Summary
Background
Maternal exposure to particulate air pollution during pregnancy has been linked to multiple adverse birth outcomes causing burden of disease later in the child's life. To date, there is a paucity of data on whether or not ambient particles can both reach and cross the human placenta to exert direct effects on fetal organ systems during gestation.
Methods
In this analysis, we used maternal-perinatal and fetal samples collected within the framework of two independent studies: the ENVIRONAGE (Environmental Influences on Ageing in Early Life) birth cohort of mothers giving birth at the East-Limburg Hospital in Genk, Belgium, and the SAFeR (Scottish Advanced Fetal Research) cohort of terminated, normally progressing pregnancies among women aged 16 years and older in Aberdeen and the Grampian region, UK. From the ENVIRONAGE study, we included 60 randomly selected mother-neonate pairs, excluding all mothers who reported that they ever smoked. From the SAFeR study, we included 36 fetuses of gestational age 7–20 weeks with cotinine concentrations indicative of non-smoking status. We used white light generation under femtosecond pulsed illumination to detect black carbon particles in samples collected at the maternal-fetal interface. We did appropriate validation experiments of all samples to confirm the carbonaceous nature of the identified particles.
Findings
We found evidence of the presence of black carbon particles in cord blood, confirming the ability of these particles to cross the placenta and enter the fetal circulation system. We also found a strong correlation (r ≥0·50; p<0·0001) between the maternal-perinatal particle load (in maternal blood [n=60], term placenta [n=60], and cord blood [n=60]) and residential ambient black carbon exposure during pregnancy. Additionally, we found the presence of black carbon particles in first and second trimester tissues (fetal liver [n=36], lung [n=36], and brain [n=14]) of electively terminated and normally progressing pregnancies from an independent study.
Interpretation
We found that maternally inhaled carbonaceous air pollution particles can cross the placenta and then translocate into human fetal organs during gestation. These findings are especially concerning because this window of exposure is key to organ development. Further studies are needed to elucidate the mechanisms of particle translocation.
Funding
European Research Council, Flemish Scientific Research Foundation, Kom op Tegen Kanker, UK Medical Research Council, and EU Horizon 2020.
Introduction
Combustion-derived particulate matter, including black carbon, is produced during the incomplete combustion of fuels and biomass1 and is ubiquitous in the air we breathe, hereby contributing to non-communicable disease burdens.2 Maternal exposure to combustion-derived particulate matter during pregnancy is an important component of the exposome3 that can adversely affect pregnancy4, 5 and birth outcomes6, 7, 8 and is linked to molecular alterations9, 10, 11 that might influence disease risk later in the life of the infant. Nevertheless, the underlying mechanisms and the developmental stages at which adverse effects might be initiated remain unknown. A plausible explanation comprises transplacental particle translocation towards the fetal circulation system during gestation. The placenta is a multifunctional and layered organ that serves as an interface between mother and fetus. However, a range of xenobiotics, such as medication,12 alcohol,13 and cigarette compounds (including nicotine and polycyclic aromatic hydrocarbons)14 can cross the placenta and affect fetal development. Previously, we found that ambient black carbon particles transfer towards the human placenta;15 however, this study was criticised16 because neither transplacental transfer nor fetal exposure was proven. Our follow-up mechanistic study17 in perfused human placentae further corroborates the translocation and accumulation of combustion-derived particles by providing quantitative and kinetic data on maternal–fetal transfer and localisation within important placental cell types, such as endothelial cells of the fetal capillaries.
Research in context.
Evidence before this study
We searched PubMed and Web of Science for studies published in English from database inception up to Jan 31, 2022, that investigated the translocation of particulate air pollution particles from mother to fetus during gestation. We used the search terms (“particulate matter” OR “air pollution”) AND (“placenta” OR “fetus”) AND “translocation” AND (“gestational” OR “prenatal” OR “pregnancy”). Although studies have shown the transfer of air polluting particles to the human placenta, no direct fetal exposure has been shown to date.
Added value of this study
This study provides direct evidence that maternally inhaled carbonaceous air pollution particles cross the placenta to reach the fetal circulation. This observation is further substantiated by the presence of these particles in first and second trimester tissues (fetal liver, lung, brain, and preterm placenta) of terminated normally progressing pregnancies from an independent study.
Implications of all the available evidence
Finding black carbon particles in human cord blood and human fetal organs from two independent studies shows the ubiquity of this environmental pollutant and proves that ambient particulates can be transported directly to fetuses in utero. Accordingly, cord blood black carbon load could be used as a novel and accessible marker of prenatal air pollution exposure in studies on human fetal programming and will assist in unravelling the complexity of particulate air pollution-related health effects in early life. Therefore, black carbon accumulation in the fetal circulation system and organs might be directly responsible for observed adverse health effects during early life and might have a role in the developmental origins of health and disease.
We aimed to determine whether maternally inhaled black carbon particles translocate to the placenta and reach the fetal circulation system and organs under real-life exposure conditions. Maternal-perinatal and fetal samples were collected within the framework of two independent studies for the purpose of this work: the mother-neonate Environmental Influences on Ageing in Early Life (ENVIRONAGE) birth cohort18 and the Scottish Advanced Fetal Research (SAFeR) cohort19 of terminated normally progressing pregnancies.
Methods
Study design, population, and samples
In this analysis, we used a subset of samples collected within the ENVIRONAGE birth cohort18 and SAFeR study.19
The ENVIRONAGE birth cohort study is an ongoing population-based prospective study recruiting pregnant women (with no age restriction) giving birth in the East-Limburg Hospital (ZOL; Genk, Belgium). Mothers were asked to fill out a questionnaire to obtain lifestyle information. Women who were able to complete questionnaires in Dutch, provide written consent, and without a planned caesarean section were considered eligible for participation in the ENVIRONAGE birth cohort study. Because cigarette smoke is a source of black carbon,20 we excluded participating mothers who reported that they ever smoked.
The SAFeR study is an ongoing study recruiting women across Aberdeen in the Grampian region in Scotland, UK, undergoing non-medical, elective terminations by medical (ie, non-surgical) means. The overarching objective of SAFeR is to study the human fetus during a normal pregnancy versus pregnancies with challenging maternal lifestyle (eg, smoking) or environmental (eg, pollution) factors. In the study, women seeking elective termination of pregnancy (between 7 and 20 weeks of gestation) for non-medical reasons registered at Aberdeen Community Health Care Village were given a SAFeR study patient information sheet to take away with them. When they attended Aberdeen Royal Infirmary on the morning of their medical (non-surgical) termination, women older than 16 years were recruited and full written informed consent was obtained by nurses working independently of the study. Women are eligible for inclusion if they have a clear understanding of English language, are older than 16 years, and have a normally progressing pregnancy (determined by ultrasound scan) that was at 7–20 weeks of gestation (important stage of fetal development). Absence of fetal anomaly and gestational stage were determined by foot length as measured by ultrasound scan at enrolment.21 There was no change in patient treatment or care, and women were able to withdraw from the study at any point. Additionally, maternal morphological data (eg, age and BMI) and fetal morphological data (eg, age, bodyweight, and crown–rump length) were recorded. For our analysis, we only included fetuses with blood or placental cotinine concentrations indicative of non-smoking status, to exclude active and passive smoke exposure during gestation.
The ENVIRONAGE birth cohort study is approved by the Ethics Committee of Hasselt University and East-Limburg Hospital. For the SAFeR study, the collection of fetal material was approved by the National Health Service Grampian Research Ethics Committee (REC 15/NS/0123). Both studies are being conducted according to the guidelines of the Declaration of Helsinki.
Procedures
Within the ENVIRONAGE birth cohort, fresh term placentae were collected and deep-frozen at –20°C within 10 min after delivery. Samples were then processed and analysed at Hasselt University (Hasselt, Belgium). After minimally thawing, biopsy samples were taken at four standardised sites near the chorionic plate of the placenta across the middle region, approximately 4 cm away from the umbilical cord and under the chorion-amniotic membrane and stored at –80°C. Plastic BD Vacutainer Lithium Heparin tubes (BD, Franklin Lakes, NJ, USA) were used to collect 8 mL of cord blood immediately after delivery and 8 mL of maternal blood 1 day after delivery, and these samples were frozen at –80°C until analysis. Placental biopsy samples were fixed in 4% formaldehyde for a minimum of 24 h and embedded in paraffin. 4 μm sections were cut using a microtome (Leica Microsystems, Wetzlar, Germany), floated onto charged glass slides (Super-Frost Plus, Fisher Scientific, Waltham, MA, USA), dried overnight at 37°C, and stored at room temperature until analysis. Blood-filled imaging chambers were constructed by placing a glass coverslip (24 × 24 mm, #1·5, VWR, Radnor, PA, USA) on a microscopic glass slide (75 × 25 mm, VWR) merged with 100 μm thick double-sided tape (4959, Tesa, Germany). The imaging chambers were air-sealed to prevent drying and prepared before analysis.
To assess the correlation between the residential black carbon exposure of mothers (all non-smokers) during pregnancy and accumulation of black carbon in blood and placenta, we randomly selected 60 mother-neonate pairs within three predefined exposure groups (20 pairs per group) from the ENVIRONAGE biobank. The groups were defined as follows: whole pregnancy exposure to residential black carbon below the 25th percentile (0·97 μg/m3) was defined as low exposure, between the 25th and 75th percentile (0·97–1·43 μg/m3) as intermediate exposure, and above the 75th percentile (1·43 μg/m3) as high exposure to residential black carbon during pregnancy. These definitions of exposure were based on black carbon exposure in Belgium. We determined the ambient black carbon exposure averaged over the pregnancy period of the mothers on the basis of their residential address, using a validated spatial-temporal interpolation method.22, 23 This method combines land cover data obtained from satellite images (CORINE land cover dataset) and pollution data from fixed monitoring stations. This model chain provides daily exposure values in a high-resolution receptor grid coupled with a dispersion model that uses emissions from point sources and line sources. We assessed overall performance of this model using leave-one-out cross-validation, including 16 monitoring points for black carbon. Validation statistics of the interpolation tool gave a spatiotemporal explained variance of more than 0·74 for black carbon.
For the sample collection in the SAFeR study, fetuses were transported to the laboratory within 30 min of delivery (in almost all cases they were intact), weighed, crown–rump length recorded, and sex determined. The fetal liver, lung, brain, and preterm placenta were weighed and part of the tissue was immediately snap-frozen at –85°C and stored at –80°C until further analysis, and the rest of the sample was fixed overnight in neutral-buffered formaldehyde 10%, transferred to 70% ethanol, and processed for histology and black carbon detection. The integrity of each fetal and placental tissue morphology was assessed by examination of a haematoxylin-eosin stained section before black carbon detection.
For this analysis, we selected fetuses from the SAFeR cohort and excluded all fetuses with cotinine concentrations above non-smoking limits, regardless of maternal smoking statement. Next, 36 representative fetuses with availability of suitably fixed placenta, liver, and lung samples were sex-matched across gestational age so that mean fetal and maternal ages were balanced between females and males. We identified a subset of 14 fetuses with additional availability of suitably fixed brains among these 36 fetuses. No information on modelled maternal black carbon exposure is present within the SAFeR study.
We detected black carbon particles present in term placental tissue, maternal blood, and cord blood from the ENVIRONAGE study and preterm placental and fetal tissue samples from the SAFeR study using a specific and sensitive detection technique based on the non-incandescence related white light generation of carbonaceous particles under femtosecond pulsed illumination, as previously reported15, 24 and further detailed in the appendix (p 1). We analysed the present black carbon particles on the basis of two of the characteristic white light features: (1) the emission signals saturate compared with other label-free signals, including second harmonic generation and two-photon excited autofluorescence, which allows thresholding of the particles in each of the respective detection channels, and (2) the emitted white light ranges over the whole visible spectrum. Hence, the black carbon particles were detected with pixels thresholded in both channels simultaneously. We confirmed the carbonaceous nature and intra-tissue localisation of the identified black carbon particles using rigorous validation experiments (appendix pp 5–7). Additional considerations with regard to the size distribution of the identified particles in the fetal tissues are in the appendix (p 3).
Statistical analysis
We present all black carbon particle data as geometric means with the 95% CIs, which we analysed using GraphPad (GraphPad Prism 8). We log10 transformed the black carbon load to normalise the distribution. We used two-sided Pearson correlations to estimate the correlation between (1) the residential black carbon exposure of mothers during pregnancy and the determined blood and placental black carbon load, (2) the black carbon load in the different samples (ie, term placenta, cord blood, and maternal blood) collected within ENVIRONAGE, and (3) the black carbon load in the fetal organ samples collected within SAFeR. We expressed differences in blood and tissue black carbon load as a fold change and analysed them using the two-sided paired t test. We present study characteristics as mean (10th–90th percentile) or number of participants (%). We considered a p value of less than 0·05 to be significant. We generated heat maps using R (version 3.6.2).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
To study transplacental black carbon particle translocation towards the fetus, we included 60 mother-neonate pairs from the ENVIRONAGE birth cohort. Of these pairs, maternal-perinatal samples (ie, maternal blood, term placenta, and cord blood) were screened for the presence of ambient black carbon particles.
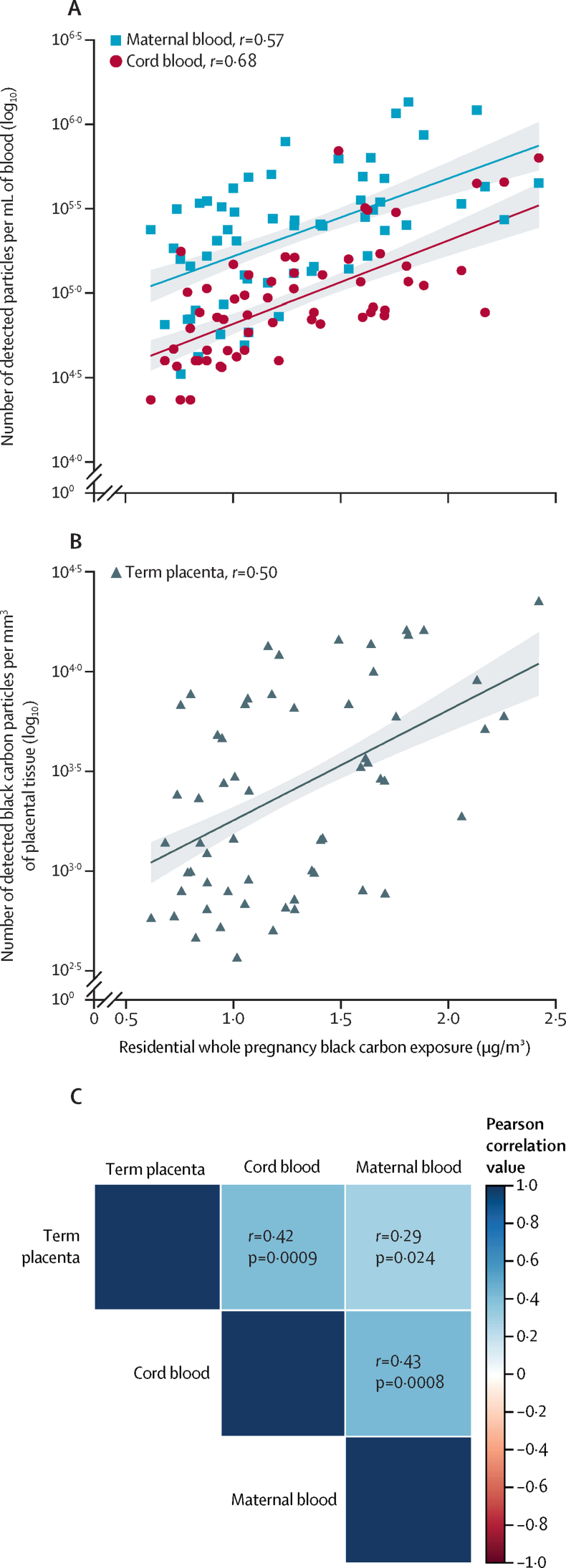
For the 60 mother-neonate pairs included in our analysis (recruited between Feb 18, 2012, and Oct 6, 2016), the mean maternal age was 30·3 years (10th–90th percentile 25·0–35·9) and the mean BMI before pregnancy was 24·1 kg/m2 (10th–90th percentile 19·1–31·6). During pregnancy four (7%) of 60 mothers were diagnosed with gestational diabetes, two (3%) with hypothyroidism, two (3%) with asthma, one (2%) with hypertension, and one (2%) with vaginal bleeding, while the other 50 (83%) mothers had no medical problems during pregnancy. Based on the International Standard Classification of Occupations, 16 (27%) of 60 mothers were employed as technicians and associate professionals, 15 (25%) as professionals, 12 (20%) as service and sales workers, seven (12%) as managers, four (7%) as clerical support workers, and two (3%) as plant or machine operators. Four (7%) mothers were not employed. The neonates were all singletons, of whom 26 (43%) of 60 were female and 34 (57%) were male, had a mean gestational age of 39·6 weeks (10th–90th percentile 38·0–41·0) and comprised 29 (48%) primiparous and 31 (52%) multiparous neonates. 52 (87%) of 60 newborn babies were European and of White race (appendix p 2). Black carbon particles were present in all maternal and neonate samples analysed. Black carbon loads were strongly and positively associated with the mothers' residential exposure averaged over the entire pregnancy (ranging between 0·63 and 2·34 μg/m3; figure 1); maternal blood (r=0·57; p<0·0001), term placental tissue (r=0·50; p<0·0001), and cord blood (r=0·68; p<0·0001). This exposure corresponds to a 31·1% (95% CI 17·7–46·1; p<0·0001) higher black carbon load in maternal blood, 33·7% (15·9–54·3; p<0·0001) higher black carbon load in term placenta, and 39·8% (27·1–53·7%; p<0·0001) higher black carbon load in cord blood per 0·25 μg/m3 increase in residential black carbon exposure during pregnancy. The corresponding slopes for the various biological samples were similar and did not differ significantly (p=0·56; figure 1). All the screened maternal-perinatal samples (ie, maternal blood, term placenta, and cord blood) showed high black carbon load associations between (1) maternal blood and term placental tissue black carbon load (r=0·29; p=0·024), (2) maternal blood and cord blood black carbon load (r=0·43; p=0·0008), and (3) term placental tissue and cord blood black carbon load (r=0·42; p=0·0009; figure 1C). Black carbon particle accumulation was approximately 1·08-fold higher in the maternal blood than in cord blood (p<0·0001; appendix p 4).
Figure 1.
Maternal-perinatal black carbon load and residential black carbon exposure during pregnancy
Association between mothers' black carbon exposure during the whole pregnancy and the amount of black carbon particles present in maternal and cord blood (A) and term placental tissue (B) from 60 mother-neonate pairs of the ENVIRONAGE birth cohort study. In panels A and B, datapoints correspond to the geometric mean black carbon load in each sample, the solid lines indicate the regression lines, and shaded areas show the 95% CIs. (C) Heatmap showing association between different biological samples and black carbon load, with two-sided Pearson correlation. The stronger the positive association, the darker the colour of the blue box and similar for the red colour indicating a negative association.
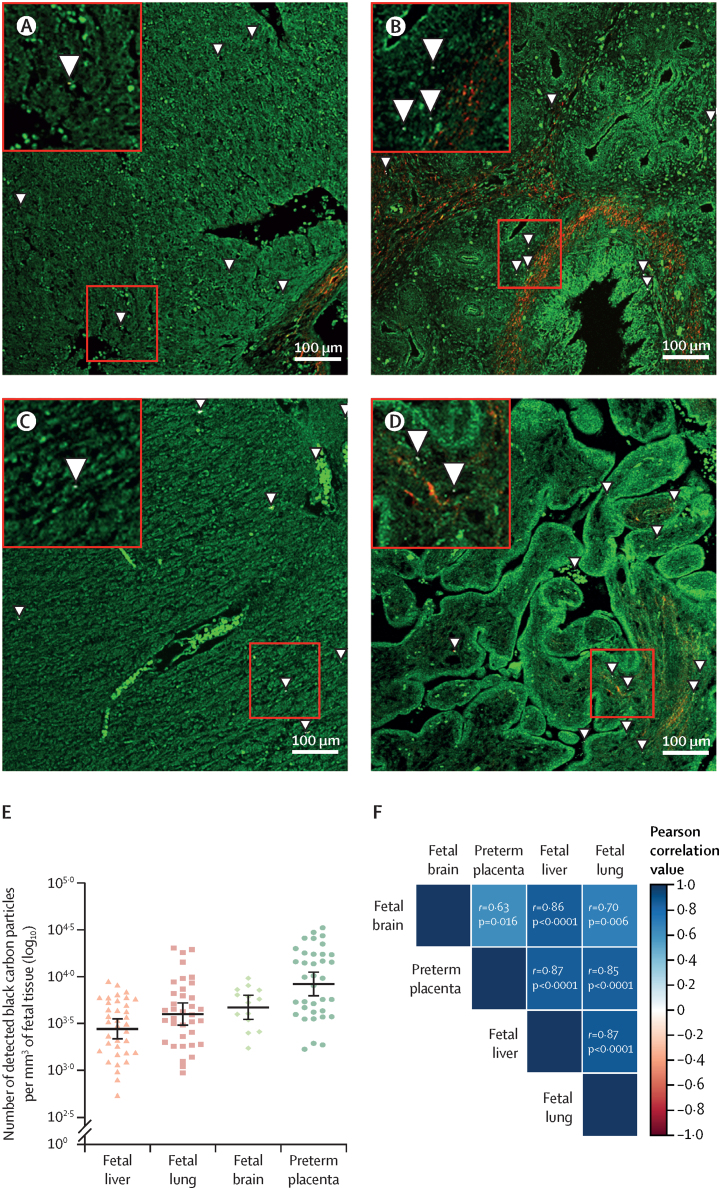
For our study, we included 36 fetuses collected from electively terminated normally progressing pregnancies in non-smoking women between June 20, 2016, and Feb 18, 2021, as part of the independent SAFeR study. We examined fetal liver, fetal lung, and preterm placenta tissues for the presence of black carbon particles, and we included fetal brain samples that were available for 14 of 36 fetuses. For the 36 fetuses in our analysis, the mean maternal age was 25·6 years (10th–90th percentile 19·0-37·0) and the mean pregnancy BMI just before termination was 27·2 kg/m2 (18·8–35·7). The fetuses, of which 18 (50%) were female and 18 (50%) were male, had a mean fetal age of 13·6 weeks (10·5–18·1) of gestation (appendix p 2). We found black carbon particles in all tissues (figure 2), and we extensively validated their carbonaceous nature plus embedment in the fetal and placental tissue (appendix pp 5–7). The highest particle load was found in preterm placental tissue, which is paralleled by an increased black carbon accumulation in the corresponding fetal brain, fetal lung, and fetal liver tissues. High black carbon load associations were found between (1) preterm placenta and fetal brain (r=0·63; p=0·016), (2) preterm placenta and fetal lung (r=0·85; p<0·0001), (3) preterm placenta and fetal liver (r=0·87; p<0·0001), (4) fetal brain and fetal lung (r=0·70, p=0·006); (5) fetal brain and fetal liver (r=0·86, p<0·0001); and (6) fetal lung and fetal liver (r=0·87, p<0·0001). Compared with the preterm placental particle load, black carbon accumulation was on average 0·88-fold lower in the fetal liver (p<0·0001), 0·92-fold lower in the fetal lung (p<0·0001), and 0·94-fold lower in the fetal brain (p=0·0026; figure 2).
Figure 2.
Fetal black carbon load
Presence of intra-tissue black carbon particles in the fetal liver (A), lung (B), brain (C), and preterm placenta (D) samples, as indicated by zoomed in squares and arrows. (E) Plot of log10-transformed number of detected black carbon particles in each sample (fetal liver n=36, lung n=36, brain n=14, and preterm placenta n=36), with datapoints corresponding to geometric mean black carbon load in each sample, with horizontal lines showing the geometric mean, and the whiskers showing the corresponding 95% CIs. (F) Heat map of association between black carbon load in different tissues, with corresponding two-sided Pearson correlation values. The stronger the positive association, the darker the colour of the blue box and similar for the red colour indicating a negative association.
Identified particle aggregates consisted of various smaller particles that translocated from the mothers' circulation system to distinct locations inside the fetal circulation and tissues (appendix p 3).
In validation experiments to determine the carbonaceous nature of the identified black carbon particles and reference carbon black particles, we found that carbonaceous particles, including the environmental pollutant black carbon and commercially engineered carbon black, generated a white light emission signal that stretched the visible spectrum under femtosecond pulsed illumination (appendix p 5). By contrast, the emission fingerprint of the background signals of the placental and fetal tissue consisted of a distinct peak that did not continuously range over all wavelengths. We also found that the recorded temporal response of the carbonaceous particles was non-resolvable from the instrument response function indicating an instantaneous response (appendix p 6). By contrast, the temporal response recorded from the placental and fetal tissue showed an exponential decay indicative of the autofluorescence lifetime decay. Finally, we checked the embedment of the black carbon particles inside the placental and fetal tissues. Optical sectioning in the z-direction throughout the placental and fetal tissues and the corresponding orthogonal projections showed that the detected black carbon particles were embedded in the tissues and were therefore not originating from external pollution during sample preparation or analysis (appendix p 7).
Discussion
We found black carbon particles in human cord blood, which provides evidence of their transfer to the fetal circulation system, and we further corroborated this finding by also identifying these particles in fetal tissue samples from electively terminated, normally progressing pregnancies. Our key findings are that black carbon particles were present in maternal blood, term placental tissues, cord blood, and first and second trimester tissues (ie, fetal liver, fetal lung, fetal brain, and preterm placenta); the black carbon particle count in cord blood is strongly and positively (r=0·68) associated with the relatively low residential ambient black carbon exposure (0·63–2·34 μg/m3) during gestational life compared with other countries and settings with very high black carbon levels; black carbon concentrations in cord blood were related to the maternal (r=0·43) and placental (r=0·42) black carbon load at term; and a higher preterm placental black carbon load is associated with an increased black carbon particle count in the corresponding fetal tissue samples (ie, brain, lung, and liver). These findings are especially concerning because this window of exposure is key to organ development.
Gestation is known as a period of heightened vulnerability for the developing fetus during which organ systems have increased cell proliferation rates, changes in metabolic capabilities, and a restricted capacity for DNA repair.25, 26 It is the life stage during which susceptibility for many diseases later in life is programmed.27, 28 The placenta forms the protective interface between mother and fetus, and impairment of its function (eg, reduced transplacental oxygen and nutrient transport) is a potential mechanism through which air pollution particulates and associated compounds might affect fetal and subsequent infant development and health.29 In this regard, maternal or placental mediated indirect effects have already been documented, such as higher inflammatory stress and molecular alterations in the placenta after gestational air pollution particle exposure.9, 30, 31 Here, we propose a novel mechanism through which particles do not only reach and accumulate in the placenta but also directly enter the fetal system to reach developing organs. Based on real-life exposure conditions, these observations are further supported by previously reported ex-vivo17 and in-vivo32, 33 studies on transplacental combustion-derived particle transfer.34 Together, these data support the proposed pathway of direct fetotoxic effects after placental particle translocation and provide new avenues to explain observed developmental impairments after ambient exposure to particulate air pollution. Nevertheless, the exact impact of direct fetal black carbon exposure requires clarification and must be further elucidated in follow-up studies.
In our study sample of mother-neonate pairs, the residential black carbon exposure ranged between 0·63 and 2·34 μg/m3 in the study population area across the whole pregnancy period, such that a maternal blood concentration between 11 and 42 ng could be reached after 24 h of exposure, assuming a daily inhaled air volume of 20 m3, a deposited fraction of 30% in the lungs, and a lung blood translocation of 0·3%.35 Additionally, because maternal minute ventilation increases by up to 48% during pregnancy while respiration rate remains unchanged,36 total daily exposure and consequently the estimated black carbon blood concentration is likely to be even higher than this estimation. Additionally, long-term retention and chronic accumulation of inhaled ultrafine carbon particles in the maternal lung is expected because of their stability.37, 38 Accordingly, the smallest particles are believed to be in a steady-state because they are retained for a longer time than larger particles and exhibit extra-pulmonary translocation. Although these factors might be expected to increase the inter-individual variability in black carbon load, we found, in a relatively narrow exposure range, a strong association between ambient concentrations and particles in fetal circulation.
Regarding the maternal-fetal particle transfer, we observed the highest black carbon particle load in the placenta, which is to be expected because of its barrier function. Through comparison of the prenatal (SAFeR) and term (ENVIRONAGE) placental black carbon loads, we found no indication (with the proviso that we assessed two different populations from different countries) that term placenta levels are higher than those of the prenatal placentas. Therefore, maximal transfer of black carbon to the fetus might already be reached in the first and second trimester. We can only speculate about factors leading to the differential black carbon load in the fetal tissues with the available data. However, we expect varying vulnerability to the accumulation of black carbon particles in the fetal organs because the window of susceptibility for fetal organ development and function is organ specific.39, 40, 41 Biokinetic studies are needed to investigate the distribution of these black carbon particles in the fetal organs and will help to increase understanding of the involved mechanisms and pathways during development.
Our study has several strengths. First, we present evidence of transplacental transport of black carbon particles from mother to fetus. Our established detection method has several advantages over conventionally used techniques such as light and electron microscopy.42, 43 In summary, our biocompatible and label-free detection technique allows the specific and sensitive detection of ambient black carbon particles. With this technique, direct visualisation of black carbon particles in their biological context is possible without the need for sample preparation (eg, macrophage isolation) or labelling. Second, because of our analytical approach, we can measure real-life black carbon exposures in cord blood and maternal blood and within placentas of mother-neonate pairs exposed to relatively low annual ambient residential black carbon concentrations. Even at these relatively low ambient exposures, we found a strong and positive correlation between gestational ambient black carbon exposure at the residential address and the black carbon load in maternal blood, term placental tissue, and the fetus (ie, cord blood). Third, a major advantage of our work was the inclusion of two independent studies resulting in substantial and complementary results. Unfortunately, there was no information on maternal black carbon exposure for the SAFeR samples; hence, we could not study the association between modelled gestational exposure and the fetal tissue black carbon load. Nevertheless, Scotland's air quality is one of the best in Europe, with a geometric mean PM2·5 concentration of 7·4 μg/m3 in 2018 in Scotland44 (no black carbon data are available). Even at these low levels, we found uptake of air pollution particles into different fetal organs. Finally, in terms of methods, we confirmed the carbonaceous nature of the identified black carbon particles and reference carbon black particles through validation experiments (ie, emission signals stretched in the visible spectrum and there was an instantaneous response) and excluded external contamination of the tissues (ie, the black carbon particles were within the tissue sections). One might question the possibility of false positives via non-carbonaceous particles generating a signal in the two detection channels; however, the spectral characteristics combined with the temporal behaviour (ie, instantaneous relaxation) allowed us to discriminate between carbonaceous particles and other types of particles.45 Moreover, maternal exposure to cigarette smoke could be excluded because we only included non-smoking mothers and fetal cotinine levels were determined for the SAFeR study to exclude both passive and active smoke exposure. However, we cannot claim that all black carbon particles present in the samples were detected by our method because of the possibility for a bias towards detection of larger particles. Moreover, our study might not be representative for cord blood black carbon distribution in other geographical settings because of the restricted geographical range and relatively low residential black carbon levels of the included mother-neonate pairs. Nevertheless, our conclusion that ambient black carbon particles reach the fetal circulation and organs still stands.
In summary, our study provides compelling evidence for the presence of black carbon particles originating from ambient air pollution in the fetal circulation system during gestation. We provide direct evidence that fetal organ exposure to these particulates occurs during the most susceptible period of life—in utero—and show that human fetuses have a black carbon load before they even take their first breath. The evidence of particle translocation into the fetus is probably a crucial component to explain the observed detrimental effects of ambient particulate air pollution on fetal development over and beyond the increased systemic inflammation in response to particulate accumulation in the maternal lungs. Further research will need to elucidate mechanisms by which particle translocation into the fetus and consequent black carbon accumulation in fetal organs could be directly responsible for observed adverse health effects during early life.
Data sharing
The data used in this study are not publicly available because they contain information that could compromise research participant privacy, but are available within General Data Protection Regulation restrictions from the corresponding author upon reasonable request.
Declaration of interests
HB, MBJR, MA, and TSN declare that aspects of the work mentioned in the paper are the subject of an awarded patent (Method for detecting or quantifying carbon black and/or black carbon particles, reference codes: EP3403068B1 and US11002679B2) filed by Hasselt University (Hasselt, Belgium) and KU Leuven (Leuven, Belgium). All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The ENVIRONAGE birth cohort was initiated by the European Research Council (ERC-2012-StG 310898) and received additional funding from the Flemish Scientific Research Foundation and Kom op Tegen Kanker (KoTK). The detection equipment was funded by the METHUSALEM Program and the INCALO project (ERC-PoC). We acknowledge the Flemish Scientific Research Foundation (FWO; 1150920N to EB and G082317N). The SAFeR study was funded by the UK Medical Research Council (MR/L010011/1 and MR/P011535/1) and the EU's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie project PROTECTED (grant agreement number 722634) and FREIA project (grant agreement number 825100) as well as by NHS Grampian Endowments grants (16/11/056, 17/034, 18/14, 19/029, and 20/031) to PAF. We thank the midwives from the maternity ward of the East-Limburg Hospital in Genk, Belgium, for coordinating and supporting the study at the ward. We thank the Advanced Optical Microscopy Centre for the maintenance of the microscopic instruments. Moreover, we thank our colleagues from the Centre for Environmental Sciences for their hard work in collecting and processing the samples for the ENVIRONAGE birth cohort. Additionally, we thank the NHS Grampian Research Nurses and NHS Grampian R&D for their tireless recruitment work for the SAFeR study. We thank the past and present SAFeR team for their hard work with the fetuses and placentae. Finally, we thank the NHS Grampian Biorepository for their oversight role in SAFeR and assistance in processing and preparation of tissue sections.
Contributors
TSN designed the ENVIRONAGE birth cohort and PAF designed the SAFeR study. EB, LLL, TSN, and PAF designed the current research. EB, HB, MBJR, and MA developed the protocol for black carbon measurements in blood and tissue. EB did all measurements. EB and LLL wrote the first draft of the manuscript, with the help of TSN and PAF. All authors critically reviewed and approved the manuscript. EB and TSN have directly accessed and verified the underlying data in this research article and all authors had access to the data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Bond TC, Doherty SJ, Fahey DW, et al. Bounding the role of black carbon in the climate system: a scientific assessment. J Geophys Res Atmos. 2013;118:5380–5552. [Google Scholar]
- 2.Southerland VA, Brauer M, Mohegh A, et al. Global urban temporal trends in fine particulate matter (PM2·5) and attributable health burdens: estimates from global datasets. Lancet Planet Health. 2022;6:e139–e146. doi: 10.1016/S2542-5196(21)00350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeulen R, Schymanski EL, Barabási A-L, Miller GW. The exposome and health: where chemistry meets biology. Science. 2020;367:392–396. doi: 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen M, Stayner L, Slama R, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. 2014;64:494–500. doi: 10.1161/HYPERTENSIONAHA.114.03545. [DOI] [PubMed] [Google Scholar]
- 5.Mozzoni P, Iodice S, Persico N, et al. Maternal air pollution exposure during the first trimester of pregnancy and markers of inflammation and endothelial dysfunction. Environ Res. 2022;212 doi: 10.1016/j.envres.2022.113216. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen M, Giorgis-Allemand L, Bernard C, et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE) Lancet Respir Med. 2013;1:695–704. doi: 10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Huang S, Jiao A, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ Pollut. 2017;227:596–605. doi: 10.1016/j.envpol.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 8.Dadvand P, Parker J, Bell ML, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121:267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saenen ND, Vrijens K, Janssen BG, et al. Placental nitrosative stress and exposure to ambient air pollution during gestation: a population study. Am J Epidemiol. 2016;184:442–449. doi: 10.1093/aje/kww007. [DOI] [PubMed] [Google Scholar]
- 10.Grevendonk L, Janssen BG, Vanpoucke C, et al. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ Health. 2016;15:10. doi: 10.1186/s12940-016-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martens DS, Cox B, Janssen BG, et al. Prenatal air pollution and newborns' predisposition to accelerated biological aging. JAMA Pediatr. 2017;171:1160–1167. doi: 10.1001/jamapediatrics.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman CG. The thalidomide syndrome: risks of exposure and spectrum of malformations. Clin Perinatol. 1986;13:555–573. [PubMed] [Google Scholar]
- 13.Bosco C, Diaz E. Placental hypoxia and foetal development versus alcohol exposure in pregnancy. Alcohol Alcohol. 2012;47:109–117. doi: 10.1093/alcalc/agr166. [DOI] [PubMed] [Google Scholar]
- 14.O'Shaughnessy PJ, Monteiro A, Bhattacharya S, Fowler PA. Maternal smoking and fetal sex significantly affect metabolic enzyme expression in the human fetal liver. J Clin Endocrinol Metab. 2011;96:2851–2860. doi: 10.1210/jc.2011-1437. [DOI] [PubMed] [Google Scholar]
- 15.Bové H, Bongaerts E, Slenders E, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10 doi: 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holder B, Aplin JD, Gomez-Lopez N, et al. ‘Fetal side’ of the placenta: anatomical mis-annotation of carbon particle ‘transfer’ across the human placenta. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bongaerts E, Aengenheister L, Dugershaw BB, et al. Label-free detection of uptake, accumulation, and translocation of diesel exhaust particles in ex vivo perfused human placenta. J Nanobiotechnology. 2021;19:144. doi: 10.1186/s12951-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen BG, Madhloum N, Gyselaers W, et al. Cohort profile: the ENVIRonmental influence ON early AGEing (ENVIRONAGE): a birth cohort study. Int J Epidemiol. 2017;46:1386–1387m. doi: 10.1093/ije/dyw269. [DOI] [PubMed] [Google Scholar]
- 19.Johnston ZC, Bellingham M, Filis P, et al. The human fetal adrenal produces cortisol but no detectable aldosterone throughout the second trimester. BMC Med. 2018;16:23. doi: 10.1186/s12916-018-1009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kheradmand F, You R, Hee Gu B, Corry DB. Cigarette smoke and DNA cleavage promote lung inflammation and emphysema. Trans Am Clin Climatol Assoc. 2017;128:222–233. [PMC free article] [PubMed] [Google Scholar]
- 21.Evtouchenko L, Studer L, Spenger C, Dreher E, Seiler RW. A mathematical model for the estimation of human embryonic and fetal age. Cell Transplant. 1996;5:453–464. doi: 10.1177/096368979600500404. [DOI] [PubMed] [Google Scholar]
- 22.Janssen S, Dumont G, Fierens F, Mensink C. Spatial interpolation of air pollution measurements using CORINE land cover data. Atmos Environ. 2008;42:4884–4903. [Google Scholar]
- 23.Saenen ND, Bové H, Steuwe C, et al. Children's urinary environmental carbon load. A novel marker reflecting residential ambient air pollution exposure? Am J Respir Crit Care Med. 2017;196:873–881. doi: 10.1164/rccm.201704-0797OC. [DOI] [PubMed] [Google Scholar]
- 24.Bové H, Steuwe C, Fron E, et al. Biocompatible label-free detection of carbon black particles by femtosecond pulsed laser microscopy. Nano Lett. 2016;16:3173–3178. doi: 10.1021/acs.nanolett.6b00502. [DOI] [PubMed] [Google Scholar]
- 25.Srám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113:375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pachkowski BF, Guyton KZ, Sonawane B. DNA repair during in utero development: a review of the current state of knowledge, research needs, and potential application in risk assessment. Mutat Res. 2011;728:35–46. doi: 10.1016/j.mrrev.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 28.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 29.Dugershaw BB, Aengenheister L, Hansen SSK, Hougaard KS, Buerki-Thurnherr T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part Fibre Toxicol. 2020;17:31. doi: 10.1186/s12989-020-00359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luyten LJ, Saenen ND, Janssen BG, et al. Air pollution and the fetal origin of disease: a systematic review of the molecular signatures of air pollution exposure in human placenta. Environ Res. 2018;166:310–323. doi: 10.1016/j.envres.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Neven KY, Saenen ND, Tarantini L, et al. Placental promoter methylation of DNA repair genes and prenatal exposure to particulate air pollution: an ENVIRONAGE cohort study. Lancet Planet Health. 2018;2:e174–e183. doi: 10.1016/S2542-5196(18)30049-4. [DOI] [PubMed] [Google Scholar]
- 32.Valentino SA, Tarrade A, Aioun J, et al. Maternal exposure to diluted diesel engine exhaust alters placental function and induces intergenerational effects in rabbits. Part Fibre Toxicol. 2016;13:39. doi: 10.1186/s12989-016-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernal-Meléndez E, Lacroix M-C, Bouillaud P, et al. Repeated gestational exposure to diesel engine exhaust affects the fetal olfactory system and alters olfactory-based behavior in rabbit offspring. Part Fibre Toxicol. 2019;16:5. doi: 10.1186/s12989-018-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bongaerts E, Nawrot TS, Van Pee T, Ameloot M, Bové H. Translocation of (ultra)fine particles and nanoparticles across the placenta; a systematic review on the evidence of in vitro, ex vivo, and in vivo studies. Part Fibre Toxicol. 2020;17:56. doi: 10.1186/s12989-020-00386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreyling WG, Holzwarth U, Haberl N, et al. Quantitative biokinetics of titanium dioxide nanoparticles after intratracheal instillation in rats: part 3. Nanotoxicology. 2017;11:454–464. doi: 10.1080/17435390.2017.1306894. [DOI] [PubMed] [Google Scholar]
- 36.LoMauro A, Aliverti A. Respiratory physiology of pregnancy: physiology masterclass. Breathe (Sheff) 2015;11:297–301. doi: 10.1183/20734735.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Möller W, Felten K, Sommerer K, et al. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- 38.Brauer M, Avila-Casado C, Fortoul TI, Vedal S, Stevens B, Churg A. Air pollution and retained particles in the lung. Environ Health Perspect. 2001;109:1039–1043. doi: 10.1289/ehp.011091039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schittny JC. Development of the lung. Cell Tissue Res. 2017;367:427–444. doi: 10.1007/s00441-016-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virgintino D, Errede M, Girolamo F, et al. Fetal blood-brain barrier P-glycoprotein contributes to brain protection during human development. J Neuropathol Exp Neurol. 2008;67:50–61. doi: 10.1097/nen.0b013e31815f65d9. [DOI] [PubMed] [Google Scholar]
- 41.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Liu NM, Miyashita L, Maher BA, et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.142235. [DOI] [PubMed] [Google Scholar]
- 43.Calderón-Garcidueñas L, Pérez-Calatayud ÁA, González-Maciel A, et al. Environmental nanoparticles reach human fetal brains. Biomedicines. 2022;10:410. doi: 10.3390/biomedicines10020410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobson R, Semple S. Changes in outdoor air pollution due to COVID-19 lockdowns differ by pollutant: evidence from Scotland. Occup Environ Med. 2020;77:798–800. doi: 10.1136/oemed-2020-106659. [DOI] [PubMed] [Google Scholar]
- 45.Aslam I, Roeffaers MBJ. Unique emissive behavior of combustion-derived particles under illumination with femtosecond pulsed near-infrared laser light. Nanoscale Adv. 2021;3:5355–5362. doi: 10.1039/d1na00248a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are not publicly available because they contain information that could compromise research participant privacy, but are available within General Data Protection Regulation restrictions from the corresponding author upon reasonable request.