Abstract
Background:
Metabolic dysfunction links to cognitive deficits in Alzheimer’s disease (AD). Leptin is an anti-obesity hormone that modulates energy homeostasis and memory function. Although leptin deregulation is implicated in mouse models of AD-like brain pathology, clinical studies have shown inconsistent results regarding an association of leptin with the development of this neurodegenerative disorder.
Objective:
We investigated the changes of plasma leptin and the correlation of sex-stratified circulating leptin with cognitive performance, AD-related biological markers, and metabolic status in patients with AD and cognitively unimpaired (CU) counterparts.
Methods:
We used nonobese AD patients and CU controls in a University of Kansas Medical Center (KUMC) cohort. Plasma leptin levels, circulating AD-related molecules and metabolic profiles were examined and analyzed.
Results:
In contrast to unchanged circulating leptin in females, male patients exhibited decreased plasma leptin levels compared with male CU counterparts. Moreover, plasma leptin showed no correlation with cognitive performance and AD blood biomarkers in patients with either sex. Of note, females but not males demonstrated an association of plasma leptin with body mass index, high density lipoprotein-cholesterol and its ratio with total cholesterol and triglycerides.
Conclusion:
Our findings suggest that leptin deficiency is associated with nonobese male AD patients, supporting systemic dysmetabolism in the development of this neurodegenerative disorder in certain populations. Although plasma leptin may have limited capacity to reflect disease severity or progression, future mechanistic studies on the regulation of leptin in nonobese patients with AD would deepen our understanding of the sex-related disparity of AD etiopathogenesis.
Keywords: Alzheimer’s disease, blood biomarker, metabolism, plasma leptin, sex
INTRODUCTION
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder in which cognitive decline and systemic changes converge [1, 2]. In consistency with clinical findings of dysmetabolism in patients, increasing evidence accentuates a contribution of metabolic dysfunction in the development of this devastating neurological disorder [3-6]. Leptin is a metabolic hormone primarily produced by adipocytes and plays a crucial role in controlling fat metabolism [7]. In recent years, a critical role of leptin in the regulation of cognition and behavior through the brain-gut axis is emerging. Previous studies have linked leptin to the modulation of hippocampal synaptic activity and highlighted leptin’s regulation of memory-related long-term potentiation and long-term depression as well as hippocampal neurogenesis [8-11]. The neurotrophic effect of leptin and decreased leptin production with age [12] implicate a relevance of leptin deregulation to age-related cognitive disorders such as AD. In addition, because obesity constitutes a key risk factor for AD [13], elevated circulating leptin in response to increased body mass index (BMI) and fat mass [7] seems to support leptin as a potential anti-dementia agent [14, 15].
Studies using rodent and cell models have reported protective effects of leptin in ameliorating AD-like pathologies, especially through the inhibition of amyloid-β (Aβ) generation and pathological tau formation [16-19]. Although these preclinical findings endorse an involvement of leptin deficiency in the pathogenesis of AD, clinical studies have shown inconsistent results regarding leptin levels in the blood and/or cerebrospinal fluid (CSF) from patients with AD and mild cognitive impairment (MCI) [20-25]. The discrepancy in leptin levels in biofluids from AD patients may arise from many confounding factors such as patient base composition. The inclusion of obese subjects with mixed sexes may complicate the results in view of the intertwined relationship between leptin production and body fat mass [26] as well as sex-based differences in leptin production and metabolism [27, 28]. To this end, in this study we sought to compare plasma leptin between nonobese patients with AD and their cognitively unimpaired (CU) controls. In addition, we performed sex-stratified analysis and examined an association of circulating leptin with cognitive performance, AD-related biological markers as well as metabolic status. Our results showed that only male patients with AD demonstrated decreased plasma leptin. Moreover, in contrast to females, no association of circulating leptin with BMI or lipid metabolism was detected with males. Lastly, plasma leptin had a poor correlation with cognitive performance or AD-related biomarkers regardless of biological sex. The simplest interpterion of our findings is that nonobese male AD patients are vulnerable to leptin signaling deregulation. Although plasma leptin might have limited capacity to predict AD severity and progression, the decreased circulating leptin in male patients supports a potential sex-based disparity in the etiopathogenesis of this neurodegenerative disorder.
MATERIALS AND METHODS
Participants
Frozen human plasma samples were requested from University of Kansas (KU) Alzheimer’s Disease center under a protocol approved by KU Medical Center supported by NIH GRANT (P30 AG035982). Informed consent was collected from all subjects and the study adhered to the Declaration of Helsinki principles. Subjects with metabolic and endocrinal disorders including hypothalamic disorders, diabetes, familial hypercholesterolemia and hyperlipidemia, and hyper- or hypo-thyroidism, inflammatory diseases, liver and renal disorders as well as those under treatment with weight-control drugs, lipid-lowering agents, amylin mimetics, and leptin analogues were excluded from the study.
ELISA assay
Patients with BMI <25 kg/m2 were chosen and the plasma were subjected to the following tests using commercially available kit: leptin (RAB0333, Millipore Sigma), glucose (TR15421, Thermo Fisher), cholesterol (MAK043, Sigma-Aldrich), triglyceride (10010303, Cayman), HDL (80059, Crystal Chem), Aβ40 (KHB3481, Invitrogen), Aβ42 (KHB3441, Invitrogen), p-Taul81 (KHO0631, Invitrogen). All tests were performed following standard protocols.
Statistical analysis
Statistical analyses were performed using Graph-Pad Prism 8 software. Unpaired Student’s t test was applied in data analysis. Correlation was calculated using Pearson’s correlation coefficient. All data were expressed as means and 95% CI. Significance was concluded when the p value was less than 0.05. Significance was indicated by *p < 0.05, **p < 0.01.
RESULTS
Sex difference in plasma leptin in nonobese patients with AD
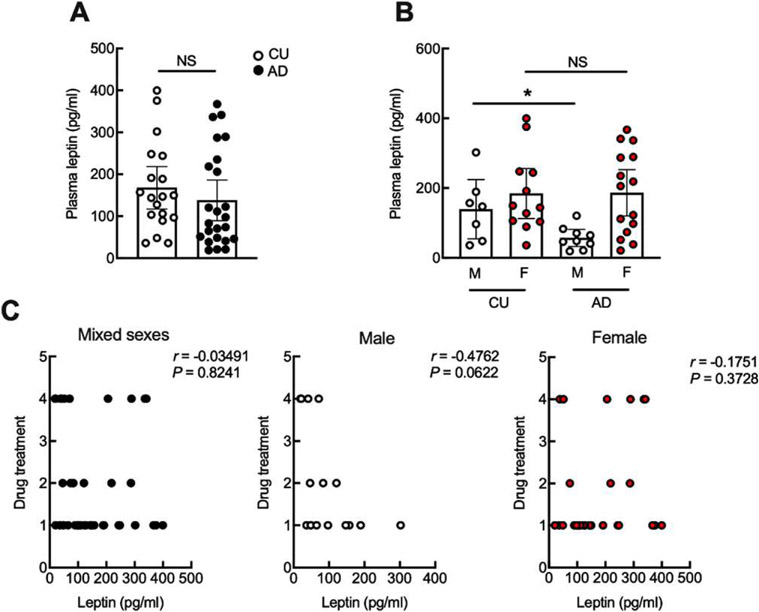
Twenty-four participants diagnosed with AD and 19 CU controls with a BMI lower than 25 kg/m2 in a University of Kansas Medical Center (KUMC) cohort were included in the present study. Participant characteristics are presented in Table 1. The two study groups were comparable regarding age, sex, race, educational level, and BMI. In comparison with the CU subjects, the AD group showed decreased Mini-Mental State Exam (MMSE) scores (CU: 29.06 ± 0.372 versus AD: 21.72 ± 2.026, p < 0.0001). Although ELISA assay for plasma leptin showed no change between AD and CU subjects (Fig. 1A), an association between circulating leptin and biological sex was observed (Table 2). We therefore stratified the data and performed a sex-based analysis. Despite comparable baseline characteristics including age, BMI, and MMSE scores across the two biological sexes (Table 1), male patients with AD exhibited a reduction in plasma leptin as compared with their CU counterparts (Fig. 1B). In contrast, there was no disease-related change in plasma leptin levels between female patients and their control subjects (Fig. 1B). Furthermore, circulating leptin in patients of either sex was not affected by conventional AD symptom-modifying therapies including single or combined treatment using acetylcholinesterase inhibitors and/or glutamate receptor antagonists (Fig. 1C), indicating that the reduction of leptin in nonobese male patients is a disease-related pathological change. These findings suggest a sex difference in plasma leptin deregulation in nonobese patients with AD.
Table 1.
Demographic characteristics of human subjects
| CU (95%CI) | AD (95%CI) | F | p | |
|---|---|---|---|---|
| Age (y) | 73.89 ± 3.99 | 72.58 ± 11.38 | 1.14 | 0.646 |
| Sex | 1.004 | 0.966 | ||
| Male | 8 (40%) | 9 (36%) | ||
| Female | 12 (60%) | 16 (64%) | ||
| Race | 12.42 | 0.0801 | ||
| Caucasian | 67% | 90% | ||
| African American | 27% | 10% | ||
| Asian | 6% | 0% | ||
| Education (y) | 16.87 ± 0.913 | 15.05 ± 2.36 | 2.024 | 0.0439* |
| Height (in) | 65.42 ± 1.54 | 66.11 ± 1.55 | 1.277 | 0.546 |
| Weight (lbs) | 148.89 ± 10.99 | 151.79 ± 10.72 | 1.202 | 0.716 |
| BMI | 24.34± 1.15 | 24.28 ± 1.072 | 1.081 | 0.943 |
| MMSE score | 29.06 ± 0.372 | 21.72 ± 2.026 | 37.5 | <0.0001 |
Demographic characters including age, sex, race, education, height, weight, BMI and MMSE score are evaluated. Unpaired Student’s t test,
p < 0.05. n = 19 cognitively unimpaired (CU) controls, 24 AD.
Fig. 1.
Plasma leptin shows sex difference in nonobese patients with AD. A) No difference of plasma leptin between cognitively unimpaired (CU) controls and AD patients. Unpaired Student’s t test, n = 19 CU, 24 AD. B) Plasma leptin shows significant decrease in male patients with AD as compared with cognitively unimpaired controls. Unpaired Student’s t test, *p < 0.05. n = 12 CU females, 15 AD female, 7 CU males, 9 AD males. C) is the correlation between plasma leptin and AD symptomatic treatments. 1 represents no treatment, 2 represents cholinesterase inhibitors treatment, 3 represents N-methl-D-aspartate receptor antagonist treatment, 4 represents the co-treatment of cholinesterase inhibitors and N-methyl-D-aspartate receptor antagonist in mixed or separated sex. Two-tailed Pearson correlation coefficients. n = 43 human subjects, 16 males, 27 females.
Table 2.
Correlation between plasma leptin and demographic characters
| r | p | |
|---|---|---|
| Age | −0.1776 | 0.2546 |
| Race | 0.1447 | 0.4069 |
| Education | −0.1807 | 0.2988 |
| Sex | 0.4096 | 0.0064** |
The correlation between plasma leptin and age, race, education, and sex. Two-tailed Pearson correlation coefficients,
p < 0.01. n = 19 cognitively unimpaired (CU) controls, 24 AD.
No association of circulating leptin with plasma AD-related molecules
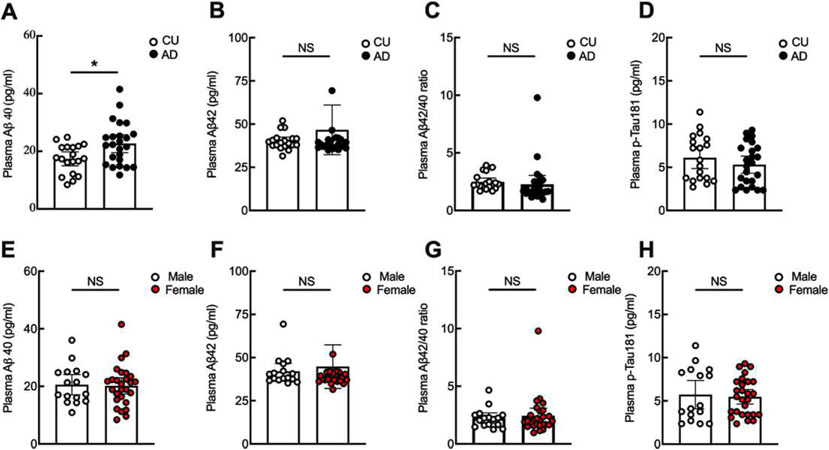
To determine whether plasma leptin levels are affected by AD-associated pathological molecules, we examined plasma Aβ40, Aβ42, and p-Taul81 using ELISA assays. Except for increased plasma Aβ40 (Fig. 2A), AD patients in the tested cohort demonstrated no change in plasma Aβ42 (Fig. 2B), Aβ42/40 ratio (Fig. 2C), or p-Taul81 (Fig. 2D) as compared with their CU controls. Moreover, there were no sex-dependent changes in plasma Aβ40 (Fig. 2E), Aβ42 (Fig. 2F), Aβ42/40 ratio (Fig. 2G), or p-Taul81 (Fig. 2H) between male and female patients. Further correlation analysis showed no association of plasma leptin with Aβ40, Aβ42, Aβ42/40 ratio, or p-Taul81 in studied groups of mixed sexes or separated by sex (Table 3). The results implicate no interaction of leptin production with circulating pathological biological markers of AD.
Fig. 2.
Plasma Aβ40 significantly increased in patients with AD. Plasma AD-related molecules including Aβ40 (A), Aβ42 (B), Aβ42/40 (C), p-Taul81 (D) were examined and compared between cognitively unimpaired (CU) controls and AD. Unpaired Student’s t test, *p < 0.05. n = 19 CU, 24 AD. Sex effect on Aβ40 (E), Aβ42 (F), Aβ42/40 (G), p-Taul81 (H) were compared. Unpaired Student’s t test. n = 16 males, 27 females.
Table 3.
Correlation between plasma leptin and AD-related molecules
| All population |
Male |
Female |
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Aβ40 (pg/ml) | 0.08647 | 0.5814 | −0.1196 | 0.659 | 0.1932 | 0.3247 |
| Aβ42 (pg/ml) | 0.2749 | 0.0744 | −0.01831 | 0.9463 | 0.32 | 0.0969 |
| Aβ42/40 ratio | 0.1578 | 0.3122 | 0.0244 | 0.9285 | 0.1710 | 0.3842 |
| p-Taul81 (pg/ml) | 0.1458 | 0.3507 | 0.2413 | 0.3678 | 0.1868 | 0.3411 |
The correlation between plasma leptin and Aβ40, Aβ42, Aβ42/40 ratio, and p-Taul81. Two-tailed Pearson correlation coefficients. n = 19 cognitively unimpaired (CU) controls, 24 AD.
No association of circulating leptin with cognitive performance
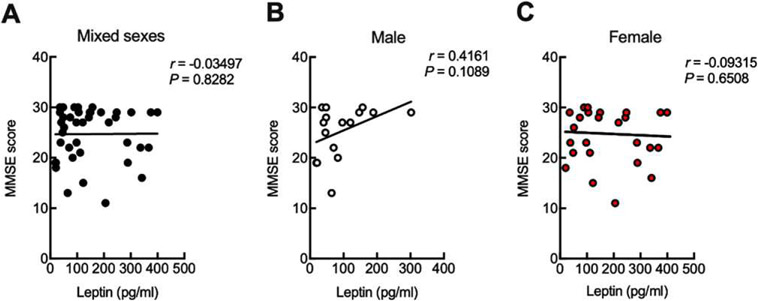
Previous studies reported inconsistent results regarding the correlation between leptin and cognition in patients with AD or MCI [20-24]. To determine whether leptin is associated with cognitive performance in the nonobese cohort, we examined the relationship between plasma leptin and patients’ cognitive performance, by proxy, the MMSE scores. Further analysis showed no correlation between plasma leptin and MMSE scores in the tested groups of mixed sexes (Fig. 3A), male only (Fig. 3B), or female only (Fig. 3C).
Fig. 3.
Plasma leptin is uncorrelated to cognitive performance. Correlation between MMSE score and leptin in mixed sexes (A), male (B), and female (C) were calculated using two-tailed Pearson correlation coefficients. n = 43 human subjects, 16 males, 27 females.
Sex difference in the association of metabolic profiles with plasma leptin
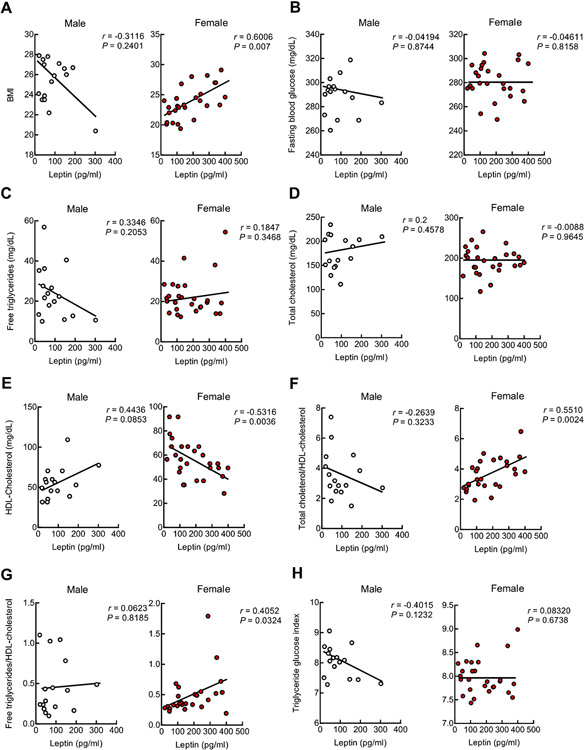
The production of leptin is regulated by metabolic status and is consequently responsive to glucose and lipid metabolism [29]. BMI has been used to measure metabolism but suffers from inaccuracy in reflecting metabolic profiles [30]. To this end, in addition to BMI, we examined total free triglycerides (TG), total cholesterol (TC), and high-density lipoprotein (HDL)-cholesterol (HDL-C) and calculated the lipid-related indexes including TC/HDL-C ratio (TC/HDL-C) and TG/HDL-C ratio (TG/HDL-C) to reflect lipid metabolism (Table 4). In addition, we tested fasting blood glucose (FG) and TG-FG (TyG) index to reflect glucose regulation and insulin resistance [31] (Table 4). Neither disease-associated nor sex-related effects on these metabolic parameters were present (Table 4). Further analysis showed that male subjects had no correlation of plasma leptin with BMI (Fig. 4A), FG (Fig. 4B), TG (Fig. 4C), TC (Fig. 4D), HDL-C (Fig. 4E), TC/HDL-C (Fig. 4F), TG/HDL-C (Fig. 4G), or TyG (Fig. 4H); whereas a positive relationship of circulating leptin with BMI (Fig. 4A), TC/HDL-C (Fig. 4F), and TG/HDL-C (Fig. 4G) as well as a negative association of circulating leptin with HDL-C (Fig. 4E) were observed with female subjects. In view of the impact of leptin on lipid metabolism [32], the results suggest that the metabolism-regulating effect of leptin is preserved, at least, in nonobese aged females regardless of AD diagnosis.
Table 4.
Plasma metabolic parameters
| CU (95%CI) | AD (95%CI) | F | p | CU versus AD |
Male versus Female |
|||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | |||||
| FG (mg/dL) | 283.11 ± 7.66 | 283.33 ± 6.04 | 1.272 | 0.9646 | 0.007 | 0.96 | −0.3 | 0.05 |
| TG (mg/dL) | 23.20 ± 5.45 | 23.37 ± 3.983 | 1.484 | 0.9590 | 0.008 | 0.96 | −0.06 | 0.7 |
| TC (mg/dL) | 186.18 ± 17.36 | 188.84 ± 12.82 | 1.451 | 0.8062 | 0.038 | 0.81 | 0.23 | 0.14 |
| HDL/C (mg/dL) | 58.09 ± 9.72 | 54.07 ± 5.1738 | 2.795 | 0.4532 | −0.12 | 0.45 | 0.004 | 0.98 |
| TG/HDL-C | 0.4730 ± 0.143 | 0.4786 ± 0.1358 | 1.132 | 0.9569 | 0.008 | 0.96 | −0.17 | 0.29 |
| TC/HDL-C | 3.576 ± 0.6139 | 3.697 ± 0.4561 | 1.434 | 0.7545 | 0.05 | 0.75 | 0.039 | 0.801 |
| TyG index | 7.974 ± 0.2207 | 8.027 ± 0.1542 | 1.621 | 0.6944 | 0.062 | 0.69 | −0.04 | 0.81 |
Plasma level of fasting blood glucose (FG), free triglycerides (TG), total cholesterol (TC) high density lipoprotein (HDL)-cholesterol, total cholesterol/HDL-cholesterol ratio (TC/HDL-C), triglycerides/HDL-cholesterol ratio (TG/HDL-C), and triglycerides and glucose (TyG) index were measured or calculated then compared between cognitively unimpaired (CU) controls and AD patients. Unpaired Student’s t test, n = 19 CU, 24 AD. Correlation between the above parameters and AD or sex were performed using two-tailed Pearson correlation coefficients.
Fig. 4.
Plasma leptin metabolic regulation is preserved in female. Correlation between leptin and BMI (A), fasting blood glucose (B), free triglyceride (C), total cholesterol (D), HDL-cholesterol (E), ratio of total cholesterol/HDL-cholesterol (F), ratio of free triglyceride/HDL-cholesterol (G) and Triglyceride glucose index (H) were calculated in male and female using two-tailed Pearson correlation coefficients. Triglyceride glucose index was calculated as ln[fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2]. n = 16 males, 27 females.
DISCUSSION
Although leptin has been associated with cognitive function in aged population [33], the role of leptin deregulation in the development of age-related cognitive disorders such as AD remains as a long-standing scientific question. Previous studies using genetic leptin receptor depleted diabetic (DB/DB) mice have established a link between obesity, diabetes, and AD-like brain pathologies and further implicates a contribution of leptin signaling perturbations to the development of AD [34, 35]. A direct association of leptin with AD-related brain damages is supported by findings that leptin functions through its influence on β-secretase and γ-secretase, resulting in reduced Aβ production in cell and animal models of AD [16-19]. Moreover, leptin also attenuates tau phosphorylation via AMP-activated protein kinase [36] and Wnt signaling [37]. Those preclinical data suggest leptin deficiency as a contributing factor to the development of AD and further underscore a therapeutic potential of leptin-boosting agents for the treatment of this neurodegenerative disorder. However, there is thus far no consensus in clinical studies on whether leptin deficiency constitutes a characteristic AD pathology or predicts the risk of AD dementia in aged population. A previous study showed lowered leptin levels in the plasma and CSF samples from patients with MCI and AD [25]. Moreover, females exhibited increased baseline levels of circulating leptin [25]. In consistency, Holden and colleagues examined circulating leptin and cognitive changes in cognitive norms [12]. After adjusting for BMI and body fat mass, they found a negative relationship between baseline serum leptin and cognitive decline within a 4-year observation window [12]. These results imply a deleterious impact of leptin deficiency on the development of cognitive impairment in aged individuals at risk of AD, which agrees with studies done by Littlejohns in Italian populations [38] as well as by Lieb using the Framingham Original cohort [20]. Moreover, Yaffe and colleagues reported an increased risk of developing AD in aged nonobese females with lowered circulating leptin [22]. In contrast, McEvoy did not detect altered plasma leptin levels or correlation of leptin levels with cognitive function in patients with MCI [23]. Furthermore, another study on nonobese patients with AD showed no association of plasma leptin with disease diagnosis, MMSE score, or brain atrophy [21]. The results agree with our observations of unchanged plasma leptin levels in nonobese AD patients with mixed sexes. However, we found that male, but not female patients exhibited decreased circulating leptin, implicating a sex-based difference in leptin regulation accompanying AD. In addition, we found a marginal increase in plasma leptin levels in females regardless of AD diagnosis, which is in agreement with Johnston’s report [25]. But Johnston’s study showed no sex difference in disease-associated plasma leptin reduction [25], which is inconsistent with our observation of preserved leptin in nonobese female patients. Such a discrepancy regarding leptin levels in female patients may arise from selection of patients. In contrast to the majority of patients with MCI in Johnston’s study [25], we examined plasma leptin in patients with diagnosed AD. In addition, we only recmited nonobese (that is, BMI <25 kg/m2) patients and there was no difference regarding the BMI between CU and AD subjects. However, in Johnston’s study the control groups had a higher level of BMI [25]. Because of a positive relationship between leptin production and BMI [39], it cannot be excluded the possibility that the decreased leptin levels in female patients is, at least in part, reflects a difference of BMI in the tested groups. Indeed, we cannot exclude the possibility that aged subjects of both sexes with lowered baseline levels of plasma leptin may have higher odds of developing AD or MCI in later years as shown in previous studies [12]. Further follow-up studies will help to address this question.
Another finding merits discussion is that nonobese females, but not males exhibited a strong relationship between circulating leptin and BMI, HDL-C, and TC/HDL-C as well as TG/HDL-C. Such a correlation of leptin with the lipid metabolism-related parameters indicates that aged nonobese females have preserved leptin regulation to lipid metabolism, which corroborates previous findings [40, 41] and further indicates impaired leptin regulation in nonobese males with AD. Previous studies have determined a sex-based disparity in leptin metabolism and reported increased leptin in females independent of fat distribution or adipocyte size [28, 42-44]. Furthermore, females have increased leptin production per fat mass as compared with males in health [28] and enhanced leptin secretion rate by adipose tissues [44]. In this regard, it is possible that AD is associated with decreased leptin production by adipocytes, and females are more resistant to such a disease-related change of adipocyte function. Aside from adipocyte regulation, the impact of sex hormones should not be overlooked. It is well-documented that postmenopausal females have higher odds to develop dysmetabolism and AD dementia as compared with premenopausal females and age-matched males, indicating a critical influence of sex hormone deregulation in the development of AD, especially in females [45, 46]. Previous studies have suggested a complicated interaction between leptin, estrogen, and follicle-stimulating hormone (FSH) [47, 48]. Elevated FSH has been associated with females with AD [49] and blockade of FSH signaling is protective against AD symptoms in mouse models [50]. In this regard, the maintained levels of leptin may potentially be a result of and, meanwhile, a promoting factor for gonadotropin perturbations in female AD patients. In this context, unchanged leptin in female patients may instead serve as a demonstration of an AD-associated pathological change resulting from a perplexing mixture of dysmetabolism, sex hormone deregulation, and aging process. Further elucidation of the sex-related leptin regulation in AD patients and subjects at risk to develop AD will have the impact to advance our understanding of the sex disparity in AD pathogenesis and address potential mechanisms of sex difference in vulnerability to this devastating disease. Nevertheless, based on our current findings, nonobese male patients exhibited increased sensitivity to suppressed leptin production, which may potentially lead to deleterious consequences of both metabolic aberrations and cognitive decline with disease progression.
In our current study, we also examined potential association of circulating leptin with blood-based biological markers of AD including Aβ40, Aβ42, and Aβ42/40 ratio as well as p-Taul81. Our results showed no association of plasma leptin with these blood biomarkers of AD. These findings are consistent with our observation of lack of correlation between leptin and cognitive performance in patients with AD of either sex. In addition, our assays for the association of AD-related biomarkers with leptin also supplement previous studies, which primarily focused on brain volume assay with little information about plasma Aβ40, Aβ42, Aβ42/40 ratio, and p-Taul81 in the tested subjects [20, 21]. Although the capacity of blood Aβ in reflecting AD severity and brain pathology is still under debate [51], our results suggest limited impact of circulating AD-related pathological molecules on leptin production. Moreover, the results have undermined the capacity of plasma leptin to predict AD and/or monitor disease progression to some extent.
In summary, our current study showed a sex difference in circulating leptin in nonobese patients with AD. These findings warrant our further investigation into the detailed mechanisms of leptin downregulation in nonobese male patients and the contribution of leptin deficiency to the development of AD. Several limitations of this study are present such as a relatively small cohort size. Moreover, whether plasma leptin levels are associated with disease progression and the potential risk of CU controls with low leptin to develop AD in our tested cohort remains unknown. These questions will be addressed in our future longitudinal study on a large-scale cohort. Lastly, a previous clinical study showed elevated leptin in the CSF samples from AD patients alongside decreased leptin receptor mRNA levels in the AD brains, while female patients displayed substantially increased CSF leptin [24]. These results implicate a possibility of “leptin resistance” accompanying AD. Indeed, “insulin resistance” and “ghrelin resistance” have previously been associated with AD brain damages [52-55], which highlights an involvement of dysfunctional metabolic hormone receptors in the development of synaptic injuries and cognitive impairment in AD. In current study, we only included nonobese subjects and excluded those with obesity and diagnosed diabetes. Moreover, we did not observe insulin resistance in the tested subjects. Previous studies have established an association between leptin deregulation and insulin resistance [39, 56] and determined shared downstream signaling between insulin and leptin in neural cells [57]. It is possible that leptin may play a distinct role in patients with insulin resistance from those without glucose and insulin deregulation, which forms the ground-work for our future study. Moreover, leptin-boosting agents may benefit AD patients from their “anti-diabetic” and “anti-obesity” effects. However, such a therapeutic effect of leptin analogues for the treatment and prevention of AD may be diminished by the development of leptin resistance in patients. To this end, further efforts should be put forth towards the functional status of leptin receptor in AD patients, which will deepen our understanding of leptin signaling deregulation in AD and help to reconcile current discrepancy regarding the capacity of leptin in mitigating AD symptoms in clinical settings. Therefore, the most parsimonious interpretation of our results is that reduced plasma leptin is associated with nonobese male patients with AD. Although circulating leptin may not accurately reflect disease severity, changes of circulating leptin in nonobese male patients not only implicate a potential contribution of systemic dysmetabolism to the development of AD in certain populations, but also support the heterogenous nature of this neurodegenerative disorder. Further mechanistic study on leptin regulation in patients would foster a better understanding of sex-related disparity in the etiopathogenesis of AD.
ACKNOWLEDGMENTS
This work was supported by research fundings from NIH (R01AG053588, R01AG059753, and R01AG075108 to HD; P30 AG035982 to RS), Higuchi Biosciences Center research grant to HD and RS, as well as Brightfocus Foundation research grant (A20201159S) to HD, KUMC research grant and KU career development grant to LG.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0447rl).
REFERENCES
- [1].Morris JK, Honea RA, Vidoni ED, S werdlow RH, Burns JM (2014) Is Alzheimer’s disease a systemic disease? Biochim Biophys Acta 1842, 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Giordano V, Peluso G, Iannuccelli M, Benatti P, Nicolai R, Calvani M (2007) Systemic and brain metabolic dysfunction as a new paradigm for approaching Alzheimer’s dementia. Neurochem Res 32, 555–567. [DOI] [PubMed] [Google Scholar]
- [3].Tian J, Guo L, Sui S, Driskill C, Phensy A, Wang Q, Gauba E, Zigman JM, Swerdlow RH, Kroener S, Du H (2019) Disrupted hippocampal growth hormone secretagogue receptor lalpha interaction with dopamine receptor D1 plays a role in Alzheimer’s disease. Sci Transl Med 11, eaav6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rosenbloom MH, Barclay TR, Pyle M, Owens BL, Cagan AB, Anderson CP, Frey WH 2nd, Hanson LR (2014) A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs 28, 1185–1189. [DOI] [PubMed] [Google Scholar]
- [5].Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N (2009) Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett 455, 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fu W, Ruangkittisakul A, MacTavish D, Shi JY, Ballanyi K, Jhamandas JH (2012) Amyloid beta (Abeta) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. J Biol Chem 287, 18820–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Perakakis N, Farr OM, Mantzoros CS (2021) Leptin in leanness and obesity: JACC state-of-the-art review. J Am Coll Cardiol 77, 745–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yook JS, Rakwal R, Shibato J, Takahashi K, Koizumi H, Shima T, Ikemoto MJ, Oharomari LK, McEwen BS, Soya H (2019) Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc Natl Acad Sci U S A 116, 10988–10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J (2002) Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol 545, 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K (2006) Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides 27,2738–2749. [DOI] [PubMed] [Google Scholar]
- [11].Shanley LJ, Irving AJ, Harvey J (2001) Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci 21, RC186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K, Health ABCs (2009) Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging 30, 1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alford S, Patel D, Perakakis N, Mantzoros CS (2018) Obesity as a risk factor for Alzheimer’s disease: Weighing the evidence. Obes Rev 19, 269–280. [DOI] [PubMed] [Google Scholar]
- [14].Johnston JM, Greco SJ, Hamzelou A, Ashford JW, Tezapsidis N (2011) Repositioning leptin as a therapy for Alzheimer’s disease. Therapy 8, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beccano-Kelly D, Harvey J (2012) Leptin: A novel therapeutic target in Alzheimer’s disease? Int J Alzheimers Dis 2012, 594137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N (2004) Obesity-related leptin regulates Alzheimer’s Abeta. FASEB J 18, 1870–1878. [DOI] [PubMed] [Google Scholar]
- [17].Niedowicz DM, Studzinski CM, Weidner AM, Platt TL, Kingry KN, Beckett TL, Bruce-Keller AJ, Keller JN, Murphy MP (2013) Leptin regulates amyloid beta production via the gamma-secretase complex. Biochim Biophys Acta 1832, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marwarha G, Raza S, Meiers C, Ghribi O (2014) Leptin attenuates BACE1 expression and amyloid-beta genesis via the activation of SIRT1 signaling pathway. Biochim Biophys Acta 1842, 1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Greco SJ, Hamzelou A, Johnston JM, Smith MA, Ashford JW, Tezapsidis N (2011) Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and beta-amyloid in neurons. Biochem Biophys Res Commun 414, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenofif R, Auerbach S, DeCarli C, Wolf PA, Seshadri S (2009) Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 302, 2565–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Teunissen CE, van der Flier WM, Scheltens P, Duits A, Wijnstok N, Nijpels G, Dekker JM, Blankenstein RM, Heijboer AC (2015) Serum leptin is not altered nor related to cognitive decline in Alzheimer’s disease. J Alzheimers Dis 44, 809–813. [DOI] [PubMed] [Google Scholar]
- [22].Zeki Al Hazzouri A, Stone KL, Haan MN, Yaffe K (2013) Leptin, mild cognitive impairment, and dementia among elderly women. J Gerontol A Biol Sci Med Sci 68, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oania R, McEvoy LK (2015) Plasma leptin levels are not predictive of dementia in patients with mild cognitive impairment. Age Ageing 44, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bonda DJ, Stone JG, Torres SL, Siedlak SL, Perry G, Kryscio R, Jicha G, Casadesus G, Smith MA, Zhu X, Lee HG (2014) Dysregulation of leptin signaling in Alzheimer disease: Evidence for neuronal leptin resistance. J Neurochem 128, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johnston JM, Hu WT, Fardo DW, Greco SJ, Perry G, Montine TJ, Trojanowski JQ, Shaw LM, Ashford JW, Tezapsidis N, Alzheimer’s Disease Neuroimaging Initiative (2014) Low plasma leptin in cognitively impaired ADNI subjects: Gender differences and diagnostic and therapeutic potential. Curr Alzheimer Res 11, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Considine RV (2001) Regulation of leptin production. Rev Endocr Metab Disord 2, 357–363. [DOI] [PubMed] [Google Scholar]
- [27].Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT (1997) The metabolic significance of leptin in humans: Gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab 82, 1293–1300. [DOI] [PubMed] [Google Scholar]
- [28].Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, Houmard JA, Marks RH, Caro JF (1996) Gender differences in serum leptin levels in humans. Biochem Mol Med 59, 1–6. [DOI] [PubMed] [Google Scholar]
- [29].Wolsk E, Mygind H, Grondahl TS, Pedersen BK, van Hall G (2011) The role of leptin in human lipid and glucose metabolism: The effects of acute recombinant human leptin infusion in young healthy males. Am J Clin Nutr 94, 1533–1544. [DOI] [PubMed] [Google Scholar]
- [30].Weber DR, Leonard MB, Shults J, Zemel BS (2014) A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab 99, 3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kheirollahi A, Teimouri M, Karimi M, Vatannejad A, Moradi N, Borumandnia N, Sadeghi A (2020) Evaluation of lipid ratios and triglyceride-glucose index as risk markers of insulin resistance in Iranian polycystic ovary syndrome women. Lipids Health Dis 19, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hynes GR, Jones PJ (2001) Leptin and its role in lipid metabolism. Curr Opin Lipidol 12, 321–327. [DOI] [PubMed] [Google Scholar]
- [33].Zonneveld MH, Noordam R, van der Grond J, van Heemst D, Mooijaart SP, Sabayan B, Jukema JW, Trompet S (2021) Interplay of circulating leptin and obesity in cognition and cerebral volumes in older adults. Peptides 135, 170424. [DOI] [PubMed] [Google Scholar]
- [34].Niedowicz DM, Reeves VL, Platt TL, Kohler K, Beckett TL, Powell DK, Lee TL, Sexton TR, Song ES, Brewer LD, Latimer CS, Kraner SD, Larson KL, Ozcan S, Norris CM, Hersh LB, Porter NM, Wilcock DM, Murphy MP (2014) Obesity and diabetes cause cognitive dysfunction in the absence of accelerated beta-amyloid deposition in a novel murine model of mixed or vascular dementia. Acta Neuropathol Commun 2, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ernst A, Sharma AN, Elased KM, Guest PC, Rahmoune H, Bahn S (2013) Diabetic db/db mice exhibit central nervous system and peripheral molecular alterations as seen in neurological disorders. Transl Psychiatry 3, e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Greco SJ, Sarkar S, Johnston JM, Tezapsidis N (2009) Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biopkys Res Commun 380, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang Z, Guo M, Zhang J, Du C, Xing Y (2016) Leptin regulates tau phosphorylation through Wnt signaling pathway in PC12 cells. Neurosignals 24, 95–101. [DOI] [PubMed] [Google Scholar]
- [38].Littlejohns TJ, Kos K, Henley WE, Cherubini A, Ferrucci L, Lang IA, Langa KM, Melzer D, Llewellyn DJ (2015) Serum leptin and risk of cognitive decline in elderly Italians. J Alzheimers Dis 44, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kumar R, Mai K, Razaq MK, Magsi M, Memon MK, Memon S, Afroz MN, Siddiqui HF, Rizwan A (2020) Association of leptin with obesity and insulin resistance. Cureus 12, e12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith J, Al-Amri M, Sniderman A, Cianflone K (2006) Leptin and adiponectin in relation to body fat percentage, waist to hip ratio and the apoB/apoA1 ratio in Asian Indian and Caucasian men and women. Nutr Metab (Lond) 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Idris S, Sunitha S (2014) Assessment of BMI, serum leptin levels and lipid profile in patients with skin tags. J Clin Diagn Res 8, CC01–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wauters M, Van Gaal L (1999) Gender differences in leptin levels and physiology: A role for leptin in human reproduction. J Gend Specif Med 2, 46–51. [PubMed] [Google Scholar]
- [43].Flanagan DE, Vaile JC, Petley GW, Phillips DI, Godsland IF, Owens P, Moore VM, Cockington RA, Robinson JS (2007) Gender differences in the relationship between leptin, insulin resistance and the autonomic nervous system. Regul Pept 140, 37–42. [DOI] [PubMed] [Google Scholar]
- [44].Hellstrom L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P (2000) Mechanisms behind gender differences in circulating leptin levels. J Intern Med 247, 457–462. [DOI] [PubMed] [Google Scholar]
- [45].Wang G, Li W (2021) Sex as a risk factor for developing cognitive impairments in National Alzheimer’s Coordinating Center Participants. J Alzheimers Dis Rep 5, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhao N, Ren Y, Yamazaki Y, Qiao W, Li F, Felton LM, Mahmoudiandehkordi S, Kueider-Paisley A, Sonoustoun B, Arnold M, Shue F, Zheng J, Attrebi ON, Martens YA, Li Z, Bastea L, Meneses AD, Chen K, Thompson JW, St John-Williams L, Tachibana M, Aikawa T, Oue H, Job L, Yamazaki A, Liu CC, Storz P, Asmann YW, Ertekin-Taner N, Kanekiyo T, Kaddurah-Daouk R, Bu G (2020) Alzheimer’s risk factors age, APOE genotype, and sex drive distinct molecular pathways. Neuron 106, 727–742 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Geber S, Brandao AH, Sampaio M (2012) Effects of estradiol and FSH on leptin levels in women with suppressed pituitary. Reprod Biol Endocrinol 10, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ogura K, Irahara M, Kiyokawa M, Tezuka M, Matsuzaki T, Yasui T, Kamada M, Aono T (2001) Effects of leptin on secretion of LH and FSH from primary cultured female rat pituitary cells. Eur J Endocrinol 144, 653–658. [DOI] [PubMed] [Google Scholar]
- [49].Short RA, Bowen RL, O’Brien PC, Graff-Radford NR (2001) Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc 76, 906–909. [DOI] [PubMed] [Google Scholar]
- [50].Xiong J, Kang SS, Wang Z, Liu X, Kuo TC, Korkmaz F, Padilla A, Miyashita S, Chan P, Zhang Z, Katsel P, Burgess J, Gumerova A, Ievleva K, Sant D, Yu SP, Muradova V, Frolinger T, Lizneva D, Iqbal J, Goosens KA, Gera S, Rosen CJ, Haroutunian V, Ryu V, Yuen T, Zaidi M, Ye K (2022) FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 603, 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zetterberg H, Blennow K (2006) Plasma Abeta in Alzheimer’s disease–up or down? Lancet Neurol 5, 638–639. [DOI] [PubMed] [Google Scholar]
- [52].Woodfield A, Porter T, Gilani I, Noordin S, Li QX, Collins S, Martins RN, Maruff P, Masters CL, Rowe CC, Villemagne VL, Dore V, Newsholme P, Laws SM, Verdile G, Group AR (2022) Insulin resistance, cognition and Alzheimer’s disease biomarkers: Evidence that CSF Abeta42 moderates the association between insulin resistance and increased CSF tau levels. Neurobiol Aging 114, 38–48. [DOI] [PubMed] [Google Scholar]
- [53].Sedzikowska A, Szablewski L (2021) Insulin and insulin resistance in Alzheimer’s disease. Int J Mol Sci 22, 9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tian J, Wang T, Wang Q, Guo L, Du H (2019) MK0677, a ghrelin mimetic, improves neurogenesis but fails to prevent hippocampal lesions in a mouse model of Alzheimer’s disease pathology. J Alzheimers Dis 72, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang Y, Ji G, Li G, Hu Y, Liu L, Jin Q, Meng Q, Zhao J, Yuan K, Liu J, von Deneen KM, Chen A, Cui G, Wang H, Zhao Q, Wu K, Tian J, Manza P, Tomasi D, Volkow ND, Nie Y, Wang GJ (2019) Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes (Lond) 43, 842–851. [DOI] [PubMed] [Google Scholar]
- [56].Esteghamati A, Khalilzadeh O, Anvari M, Rashidi A, Mokhtari M, Nakhjavani M (2009) Association of serum leptin levels with homeostasis model assessment-estimated insulin resistance and metabolic syndrome: The key role of central obesity. Metab Syndr Relat Disord 7, 447–452. [DOI] [PubMed] [Google Scholar]
- [57].Benomar Y, Roy AF, Aubourg A, Djiane J, Taouis M (2005) Cross down-regulation of leptin and insulin receptor expression and signalling in a human neuronal cell line. Biochem J 388, 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]