Abstract
Aim
Several studies emphasized the antiviral properties of many natural compounds enclosed in nutraceuticals formulas and quite effective to prevent the respiratory infections. The rationale of our investigation has been to achieve protection from common cold viruses' infection of the upper airways pooling together and dispensing different active principles on a multistep defense basis. Material and Methods. 30 patients affected by sudden aspecific viral-induced sore throat rhinitis were divided in two groups: (1) the first group included 15 patients which were administered with our spray formula and (2) the second group included 15 patients with the commercial nasal lavage kit. The mucous smear was stained with May Grunwald-Giemsa to exclude eosinophilic infiltrate and confirm the prevalence of granulocytes and lympho-monocytes typical of viral seasonal inflammatory upper airways conditions.
Results
The symptomatic relieve is remarkedly evident in the treated group with our spray compared to the second group treated with commercial nasal lavage kit.
Conclusions
The open case-control retrospective observational study showed a definite benefit of the spray based on natural herbal extracts to take control of the upper airways respiratory distress due to viral infections.
1. Introduction
Many viral pathogenic agents enclosing influential respiratory syncytial rhinovirus, herpes, and several other agents (such as SARS-CoV-2) harbor usually along the upper airways mucosae and spread the infection into the trachea, bronchus, and lungs and even the bowel with the possible renown clinical complications (Tables 1 and 2).
Table 1.
Common pathogens concerned in respiratory tract infections (bacteria, viruses, and fungi).
| Pathogen | Type gram | Pathology | Ref. |
|---|---|---|---|
| Streptococcus pneumoniae | + | Bacterial pneumonia | [1, 2] |
| Haemophilus influenzae | − | Pneumonia, sinusitis | [3, 4] |
| Chlamydophila pneumoniae | Obligate intracellular bacterium | Atypical pneumonia | [5, 6] |
| Staphylococcus aureus | + | Sinusitis, pneumonia | [7, 8] |
| Pseudomonas aeruginosa | − | Sinusitis, pneumonia | [9] |
| Legionella pneumophila | − | Cough with sputum, bronchiolitis | [10, 11] |
| Moraxella catarrhalis | − | Bronchitis, sinusitis, and bronchopneumonia | [12] |
| Rhinoviruses | Enterovirus | Common cold and sinusitis | [13] |
| Coronaviruses | Coronavirinae | Pneumonia | [14, 15] |
| Respiratory syncytial virus | Pneumovirus | Bronchiolitis | [16] |
| Adenovirus | Adenoviridae | Pneumonia and tonsillitis, | [17] |
| Herpes simplex virus | Respirovirus | Pneumonia | [18, 19] |
| Histoplasma capsulatum | Histoplasma (dimorphic fungi) | Pneumonia | [20] |
| Cryptococcus neoformans | Cryptococcus (yeast) | Pneumonia | [21, 22] |
| Coccidioides immitis | Coccidioides (pathogenic fungus) | Pneumonia | [23, 24] |
| Pneumocystis jirovecii | Pneumonia | [25, 26] |
Table 2.
Estimated annual proportion of clinical case based on virus' type.
| Virus's type | Estimated annual proportion of cases |
|---|---|
| Rhinovirus | 30–50% |
| Coronavirus | 10–15% |
| Influenza virus | 5–15% |
| Respiratory syncytial virus | 5% |
| Parainfluenza virus | 5% |
| Adenovirus | <5% |
| Enterovirus | <5% |
| Metapneumovirus | Unknown |
| Unknown | 20–30% |
Furthermore, sometimes even in healthy carriers, the nasopharyngeal diagnostic swab can remain virus positive even for weeks or months after the complete resolution of the symptoms.
Several clinical studies faced in the last 3 years the prevention of virus entrance through the nostrils and the throat by means of nasal irrigation with antiseptics, drops, or sprays to inactivate the pathogenic agents. As to the different marketing available tools, the nasal sprays are based on antiseptics (chlorhexidine and iodine povidone), antihistaminic, and vasoconstrictors, while the oral ones being mainly based on generic antiseptics but also anti-inflammatory and natural compounds such as propolis.
In this perspective, the antiviral properties of many natural compounds have been investigated as effective nutraceuticals formulas to achieve preventive control of the respiratory infections.
Our proposal is based on a mix of herbal extracts such as Phyllantus and Andrographis paniculata, added with copper ions and zinc. The activity of each herbal strain can be summarized as follows:
Phyllanthus family involves Niruri and/or Phyllantus Emblica, Amarus, and urinaria., leaves extract to concentration range among 2 and 15%. This extract is composed by secondary metabolites including flavonoids, tannins, lignins, polyphenols, and triterpenes, and displays several ethnopharmacological actions, e.g., anti-inflammatory, hepatoprotective, nephroprotective, anticancer, antioxidant, and antiviral, particularly in conditions including diarrhea, dysentery, dropsy, running nose, winter common colds [27, 28]. Yang et al. [29] enhanced the antiviral role of extracts of Phyllanthus urinaria against Herpes Simplex Virus 1 (HSV-1), strain KOS, and Herpes Simplex Virus 2 (HSV-2). In particular, the acetone (31.4%), ethanolic (40.4%), and methanolic (41.3%) extractants inhibited more than 90% of HSV-2 plaques. HSV-2 were destroyed when the compound was added to cells just after viral infection rather than added pre- or postviral infection
Andrographis paniculata is widely used in the eastern folk medicine for relieving and decreasing the typical symptoms of flu, such as fever, cough and sore throats, in vitro, it suppresses avian influenza A (H9N2 and H5N1) and human influenza A H1N1 viruses, maybe through blocking the binding of viral hemagglutinin to cells [30], or by inhibiting H1N1 virus-induced cell death [31]. A. paniculata extracts were analyzed against a wide variety of pathogens, such as several antibiotic-resistant species, e.g., Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella spp., Candida spp., and Streptococcus pneumoniae. Fifty-nine invasive microbes have been used to analyze the antimicrobial efficacy of A. paniculata extracts and/or their isolated pure compounds. Its active components named “andrographolides” include the diterpene, lactones which provided anti-inflammatory, antiviral, and immune-stimulatory activities [32]. They inhibit platelet-activating factor mediated inflammatory response [33], reduce expression of proinflammatory proteins (e.g., cyclooxygenase-2) [34, 35], and demonstrate analgesic effects, in addition to antipyretic effects comparable to paracetamol [36]
Copper (Cu) ions are very active against several viruses responsible of pneumonitis, enclosing Polio, and the SARS-CoV-2. Adhesion to the respiratory mucosae is enhanced by poloxamer or carboxymethyl cellulose. Cu modulates the morphology and action of neutrophils, NK cells, macrophages, T helper cells, and B cells, on killing infectious pathogens, activation of cell-mediated immunity, and secretion of specific antibodies. Neutropenia and lymphopenia have been studied in Cu deficient cases. Subsequently, insufficient Cu status leads to immunosuppression, rising susceptibility to infectious diseases [37]. A raised serum Cu content exists in response to inflammation, provoking Cu to accumulate at inflammatory sites. Cu may suppress the production of inflammatory cytokines, chemokines, and adhesion molecules by downregulating the expression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which is generally activated by virus-induced ROS [38, 39]. Cu is a powerful virucidal element, on many infectious viruses, such as the bronchitis virus, influenza virus, HIV type 1, and other, single- or double-stranded DNA and RNA viruses [40, 41]. During viral infection, Cu acts as an essential micronutrient for both pathogens and animal hosts but not at high toxic levels triggering undue redox reactions. This occurrence can be explained by the fact that macrophages can attack pathogens at high Cu load during an infection, but higher Cu levels have been found in the sites of lung infection [42, 43]. Reliably, Cu inhibits influenza virus replication by stopping the action of RNA-dependent RNA polymerase (RdRP) or damaging its negative-sense RNA [40]. Copper iodide induces the production of free radicals (-OH and -O2), which exhibit virucidal actions by the degradation of viral proteins, e.g., hemagglutinin and neuraminidase [44]. Cu meddles with main proteins of the virus. In vitro studies have reported that Cu action caused 99% inactivation of viruses after 30 minutes, and the effect was observed to be more obvious in enveloped viruses [45]. Oxidized Cu oxide (CuO) nanoparticles are utilized as catalysts that determine inactivation of viruses. Similarly, nano-sized Cu iodide particles have also been presented to display the inactivation of the H1N1 influenza virus. Cu exerts a stimulatory role on ceruloplasmin expression, a major copper-carrying protein in the blood that boosts the immune response during inflamed/infectious actions
Zinc (Zn) has many direct and indirect antiviral properties. Zn homeostasis is connected with infections related to coronaviridae [46], picornavirus [47], papilloma virus [48], rhinovirus [49], herpes simplex virus [50], varicella-zoster virus [51], respiratory syncytial virus (RSV) [52], human immunodeficiency virus (HIV) [53], and hepatitis C virus (HCV) [54]. Zn shows antiviral actions in physical phases, e.g., the infection and replication of virus [55, 56]. Zn is involved in the expression of different cellular enzymes and transcription factors being cofactor of many viral proteins as well as rendering easier and faster the polyproteins misfolding of viral proteins, which alters the architecture of the virus modulating its protease activity, as shown in the picorna and polioviruses [47, 57, 58]. An in vitro study displayed that Zn can inactivate the free varicella-zoster virus [51]. Zn salts facilitate viral destruction by abolishing viral entry, polyprotein processing, or viral RNA-dependent RNA polymerase (RdRP) activity in other viruses, such as SARS-CoV [46], rhinovirus [49], HSV [50], HIV [53], and vaccinia virus [59]. Zn behaves as a membrane stabilizer that may prevent the entry of the virus into the cell. [55]. The usage of Zn also reduces the oxidative stress induced by the RSV and influenza virus by triggering metallothioneins (MTs) to release Zn into the cytoplasm, which maintains the cellular redox state [60]. A significant reduction in the levels of plasma Zn levels in patients with acute respiratory distress syndrome (ARDS) suggests that Zn could be helpful in improving the clinical conditions of mechanically ventilated patients [61]
Poloxamer (Pluronic) is part of the family of hydrogels polymeric materials provided of a three-dimensional network that can retain a large amount of water or biological fluid under physiological conditions. They could be used as delivery systems due to the unique properties of sol–gel conversion that is modulated by a specific biological stimulus [62]. In our case, poloxamer 407 (P407) is used for its good solubilizing capacity, low toxicity, good drug-release characteristics, and its compatibility with several natural compounds and excipients [63]. P407 is useful mucosal drug delivery systems, due to its coating a thin layer on the biomembranes, thus delivering active principles along the compartment, at a long-term steady concentration. Poloxamer molecules produce entanglements or noncovalent bonds with mucus, thus intensively interacting with the epithelial background in a safe and effective barrier against noxious agents
Our strategy has been to share protection from common cold viruses' infection of the upper airways pooling together and dispensing different active principles on a multistep defense basis.
2. Materials and Methods
The study was done using 30 cases of infected patients by sudden aspecific viral-induced sore throat rhinitis excluding bacterial origin by preliminary microbiological swab ruling out infections of Streptococcus, Staphylococcus aureus, Hemophilus, etc., just limiting the diagnosis and patients' selection to the common cold viruses' group such as influential, rhinovirus, and adenovirus. The study is a simple open investigation comparing two groups of subjects admitted to the Second Opinion Medical Consulting Network at the first arousal of the symptoms and submitted to diagnostic nasal and oral swab. The Second Opinion Medical Consulting Network is a medical consultation referral web, quickly recruiting a wide panel of real-time available specialists, to whom any patient affected by any disease or syndrome not adequately satisfied by the first diagnosis or therapy, can apply for an individual clinical audit [64–68]. We usually after a careful review of the diagnosis plan for the patient's tailored proper therapeutic strategy with chemical or natural drugs or galenic formulations prepared by the pharmacists or nutraceutical manufacturing companies∗.
The patients were divided in two groups comparable in terms of age, clinical history, symptoms: (1) the first group included 15 patients, which were administered with our spray formula, and (2) the second group included 15 patients with a commercial nasal lavage kit (Table 3). The mucous smear was stained with May Grunwald-Giemsa to exclude eosinophilic infiltrate and confirm the prevalence of granulocytes and lympho-monocytes typical of viral seasonal inflammatory upper airways conditions.
Table 3.
Characteristic of patients.
| First group | Second group | |||||
|---|---|---|---|---|---|---|
| Patient ID | Age (years) | Sex | Patient ID | Age (years) | Sex | |
| #1 | P.E. | 76 | M | D.B. | 36 | F |
| #2 | M.G. | 64 | M | M.C. | 73 | M |
| #3 | F.W. | 55 | F | A.C. | 62 | M |
| #4 | V.F. | 61 | F | S.E. | 76 | M |
| #5 | B.C. | 66 | M | A.U. | 56 | F |
| #6 | S.V. | 78 | F | B.A. | 59 | M |
| #7 | C.A. | 56 | M | F.M. | 68 | M |
| #8 | P.M. | 51 | F | M.C. | 49 | M |
| #9 | C.M. | 62 | M | S.E. | 61 | M |
| #10 | R.S. | 38 | M | V.K. | 76 | M |
| #11 | B.M. | 47 | M | A.G. | 64 | F |
| #12 | L.A. | 61 | F | L.P. | 45 | F |
| #13 | E.R. | 54 | F | A.T. | 51 | F |
| #14 | M.B. | 66 | F | D.E. | 38 | F |
| #15 | A.A. | 36 | M | I.F. | 53 | F |
They were then instructed to take daily record of the symptoms and to use the spray (our spray and commercial spray, respectively, in the first and second group) every 8 hours for 5 days, unless remission had been detected in advance.
Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA). The data were examined using an unpaired t-test with Welch's correction. p < 0.05 was considered significant.
∗In this study, the nasal spray samples conventionally labeled “Trivir” were kindly prepared free of charge on our prescription by Phytoitalia (Milan, Italy).
3. Results
The results in Table 3 report the symptoms recorded by the first group of patients, treated with our spray. A significative reduction of the main influential symptoms was detected (Table 4, Figure 1).
Table 4.
Description of symptoms in the first group (our spray) after treatment.
| First group | Symptoms posttreatment | ||||||
|---|---|---|---|---|---|---|---|
| Nasal obstruction | Blow the nose | Sneezing | Mucous discharge | Pharyngeal soreness | Throat scraping | Laryngeal cough | |
| P.E. | Rare | Intermittent | Absent | Absent | Absent | Rare | Absent |
| M.G. | Rare | Absent | Intermittent | Absent | Persistent | Intermittent | Absent |
| F.W. | Absent | Absent | Rare | Intermittent | Intermittent | Rare | Absent |
| V.F. | Absent | Absent | Rare | Persistent | Rare | Rare | Rare |
| B.C. | Rare | Rare | Absent | Absent | Intermittent | Absent | Intermittent |
| S.V. | Rare | Absent | Intermittent | Absent | Rare | Rare | |
| C.A. | Absent | Intermittent | Absent | Persistent | Rare | Rare | Absent |
| P.M. | Absent | Rare | Rare | Intermittent | Rare | Absent | Intermittent |
| C.M. | Rare | Intermittent | Absent | Intermittent | Absent | Rare | Rare |
| R.S. | Absent | Absent | Rare | Intermittent | Persistent | Intermittent | Persistent |
| B.M. | Rare | Absent | Absent | Absent | Absent | Rare | Intermittent |
| L.A. | Intermittent | Rare | Intermittent | Absent | Rare | Rare | Absent |
| E.R. | Rare | Absent | Absent | Rare | Rare | Absent | Absent |
| M.B. | Absent | Intermittent | Rare | Intermittent | Intermittent | Absent | Absent |
| A.A. | Intermittent | Rare | Rare | Rare | Absent | Intermittent | Rare |
Figure 1.
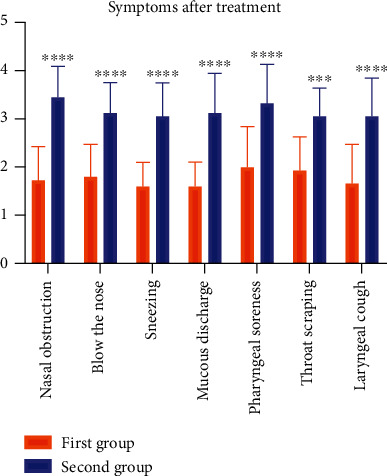
Graphical representation of symptoms in first and second group after treatment. There were significant differences. ∗∗∗∗P < 0.0001 first- vs. second group.
The patients of the second group recovered more slowly and with persistent symptoms compared with the first group (Table 5, Figure 1).
Table 5.
Description of symptoms in the second group (commercial spray) after treatment.
| Second group | Symptoms post-treatment | ||||||
|---|---|---|---|---|---|---|---|
| Nasal obstruction | Blow the nose | Sneezing | Mucous discharge | Pharyngeal soreness | Throat scraping | Laryngeal cough | |
| D.B. | Persistent | Intermittent | Absent | Absent | Persistent | Rare | Persistent |
| M.C. | Rare | Absent | Intermittent | Absent | Persistent | Intermittent | Absent |
| A.C. | Rare | Intermittent | Persistent | Rare | Intermittent | Rare | Absent |
| S.E. | Intermittent | Absent | Rare | Persistent | Persistent | Intermittent | Rare |
| A.U. | Absent | Rare | Persistent | Absent | Intermittent | Persistent | Intermittent |
| B.A. | Intermittent | Persistent | Intermittent | Absent | Intermittent | Rare | Persistent |
| F.M. | Persistent | Intermittent | Absent | Persistent | Rare | Rare | Absent |
| M.C. | Absent | Persistent | Rare | Intermittent | Rare | Persistent | Intermittent |
| S.E. | Persistent | Intermittent | Absent | Absent | Persistent | Rare | Persistent |
| V.K. | Absent | Absent | Intermittent | Absent | Persistent | Intermittent | Absent |
| A.G. | Persistent | Intermittent | Persistent | Rare | Intermittent | Rare | Absent |
| L.P. | Intermittent | Absent | Rare | Persistent | Persistent | Intermittent | Rare |
| A.T. | Rare | Rare | Persistent | Absent | Intermittent | Rare | Intermittent |
| D.E. | Intermittent | Persistent | Intermittent | Persistent | Intermittent | Rare | Persistent |
| I.F. | Persistent | Intermittent | Absent | Persistent | Rare | Rare | Absent |
Anecdotally, we showed in (Table 6) how further 10 healthy carriers of SARS-CoV-2 in the nostrils and upper airways become quickly negative using our spray and reporting the diagnostic swab every three days.
Table 6.
Case report of 10 healthy voluntaries, carriers of SARS-CoV-2, treated with our spray.
| Case report | Sex | Age | 2 consecutive swab before | Day 3 | Day 5 | |
|---|---|---|---|---|---|---|
| Patient ID | Swab1 | Swab2 | Swab3 | Swab4 | ||
| D.B. | F | 36 | + + | + + | Negative | Negative |
| M.C. | M | 73 | + + | + + | Negative | Negative |
| A.C. | M | 62 | + + | + + | Negative | Negative |
| S.E. | M | 76 | + + | + + | Negative | Negative |
| A.U. | F | 56 | + + | + + | Negative | Negative |
| B.A. | M | 59 | + + | + + | + + | Negative |
| F.M. | M | 68 | + + | + + | + + | Negative |
| M.C. | M | 49 | + + | + + | Negative | Negative |
| S.E. | M | 61 | + + | + + | Negative | Negative |
| V.K. | M | 76 | + + | + + | + + | Negative |
| Positivity index | 10/10 | 10/10 | 3/10 | 0/10 | ||
4. Discussion and Conclusions
The open case-control retrospective observational investigation showed a definite benefit of the spray based on natural herbal extracts to take control of the upper airways respiratory distress due to viral infections, as a matter of fact, the symptomatic relief is reported in Table 6. The results are remarkably evident in the treated group with the spray treatment compared to the control group, to whom oral medications antihistamines, vasoconstrictors, salicylates, or local antigrippal formulas had been prescribed.
This study is very basic in terms of design and concepts; however, the lesson that folk medicine taught us about natural products has been very helpful. In fact, the control of the seasonal cold symptoms, achieved in the present study with herbal extracts plus divalent cations, is worth of further investigation.
The main limitation of our study was the small cohort of patients, consequently we cannot exclude error rates (Type 1 and Type 2 errors) and cannot ensure that our results may be replicated in future research with a major patients group. But this preliminary observation and the positive outcomes are very promising and recommend further major evidence-based clinical trial.
Obviously, the pharmaceutical technology of adhesion molecules, namely, the poloxamer steadily and firmly coating the herbal active principles along the mucosal surfaces ideally integrates the antivirus action mechanism fulfilling the scope of the formula.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Ethical Approval
Approval was obtained from the local ethics committee Second Opinion Local Institutional Review Board (IRB), named: Second Opinion Medical Network, the number of Approval is 7/2021.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Authors' Contributions
The authors confirm contribution to the paper as follows: study conception and design was done by MV and data collection, proof, and writing was done by BP.
References
- 1.Madhi S. A., Klugman K. P., The Vaccine Trialist Group A role for _Streptococcus pneumoniae_ in virus-associated pneumonia. Nature medicine. . 2004;10(8):811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan T. Q., Mason E. O., Jr., Wald E. R., et al. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics . 2002;110(1):1–6. doi: 10.1542/peds.110.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Cordero E., Pachón J., Rivero A., et al. Haemophilus influenzae pneumonia in human immunodeficiency virus-infected patients. The Grupo Andaluz para el Estudio de las Enfermedades Infecciosas. Clinical Infectious Diseases . 2000;30(3):461–465. doi: 10.1086/313690. [DOI] [PubMed] [Google Scholar]
- 4.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clinical Microbiology Reviews . 2000;13(2):302–317. doi: 10.1128/CMR.13.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelow I. C., Olsen K., Lozano J., et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics . 2004;113(4):701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 6.Mandell L. A., Wunderink R. G., Anzueto A., et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical Infectious Diseases . 2007;44(Supplement 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinstein E., Kollef M. H., Nathwani D. Pneumonia caused by Methicillin‐ResistantStaphylococcus aureus. Clinical Infectious Diseases . 2008;46(Suppl 5):S378–S385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]
- 8.Biel M. A., Brown C. A., Levinson R. M., et al. Evaluation of the microbiology of chronic maxillary sinusitis. The Annals of Otology, Rhinology, and Laryngology. . 1998;107, 11, Part 1:942–945. doi: 10.1177/000348949810701107. [DOI] [PubMed] [Google Scholar]
- 9.Bendouah Z., Barbeau J., Hamad W. A., Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngology--Head and Neck Surgery . 2006;134(6):991–996. doi: 10.1016/j.otohns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Beigel F., Jürgens M., Filik L., et al. Severe legionella pneumophila pneumonia following infliximab therapy in a patient with Crohn's disease. Inflammatory Bowel Diseases. . 2009;15(8):1240–1244. doi: 10.1002/ibd.20866. [DOI] [PubMed] [Google Scholar]
- 11.Carratala J., Gudiol F., Pallares R., et al. Risk factors for nosocomial Legionella pneumophila pneumonia. American Journal of Respiratory and Critical Care Medicine . 1994;149(3):625–629. doi: 10.1164/ajrccm.149.3.8118629. [DOI] [PubMed] [Google Scholar]
- 12.Karalus R., Campagnari A. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes and Infection . 2000;2(5):547–559. doi: 10.1016/S1286-4579(00)00314-2. [DOI] [PubMed] [Google Scholar]
- 13.Winther B. Rhinovirus infections in the upper airway. Proceedings of the American Thoracic Society . 2011;8(1):79–89. doi: 10.1513/pats.201006-039RN. [DOI] [PubMed] [Google Scholar]
- 14.Woo P. C., Lau S. K., Tsoi H. W., et al. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. The Lancet . 2004;363(9412):841–845. doi: 10.1016/S0140-6736(04)15729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiris J. S., Chu C. M., Cheng V. C., et al. Clinical progression and viral load in a community outbreak of coronavirus- associated SARS pneumonia: a prospective study. The Lancet . 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willson D. F., Landrigan C. P., Horn S. D., Smout R. J. Complications in infants hospitalized for bronchiolitis or respiratory syncytial virus pneumonia. The Journal of Pediatrics . 2003;143(5 Suppl):S142–S149. doi: 10.1067/S0022-3476(03)00514-6. [DOI] [PubMed] [Google Scholar]
- 17.Siegal F. P., Dikman S. H., Arayata R. B., Bottone E. J. Fatal disseminated adenovirus 11 pneumonia in an agammaglobulinemic patient. The American Journal of Medicine . 1981;71(6):1062–1067. doi: 10.1016/0002-9343(81)90343-0. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey P. G., Fife K. H., Hackman R. C., Meyers J. D., Corey L. Herpes simplex virus Pneumonia. Annals of Internal Medicine . 1982;97(6):813–820. doi: 10.7326/0003-4819-97-6-813. [DOI] [PubMed] [Google Scholar]
- 19.Luyt C. E., Combes A., Deback C., et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. American Journal of Respiratory and Critical Care Medicine . 2007;175(9):935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 20.Tinelli M., Michelone G., Cavanna C. Recurrent Histoplasma capsulatum pneumonia: a case report. Microbiologica . 1992;15(1):89–93. [PubMed] [Google Scholar]
- 21.Levitz S. M. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Reviews of Infectious Diseases . 1991;13(6):1163–1169. doi: 10.1093/clinids/13.6.1163. [DOI] [PubMed] [Google Scholar]
- 22.Jensen W. A., Rose R. M., Hammer S. M., Karchmer A. W. Serologic diagnosis of focal pneumonia caused by Cryptococcus neoformans. The American Review of Respiratory Disease . 1985;132(1):189–191. doi: 10.1164/arrd.1985.132.1.189. [DOI] [PubMed] [Google Scholar]
- 23.Lopez A. M., Williams P. L., Ampel N. M. Acute pulmonary coccidioidomycosis mimicking bacterial pneumonia and septic shock: a report of two cases. The American Journal of Medicine . 1993;95(2):236–239. doi: 10.1016/0002-9343(93)90267-S. [DOI] [PubMed] [Google Scholar]
- 24.Swartz J., Stoller J. K. Acute eosinophilic pneumonia complicating <i>Coccidioides immitis</i> pneumonia: a case report and literature review. Respiration . 2009;77(1):102–106. doi: 10.1159/000109977. [DOI] [PubMed] [Google Scholar]
- 25.Kanemoto H., Morikawa R., Chambers J. K., Kasahara K., Hanafusa Y., Uchida K., et al. Common variable immune deficiency in a Pomeranian with pneumocystis carinii pneumonia. The Journal of Veterinary Medical Science . 2015;77(6):715–719. doi: 10.1292/jvms.14-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linke M. J., Rebholz S., Collins M., Tanaka R., Cushion M. T. Noninvasive method for monitoring pneumocystis carinii pneumonia. Emerging Infectious Diseases . 2003;9(12):1613–1616. doi: 10.3201/eid0912.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel J. R., Tripathi P., Sharma V., Chauhan N. S., Dixit V. K. _Phyllanthus amarus_ : Ethnomedicinal uses, phytochemistry and pharmacology: A review. Journal of Ethnopharmacology . 2011;138(2):286–313. doi: 10.1016/j.jep.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 28.Calixto J. B., Santos A. R., Cechinel Filho V., Yunes R. A. A review of the plants of the genusPhyllanthus: their chemistry, pharmacology, and therapeutic potential. Medicinal Research Reviews . 1998;18(4):225–258. doi: 10.1002/(SICI)1098-1128(199807)18:4<225::AID-MED2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 29.Yang C. M., Cheng H. Y., Lin T. C., Chiang L. C., Lin C. C. Hippomanin a from acetone extract of Phyllanthus urinaria inhibited HSV-2 but not HSV-1 infection in vitro. Phytotherapy Research . 2007;21(12):1182–1186. doi: 10.1002/ptr.2232. [DOI] [PubMed] [Google Scholar]
- 30.Chen J. X., Xue H. J., Ye W. C., et al. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biological & Pharmaceutical Bulletin . 2009;32(8):1385–1391. doi: 10.1248/bpb.32.1385. [DOI] [PubMed] [Google Scholar]
- 31.Yu B., Dai C. Q., Jiang Z. Y., et al. Andrographolide as an anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway. Chinese Journal of Integrative Medicine . 2014;20(7):540–545. doi: 10.1007/s11655-014-1860-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhong M.-G., Xiang Y.-F., Qiu X.-X., Liu Z., Kitazato K., Wang Y.-F. Natural products as a source of anti-herpes simplex virus agents. RSC Advances . 2013;3(2):313–328. doi: 10.1039/C2RA21464D. [DOI] [Google Scholar]
- 33.Naithani R., Huma L. C., Holland L. E., et al. Antiviral activity of phytochemicals: a comprehensive review. Mini Reviews in Medicinal Chemistry . 2008;8(11):1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y.-C., Chen C.-F., Chiou W.-F. Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect. British Journal of Pharmacology . 2002;135(2):399–406. doi: 10.1038/sj.bjp.0704493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Y. F., Ye B. Q., Li Y. D., et al. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. Journal of Immunology . 2004;173(6):4207–4217. doi: 10.4049/jimmunol.173.6.4207. [DOI] [PubMed] [Google Scholar]
- 36.Suebsasana S., Pongnaratorn P., Sattayasai J., Arkaravichien T., Tiamkao S., Aromdee C. Analgesic, antipyretic, anti-inflammatory and toxic effects of andrographolide derivatives in experimental animals. Archives of Pharmacal Research . 2009;32(9):1191–1200. doi: 10.1007/s12272-009-1902-x. [DOI] [PubMed] [Google Scholar]
- 37.Percival S. S. Copper and immunity. The American Journal of Clinical Nutrition . 1998;67(5):1064s–1068s. doi: 10.1093/ajcn/67.5.1064S. [DOI] [PubMed] [Google Scholar]
- 38.Satake H., Suzuki K., Aoki T., et al. Cupric ion blocks NFκB activation through inhibiting the signal-induced phosphorylation of IκBα. Biochemical and Biophysical Research Communications . 1995;216(2):568–573. doi: 10.1006/bbrc.1995.2660. [DOI] [PubMed] [Google Scholar]
- 39.Kenneth N. S., Hucks G. E., Jr., Kocab A. J., McCollom A. L., Duckett C. S. Copper is a potent inhibitor of both the canonical and non-canonical NFκB pathways. Cell Cycle . 2014;13(6):1006–1014. doi: 10.4161/cc.27922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noyce J. O., Michels H., Keevil C. W. Inactivation of influenza a virus on copper versus stainless steel surfaces. Applied and Environmental Microbiology . 2007;73(8):2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borkow G., Gabbay J. Copper as a biocidal tool. Current Medicinal Chemistry . 2005;12(18):2163–2175. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- 42.Besold A. N., Culbertson E. M., Culotta V. C. The yin and Yang of copper during infection. Journal of Biological Inorganic Chemistry . 2016;21(2):137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C. X., Gleason J. E., Zhang S. X., Bruno V. M., Cormack B. P., Culotta V. C. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proceedings of the National Academy of Sciences of the United States of America . 2015;112(38):E5336–E5342. doi: 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimori Y., Sato T., Hayata T., et al. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Applied and Environmental Microbiology . 2012;78(4):951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horie M., Ogawa H., Yoshida Y., et al. Inactivation and morphological changes of avian influenza virus by copper ions. Archives of Virology . 2008;153(8):1467–1472. doi: 10.1007/s00705-008-0154-2. [DOI] [PubMed] [Google Scholar]
- 46.te Velthuis A. J., van den Worm S. H., Sims A. C., Baric R. S., Snijder E. J., van Hemert M. J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathogens . 2010;6(11, article e1001176) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanke K., Krenn B. M., Melchers W. J. G., Seipelt J., van Kuppeveld F. J. M. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. The Journal of General Virology . 2007;88(4):1206–1217. doi: 10.1099/vir.0.82634-0. [DOI] [PubMed] [Google Scholar]
- 48.Beerheide W., Bernard H. U., Tan Y. J., Ganesan A., Rice W. G., Ting A. E. Potential drugs against cervical cancer: zinc-ejecting inhibitors of the human papillomavirus type 16 E6 oncoprotein. Journal of the National Cancer Institute . 1999;91(14):1211–1220. doi: 10.1093/jnci/91.14.1211. [DOI] [PubMed] [Google Scholar]
- 49.Korant B. D., Kauer J. C., Butterworth B. E. Zinc ions inhibit replication of rhinoviruses. Nature . 1974;248(5449):588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- 50.Wahba A. Topical application of zinc-solutions: a new treatment for herpes simplex infections of the skin? Acta Dermato-Venereologica . 1980;60(2):175–177. [PubMed] [Google Scholar]
- 51.Shishkov S., Varadinova T., Bontchev P., Nachev C., Michailova E. Complexes of zinc with picolinic and aspartic acids inactivate free varicella-zoster virions. Metal-Based Drugs . 1996;3(1) doi: 10.1155/MBD.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suara R. O., Crowe J. E., Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrobial Agents and Chemotherapy . 2004;48(3):783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haraguchi Y., Sakurai H., Hussain S., Anner B. M., Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Research . 1999;43(2):123–133. doi: 10.1016/S0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 54.Grüngreiff K., Reinhold D. Zinc: a complementary factor in the treatment of chronic hepatitis C? (review) Molecular Medicine Reports . 2010;3(3):371–375. doi: 10.3892/mmr_00000267. [DOI] [PubMed] [Google Scholar]
- 55.Read S. A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Advances in Nutrition . 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rani I., Goyal A., Bhatnagar M., et al. Potential molecular mechanisms of zinc- and copper-mediated antiviral activity on COVID-19. Nutrition Research . 2021;92:109–128. doi: 10.1016/j.nutres.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kümel G., Schrader S., Zentgraf H., Daus H., Brendel M. The mechanism of the antiherpetic activity of zinc sulphate. The Journal of General Virology . 1990;71(12):2989–2997. doi: 10.1099/0022-1317-71-12-2989. [DOI] [PubMed] [Google Scholar]
- 58.Liu C. Y., Kielian M. Identification of a specific region in the e1 fusion protein involved in zinc inhibition of semliki forest virus fusion. Journal of Virology . 2012;86(7):3588–3594. doi: 10.1128/JVI.07115-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz E., Margalith E. Inhibition of vaccinia virus maturation by zinc chloride. Antimicrobial Agents and Chemotherapy . 1981;19(2):213–217. doi: 10.1128/AAC.19.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan N. A., Singla M., Samal S., Lodha R., Medigeshi G. R. Respiratory syncytial virus-induced oxidative stress leads to an increase in labile zinc pools in lung epithelial cells. mSphere . 2020;5(3) doi: 10.1128/mSphere.00447-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boudreault F., Pinilla-Vera M., Englert J. A., et al. Zinc deficiency primes the lung for ventilator-induced injury. JCI Insight . 2017;2(11) doi: 10.1172/jci.insight.86507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agrawal A. K., Das M., Jain S. In situgel systems as 'smart' carriers for sustained ocular drug delivery. Expert Opinion on Drug Delivery . 2012;9(4):383–402. doi: 10.1517/17425247.2012.665367. [DOI] [PubMed] [Google Scholar]
- 63.Dumortier G., Grossiord J. L., Agnely F., Chaumeil J. C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharmaceutical Research . 2006;23(12):2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 64.Wunsch A. P. B. The role of second opinion in oncology: an update. European Journal of Oncology . 2014;18(3):117–120. [Google Scholar]
- 65.Palmieri B. L. C., Vadala M. The “second opinion medical network”. International Journal of Pathology and Clinical Research . 2017;3:1–7. [Google Scholar]
- 66.Palmieri B., Iannitti T. The web babel syndrome. Patient Education and Counseling . 2011;85(2):331–333. doi: 10.1016/j.pec.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 67.Palmieri B., Iannitti T., Capone S., Fistetto G., Arisi E. Second opinion clinic: is the web babel syndrome treatable? La Clinica Terapeutica . 2011;162(6):575–583. [PubMed] [Google Scholar]
- 68.Di Cerbo A., Palmieri B. The economic impact of second opinion in pathology. Saudi Medical Journal . 2012;33(10):1051–1052. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.


