PURPOSE
Orteronel (TAK-700) is a nonsteroidal 17,20-lyase inhibitor suppressing androgen synthesis. We evaluated the clinical benefit of orteronel when added to androgen deprivation therapy (ADT) in patients with newly diagnosed metastatic hormone-sensitive prostate cancer.
METHODS
In this open-label randomized phase III study, patients with metastatic hormone-sensitive prostate cancer were randomly assigned 1:1 to ADT with orteronel (300 mg oral twice daily; experimental arm) or ADT with bicalutamide (50 mg oral once daily; control arm). The primary objective was the comparison of overall survival (OS), targeting a 33% improvement in median survival. A stratified log-rank test with a one-sided P ≤ .022 would indicate statistical significance. Secondary end points were progression-free survival (PFS), prostate-specific antigen (PSA) level at 7 months (≤ 0.2 v 0.2 to ≤ 4 v > 4 ng/mL), and adverse event profile.
RESULTS
Among 1,279 patients included in the analysis, 638 were randomly assigned to the ADT plus orteronel arm and 641 to the control arm. The median age was 68 years; 49% had extensive disease. After a median follow-up of 4.9 years, there was a significant improvement in PFS (median 47.6 v 23.0 months, hazard ratio 0.58; 95% CI, 0.51 to 0.67; P < .0001) and PSA response at 7 months (P < .0001), but not in OS (median 81.1 v 70.2 months, hazard ratio 0.86; 95% CI, 0.72 to 1.02; P = .040, one-sided). More grade 3/4 adverse events occurred in the experimental versus the control arms (43% v 14%). Postprotocol life-prolonging therapy was received by 77.4% of patients in the control arm and 61.3% of patients in the orteronel arm.
CONCLUSION
The study did not meet the primary end point of improved OS with orteronel. The lack of correlation of PFS and PSA response with OS raises concerns over assumption of their consistent surrogacy for OS in the context of extensive postprotocol therapy in this setting.
INTRODUCTION
The cornerstone of treatment for metastatic hormone-sensitive prostate cancer (mHSPC) is androgen deprivation therapy (ADT) with medical or surgical castration.1 However, disease progression universally occurs on ADT, mediated by extragonadal production of androgens or androgen-independent activation of androgen receptor.1 The treatment paradigm of mHSPC has evolved over the past decade with multiple trials showing improved survival by intensification of ADT with either docetaxel chemotherapy or abiraterone, which inhibits a pivotal enzyme in intra- and extragonadal androgen synthesis (ie, CYP17 [CYP17 hydroxylase, CYP17, 20 lyase]) located in the testes and adrenal glands.1-5 More recently, two direct, potent, and specific inhibitors of the androgen receptor, apalutamide and enzalutamide, were approved on the basis of improved survival in phase III trials.1,6,7 Currently, the most common sequencing of these agents in the treatment of metastatic prostate cancer is abiraterone acetate followed by enzalutamide or docetaxel.8 One challenge with abiraterone acetate is the requirement for coadministration of corticosteroids because of inhibition of CYP17 hydroxylase.1 This results in diminished cortisol production and leads to secondary mineralocorticoid excess, with symptoms such as electrolyte abnormalities, hypertension, and edema.1
CONTEXT
Key Objective
We examined if adding orteronel, a novel androgen axis inhibitor, to androgen deprivation therapy (ADT) would improve outcomes compared with ADT plus bicalutamide in men with metastatic hormone-sensitive prostate cancer in a phase III trial.
Knowledge Generated
Addition of orteronel to ADT resulted in a significant improvement in progression-free survival and prostate-specific antigen response but not in overall survival (OS), the primary end point. Interestingly, the median OS of patients in the control arm of SWOG-1216 was 24 months higher than that in patients with metastatic hormone-sensitive prostate cancer on the SWOG-9346 trial (reported in 2013) who had similar disease severity and also received ADT plus bicalutamide (70.2 v 46 months).
Relevance
These results indicate that orteronel is likely not an effective androgen axis inhibitor compared with recently approved agents in this setting. Higher than anticipated OS of patients in the control arm is reflective of therapeutic advancements made over the past decade in men with metastatic prostate cancer.
Orteronel (TAK-700) is a novel CYP17 inhibitor. In comparison with abiraterone acetate, orteronel inhibits CYP17, 20 lyase with greater specificity compared with CYP17 hydroxylase and does not generally lead to the syndrome of secondary mineralocorticoid excess.9,10 We hypothesized that the addition of orteronel to ADT would improve overall survival (OS), progression-free survival (PFS), and prostate-specific antigen (PSA) response relative to bicalutamide with ADT among men with mHSPC.
METHODS
Trial Design and Conduct
The S1216 trial was a phase III, randomized, open-label, multicenter trial involving patients with mHSPC. The trial was designed by the SWOG study team and was approved by the National Cancer Institute (NCI). Patients were enrolled from 248 academic and community centers throughout the United States, incorporating three other NCI-funded cooperative groups (ECOG-ACRIN, ALLIANCE, and NRG). The NCI Central Institutional Review Board approved the initial version of the Protocol (online only) and all subsequent amendments. The study was conducted in accordance with the International Conference on Harmonization of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Signed written consent was obtained from all patients. First and last authors (N.A. and D.I.Q.) and the statisticians (C.M.T. and M.P.) among the authors assume responsibility for the completeness and accuracy of the data and analyses and for the fidelity of the trial to the Protocol.
Patients and Interventions
Eligible patients were required to have histologically confirmed adenocarcinoma of the prostate and metastatic disease as evidenced by soft tissue and/or bony metastases. Enrolled patients had a Zubrod performance status of 0-2; a Zubrod performance status of 3 was allowed if from bone pain only and a PSA ≥ 2.0 ng/mL. No other prior systemic therapy for metastatic prostate cancer was allowed (with the exception of up to 30 days of ADT for metastatic disease before registration), and at least 6 months must have elapsed since completion of prior neoadjuvant and/or adjuvant ADT. Concomitant radiotherapy was allowed only for baseline symptoms per investigator's clinical judgment during the first 4 months of protocol treatment. Patients who had not started any therapy with luteinizing hormone-releasing hormone (LHRH) agonist, antagonist, or orchiectomy (early induction group) and patients who had already started therapy with LHRH agonist, antagonist, or orchiectomy within 30 days before registration (late induction group) were eligible. Patients who were deemed to have extensive mHSPC were eligible for enrollment if, on the basis of the judgment of the treating physician, they were unsuitable candidates for docetaxel or if they declined docetaxel therapy.11
Exclusion criteria included brain metastases; prior therapy with ketoconazole, abiraterone acetate, or enzalutamide; New York Heart Association class III or IV heart failure at screening or thromboembolic event; unstable angina pectoris; myocardial infarction; or serious uncontrolled cardiac arrhythmia ≤ 6 months before registration.
Patients were randomly assigned in a 1:1 ratio to receive orteronel (300 mg) orally twice daily or bicalutamide (50 mg) administered orally once daily, in addition to continuous ADT. Patient assignment was dynamically balanced on three stratification factors: severity of disease (minimal v extensive), Zubrod performance status (0-1 v 2-3), and preregistration treatment status (early v late induction). Severity (or risk) of disease criteria in this study was originally reported in 1989, and since then, it has been used in all SWOG trials in the mHSPC setting to date.11-13 Minimal disease was defined as involvement of vertebrae and/or pelvic bones and/or lymph nodes, and extensive disease was defined as that with greater than minimal involvement. Dynamic balancing is a general procedure for treatment assignment, which concentrates on minimizing imbalance in the distribution of treatment assignment within the levels of each individual prognostic factor.14
End Points
The primary end point was OS, which was defined as the time from random assignment to the date of death from any cause. Secondary end points were PFS, PSA response rates, and adverse events (AEs) rate. PFS was defined as the time from random assignment to first documentation of PSA progression (≥ 25% increase and an absolute increase of at least 2 ng/mL from the nadir PSA), radiologic progression (two or more new lesions on radionuclide bone scans, per Prostate Cancer Working Group 2 [PCWG2] criteria, and/or soft tissue progression per RECIST version 1.1), clinical progression (symptomatic deterioration) or death, whichever occurred first. PSA response rates were divided into complete response (CR; PSA < 0.2 ng/mL), partial response (PR; PSA between 0.2 and 4.0 ng/mL), and no response (NR; PSA > 4.0 ng/mL) at a 7-month landmark after random assignment.
Assessments
Patients were assessed for efficacy according to the modified RECIST version 1.1 with the use of computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis during screening (≤ 6 weeks before random assignment) and according to PCWG2 criteria with the use of bone scanning.15 Progression events were assessed by the treating investigator.
Statistical Analysis
The median survival of patients randomly assigned to ADT plus bicalutamide was assumed to be 54 months on the basis of revised estimates from previous SWOG studies (48 months on the basis of the median from SWOG-S9346, the intermittent v continuous ADT phase III trial, and an additional 6 months from the newer drug approvals in the castrate-resistant setting, which may extend survival).11 With 2.75 years to accrue 1,186 eligible patients and an additional follow-up of 3 years, we had a 90% power to determine a 33% improvement in median OS from 54 to 72 months (one-sided α = .025). A final analysis was prespecified after 523 deaths in the combined arms using a one-sided α = .022 to account for interim analyses.
Demographic and clinical characteristics at baseline were summarized with the use of descriptive statistics. The primary statistical method comparing time-to-event end points was a stratified log-rank test, with stratification according to prespecified factors. The Kaplan-Meier product-limit method was used to generate the survival and PFS curves in Figures 1, 2, Appendix Figure A2 (online only), and the Cox proportional hazards model with covariate adjustment for stratification factors was used to estimate treatment hazard ratios (HRs) and associated CIs for survival and PFS. Homogeneity of treatment effect was descriptively evaluated with a forest plot showing the survival HR and 95% CI for each subgroup defined by the stratification factors and race using a Cox regression model with no covariate adjustment. The interaction of the stratification factor with treatment, evaluating heterogeneity between levels of a factor, was tested using a residual chi-square from the Cox model. A two-sided Cochrane-Mantel-Haenszel test was used to compare three-category PSA response rates between the two treatment arms.
FIG 1.
Intention-to-treat comparison of overall survival by arm. HR, hazard ratio; LHRH, luteinizing hormone-releasing hormone.
FIG 2.
Intention-to-treat comparison of progression-free survival by arm. HR, hazard ratio; LHRH, luteinizing hormone-releasing hormone.
RESULTS
Patients
Between March 1, 2013, and July 15, 2017, 1,313 patients were randomly assigned and 1,279 were included in the intention-to-treat analysis (32 patients were ineligible, and two patients withdrew all consent before starting treatment—see Appendix Fig A1 [online only] for details). Six hundred thirty-eight patients were randomly assigned to the orteronel arm, and 641 were randomly assigned to the control arm. At the cutoff date (February 5, 2021) for the final analysis after 525 deaths (and 787 PFS events), the median follow-up time was 4.9 years. Demographic and clinical characteristics at baseline were well balanced (Table 1). The median age of the patients for the orteronel and control arms was 67.6 and 68.1 years, respectively. Across both groups, approximately 10% of the patients were African American, 49% had extensive disease, and 96% had a Zubrod performance status of 0 or 1.
TABLE 1.
Baseline Characteristics of Patients Included in the Primary Intention-To-Treat Analysis (N = 1,279)
Primary End Point
Analysis for OS occurred after the prespecified 525 deaths were observed (251 in the orteronel group and 274 in the control group). The median OS in the orteronel group was 81.1 months as compared with 70.2 months in the control group. The OS rate at 5 years was 59.7% in the orteronel group and 57.9% in the control group (HR for death 0.86; 95% CI, 0.72 to 1.02; P = .040, one-sided). No statistically significant improvement in OS was observed with orteronel compared with control (Fig 1). This lack of treatment effect on OS seemed consistent across stratification factors, including disease severity, and by race (Fig 3 and Appendix Table A3, online only).
FIG 3.
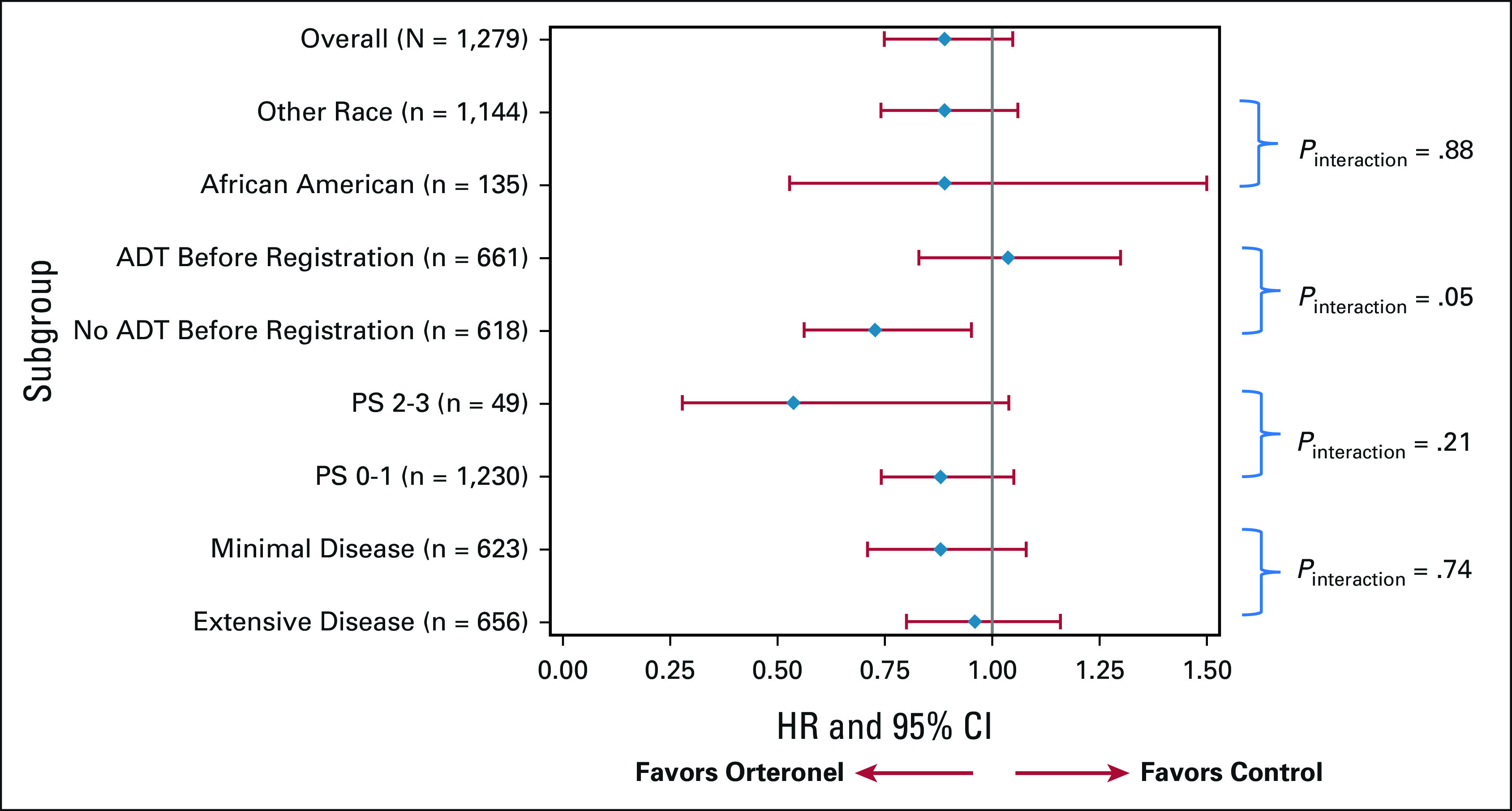
Estimated treatment HRs for overall survival by subgroup (survival forest plot for S1216). ADT, androgen deprivation therapy; HR, hazard ratio; PS, performance score.
Secondary End Points
Analysis of PFS occurred after 787 events were observed (343 in the orteronel group and 444 in the control group). The median PFS in the orteronel group was 47.6 months compared with 23.0 months in the control group (Fig 2). The PFS at 4 years was 50.2% in the orteronel group and 33.9% in the control group (HR, 0.58; 95% CI, 0.51 to 0.67; P < .001).
The PSA response rates at month 7 were significantly improved with orteronel (CR 58%; PR: 22%, NR: 19%) versus bicalutamide (CR: 44%; PR: 31%; NR: 25%; P < .0001).
Safety
More grade 3 and 4 AEs occurred in the orteronel group versus the control group (43% v 13%). Notable differences included the frequency of hypertension (20% with orteronel v 5% with control) and fatigue (5% in orteronel v 2% in the control group). Five patients in the experimental arm and one patient in the control arm had grade 5 AEs, including two patients experiencing myocardial infarction and one experiencing a stroke in the orteronel arm (Table 2).
TABLE 2.
No. and Percent of Patients With a Given Type and Grade of AE
Subsequent Life-Prolonging Anticancer Therapy
Treatment after discontinuation of protocol therapy was initiated per patient's choice and as per local standard of care. At the time of this report, receipt of at least one approved life-prolonging therapy after discontinuation of protocol therapy was evaluable in 331 patients in the orteronel arm and 402 patients in the control arm. A higher proportion of patients in the control arm received postprotocol therapy, which was consistent with a higher incidence of disease progression in the control arm. Of these, 203 (61.3%) patients in the orteronel arm and 311 (77.4%) patients in the control arm received one or more life-prolonging anticancer therapies (Appendix Table A1, online only).
DISCUSSION
In this study, addition of orteronel to ADT compared with bicalutamide led to a statistically significant improvement in PFS and PSA response. Although the approximately 11-month improvement in median OS with orteronel appears clinically meaningful, it did not reach the prespecified requirement for statistical significance. The assumption of 54-month OS for the control arm was a substantial underestimate, exceeded by 16 months in the actual results, challenging the probability of a statistically significant result.
The discrepancy between improvement in PFS but not in OS might have occurred because of receipt of subsequent life-prolonging therapy in a large proportion of patients in the control arm. The primary end point of OS evaluated not only the benefit of early addition of orteronel but also the impact of subsequent life-prolonging therapies including abiraterone acetate, enzalutamide, docetaxel, cabazitaxel, sipuleucel-T, and radium-223.16-23 In this study, 77.4% of the patients who progressed in the control arm received subsequent life-prolonging therapies compared with 21%-64% of patients in phase III studies in this setting reported since 2013 where the control arm comprised ADT only without recently approved novel agents (nonintensified ADT; Appendix Table A2, online only). The receipt of subsequent life-prolonging therapies by the majority of patients on the control arm might have also resulted in an absolute median OS of 70 months, the highest ever reported for patients on a nonintensified ADT control arm of contemporary trials in mHSPC. It is also possible that the results from the CHAARTED trial, which reported approximately 1 year after activation of SWOG-1216 and showed significant improvement in OS with docetaxel in patients with high-volume disease, prompted a preferential recruitment of patients with low-volume disease on the SWOG-1216 trial, leading to an unexpected improvement in the OS in both arms, especially in the control arm. For example, the median OS in patients with low-volume mHSPC in the CHAARTED trial had not been reached after a median follow-up of 53.7 months and was 83.4 months in the GETUG AFU-15 trial.24,25 Nonetheless, when compared with the median OS of men on the SWOG-9346 trial in the mHSPC setting (reported in 2013), where all patients received ADT plus bicalutamide and which had almost an identical proportion of men with extensive disease by SWOG criteria of disease severity (48% v 49%), the median OS in the control arm of SWOG-1216 was 24 months higher (46 v 70.2 months) (Appendix Figure A2).11
SWOG-1216 is the third phaseIII trial investigating the use of orteronel in the metastatic prostate cancer setting after the ELM-PC5 and ELM-PC4 trials failed to meet their primary end point of OS. The ELM-PC5 trial was conducted in the postdocetaxel metastatic castration-resistant prostate cancer (mCRPC) setting, whereas ELM-PC4 trial was conducted in the predocetaxel mCRPC setting.26,27 Results of these three trials, which combined and enrolled more than 3000 patients with metastatic prostate cancer, suggest that orteronel is likely not an effective androgen axis inhibitor compared with recently approved agents such as abiraterone, apalutamide, and enzalutamide. Furthermore, both PFS and OS were significantly improved with addition of abiraterone, apalutamide, and enzalutamide to ADT compared with ADT alone in the LATITUDE, TITAN, and the ARCHES trials, respectively, all conducted in the mHSPC setting (Appendix Table A2).
The strengths of this study include enrollment of patients from academic and community centers across the United States, proportionate representation of African-American patients (highest to date among all contemporary trials), and a representative patient population as evident by the risk status (approximately 50% high risk). These results emphasize the need to account for receipt of subsequent life-prolonging therapy and a need for validated surrogate intermediate end points to expedite drug development in a time where the treatment paradigm in the mCRPC setting is rapidly changing. Given the improved OS in men with mHSPC receiving nonintensified therapy, it may also be prudent to focus on designing trials for those at the highest risk of early disease progression and death. The results of SWOG-1216 indicate that access to life-prolonging therapies, approved over the past decade, has significantly improved outcomes in patients with metastatic prostate cancer. Furthermore, this trial provides survival estimates in the contemporary era when patients with mHSPC have access to multiple approved life-prolonging therapies. These contemporary survival data will be invaluable for counseling of patients with newly diagnosed mHSPC and for design of future clinical trials in this setting.
APPENDIX
TABLE A1.
Post Hoc Analysis of Subsequent Life-Prolonging Therapy
TABLE A2.
Comparison of Contemporary Phase III Trials
TABLE A3.
Treatment Outcomes by Disease Severity
FIG A1.

CONSORT diagram for the S1216 trial. ADT, androgen deprivation therapy; ITT, intention-to-treat; LHRHa, luteinizing hormone-releasing hormone agonist; QTc, corrected QT interval.
FIG A2.
Overall survival curves of different SWOG trials (stratified by trial, follow-up truncated at 6 years). Bical, bicalutamide; LHRHa, luteinizing hormone-releasing hormone agonist; Pats, patients.
Neeraj Agarwal
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Pfizer, Medivation/Astellas, Bristol Myers Squibb, AstraZeneca, Nektar, Lilly, Bayer, Pharmacyclics, Foundation Medicine, Astellas Pharma, Exelixis, Merck, Novartis, Eisai, Seattle Genetics, EMD Serono, Janssen Oncology, AVEO, Calithera Biosciences, MEI Pharma, Genentech, Gilead Sciences
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Takeda (Inst), Pfizer (Inst), Exelixis (Inst), Amgen (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Celldex (Inst), Eisai (Inst), Genentech (Inst), Immunomedics (Inst), Janssen (Inst), Merck (Inst), Lilly (Inst), Nektar (Inst), ORIC Pharmaceuticals (Inst), CRISPR therapeutics (Inst), Arvinas (Inst)
Maha H.A. Hussain
Honoraria: Research to Practice, Astellas Pharma, AstraZeneca, OncLive, UroToday, Merck, Astellas Pharma, Precisca
Consulting or Advisory Role: BMS, Pfizer, Novartis, Merck, Janssen, Tempus, Bayer
Research Funding: Genentech (Inst), Pfizer (Inst), PCCTC (Inst), AstraZeneca (Inst), Bayer (Inst), Arvinas (Inst)
Patents, Royalties, Other Intellectual Property: TITLE: Systems and Methods for Tissue Imaging, 3676 Our File: Serial Number: UM-14437/US-1/PRO 60/923,385 UM-14437/US-2/ORD 12/101,753 US 8,185,186 (US patent number); Systems and methods for tissue imaging (issued patent) EP 08745653.9 (EP application number); Systems and methods for tissue imaging (pending) CA 2683805 (Canadian application number); Systems and methods for tissue imaging (pending) US 13/362,500 (US application number); Systems and Methods for Tissue Imaging (continuation application of US 8,185,186); TITLE: Method of Treating Cancer Docket No: Serial Number: 224990/10-016P2/311733 61/481/671 Application Filed on 5/2/2011; and TITLE: Dual Inhibition of MET and VEGF for the treatment of castration-resistant prostate cancer and osteoblastic bone metastases. Applicant/Proprietor Exelixis, Inc Application No./Patent No. 11764665.4- 1464 Application No./Patent No. 11764656.2-1464 Application Filed on 26/9/2011
Open Payments Link: https://openpaymentsdata.cms.gov/physician/146932/summary
Shilpa Gupta
Stock and Other Ownership Interests: Nektar, Moderna Therapeutics
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Gilead Sciences, Guardant Health, AVEO, EMD Serono, Pfizer, Merck, Loxo/Lilly
Speakers' Bureau: Bristol Myers Squibb, Janssen Oncology
Primo N. Lara
Consulting or Advisory Role: Janssen, Calithera Biosciences (Inst)
Research Funding: Janssen Biotech (Inst), Taiho Pharmaceutical (Inst)
Przemyslaw W. Twardowski
Honoraria: Astellas Medivation, Bayer, Janssen, Genentech/Roche, Sanofi/Aventis, AVEO, Pfizer, AstraZeneca
Consulting or Advisory Role: Sanofi/Aventis, Janssen, AstraZeneca
Speakers' Bureau: Astellas Pharma, Bayer, Janssen, Genentech/Roche, Sanofi, AstraZeneca
Channing J. Paller
Consulting or Advisory Role: Dendreon, Omnitura, Exelixis
Research Funding: Lilly (Inst)
Dylan Zylla
Research Funding: Celgene (Inst), Novartis (Inst), Exact Sciences (Inst), Roche (Inst), Amgen (Inst), AstraZeneca (Inst), Innate Pharma (Inst), MERCK (Inst), Nektar (Inst), Seattle Genetics/Astellas (Inst)
Matthew R. Zibelman
Honoraria: Pfizer
Consulting or Advisory Role: EMD Serono, Pfizer, Janssen, Exelixis, AVEO, Blue Earth Diagnostics
Research Funding: Horizon Pharma (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst)
Other Relationship: Association of Community Cancer Centers (ACCC)
Ellis Levine
Research Funding: Oncolytics (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Bruce J. Roth
Consulting or Advisory Role: Seattle Genetics, Merck, Secura Bio
Research Funding: Medivation (Inst)
Amir Goldkorn
Research Funding: Thermo Fisher Scientific, RareCyte, Menarini Silicon Biosystems
Patents, Royalties, Other Intellectual Property: I am listed as a coinventor on a patent held jointly by USC and Caltech for a microfilter that we developed for capturing live circulating tumor cells from blood
Daniel A. Vaena
Honoraria: HMP, OneOncology
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Genomic Health, Natera, Seattle Genetics, Exelixis, Bayer, EMD Serono, Immunomedics, Bristol Myers Squibb/Celgene, Eisai, AVEO
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), BioClin Therapeutics (Inst), Aeglea BioTherapeutics (Inst), Nektar (Inst), Calithera Biosciences (Inst), Tizona Therapeutics, Inc (Inst), TG Therapeutics (Inst), Merck (Inst), Compugen (Inst), OBI Pharma (Inst), Incyte (Inst), Seattle Genetics (Inst), Roche/Genentech (Inst), Blueprint Medicines (Inst)
Travel, Accommodations, Expenses: Tempus
Manish Kohli
Employment: nference
Honoraria: Advanced Accelerator Applications
Consulting or Advisory Role: Bristol Myers Squibb/Celgene, Genapsys, Tempus
Patents, Royalties, Other Intellectual Property: Patent number: 10982286. Algorithmic approach for determining the plasma genome abnormality PGA and the urine genome abnormality UGA scores based on cell-free cfDNA copy number variations in plasma and urine
Travel, Accommodations, Expenses: Celgene
Nicholas J. Vogelzang
Employment: US Oncology
Stock and Other Ownership Interests: Caris Life Sciences
Honoraria: UpToDate, Pfizer, Novartis, Merck
Consulting or Advisory Role: Pfizer, Bayer, Genentech/Roche, AstraZeneca, Caris Life Sciences, Tolero Pharmaceuticals, Merck, Astellas Pharma, Boehringer Ingelheim, Corvus Pharmaceuticals, Modra Pharmaceuticals, Clovis Oncology, Janssen Oncology, Eisai, on quality, Myovant Sciences
Speakers' Bureau: Bayer, Sanofi, Genentech/Roche, Bristol Myers Squibb, Seattle Genetics/Astellas, Clovis Oncology, AVEO, Myovant Sciences, AstraZeneca
Research Funding: US Oncology (Inst), Endocyte (Inst), Merck (Inst), Suzhou Kintor Pharmaceuticals (Inst)
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Genentech/Roche, US Oncology, Pfizer, Bayer/Onyx, Exelixis, AstraZeneca/MedImmune, Sanofi/Aventis
Ian M. Thompson Jr
Consulting or Advisory Role: MagForce, Profound Medical
Research Funding: MagForce
Patents, Royalties, Other Intellectual Property: I have several patents with colleagues involving novel biomarkers for cancer and two devices for sexual dysfunction and urinary incontinence. No revenues at this time, and our University IP office is working with industry to determine if these can be commercialized
David I. Quinn
Employment: AbbVie
Honoraria: Bayer, Pfizer, Genentech/Roche, Merck Sharp & Dohme, Bristol Myers Squibb, Exelixis, Seattle Genetics, Myovant Sciences, AVEO, Clinigen Group
Consulting or Advisory Role: Pfizer, Bristol Myers Squibb, Genentech/Roche, Merck Sharp & Dohme, Bayer, Exelixis, Eisai, US Biotest, Seattle Genetics, Myovant Sciences, AVEO, Clinigen Group
Research Funding: Genentech/Roche (Inst), Merck (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Merck, Roche, Bayer, Exelixis
Uncompensated Relationships: Eisai, US Biotest
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of Millennium Pharmaceuticals, Inc (Takeda Pharmaceutical Company LTD).
PRIOR PRESENTATION
Presented in part at the ASCO Virtual Annual Meeting, June 4-8, 2021.
SUPPORT
Supported by NIH/NCI/NCTN under grant No. U10CA180888, U10CA180819, U10CA180820, and U10CA180821 and in part by Millennium Pharmaceuticals, Inc (Takeda Pharmaceutical Company LTD).
CLINICAL TRIAL INFORMATION
NCT01809691 (SWOG S1216)
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.02517.
AUTHOR CONTRIBUTIONS
Conception and design: Neeraj Agarwal, Catherine M. Tangen, Maha H.A. Hussain, Primo N. Lara, Przemyslaw W. Twardowski, Amir Goldkorn, Tony Crispino, Nicholas J. Vogelzang, Ian M. Thompson Jr
Administrative support: Shilpa Gupta, David I. Quinn
Provision of study materials or patients: Maha H.A. Hussain, Shilpa Gupta, Primo N. Lara, Andrea L. Harzstark, Channing J. Paller, Matthew R. Zibelman, Ellis Levine, Bruce J. Roth, Amir Goldkorn, Daniel A. Vaena, Manish Kohli, Nicholas J. Vogelzang, David I. Quinn
Collection and assembly of data: Neeraj Agarwal, Catherine M. Tangen, Shilpa Gupta, Melissa Plets, Primo N. Lara, Andrea L. Harzstark, Channing J. Paller, Amir Goldkorn, Daniel A. Vaena, Manish Kohli, Tony Crispino, Nicholas J. Vogelzang, Ian M. Thompson Jr, David I. Quinn
Data analysis and interpretation: Neeraj Agarwal, Catherine M. Tangen, Maha H.A. Hussain, Shilpa Gupta, Primo N. Lara, Andrea L. Harzstark, Channing J. Paller, Dylan Zylla, Matthew R. Zibelman, Ellis Levine, Bruce J. Roth, Daniel A. Vaena, Manish Kohli, Tony Crispino, Nicholas J. Vogelzang, Ian M. Thompson Jr, David I. Quinn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Orteronel for Metastatic Hormone-Sensitive Prostate Cancer: a Multicenter, Randomized, Open-Label Phase III Trial (SWOG-1216)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Neeraj Agarwal
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Pfizer, Medivation/Astellas, Bristol Myers Squibb, AstraZeneca, Nektar, Lilly, Bayer, Pharmacyclics, Foundation Medicine, Astellas Pharma, Exelixis, Merck, Novartis, Eisai, Seattle Genetics, EMD Serono, Janssen Oncology, AVEO, Calithera Biosciences, MEI Pharma, Genentech, Gilead Sciences
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Takeda (Inst), Pfizer (Inst), Exelixis (Inst), Amgen (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Celldex (Inst), Eisai (Inst), Genentech (Inst), Immunomedics (Inst), Janssen (Inst), Merck (Inst), Lilly (Inst), Nektar (Inst), ORIC Pharmaceuticals (Inst), CRISPR therapeutics (Inst), Arvinas (Inst)
Maha H.A. Hussain
Honoraria: Research to Practice, Astellas Pharma, AstraZeneca, OncLive, UroToday, Merck, Astellas Pharma, Precisca
Consulting or Advisory Role: BMS, Pfizer, Novartis, Merck, Janssen, Tempus, Bayer
Research Funding: Genentech (Inst), Pfizer (Inst), PCCTC (Inst), AstraZeneca (Inst), Bayer (Inst), Arvinas (Inst)
Patents, Royalties, Other Intellectual Property: TITLE: Systems and Methods for Tissue Imaging, 3676 Our File: Serial Number: UM-14437/US-1/PRO 60/923,385 UM-14437/US-2/ORD 12/101,753 US 8,185,186 (US patent number); Systems and methods for tissue imaging (issued patent) EP 08745653.9 (EP application number); Systems and methods for tissue imaging (pending) CA 2683805 (Canadian application number); Systems and methods for tissue imaging (pending) US 13/362,500 (US application number); Systems and Methods for Tissue Imaging (continuation application of US 8,185,186); TITLE: Method of Treating Cancer Docket No: Serial Number: 224990/10-016P2/311733 61/481/671 Application Filed on 5/2/2011; and TITLE: Dual Inhibition of MET and VEGF for the treatment of castration-resistant prostate cancer and osteoblastic bone metastases. Applicant/Proprietor Exelixis, Inc Application No./Patent No. 11764665.4- 1464 Application No./Patent No. 11764656.2-1464 Application Filed on 26/9/2011
Open Payments Link: https://openpaymentsdata.cms.gov/physician/146932/summary
Shilpa Gupta
Stock and Other Ownership Interests: Nektar, Moderna Therapeutics
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Gilead Sciences, Guardant Health, AVEO, EMD Serono, Pfizer, Merck, Loxo/Lilly
Speakers' Bureau: Bristol Myers Squibb, Janssen Oncology
Primo N. Lara
Consulting or Advisory Role: Janssen, Calithera Biosciences (Inst)
Research Funding: Janssen Biotech (Inst), Taiho Pharmaceutical (Inst)
Przemyslaw W. Twardowski
Honoraria: Astellas Medivation, Bayer, Janssen, Genentech/Roche, Sanofi/Aventis, AVEO, Pfizer, AstraZeneca
Consulting or Advisory Role: Sanofi/Aventis, Janssen, AstraZeneca
Speakers' Bureau: Astellas Pharma, Bayer, Janssen, Genentech/Roche, Sanofi, AstraZeneca
Channing J. Paller
Consulting or Advisory Role: Dendreon, Omnitura, Exelixis
Research Funding: Lilly (Inst)
Dylan Zylla
Research Funding: Celgene (Inst), Novartis (Inst), Exact Sciences (Inst), Roche (Inst), Amgen (Inst), AstraZeneca (Inst), Innate Pharma (Inst), MERCK (Inst), Nektar (Inst), Seattle Genetics/Astellas (Inst)
Matthew R. Zibelman
Honoraria: Pfizer
Consulting or Advisory Role: EMD Serono, Pfizer, Janssen, Exelixis, AVEO, Blue Earth Diagnostics
Research Funding: Horizon Pharma (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst)
Other Relationship: Association of Community Cancer Centers (ACCC)
Ellis Levine
Research Funding: Oncolytics (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Bruce J. Roth
Consulting or Advisory Role: Seattle Genetics, Merck, Secura Bio
Research Funding: Medivation (Inst)
Amir Goldkorn
Research Funding: Thermo Fisher Scientific, RareCyte, Menarini Silicon Biosystems
Patents, Royalties, Other Intellectual Property: I am listed as a coinventor on a patent held jointly by USC and Caltech for a microfilter that we developed for capturing live circulating tumor cells from blood
Daniel A. Vaena
Honoraria: HMP, OneOncology
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Genomic Health, Natera, Seattle Genetics, Exelixis, Bayer, EMD Serono, Immunomedics, Bristol Myers Squibb/Celgene, Eisai, AVEO
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), BioClin Therapeutics (Inst), Aeglea BioTherapeutics (Inst), Nektar (Inst), Calithera Biosciences (Inst), Tizona Therapeutics, Inc (Inst), TG Therapeutics (Inst), Merck (Inst), Compugen (Inst), OBI Pharma (Inst), Incyte (Inst), Seattle Genetics (Inst), Roche/Genentech (Inst), Blueprint Medicines (Inst)
Travel, Accommodations, Expenses: Tempus
Manish Kohli
Employment: nference
Honoraria: Advanced Accelerator Applications
Consulting or Advisory Role: Bristol Myers Squibb/Celgene, Genapsys, Tempus
Patents, Royalties, Other Intellectual Property: Patent number: 10982286. Algorithmic approach for determining the plasma genome abnormality PGA and the urine genome abnormality UGA scores based on cell-free cfDNA copy number variations in plasma and urine
Travel, Accommodations, Expenses: Celgene
Nicholas J. Vogelzang
Employment: US Oncology
Stock and Other Ownership Interests: Caris Life Sciences
Honoraria: UpToDate, Pfizer, Novartis, Merck
Consulting or Advisory Role: Pfizer, Bayer, Genentech/Roche, AstraZeneca, Caris Life Sciences, Tolero Pharmaceuticals, Merck, Astellas Pharma, Boehringer Ingelheim, Corvus Pharmaceuticals, Modra Pharmaceuticals, Clovis Oncology, Janssen Oncology, Eisai, on quality, Myovant Sciences
Speakers' Bureau: Bayer, Sanofi, Genentech/Roche, Bristol Myers Squibb, Seattle Genetics/Astellas, Clovis Oncology, AVEO, Myovant Sciences, AstraZeneca
Research Funding: US Oncology (Inst), Endocyte (Inst), Merck (Inst), Suzhou Kintor Pharmaceuticals (Inst)
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Genentech/Roche, US Oncology, Pfizer, Bayer/Onyx, Exelixis, AstraZeneca/MedImmune, Sanofi/Aventis
Ian M. Thompson Jr
Consulting or Advisory Role: MagForce, Profound Medical
Research Funding: MagForce
Patents, Royalties, Other Intellectual Property: I have several patents with colleagues involving novel biomarkers for cancer and two devices for sexual dysfunction and urinary incontinence. No revenues at this time, and our University IP office is working with industry to determine if these can be commercialized
David I. Quinn
Employment: AbbVie
Honoraria: Bayer, Pfizer, Genentech/Roche, Merck Sharp & Dohme, Bristol Myers Squibb, Exelixis, Seattle Genetics, Myovant Sciences, AVEO, Clinigen Group
Consulting or Advisory Role: Pfizer, Bristol Myers Squibb, Genentech/Roche, Merck Sharp & Dohme, Bayer, Exelixis, Eisai, US Biotest, Seattle Genetics, Myovant Sciences, AVEO, Clinigen Group
Research Funding: Genentech/Roche (Inst), Merck (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Merck, Roche, Bayer, Exelixis
Uncompensated Relationships: Eisai, US Biotest
No other potential conflicts of interest were reported.
REFERENCES
- 1.Swami U, McFarland TR, Nussenzveig R, et al. : Advanced prostate cancer: Treatment advances and future directions. Trends Cancer 6:702-715, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Sweeney CJ, Chen YH, Carducci M, et al. : Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373:737-746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James ND, Sydes MR, Clarke NW, et al. : Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387:1163-1177, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352-360, 2017 [DOI] [PubMed] [Google Scholar]
- 5.James ND, de Bono JS, Spears MR, et al. : Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 377:338-351, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis ID, Martin AJ, Stockler MR, et al. : Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 381:121-131, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Chi KN, Agarwal N, Bjartell A, et al. : Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 381:13-24, 2019 [DOI] [PubMed] [Google Scholar]
- 8.George DJ, Sartor O, Miller K, et al. : Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical Practice setting in the United States. Clin Genitourin Cancer 18:284-294, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Kaku T, Hitaka T, Ojida A, et al. : Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg Med Chem 19:6383-6399, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Hussain M, Corn PG, Michaelson MD, et al. : Phase II study of single-agent orteronel (TAK-700) in patients with nonmetastatic castration-resistant prostate cancer and rising prostate-specific antigen. Clin Cancer Res 20:4218-4227, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Hussain M, Tangen CM, Berry DL, et al. : Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med 368:1314-1325, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford ED, Eisenberger MA, McLeod DG, et al. : A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 321:419-424, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Eisenberger MA, Blumenstein BA, Crawford ED, et al. : Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 339:1036-1042, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Pocock SJ, Simon R: Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 31:103-115, 1975 [PubMed] [Google Scholar]
- 15.Scher HI, Halabi S, Tannock I, et al. : Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the prostate cancer clinical trials working group. J Clin Oncol 26:1148-1159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995-2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138-148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scher HI, Fizazi K, Saad F, et al. : Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187-1197, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424-433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tannock IF, de Wit R, Berry WR, et al. : Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502-1512, 2004 [DOI] [PubMed] [Google Scholar]
- 21.de Bono JS, Oudard S, Ozguroglu M, et al. : Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 376:1147-1154, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Kantoff PW, Higano CS, Shore ND, et al. : Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411-422, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Parker C, Nilsson S, Heinrich D, et al. : Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369:213-223, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Kyriakopoulos CE, Chen Y, Carducci MA, et al. : Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 36:1080-1087, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gravis G, Boher J, Chen Y, et al. : Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: Further analyses of CHAARTED and GETUG-AFU15 studies. Eur Urol 73:847-855, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fizazi K, Jones R, Oudard S, et al. : Phase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or after docetaxel-based therapy: ELM-PC 5. J Clin Oncol 33:723-731, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad F, Fizazi K, Jinga V, et al. : Orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (ELM-PC 4): A double-blind, multicentre, phase 3, randomised, placebo-controlled trial. Lancet Oncol 16:338-348, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. : ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 37:2974-2986, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke NW, Ali A, Ingleby FC, et al. : Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann Oncol 30:1992-2003, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.02517.










