Abstract
Background
Obesity is a risk factor for severe airway obstruction and hypoxemia. High-flow nasal cannula (HFNC) is considered as a novel method for oxygen therapy, but the efficacy of HFNC for obese patients is controversial. This meta-analysis aimed to assess the efficacy of HFNC compared with conventional oxygen therapy (COT) in obese patients during the perioperative period.
Methods
We searched the PubMed, Embase, Web of Science, the Cochrane Library, and Google scholar databases for randomized controlled trials (RCTs) that compared the efficacy of HFNC with COT in obese patients during the perioperative period. The primary outcome was the incidence of hypoxemia, while the secondary outcomes included the lowest SpO2, the need for additional respiratory support, and the hospital length of stay (LOS).
Results
Twelve trials with 798 obese patients during the perioperative period were included. Compared with COT, HFNC reduced the incidence of hypoxemia (RR, 0.60; 95% CI, 0.43 to 0.83; P=0.002; I2 = 24%; 8 RCTs; n = 458), increased the lowest SpO2 (MD, 2.88; 95% CI, 1.53 to 4.22; P < 0.0001; I2 = 32%; 5 RCTs; n = 264), decreased the need for additional respiratory support (RR, 0.43; 95% CI, 0.21 to 0.88; P=0.02; I2 = 0%; 3 RCTs; n = 305), and shortened the hospital LOS (MD, −0.31; 95% CI, −0.57 to −0.04; P=0.02; I2 = 0%; 3 RCTs; n = 214).
Conclusions
This meta-analysis showed that compared with COT, the use of HFNC was able to reduce the incidence of hypoxemia, increase the lowest SpO2, decrease the need for additional respiratory support, and shorten the hospital LOS in obese patients during the perioperative period. Well-organized trials with large sample size should be conducted to support our findings.
1. Introduction
Obesity is defined as excessive body fat tissue accumulation that confers risks for metabolic disorders. A person with a body mass index (BMI) ≥30 kg/m2 is considered as obese [1]. Obesity and obesity-related diseases are the risk factors for cardiovascular and respiratory diseases, resulting in the decrease of life quality and expectancy [2]. Obese patients have a higher risk of difficult mask ventilation, and difficult tracheal intubation compared with the nonobese [3]. The compliance of respiratory organs, lung volumes, and a reduced functional residual capacity is decreased in obese patients [4]. Besides, obese patients are afflicted by obstructive sleep apnea [4]. These situations were exacerbated during the perioperative period and elevated risk of hypoxemia in obese patients. Therefore, appropriate oxygen therapy is crucial to the prevention of perioperative complications in obese patients. The conventional oxygen therapy (COT) is provided by nasal cannulas, or facemasks with limited flow rate (≤15 L/min). It has limited ability to meet the inspiratory demands of the obese patients with high risk of hypoxemia [5]. High-flow nasal cannula (HFNC), as a new modality oxygen therapy, is capable of delivering a high flow rate (≥20 L/min) of heated, humidified gas at an adjustable concentration without recourse to invasive or noninvasive ventilation [6]. The American clinical guideline suggested that compared to COT, HFNC as postextubation management may reduce the reintubation rates [7]. The application of HFNC is recommended in patients with hypoxemic respiratory failure. Likewise, the use of HFNC is conditionally recommended in obese patients after cardiac or thoracic surgery [8]. A recent meta-analysis investigated the efficacy of HFNC in comparison to COT or noninvasive ventilation (NIV) in obese patients in the peri- and postprocedures. The results demonstrated that the HFNC could prolong the safe apnea time, without any benefit on the reduction of hypoxemia and CO2 elimination [7]. At present, it remains unclear whether HFNC is superior to COT in obese patients in reducing hypoxemia or enhancing oxygenation. To explore the advantages of HFNC in obese patients, our meta-analysis aimed at comparing the incidence of hypoxemia, the lowest SpO2, the need for additional respiratory support, and the hospital length of stay (LOS) between obese patients receiving HFNC and those using COT.
2. Materials and Methods
2.1. Protocol and Registration
The meta-analysis was conducted in accordance with the recommendation of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [9], and registered the review protocol on INPLASY PROTOCOL (INPLASY 2021110106).
2.2. Literature Search
We searched the PubMed, Embase, Web of Science, the Cochrane Library, and Google scholar databases from inception to August 10, 2022, using the following keywords: (“HFNC” OR “HFNO” OR “NHF” OR “high flow nasal cannula” OR “high flow nasal therapy” OR “high flow nasal oxygen” OR “nasal high flow”) AND (“obesity” OR “obes∗” OR “bariatric” OR “fat” OR “corpulent”) AND (“trial”) limited to randomized controlled trials (RCTs). No restriction was imposed on language, sample size, gender, and study location. Detailed search strategies were demonstrated in Appendix 1. Besides, we reviewed the reference lists of retrieved trials for identifying additional trials, and manually searched the relevant articles by Google scholar. We included gay literature to reduce the potential publication bias by performing additional searches for conference proceeding in Web of Science (Core Collection), and registered trials in https://ClinicalTrials.gov and ChiCTR (https://www.clinicaltrials.gov, https://www.chictr.org.cn). EndNote X9 was used for managing the searched literature.
2.3. Inclusion Criteria and Exclusion Criteria
2.3.1. Inclusion Criteria
The eligibility criteria for included trials are listed below by population, intervention, comparator, outcomes, and study characteristics, according to the PICOS (Population, Intervention, Comparison, Outcomes, Study design) strategy: (a) Population: adult patients (age ≥18 years) with obesity (BMI ≥30) during the perioperative period; (b) Intervention: the application of HFNC; (c) Comparison: the use of COT [e.g., facemask or low flow nasal oxygenation]; (d) Outcomes: inclusion of at least one of the predefined outcomes: incidence of hypoxemia, the lowest SpO2, the need for additional respiratory support, and the hospital LOS. (e) Study design: RCTs.
2.3.2. Exclusion Criteria
(1) Those published as protocols, review articles, abstracts, editorials, and letters; (2) those presented as a crossover, retrospective, observational, cohort, or case-control study other than original research; (3) those ongoing or unpublished grey literature.
2.4. Outcomes and Definition
The primary outcome was the incidence of hypoxemia, while the secondary outcomes included the lowest SpO2, the need for additional respiratory support, and the hospital LOS. The definition for hypoxemia was varied in included trials, such as SpO2 < 90%, SpO2 < 92%, and SpO2 < 95%. The additional respiratory support was defined as an escalation in oxygen support therapy, including the intermittent positive-pressure ventilation, continuous positive airway pressure (CPAP), NIV, HFNC, or reintubation.
2.5. Selection Criteria and Date Extraction
2.5.1. Selection Criteria
Two authors (R. Z. and H. T. W.) examined the titles and abstracts of the retrieved trials independently, and reviewed the full texts of the potentially eligible trials based on the inclusion and exclusion criteria. Any disagreement was resolved by a third author (W. G.).
2.5.2. Date Extraction
Two authors independently extracted the trial characteristics, which were summarized in a standardized Excel file. The following information were retrieved from each trial: first author, year of publication, location, population, clinical setting, sample size, interventional time point, intervention and control details, the incidence of hypoxemia, the lowest SpO2, the need for additional respiratory support, and the hospital LOS. Disagreements were adjudicated by a third author.
2.6. Risk of Bias Assessment
The methodological quality of each trial was assessed by two authors through the Cochrane Risk of Bias Tool [10], and the risk of bias of each trial was described as “low,” “high,” or “unclear” [11]. The following domains were considered: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. We categorized the trials with low risk of bias for all domains as being at low risk of bias, the trials only owning one high bias as being at high risk, and all other trials were considered as unclear. Disagreements were settled through discussion.
2.7. Statistical Analysis
Dichotomous outcomes were presented as the relative risks (RRs), and continuous outcomes were presented as the mean differences (MDs), both with corresponding 95% confidence intervals (CIs). For continuous outcomes presented as the median (25th and 75th percentile), we converted inter-quartile ranges to standard deviation using formula conversion suggested by the Cochrane Collaboration [11]. Statistical heterogeneity was quantified by the I2tatistica, and the I2 ver 50% indicated significant heterogeneity. The intention-to-treat principle was used for performing the analyses. We adopted a priori random-effects model to pool the outcome data, on the assumption of heterogeneity across the included trials. Differences of the outcomes were graphically displayed with a forest plot, and a P value of <0.05 was considered to be statistically significant. To assess the potential impact of the findings from a single trial on the overall meta-analytical outcome, we adopted sensitivity analysis with a leave-one-out approach. The statistical analyses were performed using Review Manager, Version 5.3.
3. Results
3.1. Trial Selection
The process of trial selection is shown in Figure 1. First, the initial search yielded 183 records from the databases, and 131 records were excluded based on titles and abstracts. Of these records, 34 duplicates were excluded, and 18 records were thought to be potentially eligible. Second, after reviewing full texts in accordance with the inclusion criteria, 3 records were trial protocols, and 3 records did not include our outcomes. Finally, 12 trials were included in our meta-analysis [12–23].
Figure 1.
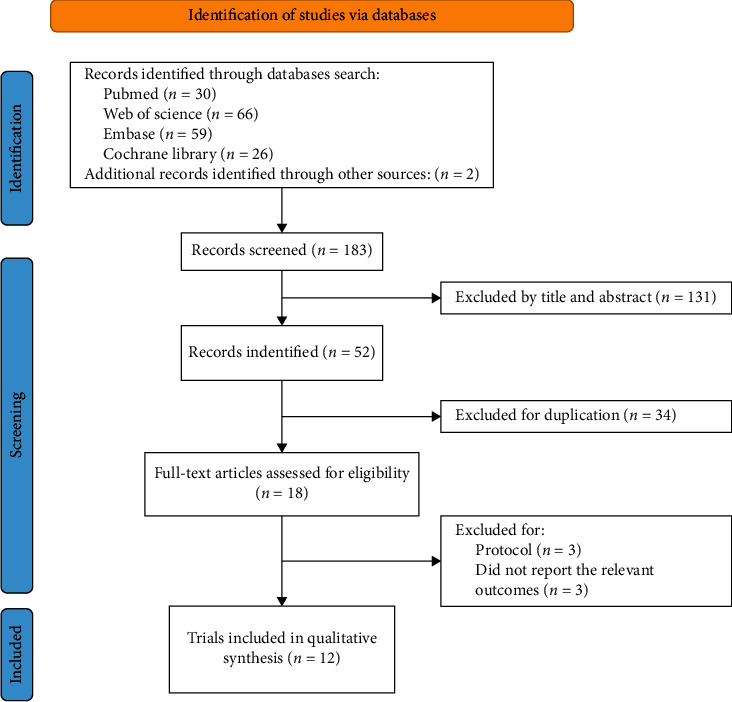
PRISMA flow diagram.
3.2. Trial Characteristics
The basic characteristics of the included trials are summarized in Table 1. These included 12 trials were published from 2015 to 2022, and the population sizes ranged from 40 to 155, with a total of 798 patients. Our meta-analysis included the cardiothoracic surgery [12, 13], bariatric surgery [14, 16, 18–23], colonoscopy [15], and elective surgery [17]. The interventional time point was varied among included trials. Of the 12 trials, 5 assessed the beneficial effect of HFNC during the postoperative period [12–14, 18, 21], while 6 trials were conducted during anesthesia induction [16, 17, 19, 20, 22, 23], 1 trial was performed at the sedation of colonoscopy [15]. The oxygen flow of HFNC ranged from 25 to 120 L/min, and in the COT group, the oxygen was delivered through facemask or nasal cannula (2∼15 L/min). Eight trials provided the incidence of hypoxemia [14, 15, 18–23]. While three trials used the definition of SpO2 < 90% [15, 19, 21], four trials used the definition of SpO2 < 92% [14, 18, 22, 23], and one trial used the definition SpO2 < 95% [20].
Table 1.
Basic characteristics of the included clinical trials.
| Author, year, location | Population | Clinical setting | Number of patients (H/C) | Intervention time point | Intervention details | Control details | Outcomes |
|---|---|---|---|---|---|---|---|
| Corley [12], 2015, Australia | Patients aged ≥18, with a BMI ≥30 | Cardiothoracic surgery | 155 (81/74) | Postextubation | The gas flow rate was 35∼50 L/min. | The gas flow rate was 2∼4 L/min via nasal cannula or 6 L/min via facemask. | ③ |
| FiO₂: NR. | FiO₂: NR. | ||||||
|
| |||||||
| Sahin [13], 2018, Turkey | Patients with a BMI > 30 | Coronary artery bypass grafting | 100 (50/50) | Postextubation | The gas flow rate was 25∼40 L/min. | The gas flow rate was 2∼4 L/min via facemask. | ③④ |
| FiO₂ = 50%. | FiO₂: NR | ||||||
|
| |||||||
| .Ferrando [14], 2019, Spain | Patients with a BMI ≥ 35 | Laparoscopic bariatric surgery | 64 (32/32) | Postoperation | The gas flow rate was 60 L/min. | The gas flow rate was 15 L/min via facemask. | ①④ |
| FiO₂ = 50%. | FiO₂ = 50%. | ||||||
|
| |||||||
| Riccio [15], 2019, USA | Patients aged 18∼80, with a BMI > 40 | Elective colonoscopy | 59 (28/31) | 5 min before and during the sedation period | The gas flow rate was 60 L/min. | The gas flow rate was 4 L/min via nasal cannula. | ①②③ |
| FiO₂ = 36∼40%. | FiO₂ = 36∼40%. | ||||||
|
| |||||||
| Wong [17], 2019, Canada | Patients aged ≥ 18, with a BMI ≥ 40 | Elective surgery | 45 (23/22) | Preoxygenation | The gas flow rate was 40 L/min. | The gas flow rate was 15 L/min via facemask. | ② |
| FiO₂ = 100% | FiO₂ = 100%. | ||||||
|
| |||||||
| Ricottilli [16], 2019, Belgium | Obese patients, BMI: (40.6±3.79) | Bariatric surgery | 40 (20/20) | Preoxygenation and duration of apnea | The gas flow rate was 50∼70 L/min. | The gas flow rate was 12 L/min via facemask. | ② |
| FiO₂ = 100%. | FiO₂ = 100%. | ||||||
|
| |||||||
| Hamp [19], 2020, Austria | Adult patients with a BMI > 40 | Bariatric surgery | 40 (20/20) | Apneic oxygenation | The gas flow rate was 120 L/min. | The gas flow rate was 10 L/min via nasal cannula. | ①② |
| FiO₂ = 100% | FiO₂ = 100%. | ||||||
|
| |||||||
| Fulton [18], 2021, Australia | Patients aged ≥ 18, with a BMI ≥30 | Elective bariatric surgery | 50 (25/25) | Postoperation | The gas flow rate was 50 L/min. | The gas flow rate was 2 L/min via nasal cannula. | ①③④ |
| FiO₂ = 50%. | FiO₂ = 50%. | ||||||
|
| |||||||
| Schutzer-Weissmann [22], 2021, UK | Patients aged 18∼65 years, with a BMI >40 | Bariatric surgery | 80 (41/39) | Preoxygenation and duration of apnea | The gas flow rate was 35∼70 L/min. | The gas flow rate was 15 L/min via facemask. | ① |
| FiO₂: NR. | FiO₂: NR. | ||||||
|
| |||||||
| Guy [20], 2021, Australia | Patients aged ≥ 18, with a BMI ≥35 | Bariatric surgery | 45 (22/23) | Apneic period | The gas flow rate was 70 L/min. | The gas flow rate was 4 L/min via nasal cannula. | ① |
| FiO₂ = 100%. | FiO₂ = 100%. | ||||||
|
| |||||||
| Rosén [21], 2022, Sweden | Patients aged 18∼60 years, with a BMI >35 | Laparoscopic bariatric surgery | 40 (20/20) | Postoperation | The gas flow rate was 40 L/min. | The gas flow rate was 2 L/min via nasal cannula. | ① |
| FiO₂ = 30%. | FiO₂ = 30%. | ||||||
|
| |||||||
| Wu [23], 2022, Taiwan, China | Patients aged 20∼65, with a BMI>30 | Laparoscopic sleeve gastrectomy | 80 (40/40) | Preoxygenation | The gas flow rate was 30∼50 L/min. | The gas flow rate was 15 L/min via facemask. | ①② |
| FiO₂ = 100% | FiO₂ = 100% | ||||||
Outcomes: ① = Hypoxemia; ② = Minimum SpO₂; ③ = Additional respiratory support; ④ = Hospital LOS; FiO₂ = fraction of inspired; SpO₂ = peripheral oxygen saturation; NR = no record.
3.3. Risk of Bias Assessment
The risks of bias of individual trials are summarized in Figures 2 and 3. Considering the impossibility of blinding among patients and medical staff, performance bias was high in all studies. In addition to the performance bias, eight trials were categorized as being at low risk of bias [12, 14, 15, 18–21, 23], and four trials as being unclear [13, 16, 17, 22].
Figure 2.
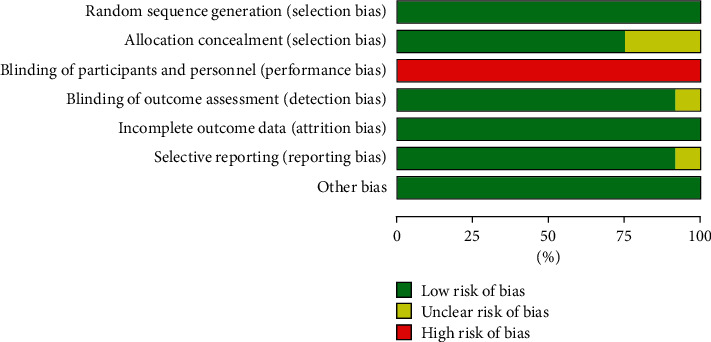
Risk of bias for each trial.
Figure 3.
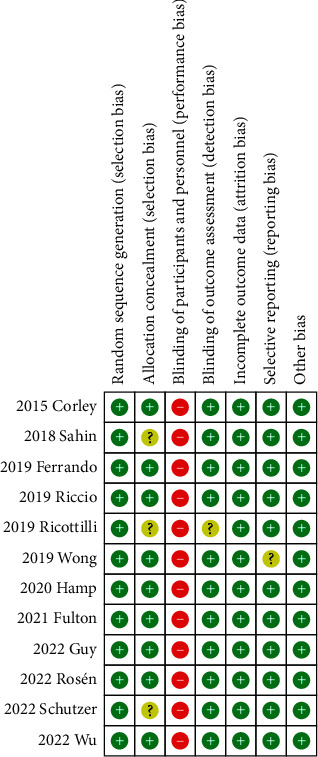
Summary of risk of bias.
3.4. Outcomes
3.4.1. Primary Outcome
Incidence of hypoxemia. Eight trials involving a total of 458 patients (HFNC group, n = 228 vs. COT group, n = 230) provided data on the incidence of hypoxemia [14, 15, 18–23]. The incidence of hypoxemia was 21.93% and 39.13% in the HFNC and COT group, respectively. Our meta-analysis revealed that the incidence of hypoxemia was lower in the HFNC compared to the COT group (RR, 0.60; 95% CI, 0.43 to 0.83; P=0.002; I2 = 24%; 8 RCTs; n = 458; Figure 4). Sensitivity analysis also demonstrated no significant influence on the incidence of hypoxemia by omitting certain trials. We did not analyze the publication bias because only eight trials were available.
Figure 4.
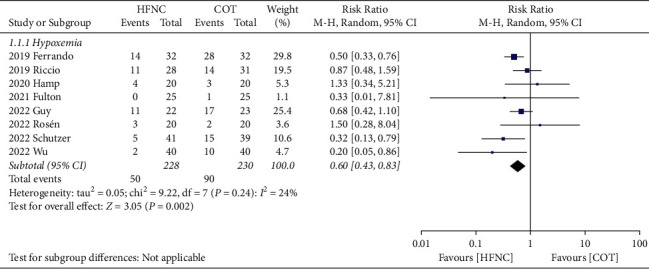
Forest plot comparing the incidence of hypoxemia between HFNC and COT.
3.4.2. Secondary Outcomes
The lowest SpO2, the need for additional respiratory support, and the hospital LOS. Our results demonstrated that the lowest SpO2 was significantly increased by HFNC compared to COT (MD, 2.88; 95% CI, 1.53 to 4.22; P < 0.0001; I2 = 32%; 5 RCTs; n = 264; Figure 5). Sensitivity analysis demonstrated consistent findings when the five trials were removed one at a time. The use of HFNC was associated with a decrease of additional respiratory support compared to COT (RR, 0.43; 95% CI, 0.21 to 0.88; P=0.02; I2 = 0%; 3 RCTs; n = 305; Figure 6). Sensitivity analysis demonstrated consistent findings when the three trials were removed one at a time. Furthermore, the HFNC was associated with the shorter hospital LOS compared to COT (MD, −0.31; 95% CI, −0.57 to −0.04; P=0.02; I2 = 0%; 3 RCTs; n = 214; Figure 7). Sensitivity analysis was not performed because only two trials were available for this outcome comparison.
Figure 5.
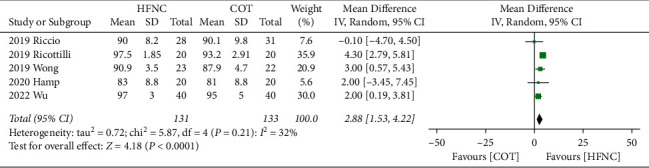
Forest plot comparing the lowest SpO2 between HFNC and COT.
Figure 6.
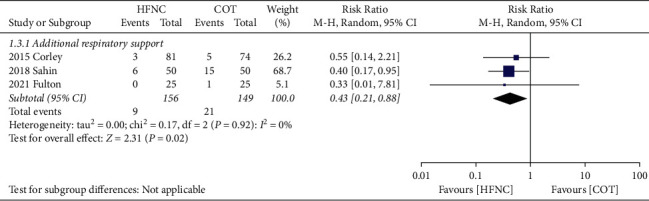
Forest plot comparing the need for additional respiratory support between HFNC and COT.
Figure 7.
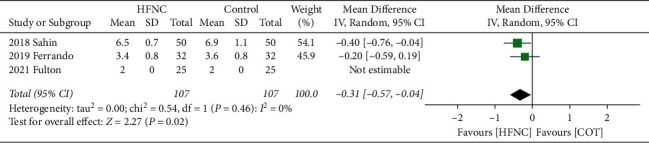
Forest plot comparing hospital LOS between HFNC and COT.
4. Discussion
4.1. Main Findings
Our study is the first meta-analysis focusing on a comparison between HFNC and COT in obese patients during the perioperative period. The results showed that the risk of hypoxemia is decreased 60% in the HFNC compared to the COT group. The application of HFNC was associated with the increased level of lowest SpO2, the decrease of the need for additional respiratory support, and a reduced hospital LOS in comparison to COT.
4.2. Comparison with Previous Meta-analyses
Previous meta-analyses assessing the efficacy of HFNC in varied clinical scenarios have been published, including patients with acute respiratory failure, COPD, obesity, and patients with planned extubation in ICU [7, 24–27]. Of these studies, only two studies explored the application of HFNC in obese patients [7, 25]. Hung's study showed HFNC might prolong the safe apnea time, but did not improve oxygenation compared to COT or NIV in obese patients during the peri- and postprocedural period [7]. Another study involving 3 trials also showed that there was no significant advantages of improving oxygenation in HFNC compared to the COT group in obese patients who underwent cardiac surgery [25]. Nevertheless, due to limited trials being included in previous studies, the conclusion might be controversial in obese patients.
Spence's meta-analysis focusing on the surgical patients during the intraoperative period showed that HFNC could reduce the risk of hypoxemia, and improve oxygenation in the intraoperative setting compared to COT [28]. In concert with this finding, we found that in obese patients, the use of HFNC could reduce the incidence of hypoxemia, and increase the lowest SpO2. Previous studies showed that compared to COT, the use of HFNC in the postoperative period may decrease the escalation of respiratory support, especially for obese patients, but it was lack of evidence [5, 29–31]. Similarly, we found a reduction of additional respiratory therapy in obese patients receiving HFNC compared with COT. The potential mechanism is that the HFNC could enhance the mucociliary clearance, and decrease the risk of patient self-inflicted lung injury [32]. However, only three trials were included in our study, and further studies were required to confirm this finding. Besides, our study demonstrated that the HFNC could shorten the hospital LOS of obese patients, but Chaudhuri's meta-analysis did not find a difference on this outcome between HFNC and COT [31]. With respect to the hospital LOS, there were relatively small sample size and limited trials in our meta-analysis, further trials were warranted to confirm it.
4.3. Implications for Clinical Practice and Mechanism
The obese patients have a higher risk for pneumonia, atelectasis, and other postoperative complications [33, 34]. HFNC with high adherence rate, comfortable experience, and lower costs has emerged as a new oxygen supportive treatment, but the efficacy of HFNC for obese patients during the perioperative period is controversial. Our meta-analysis found for obese patients the use of HFNC could reduce the incidence of hypoxemia, increase level of lowest SpO2, decrease the need for additional respiratory support, and shorten the hospital LOS.
The mechanism might be associated with the following reasons: (1) The modest amount of positive end-expiratory pressure generated by HFNC could greatly flush potential nasopharyngeal dead space, reduce the carbon dioxide levels, as well as improve ventilation and perfusion matching for the obese patients [35]. (2) HFNC could ameliorate the clearance of respiratory secretions, and reduce the incidence of upper airway obstruction [27]. (3) HFNC could reduce the work of breathing, and optimize the inspiratory air-flow dynamics and oxygenation in the obese patients [36, 37].
4.4. Strengths and Limitations
The strengths of our study lie in the population included in all obese patients during the perioperative period. Second, we only focused on the efficacy of HFNC versus COT in the obese patients, and excluded NIV, CPAP, and other oxygen treatments. Third, we prioritized the patient-centered outcomes including hypoxemia, the lowest SpO2, additional respiratory support, and the hospital LOS rather than some physiologic outcomes. Finally, we used the intention-to-treat principle and a random-effects model for a more conservative estimate accounting for clinical heterogeneity.
Our study also has limitations. First, the patient characteristics (age, medical comorbidities, obesity degree, and ASA), and clinical characteristics (the definition of hypoxemia, interventional time point, and the type of procedure) all these might affect the pooled results. We did not perform the subgroup analysis, due to the limited trials. Second, because of few included trials, the conclusions for some outcomes may not have clinical significance. Third, formula conversion was used for the data that were not represented by the meanula [11], which might affect the stability of results.
5. Conclusion
In our meta-analysis, compared with COT, the use of HFNC was able to reduce the incidence of hypoxemia, increase the lowest SpO2, and shorten the hospital LOS in obese patients during the perioperative period. Therefore, HFNC may be superior to COT for reducing hypoxemia or enhancing oxygenation in obese patients during the perioperative period, but further large-scale and well-organized clinical trials are required to confirm the efficacy of HFNC in the obese patients. Although in our study outcomes did not demonstrate statistical heterogeneity, the definition of hypoxemia and the intervention time point were varied in included trials. Therefore, for the further research, it is important for to keep consistent in the intervention time point, obese degree, the surgery type, the definition of hypoxemia, as well as the exact threshold for additional respiratory support.
Glossary
- BMI:
Body mass index
- HFNC:
High-flow nasal cannula
- COT:
Conventional oxygen therapy
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- LOS:
Length of stay
- RCT:
Randomized controlled trial
- RR:
Relative risk
- MD:
Mean difference
- CI:
Confidence interval
- NIV:
Non-invasive ventilation
- CPAP:
Continuous positive airway pressure.
Data Availability
The data used to support the findings of this study are included within the article.
Additional Points
Question: Is high-flow nasal cannula (HFNC) better than conventional oxygen therapy (COT) in obese patients during the perioperative period? Findings: HFNC could reduce the incidence of hypoxemia, increase the lowest SpO2, reduce the need for additional respiratory support, and shorten the hospital length of stay (LOS) in obese patients during the perioperative period. Meaning: HFNC may be superior to COT for reducing hypoxemia or enhancing oxygenation in obese patients during the perioperative period.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Authors' Contributions
Dr. Rong Zhou helped in the design of the work, data acquisition, data analysis, drafting the manuscript, and critical revision. Dr. Hao-Tian Wang helped in data acquisition, data analysis, drafting the manuscript, and critical revision. Wei Gu MD. helped in the design of the work, drafting the manuscript, and critical revision. Rong Zhou and Hao-Tian Wang contributed equally to this work.
Supplementary Materials
Supplement 1: Appendix 1. Search strategy.
References
- 1.De Simone G., Devereux R. B., Chinali M., et al. Prognostic impact of metabolic syndrome by different definitions in a population with high prevalence of obesity and diabetes: the Strong. Diabetes Care . 2007;30(7):1851–1856. doi: 10.2337/dc06-2152. [DOI] [PubMed] [Google Scholar]
- 2.Lin X., Li H. J. F. Obesity: epidemiology, pathophysiology, and therapeutics. Frontiers in Endocrinology . 2021;6 doi: 10.3389/fendo.2021.706978.706978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Delgado J. C., Esteve F., Manez R., et al. The influence of body mass index on outcomes in patients undergoing cardiac surgery: does the obesity paradox really exist? PLoS One . 2015;10(3) doi: 10.1371/journal.pone.0118858.e0118858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Littleton S. W., Tulaimat A. J. R. The effects of obesity on lung volumes and oxygenation. Respiratory Medicine . 2017;124:15–20. doi: 10.1016/j.rmed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Rochwerg B., Granton D., Wang D. X., et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Medicine . 2019;45(5):563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 6.Roca O., Hernández G., Díaz-Lobato S., Carratalá J., Gutiérrez R., Masclans J. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Critical Care . 2016;20(1):p. 109. doi: 10.1186/s13054-016-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung K.-C., Ko C.-C., Chang P.-C., et al. Efficacy of high-flow nasal oxygenation against peri-and post-procedural hypoxemia in patients with obesity: a meta-analysis of randomized controlled trials. Scientific Reports . 2022;12(1):6448–6511. doi: 10.1038/s41598-022-10396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochwerg B., Einav S., Chaudhuri D., et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Medicine . 2020;46(12):2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page M. J., McKenzie J. E., Bossuyt P. M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . 2021;372:p. n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J. P. T., Altman D. G., Gotzsche P. C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ . 2011;343(18 2) doi: 10.1136/bmj.d5928.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumpston M., Li T., Page M. J., et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database of Systematic Reviews . 2019;10 doi: 10.1002/14651858.ed000142.Ed000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corley A., Bull T., Spooner A. J., Barnett A. G., Fraser J. F. Direct extubation onto high-flow nasal cannulae post-cardiac surgery versus standard treatment in patients with a BMI ≥30: a randomised controlled trial. Intensive Care Medicine . 2015;41(5):887–894. doi: 10.1007/s00134-015-3765-6. [DOI] [PubMed] [Google Scholar]
- 13.Sahin M., El H., Akkoc I. Comparison of mask oxygen therapy and high-flow oxygen therapy after cardiopulmonary bypass in obese patients. Canadian Respiratory Journal . 2018;2018:7. doi: 10.1155/2018/1039635.1039635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrando C., Puig J., Serralta F., et al. High-flow nasal cannula oxygenation reduces postoperative hypoxemia in morbidly obese patients: a randomized controlled trial. Minerva Anestesiologica . 2019;85(10):1062–1070. doi: 10.23736/s0375-9393.19.13364-0. [DOI] [PubMed] [Google Scholar]
- 15.Riccio C. A., Sarmiento S., Minhajuddin A., Nasir D., Fox A. A. High-flow versus standard nasal cannula in morbidly obese patients during colonoscopy: a prospective, randomized clinical trial. Journal of Clinical Anesthesia . 2019;54:19–24. doi: 10.1016/j.jclinane.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Ricottilli F., Ickx B., Van Obbergh L. High-flow nasal cannula preoxygenation in obese patients undergoing general anaesthesia: a randomised controlled trial. British Journal Anaesthesia . 2019;123(3):e443–e444. doi: 10.1016/j.bja.2019.05.006. [DOI] [Google Scholar]
- 17.Wong D. T., Dallaire A., Singh K. P., et al. High-flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: a randomized controlled trial. Anesthesia & Analgesia . 2019;129(4):1130–1136. doi: 10.1213/ane.0000000000003966. [DOI] [PubMed] [Google Scholar]
- 18.Fulton R., Millar J. E., Merza M., et al. Prophylactic postoperative high flow nasal oxygen versus conventional oxygen therapy in obese patients undergoing bariatric surgery (OXYBAR study): a pilot randomised controlled trial. Obesity Surgery . 2021;31(11):4799–4807. doi: 10.1007/s11695-021-05644-y. [DOI] [PubMed] [Google Scholar]
- 19.Hamp T., Prager G., Baron-Stefaniak J., Muller J., Bichler C., Plochl W. Duration of safe apnea in patients with morbid obesity during passive oxygenation using high-flow nasal insufflation versus regular flow nasal insufflation, a randomized trial. Surgery for Obesity and Related Diseases . 2021;17(2):347–355. doi: 10.1016/j.soard.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Guy L., Christensen R., Dodd B., et al. The effect of transnasal humidified rapid-insufflation ventilator exchange (THRIVE) versus nasal prongs on safe apnoea time in paralysed obese patients: a randomised controlled trial. British Journal Anaesthesia . 2022;128(2):375–381. doi: 10.1016/j.bja.2021.10.048. [DOI] [PubMed] [Google Scholar]
- 21.Rosen J., Frykholm P., Fors D. Effect of high-flow nasal oxygen on postoperative oxygenation in obese patients: a randomized controlled trial. Health science reports . 2022;5(3):p. e616. doi: 10.1002/hsr2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schutzer-Weissmann J., Wojcikiewicz T., Karmali A., et al. Apnoeic oxygenation in morbid obesity: a randomised controlled trial comparing facemask and high-flow nasal oxygen delivery. British Journal Anaesthesia . 2022;S0007-0912 doi: 10.1016/j.bja.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y. M., Li C. C., Huang S. Y., et al. A comparison of oxygenation efficacy between high-flow nasal cannulas and standard facemasks during elective tracheal intubation for patients with obesity: a randomized controlled trial. Journal of Clinical Medicine . 2022;11(6) doi: 10.3390/jcm11061700.1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu C., Liu X., Zhu Q., et al. Efficiency of high-flow nasal cannula on pulmonary rehabilitation in COPD patients: a meta-analysis. Biomed Research International . 2020;2020 doi: 10.1155/2020/7097243.7097243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Zhu J., Wang X., et al. Comparison of high-flow nasal cannula (HFNC) and conventional oxygen therapy in obese patients undergoing cardiac surgery: a systematic review and meta-analysis. In Vivo . 2021;35(5):2521–2529. doi: 10.21873/invivo.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Jong A., Wrigge H., Hedenstierna G., et al. How to ventilate obese patients in the ICU. Intensive Care Medicine . 2020;46(12):2423–2435. doi: 10.1007/s00134-020-06286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldomero A. K., Melzer A. C., Greer N., et al. Wilt TJJAoim: effectiveness and harms of high-flow nasal oxygen for acute respiratory failure: an evidence report for a clinical guideline from the American college of physicians. Annals of Internal Medicine . 2021;174(7):952–966. doi: 10.7326/m20-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spence E. A., Rajaleelan W., Wong J., Chung F., Wong D. T. J. A. The effectiveness of high-flow nasal oxygen during the intraoperative period: a systematic review and meta-analysis. Anesthesia & Analgesia . 2020;131(4):1102–1110. doi: 10.1213/ane.0000000000005073. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y., Yin H., Zhang R., Wei J. High-flow nasal cannula oxygen therapy vs conventional oxygen therapy in cardiac surgical patients: a meta-analysis. Journal of Critical Care . 2017;38:123–128. doi: 10.1016/j.jcrc.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 30.Wu X., Cao W., Zhang B., Wang S. Effect of high-flow nasal cannula oxygen therapy vs conventional oxygen therapy on adult postcardiothoracic operation: a meta-analysis. Medicine . 2018;97(41) doi: 10.1097/md.0000000000012783.e12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhuri D., Granton D., Wang D. X., et al. High-flow nasal cannula in the immediate postoperative period: a systematic review and meta-analysis. Chest . 2020;158(5):1934–1946. doi: 10.1016/j.chest.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 32.Brochard L., Slutsky A., Pesenti A. Medicine cc: mechanical ventilation to minimize progression of lung injury in acute respiratory failure. American Journal of Respiratory and Critical Care Medicine . 2017;195(4):438–442. doi: 10.1164/rccm.201605-1081cp. [DOI] [PubMed] [Google Scholar]
- 33.Montané B., Toosi K., Velez-Cubian F., et al. Effect of obesity on perioperative outcomes after robotic-assisted pulmonary. Lobectomy . 2017;24(2):122–132. doi: 10.1177/1553350616687435. [DOI] [PubMed] [Google Scholar]
- 34.Dobner J., Kaser S. J. C., itopotESoC M., Diseases I. Body mass index and the risk of infection from underweight to obesity. Clinical Microbiology and Infections . 2018;24(1):24–28. doi: 10.1016/j.cmi.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Cortegiani A., Accurso G., Mercadante S., Giarratano A., Gregoretti C. High flow nasal therapy in perioperative medicine: from operating room to general ward. BMC Anesthesiology . 2018;18(1):p. 166. doi: 10.1186/s12871-018-0623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni Y. N., Luo J., Yu H., et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation? Chest . 2017;151(4):764–775. doi: 10.1016/j.chest.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Ward J. J. Rc. High-flow oxygen administration by nasal cannula for adult and perinatal patients. Respiratory Care . 2013;58(1):98–122. doi: 10.4187/respcare.01941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1: Appendix 1. Search strategy.
Data Availability Statement
The data used to support the findings of this study are included within the article.


