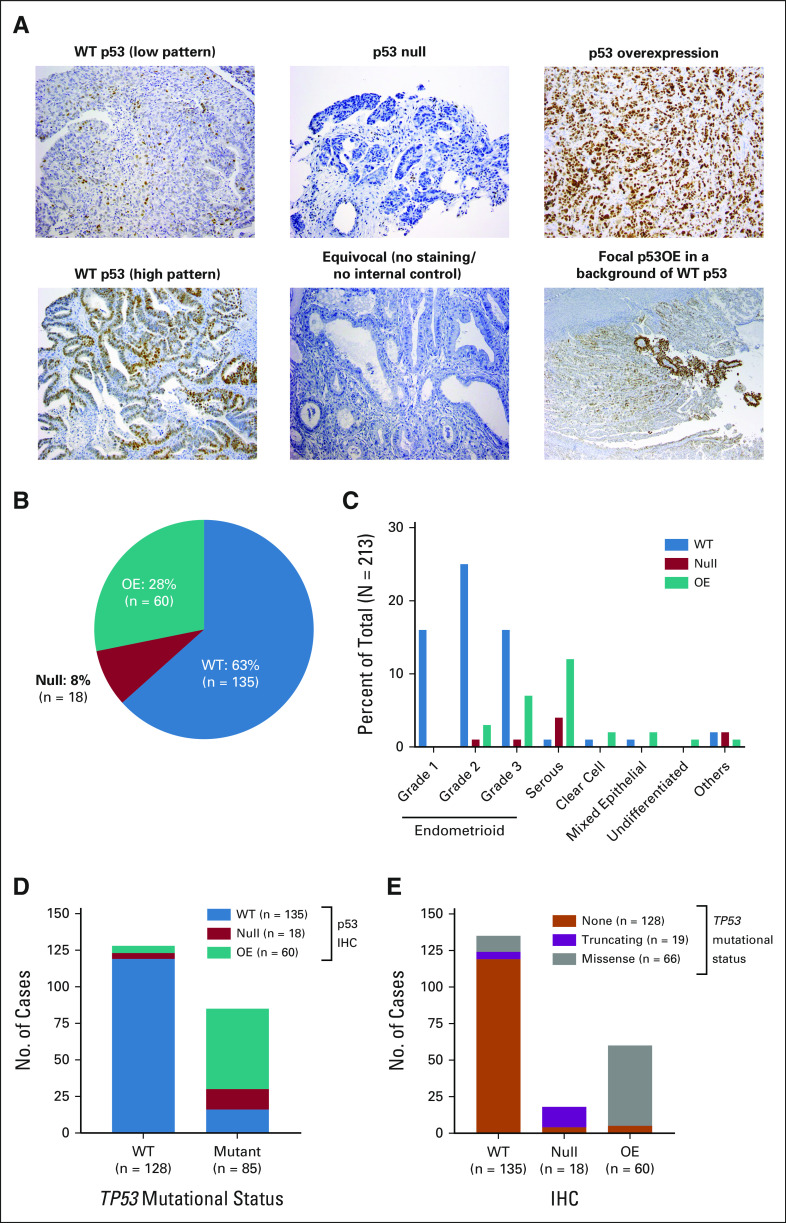
IHC for p53 (DO-7, Dako) was performed as previously described,17 and cases were assigned to one of three categories (Fig 1A): (1) WT: 1%-80% of tumor cell nuclei staining positive, usually with variable intensity; (2) null: no tumor cell nuclear staining with a positive internal control; and (3) overexpressed (OE): uniform and intense nuclear staining in at least 80% of tumor cell nuclei (estimated). Some cases did not fall into these simplified groups; examples are shown in Figure 1A. Cases were scored as equivocal when there was no nuclear signal in the absence of a positive internal control. Cases scored as focal had overall WT staining but with focal areas of clear p53 overexpression, indicating heterogeneity with respect to p53 expression.
FIG 1.
Concordance between p53 expressions by IHC with mutation in TP53. (A) Examples of WT p53 staining (both low and high patterns), null p53, and overexpression of p53. Also shown are cases with equivocal staining for p53 and focal regions of p53 overexpression. (B) Overall distribution of p53 expression by IHC in cases with available data. (C) Percent of cases with WT p53 staining, null staining, or OE by histology. (D) Concordance on the basis of the absence (WT) or presence of a TP53 mutation with p53 expression by IHC. (E) Concordance on the basis of the specific type of TP53 mutation. IHC, immunohistochemistry; OE, overexpressed; WT, wild-type.
Statistical Analyses
An exploratory analysis of biomarkers on GOG-86P was performed. Patients were grouped based on tumor TP53 mutational status, histology, and p53 IHC results. Outcomes (PFS and OS) were compared between treatment arms or between groups using proportional hazards models. Data from the two bevacizumab-containing arms (arms 1 and 3) were combined and compared with the temsirolimus-containing arm (arm 2).
RESULTS
IHC and Relationship to Histology
Of the 213 cases, 63% were WT by IHC, 28% were OE, and 8% were null (Fig 1B). The p53 null and OE cases were generally restricted to higher-grade endometrioid and serous histologies (Fig 1C).
IHC and Relationship to TP53 Sequence
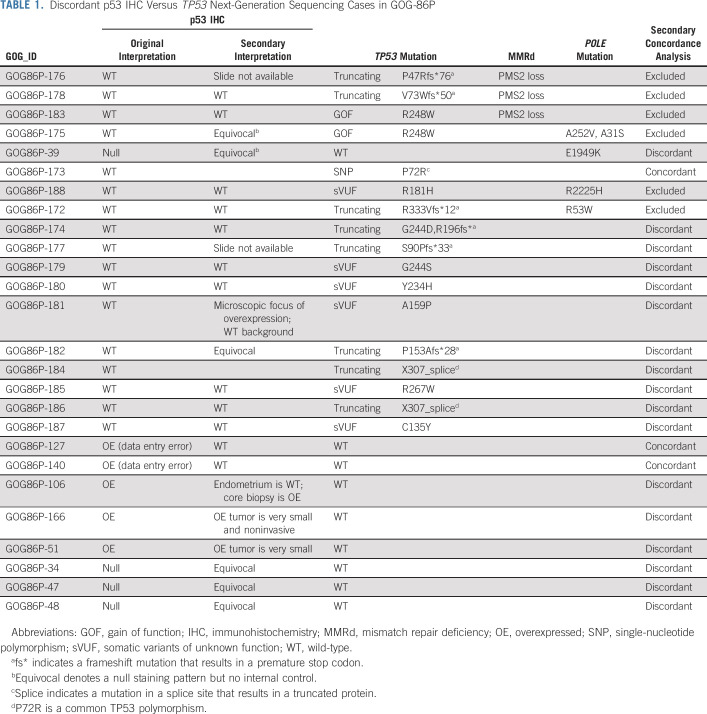
We next examined the relationship between TP53 mutational status and p53 protein expression by IHC. The overall concordance rate between p53 protein expression and TP53 mutational status was 88%. Among tumors with WT p53 by IHC, 88% also had no mutations in the TP53 gene (Fig 1D). Among the cases with mutated TP53, cases with truncating mutations were generally p53 null (78%) and cases with missense mutations were OE by IHC (92%, Fig 1E). No case with a predicted truncating mutation in TP53 was OE by IHC. The discordance rate was low—in only 26 of 213 cases (12%), the IHC did not predict the TP53 sequence (Table 1). Some of the discordant cases can be potentially explained by concurrent mutations in polymerase epsilon (POLE) or by mismatch repair deficiency (MMRd; Table 1). For other cases, p53 staining was either focal or equivocal, which confounds categorical binning. A secondary review also identified two data entry errors and one TP53 variant (P72R) that is a well-documented polymorphism.18 We therefore re-evaluated the concordance of p53 IHC with TP53 sequencing after removing the cohort with TP53 mutations and a concurrent POLE mutation and/or MMRd; we also moved the three misclassified cases from the discordant group to the concordant group. The updated concordance was 92%.
TABLE 1.
Discordant p53 IHC Versus TP53 Next-Generation Sequencing Cases in GOG-86P
We did not observe a relationship between p53 status (IHC and NGS) and race or ethnicity (Data Supplement), which may be due to the high representation of White (80%) and non-Hispanic (84%) patients in the study cohort.
Relationship of Sequence and IHC as Single Discriminators of Clinical Outcomes
GOG-86P HRs and CIs for NGS status alone.
Since TP53 mutations predominate in the serous subtype of endometrial cancer,9 we first analyzed serous histology as a predictor of response (Data Supplement). Analysis of outcomes by histology indicates improvement in PFS in nonserous cases with bevacizumab (HR: 0.74, 95% CI, 0.56 to 0.98) and a trend toward improvement in the bevacizumab arms in serous cases (HR: 0.61, 95% CI, 0.36 to 1.05).
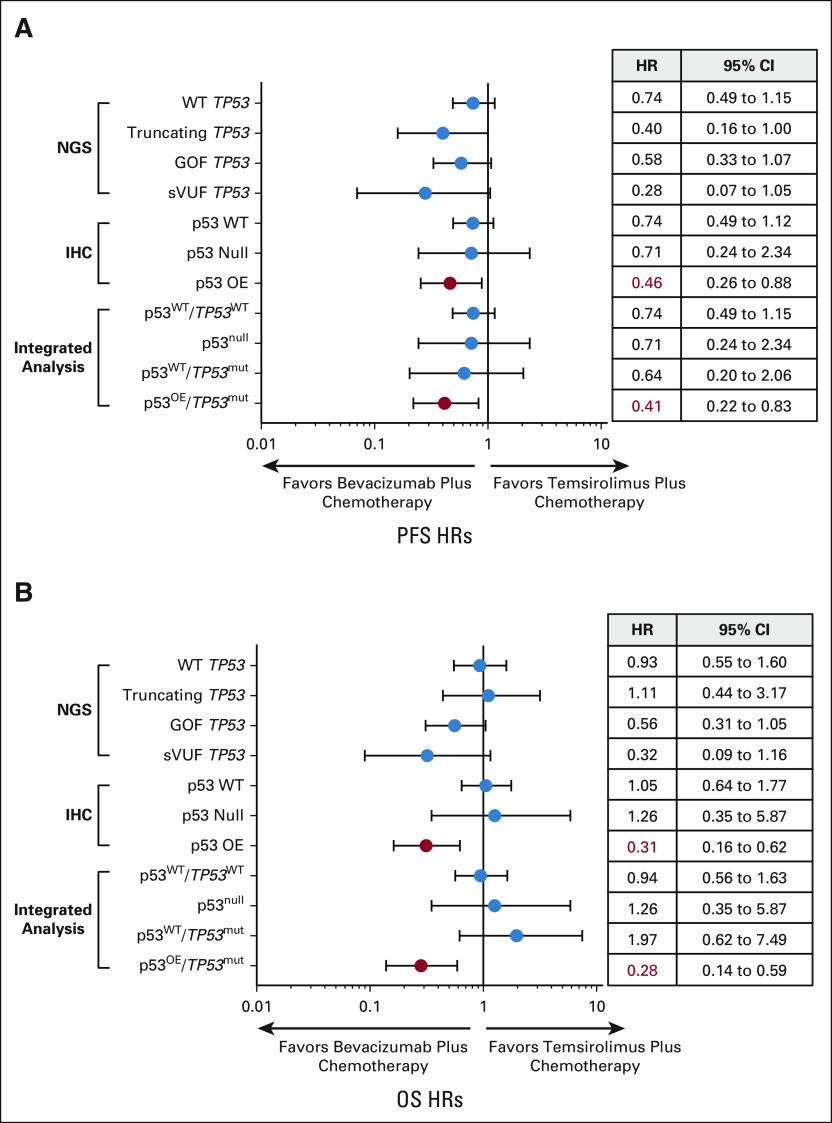
We recently reported that the PFS HR for bevacizumab plus chemotherapy versus temsirolimus plus chemotherapy in cases with mutated TP53 is 0.48 (95% CI, 0.31 to 0.74) compared with 0.87 (95% CI, 0.58 to 1.30) for WT tumors.8 For OS, the HR was 0.61 (95% CI, 0.38 to 0.96) for TP53-mutated cases versus 1.05 (95% CI, 0.64 to 1.74) for WT cases.8 We further divided the mutated cases into three categories: (1) truncating; (2) known oncogenic or GOF; and (3) missense mutations of unknown function, which we term herein sVUF. The presence of a TP53 GOF mutation was prognostic, meaning regardless of therapy such a mutation portended a worse outcome with a significantly lower PFS and OS as compared with cases with WT p53 (Data Supplement). The low number of cases in each category did not provide adequate power to make a definitive statement as to the impact of bevacizumab versus temsirolimus for each type of TP53 mutation. Nevertheless, we observed a trend toward improved PFS and OS with bevacizumab in all groups, most notably cases with missense GOF or sVUF mutations (Fig 2, Data Supplement).
FIG 2.
Comparison of HRs for bevacizumab plus chemotherapy versus temsirolimus plus chemotherapy by TP53 mutational status, expression by IHC, or integrated sequencing/IHC analysis. Forest plots of (A) PFS and (B) OS. Also show are HR and 95% CI. Significantly improved HRs are indicated in red. GOF, gain of function; HR, hazard ratio; IHC, immunohistochemistry; NGS, next-generation sequencing; OS, overall survival; PFS, progression-free survival; sVUF, somatic variants of unknown function; WT, wild-type.
GOG-86P treatment HRs and CIs for IHC status alone.
Evaluation of IHC as a prognostic factor suggests that patients with overexpression of p53 by IHC had a worse OS than cases with WT p53 (Fig 3A). However, overexpression of p53 portended longer outcomes on the bevacizumab-containing arm as compared with temsirolimus (Figs 2 and 3B). Specifically, the HR and 95% CIs for PFS on the basis of IHC alone, bevacizumab plus chemotherapy versus temsirolimus plus chemotherapy, are 0.46 (0.26 to 0.88) for OE IHC, 0.71 (0.24 to 2.34) for null IHC, and 0.74 (0.49 to 1.12) for WT by IHC (Fig 2A). For OS on the basis of IHC, the HR for OE = 0.31 (0.16 to 0.62), null = 1.26 (0.35 to 5.87), and WT = 1.05 (0.65 to 1.77, Fig 2B). There was no difference in outcomes by treatment arm for cases with a WT p53 expression pattern by IHC, and the number of cases in the p53-null category was too small to discern differences between groups (Data Supplement).
FIG 3.
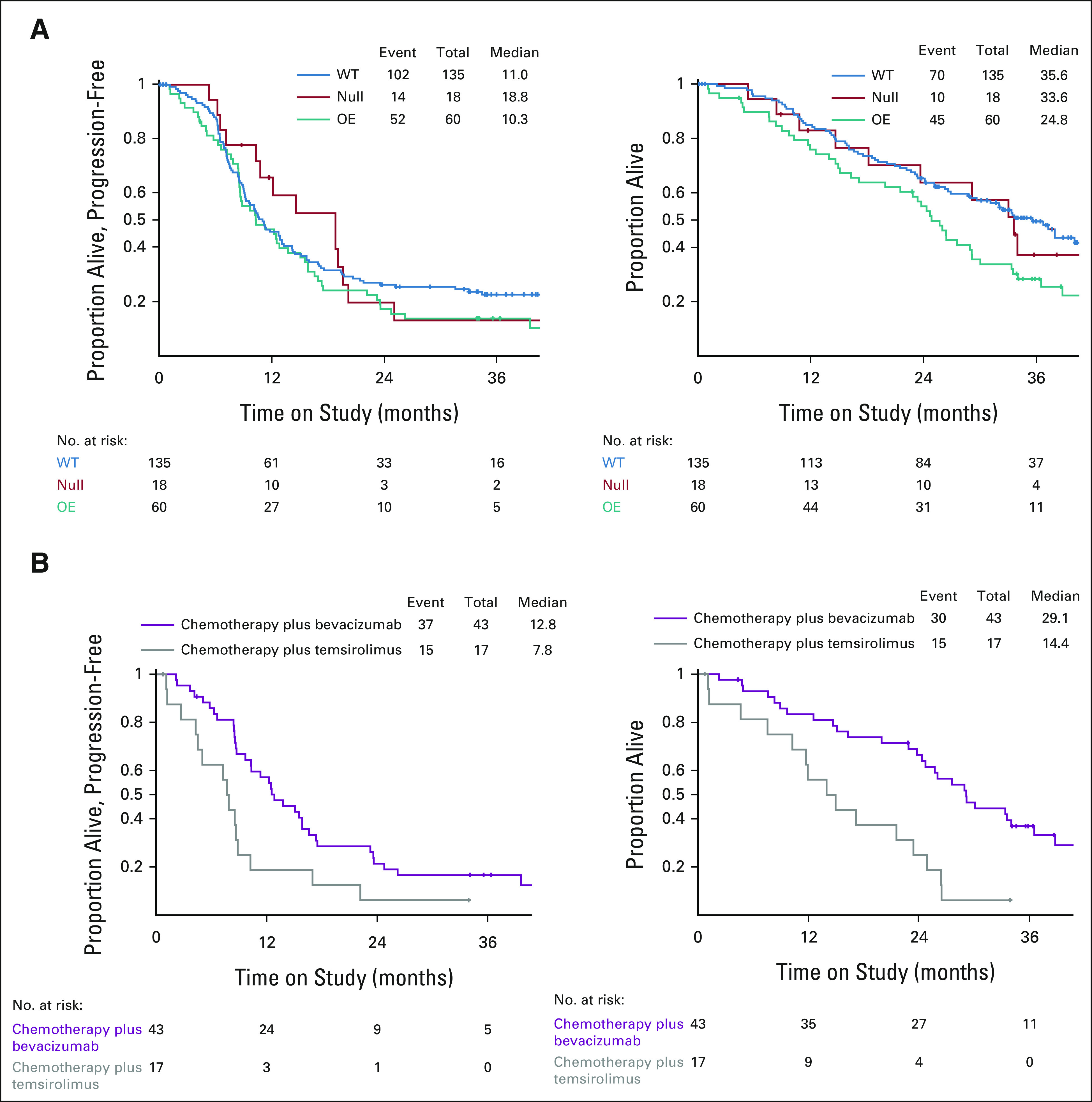
p53 overexpression by IHC predicts for response to bevacizumab plus chemotherapy. (A) Kaplan-Meier curves for PFS (left) and OS (right) on the basis of p53 status by IHC. (B) Kaplan-Meier curves for PFS (left) and OS (right) for cases with p53 overexpression by IHC by treatment arm. IHC, immunohistochemistry; OE, overexpressed; OS, overall survival; PFS, progression-free survival; WT, wild-type.
Integrated Analysis of Sequence and IHC as Discriminators of Clinical Outcomes
To improve on a p53-based classifier of patients who most benefited from bevacizumab, we designed an integrated analysis scheme to account for both IHC and TP53 sequence variables (Fig 4A).
FIG 4.
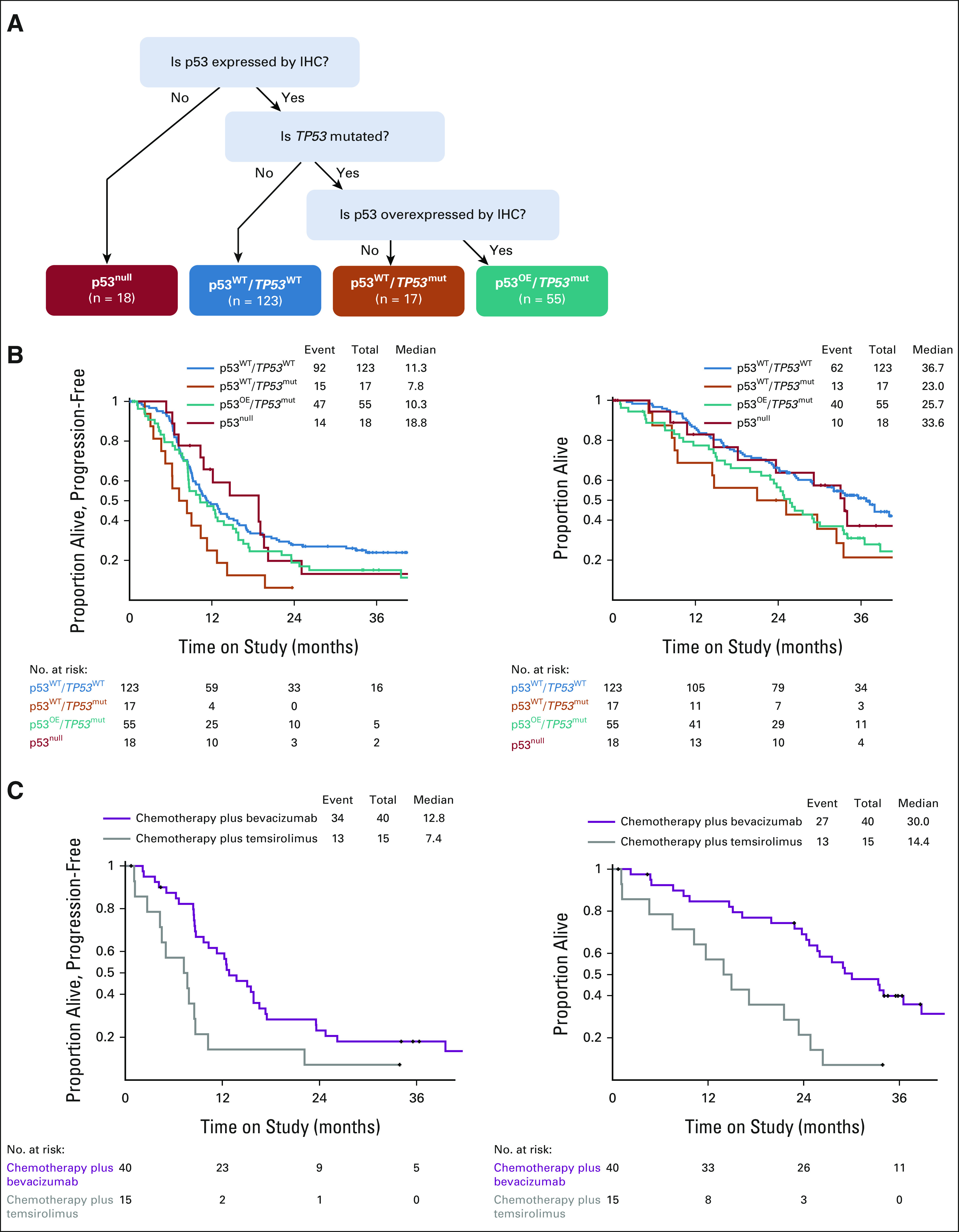
Integrated analysis of p53 using IHC and sequencing data improves the prediction of response to bevacizumab plus chemotherapy. (A) Decision tree for integrated analysis of p53 by IHC and sequencing. Kaplan-Meier curves for (B) PFS and (C) OS for the integrated categories independent of treatment. Kaplan-Meier curves for (D) PFS and (E) OS for the integrated category of p53OE/TP53mut by treatment arm. IHC, immunohistochemistry; OE, overexpressed; OS, overall survival; PFS, progression-free survival.
Similar to an analysis by IHC alone, the integrated category with OE p53 by IHC and mutation in TP53, designated the p53OE/TP53mut category, had worse OS as compared with cases with WT p53, although there was not a significant difference in PFS (Fig 4B, compare blue and teal lines; HR PFS: 0.75, 95% CI, 0.53 to 1.08; HR OS: 0.58, 95% CI, 0.39 to 0.86). However, both PFS and OS were increased for cases in the p53OE/TP53mut category when treated with bevacizumab plus chemotherapy versus temsirolimus plus chemotherapy: PFS HR = 0.41 (95% CI, 0.22 to 0.83) and OS HR = 0.28 (95% CI, 0.14 to 0.59, Fig 4C). The improvement noted for this group of patients remains significant whether the two bevacizumab arms (arms 1 and 3) are combined when compared with the temsirolimus arm (arm 2; Fig 4C and Data Supplement).
DISCUSSION
To date, it has been unclear which patients with endometrial cancer benefit from the addition of bevacizumab to chemotherapy. Patients whose tumors harbor mutated p53 have worse outcomes in general,8,9,19,20 and better treatments are needed for these individuals. We have previously reported in the GOG-86P study that while overall there was no statistically significant PFS benefit of adding bevacizumab to chemotherapy compared with historical controls, patients whose tumors harbored mutant TP53 benefited significantly from bevacizumab when added to chemotherapy in the upfront setting when compared with the nonbevacizumab experimental arm.8 But what is the best test to identify the cases with mutated p53? This question is especially timely given the integration of molecular stratification of endometrial cancer to prospectively assign patients to treatments in the PORTEC-4a trial.21,22 To our knowledge, this trial is a landmark trial for endometrial cancer because it is the first to integrate clinical and molecular prognostic factors to select adjuvant therapy, and p53 status is one of four molecular markers. The functional status of p53 in a tumor can be inferred by sequence analysis and/or by IHC.23 Herein, we sought to determine whether p53 IHC was predictive of TP53 mutations using sequence analysis and whether p53 IHC alone or integrated with TP53 sequencing was similarly predictive of outcome. By so doing, we hope to guide the most predictive yet feasible methodology to assess the p53 status of gynecologic tumors given the growing evidence that tumors with mutated p53 deserve special consideration with respect to therapy.8,19,20
The most important finding from this study was the identification of a potential new biomarker (p53 status) identifying a subset of patients on GOG-86P who benefited significantly from the addition of bevacizumab to upfront chemotherapy. p53 IHC alone had significant discriminatory power, with a PFS HR of 0.46 and an OS HR of 0.31 favoring bevacizumab arms in those with overexpression. On integrated analysis using both NGS and IHC platforms, patients whose tumors harbored a TP53 mutation by NGS accompanied by p53 protein overexpression by IHC (p53OE/TP53mut) were found to derive substantial benefits. In particular, the OS HR of 0.28 reflects a durable, long-term improvement in survival when bevacizumab is added to chemotherapy as a frontline treatment, with a median OS of 30.0 months as compared with 14.4 months in the chemotherapy plus temsirolimus arm.
Overall, concordance between TP53 mutation status and IHC was very good at 88% overall. Several studies in endometrial and other cancer types have demonstrated a strong association of p53 staining and mutational status, with a concordance of over 90%.24-26 There are a number of legitimate biologic explanations for why sequence and IHC results may not be 100% concordant in 24 discordant cases, including the presence of a POLE mutation in the tumor or MMRd (see also the Data Supplement). Indeed, the concordance rate was enhanced to 92% when the six discrepant cases with a TP53 mutation and either a POLE mutation or MMRd were excluded from the analysis.
Our findings are in line with the molecular stratification of endometrial cancer as set forth in the ProMisE algorithm (Proactive Molecular Risk Classifier for Endometrial Cancer)19 and a similar classifier developed by McAlpine and colleagues.27 Cases are first assessed for MMRd by IHC for MMR proteins PMS2 and MSH6, followed by identification of POLE hotspot mutations by NGS. The remaining cases are subjected to IHC for p53 and binned as either abnormal (p53abn) or WT. If IHC for p53 is not definitive, then NGS for mutations is performed whereby cases with mutated p53 are classified as p53abn.19 WT p53 cases that have no POLE mutations or MMRd are designated as no specific molecular profile or NSMP. The ProMisE algorithm treats both null/truncating p53 cases the same as OE/missense mutations. Our analysis of p53 goes a step further and subdivides the p53abn cases by the expression level of p53 by IHC (null, WT, or OE) and the presence or absence of a TP53 mutation, whether it be a canonical GOF mutation or an sVUF. Note that truncating mutations were mutually exclusive from p53 overexpression by IHC (Fig 1E).
A limitation of our study is the inability to comment on the specific impact of truncating mutations in TP53 and/or null cases by IHC because of the low number of such cases. Subsequent studies of p53 null cases are warranted, given that previous studies in ovarian cancer demonstrated that loss of p53 portends poorer outcomes.28,29 Other limitations of this study include that not all patients on the trial had available IHC and/or NGS data, which creates a potential for bias. In addition, the GOG-86P Protocol (online only) did not include a chemotherapy-only arm, and no IHC or NGS data are available from the trial GOG-209, which served as the historical chemotherapy alone control for PFS and OS.
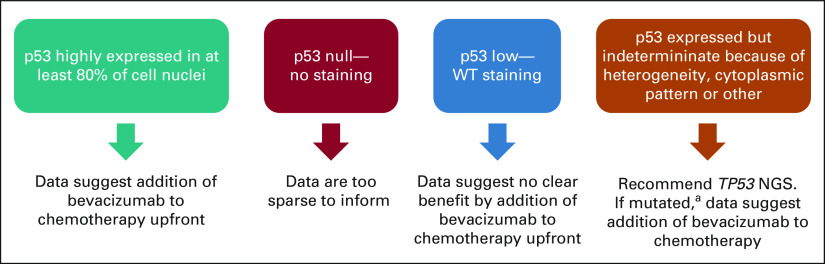
While these data are the product of a retrospective, secondary analysis and require prospective confirmation, our findings lend support to a protocol for the assessment of tumor p53 status. A stepwise approach to tumor evaluation could be instituted as outlined in Figure 5, wherein advanced endometrial cancers are first subjected to IHC for p53. If p53 is OE, a patient with the potential to benefit significantly from bevacizumab plus chemotherapy upfront is identified. However, the integration of sequence and IHC provides a slightly better prediction if both tests can be performed, particularly in those rare cases where immunostaining reveals an unusual pattern such as cytoplasmic or highly heterogeneous p53 expression. In the event that sequencing is not available as an option to the patient (eg, lack of insurance reimbursement), p53 IHC alone may be sufficient to predict for sensitivity to bevacizumab-containing chemotherapy. The ease of rapid implementation of our findings is bolstered by the standardized reporting guidelines for p53 IHC. Although the minimum percentage of tumor cells stained for a diagnosis of overexpression has varied from study to study (from 60% to > 90%), most studies emphasize the importance of the uniformity of staining intensity. Further studies have suggested that diffuse staining of uniform intensity (usually strong) is very well correlated with mutation. We selected a high threshold for this publication, using 80% or more positive nuclear staining for p53 as the definition of OE. Despite excellent concordance between mutational data and p53 immunophenotype, concordance is not perfect. We also acknowledge that the interpretation of p53 staining results can be subject to intra- and interobserver variation. A recent multi-institutional study of 50 cases stained and interpreted in three different laboratories identified eight cases, or about 16%, without uniform p53 results.30 In addition, four cases from one institution were weakly stained but diffuse in distribution, leading to an erroneous diagnosis of WT p53 expression. This type of error was mitigated in this study by having all stains performed in a single laboratory. Future studies will need to refine how cases with high cytoplasmic staining or focal nuclear overexpression of p53 in the setting of WT staining should be binned (Fig 1A). The functional consequence of these expression patterns as they relate to treatment prediction remains undefined. However, if sequencing can be performed specifically on cases with indeterminant p53 by IHC, and a missense mutation in TP53 is identified, patients could be offered bevacizumab plus chemotherapy upfront. Hence, if not available for all cases, sequencing may be a useful and predictive second-tier test specifically when IHC is indeterminant.
FIG 5.
A potential clinical management schema supported by the findings from GOG-86P. For ease and feasibility, tumor testing for p53 status can begin with standard IHC. If p53 is highly expressed in the nuclei of 80% of more of tumor cells, our data indicate that bevacizumab, when added to chemotherapy in the upfront setting, significantly improves PFS and OS. If the immunostaining is negative entirely, or null, we cannot make a strong recommendation because of the low number of cases, but we are aware that some p53 null cases appeared to benefit on the bevacizumab arms of GOG-86P. Therefore, we recommend weighing the risk of bevacizumab, including hypertensive complications, in each individual patient before adding bevacizumab to upfront chemotherapy. If immunostaining is modest and not uniform, this suggests a WT p53 expression pattern. Our data suggest no clear benefit by adding bevacizumab to chemotherapy upfront. If p53 immunostaining is indeterminant, which may occur due to high cellular heterogeneity of p53 expression, cytoplasmic instead of nuclear staining or other conditions, we recommend sequencing TP53. If results reveal a mutation (aother than the known SNP at P72), consideration should be given for adding bevacizumab to chemotherapy upfront. Note that tumors that coexpress POLE and TP53 mutations or potentially those with microsatellite instability or mismatch repair deficiency comprise a special category, and at this time, the data do not inform management. IHC, immunohistochemistry; OS, overall survival; PFS, progression-free survival; SNP, single nucleotide polymorphism; WT, wild-type.
In conclusion, our findings provide a logical framework for the assessment of p53 by IHC and NGS in routine molecular stratification of endometrial cancer. Our study has the potential to change practice for advanced endometrial cancer by emphasizing (1) the strong correlation between highly overexpressed p53 protein on IHC and the presence of a missense mutated TP53 sequence; (2) the usefulness of IHC as a cost-saving initial study that is predictive of bevacizumab plus chemotherapy benefit in the majority of cases; and (3) the use of TP53 sequencing as a means to understand p53 status when immunostaining is indeterminant. As these findings are the result of a retrospective analysis of tissues from GOG-86P, we look forward to the opportunity to confirm our findings in future prospective clinical trials.
ACKNOWLEDGMENT
We are grateful to Drs Douglas Levine, Fanny Dao, and Narciso Olvera for performing immunohistochemical analysis of p53 under the original GOG-86P protocol and sharing these data. We are grateful to Suzanne Forry, PhD, who has overseen and consulted on this research as Program Officer for the NIH NCI grant R01CA099908. Gynecologic Oncology Group member institutions mentioned in Appendix 1 (online only).
APPENDIX 1. Gynecologic Oncology Group
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, Washington University School of Medicine, Duke University Medical Center, University of California Irvine Medical Center, University of Iowa Hospitals and Clinics, Women and Infants Hospital, Ohio State University Comprehensive Cancer Center, Rush University Medical Center, Walter Reed National Military Medical Center, University of Cincinnati, Cleveland Clinic Foundation, The Hospital of Central Connecticut, UCSF-Mount Zion, Memorial Sloan Kettering Cancer Center, Mayo Clinic, Cancer Research for the Ozarks NCORP, University of Texas Southwestern Medical Center, Georgia Center for Oncology Research and Education (CORE), Northwestern University, University of North Carolina at Chapel Hill, MD Anderson Cancer Center, University of Wisconsin Hospitals and Clinics, Roswell Park Cancer Institute, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Women's Cancer Center of Nevada, University of Hawaii, Abington Memorial Hospital, University of Mississippi Medical Center, State University of New York Downstate Medical Center, Cooper Hospital University Medical Center, Carolinas Medical Center/Levine Cancer Institute, William Beaumont Hospital, Abramson Cancer Center of the University of Pennsylvania, University of Chicago, Aurora Women's Pavilion of Aurora West Allis Medical Center, Virginia Commonwealth University, Penn State Milton S. Hershey Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Stony Brook University Medical Center, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, University of Virginia, Case Western Reserve University, Yale University, University of Texas – Galveston, Michigan Cancer Research Consortium Community Clinical Oncology Program, Delaware/Christiana Care CCOP, University of Minnesota Medical Center-Fairview, University of California at Los Angeles Health System, Fred Hutchinson Cancer Research Center, University of Kentucky, Moffitt Cancer Center and Research Institute, Saint Joseph's Hospital and Medical Center, Scott and White Memorial Hospital, Kalamazoo CCOP, Northern Indiana Cancer Research Consortium, and Iowa-Wide Oncology Research Coalition NCORP.
Kristina W. Thiel
Employment: Immortagen
Stock and Other Ownership Interests: Immortagen
Patents, Royalties, Other Intellectual Property: Pending patent: Synthetically Lethal Nanoparticles for Treatment of Cancers
Virginia L. Filiaci
Other Relationship: GOG Foundation
David Mutch
Consulting or Advisory Role: lilly
Katherine Moxley
Consulting or Advisory Role: Tessa Therapeutics (Inst), Clovis Oncology (Inst), GlaxoSmithKline
Angeles Alvarez Secord
Honoraria: Myriad Genetics
Research Funding: Tesaro (Inst), AstraZeneca (Inst), Genentech (Inst), Boehringer Ingelheim (Inst), AbbVie (Inst), Merck (Inst), PharmaMar (Inst), Clovis Oncology (Inst), Eisai (Inst), Seattle Genetics (Inst), Immutep (Inst), GlaxoSmithKline (Inst), VBL Therapeutics (Inst), OncoQuest Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Uncompensated Relationships: Roche/Genentech, VBL Therapeutics, GOG Foundation, OncoQuest Pharmaceuticals, Regeneron, Aravive
Krishnansu Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Cara Mathews
Research Funding: AstraZeneca (Inst), Tesaro/GSK (Inst), Syros Pharmaceuticals (Inst), Astellas Pharma (Inst), Seattle Genetics (Inst), Deciphera (Inst), Moderna Therapeutics (Inst), Regeneron (Inst), Roche/Genentech (Inst), Pfizer (Inst), Laekna Therapeutics (Inst), EMD Serono (Inst), Merck (Inst)
Casey Cosgrove
Honoraria: UpToDate
Consulting or Advisory Role: Agenus
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, Eisai, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst), Clovis Oncology (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Association for Cancer Research Virtual Special Conference: Endometrial Cancer: New Biology Driving Research and Treatment, November 9-10, 2020.
SUPPORT
Supported by National Institutes of Health: NIH 5R01CA099908-18 (K.K.L.) and Department of Defense OC190352 (K.K.L.). Supported by National Institutes of Health/National Cancer Institute grants to NRG Oncology Statistics and Data Management Center (SDMC; U10 CA 180822), NRG Operations (U10 CA180868), and NRG Specimen Bank (U24CA196067). Further funding was received from the Holden Comprehensive Cancer Center, the University of Iowa (NIH 2 P30 CA086862-16), the University of New Mexico Comprehensive Cancer Center (NIH P30 CA118100) and general unrestricted funds from the University of New Mexico Comprehensive Cancer Center. NRG SDMC and C.A. received funding from American Recovery and Reinvestment Act (ARRA) 3 U10 CA027469-29S1. C.A. received a Stand Up to Cancer Dream Team Translational Research Grant and a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209). Additionally, this research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
CLINICAL TRIAL INFORMATION
K.W.T., E.J.D., R.A.S., and K.K.L. contributed equally to this work.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.02506.
AUTHOR CONTRIBUTIONS
Conception and design: Kristina W. Thiel, Eric J. Devor, Virginia L. Filiaci, Carol Aghajanian, Robert A. Soslow, Kimberly K. Leslie
Financial support: Kimberly K. Leslie
Administrative support: David Mutch, Angeles Alvarez Secord, Krishnansu S. Tewari, Heather A. Lankes, Kimberly K. Leslie
Provision of study materials or patients: David Mutch, Katherine Moxley, Angeles Alvarez Secord, Cara Mathews, Casey Cosgrove, Summer Dewdney, Carol Aghajanian, Robert A. Soslow, Kimberly K. Leslie
Collection and assembly of data: Kristina W. Thiel, Eric J. Devor, Virginia L. Filiaci, David Mutch, Katherine Moxley, Angeles Alvarez Secord, Megan E. McDonald, Casey Cosgrove, Summer Dewdney, Carol Aghajanian, Heather A. Lankes, Robert A. Soslow, Kimberly K. Leslie
Data analysis and interpretation: Kristina W. Thiel, Eric J. Devor, Virginia L. Filiaci, David Mutch, Angeles Alvarez Secord, Krishnansu S. Tewari, Cara Mathews, Casey Cosgrove, Carol Aghajanian, Megan I. Samuelson, Robert A. Soslow, Kimberly K. Leslie
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
TP53 Sequencing and p53 Immunohistochemistry Predict Outcomes When Bevacizumab Is Added to Frontline Chemotherapy in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kristina W. Thiel
Employment: Immortagen
Stock and Other Ownership Interests: Immortagen
Patents, Royalties, Other Intellectual Property: Pending patent: Synthetically Lethal Nanoparticles for Treatment of Cancers
Virginia L. Filiaci
Other Relationship: GOG Foundation
David Mutch
Consulting or Advisory Role: lilly
Katherine Moxley
Consulting or Advisory Role: Tessa Therapeutics (Inst), Clovis Oncology (Inst), GlaxoSmithKline
Angeles Alvarez Secord
Honoraria: Myriad Genetics
Research Funding: Tesaro (Inst), AstraZeneca (Inst), Genentech (Inst), Boehringer Ingelheim (Inst), AbbVie (Inst), Merck (Inst), PharmaMar (Inst), Clovis Oncology (Inst), Eisai (Inst), Seattle Genetics (Inst), Immutep (Inst), GlaxoSmithKline (Inst), VBL Therapeutics (Inst), OncoQuest Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Uncompensated Relationships: Roche/Genentech, VBL Therapeutics, GOG Foundation, OncoQuest Pharmaceuticals, Regeneron, Aravive
Krishnansu Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Krishnansu S. Tewari
Honoraria: Tesaro, Clovis Oncology, Merck, Eisai, AstraZeneca, Genmab
Consulting or Advisory Role: Roche/Genentech, Tesaro, Clovis Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, Merck, Tesaro, Clovis Oncology, Eisai, Genmab
Research Funding: AbbVie (Inst), Genentech/Roche (Inst), Morphotek (Inst), Merck (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Cara Mathews
Research Funding: AstraZeneca (Inst), Tesaro/GSK (Inst), Syros Pharmaceuticals (Inst), Astellas Pharma (Inst), Seattle Genetics (Inst), Deciphera (Inst), Moderna Therapeutics (Inst), Regeneron (Inst), Roche/Genentech (Inst), Pfizer (Inst), Laekna Therapeutics (Inst), EMD Serono (Inst), Merck (Inst)
Casey Cosgrove
Honoraria: UpToDate
Consulting or Advisory Role: Agenus
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, Eisai, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst), Clovis Oncology (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cancer Facts and Figures 2022, Atlanta, GA, American Cancer Society, 2022 [Google Scholar]
- 2.Sheikh MA, Althouse AD, Freese KE, et al. : USA endometrial cancer projections to 2030: Should we be concerned? Future Oncol 10:2561-2568, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Cancer Facts and Figures 2020, Atlanta, GA, American Cancer Society, 2020 [Google Scholar]
- 5.Ferlay J, Laversanne M, Ervik M, et al. : Global Cancer Observatory. Lyon, France, International Agency for Research on Cancer, 2020 [Google Scholar]
- 6.Aghajanian C, Filiaci V, Dizon DS, et al. : A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol Oncol 150:274-281, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller DS, Filiaci VL, Mannel RS, et al. : Carboplatin and paclitaxel for advanced endometrial cancer: Final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol 38:3841-3850, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie KK, Filiaci VL, Mallen AR, et al. : Mutated p53 portends improvement in outcomes when bevacizumab is combined with chemotherapy in advanced/recurrent endometrial cancer: An NRG Oncology study. Gynecol Oncol 161:113-121, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. : Integrated genomic characterization of endometrial carcinoma. Nature 497:67-73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albitar L, Carter MB, Davies S, et al. : Consequences of the loss of p53, RB1, and PTEN: Relationship to gefitinib resistance in endometrial cancer. Gynecol Oncol 106:94-104, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Brachova P, Mueting SR, Carlson MJ, et al. : TP53 oncomorphic mutations predict resistance to platinum and taxane-based standard chemotherapy in patients diagnosed with advanced serous ovarian carcinoma. Int J Oncol 46:607-618, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brachova P, Mueting SR, Devor EJ, et al. : Oncomorphic TP53 mutations in gynecologic cancers lose the normal protein:protein interactions with the microRNA microprocessing complex. J Cancer Ther 5:506-516, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brachova P, Thiel KW, Leslie KK: The consequence of oncomorphic TP53 mutations in ovarian cancer. Int J Mol Sci 14:19257-19275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng X, Dizon DS, Yang S, et al. : Strategies for molecularly enhanced chemotherapy to achieve synthetic lethality in endometrial tumors with mutant p53. Obstet Gynecol Int 2013:828165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blandino G, Levine AJ, Oren M: Mutant p53 gain of function: Differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene 18:477-485, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Dittmer D, Pati S, Zambetti G, et al. : Gain of function mutations in p53. Nat Genet 4:42-46, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Garg K, Leitao MM, Wynveen CA, et al. : p53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes. Mod Pathol 23:80-92, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Whibley C, Pharoah PD, Hollstein M: p53 polymorphisms: cancer implications. Nat Rev Cancer 9:95-107, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Stelloo E, Nout RA, Osse EM, et al. : Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res 22:4215-4224, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Leon-Castillo A, de Boer SM, Powell ME, et al. : Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncol 38:3388-3397, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Heerik A, Horeweg N, Nout RA, et al. : PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int J Gynecol Cancer 30:2002-2007, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wortman BG, Bosse T, Nout RA, et al. : Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: Evaluation of the pilot phase of the PORTEC-4a trial. Gynecol Oncol 151:69-75, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Yemelyanova A, Vang R, Kshirsagar M, et al. : Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: An immunohistochemical and nucleotide sequencing analysis. Mod Pathol 24:1248-1253, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Kobel M, Piskorz AM, Lee S, et al. : Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Pathol Clin Res 2:247-258, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh N, Piskorz AM, Bosse T, et al. : p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J Pathol 250:336-345, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Kang EY, Cheasley D, LePage C, et al. : Refined cut-off for TP53 immunohistochemistry improves prediction of TP53 mutation status in ovarian mucinous tumors: Implications for outcome analyses. Mod Pathol 34:194-206, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talhouk A, McConechy MK, Leung S, et al. : A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 113:299-310, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahin MS, Hughes JH, Sood AK, et al. : The prognostic significance of p53 tumor suppressor gene alterations in ovarian carcinoma. Cancer 89:2006-2017, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Sood AK, Sorosky JI, Dolan M, et al. : Distant metastases in ovarian cancer: Association with p53 mutations. Clin Cancer Res 5:2485-2490, 1999 [PubMed] [Google Scholar]
- 30.Plotkin A, Kuzeljevic B, De Villa V, et al. : Interlaboratory concordance of ProMisE molecular classification of endometrial carcinoma based on endometrial biopsy specimens. Int J Gynecol Pathol 39:537-545, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.02506.