Abstract
Background and Objective
Brensocatib is an investigational, first-in-class, selective, and reversible dipeptidyl peptidase 1 inhibitor that blocks activation of neutrophil serine proteases (NSPs). The NSPs neutrophil elastase, cathepsin G, and proteinase 3 are believed to be central to the pathogenesis of several chronic inflammatory diseases, including bronchiectasis. In a phase II study, oral brensocatib 10 mg and 25 mg reduced sputum neutrophil elastase activity and prolonged the time to pulmonary exacerbation in patients with non-cystic fibrosis bronchiectasis (NCFBE). A population pharmacokinetic (PPK) model was developed to characterize brensocatib exposure, determine potential relationships between brensocatib exposure and efficacy and safety measures, and inform dose selection in clinical studies.
Methods
Pharmacokinetic (PK) data pooled from a phase I study of once-daily brensocatib (10, 25, and 40 mg) in healthy adults and a phase II study of once-daily brensocatib (10 mg and 25 mg) in adults with NCFBE were used to develop a PPK model and to evaluate potential covariate effects on brensocatib pharmacokinetics. PK–efficacy relationships for sputum neutrophil elastase below the level of quantification (BLQ) and reduction in pulmonary exacerbation and PK–safety relationships for adverse events of special interest (AESIs; periodontal disease, hyperkeratosis, and infections other than pulmonary infections) were evaluated based on model-predicted brensocatib exposure. A total of 1284 steady-state brensocatib concentrations from 225 individuals were included in the PPK data set; 241 patients with NCFBE from the phase II study were included in the pharmacodynamic (PD) population for the PK/PD analyses.
Results
The PPK model that best described the observed data consisted of two distributional compartments and linear clearance. Two significant covariates were found: age on volume of distribution and renal function on apparent oral clearance. PK–efficacy analysis revealed a threshold brensocatib exposure (area under the concentration–time curve) effect for attaining sputum neutrophil elastase BLQ and a strong relationship between sputum neutrophil elastase BLQ and reduction in pulmonary exacerbations. A PK–safety evaluation showed no noticeable trends between brensocatib exposure and the incidence of AESIs. Based on the predicted likelihood of clinical outcomes for sputum neutrophil elastase BLQ and pulmonary exacerbations, brensocatib doses of 10 mg and 25 mg once daily were selected for a phase III clinical trial in patients with NCFBE (ClinicalTrials.gov identifier: NCT04594369).
Conclusions
PPK results revealed that age and renal function have a moderate effect on brensocatib exposure. However, this finding does not warrant dose adjustments based on age or in those with mild or moderate renal impairment. The PK/PD evaluation demonstrated the clinically meaningful relationship between suppression of neutrophil elastase activity and reduction in exacerbations in brensocatib-treated patients with NCFBE, supporting further development of brensocatib for bronchiectasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-022-01147-w.
Key Points
| Brensocatib is an oral medication under investigation for the treatment of non-cystic fibrosis bronchiectasis, a chronic lung disorder in which inflammation driven by elevated levels of neutrophil elastase can increase the risk of infection and cause frequent pulmonary exacerbations. |
| Using data from a phase I study conducted in healthy adult volunteers and a phase II study in adults with non-cystic fibrosis bronchiectasis, a population pharmacokinetic model was developed to describe the pharmacokinetics of brensocatib; additionally, the pharmacokinetic/pharmacodynamic relationships for inhibition of neutrophil elastase, reduction in pulmonary exacerbations, and incidence of adverse events were evaluated. |
| A threshold of brensocatib exposure that resulted in sputum neutrophil elastase levels below the level of quantification was identified, a strong relationship between such undetectable sputum neutrophil elastase levels and a reduction in pulmonary exacerbations was found, and no significant trend between brensocatib exposure and adverse events of special interest (periodontal disease, hyperkeratosis, and infections other than pulmonary infections) was detected. |
Introduction
Bronchiectasis is a chronic inflammatory disorder marked by recurrent respiratory infections, impaired mucociliary clearance, and irreversible dilatation of the bronchi [1, 2]. Patients frequently experience chronic productive cough, dyspnea, fatigue, and hemoptysis [3]. Exacerbations of these symptoms are common and associated with poor quality of life, progressive loss of lung function, and increased morbidity and mortality [2, 4]. Current treatment guidelines aim to reduce exacerbations by suppressing or eradicating airway infection via treatment with oral, intravenous, and/or inhaled antibiotics [2]. Despite frequent antibiotics treatment, airway inflammation persists, and patients experience a high burden of disease. Currently no anti-inflammatory treatments are recommended in the international guidelines for bronchiectasis [2].
Excessive activity of neutrophil serine proteases (NSPs), which are stored in azurophil (primary) granules within neutrophils, has been implicated in the pathogenesis of bronchiectasis and other chronic inflammatory respiratory diseases, including chronic obstructive pulmonary disease and cystic fibrosis [5]. Dipeptidyl peptidase 1 (DPP-1; also known as cathepsin C) activates NSPs during neutrophil maturation in the bone marrow by cleaving the N-terminal dipeptide prior to granule assembly. While neutrophils and NSPs (including neutrophil elastase, cathepsin G, and proteinase 3) are important for the innate defense against invading pathogens, dysregulation and hyperresponsiveness of neutrophils result in excessive release of NSPs that can overwhelm natural NSP inhibitors, such as α1-antitrypsin, secretory leukocyte peptidase inhibitor, and α1-antichymotrypsin [6–8]. Such disruption of the balance between active NSPs and their natural inhibition can contribute to chronic infection and the continuation of the pathogenic cycle of bronchiectasis [9].
Brensocatib is an investigational, first-in-class, small-molecule, bioavailable, selective, and reversible DPP-1 inhibitor that prevents NSP activation. Results from a first-in-human phase I study demonstrated a dose-dependent reduction in whole-blood activity of neutrophil elastase in brensocatib-treated healthy adults [10], while results from a phase II study (the WILLOW study) in patients with non-cystic fibrosis bronchiectasis revealed that treatment with two doses of brensocatib reduced neutrophil elastase activity in sputum and prolonged the time to exacerbations compared with placebo [11].
The objectives of the current study were to develop a population pharmacokinetic (PPK) model characterizing brensocatib exposure in plasma, examine the pharmacokinetic/pharmacodynamic (PK/PD) relationships of brensocatib in patients with non-cystic fibrosis bronchiectasis, and conduct model-based simulations to assist in dose selection for brensocatib phase III studies.
Methods
PPK Analysis
Participants, Studies, and PPK Data Set
The PPK data set comprised brensocatib concentrations pooled from healthy participants in a phase I bridging study and patients with non-cystic fibrosis bronchiectasis in the phase II WILLOW study (ClinicalTrials.gov: NCT03218917; EudraCT: 2017-002533-32) [11]. A total of 1284 steady-state brensocatib concentrations were pooled from 225 individuals who participated in either study; 68 participants with intensive plasma data in the phase I study and 157 patients from the phase II study (20 with intensive PK data and 137 with sparse PK data) were included in the PPK data set.
The phase I study was a single- and repeat-dosing study conducted in two parts. Part A was a randomized, double-blind, placebo-controlled, dose-escalation phase in which healthy Japanese and White adults were randomized 4:1 to receive oral doses of brensocatib 10, 25, or 40 mg or placebo after an overnight fast of ≥ 10 h (n = 6 per dose per race); participants received a single dose on day 1 followed by daily doses on days 4–30 [12]. Blood samples for PK analysis were collected pre-dose on days 1 and 30 and post-dose at 0.5, 0.75, 1, 1.5, 2, 3, 4, 8, 12, 24, 48, and 72 h. An additional sample was drawn pre-dose on day 14. Part B was an open-label, two-period, two-sequence crossover phase in which newly enrolled healthy Japanese and White participants (n = 10 per race) were randomized 1:1 to receive single doses of brensocatib 40 mg under either fasted or fed (high-fat, high-calorie breakfast) conditions on days 1 and 8 [13]. Blood samples for PK analysis were drawn pre-dose on days 1 and 8 and post-dose at 0.25, 0.5, 1, 1.5, 2, 3, 3.5, 4, 6, 8, 10, 12, 24, 36, and 72 h. Participants in both parts A and B were nonsmoking adults (18–50 years of age) with no notable abnormalities on a 12-lead electrocardiogram, a body mass index of 18.0–30.0 (calculated as weight in kilograms divided by height in meters squared), and a body weight of ≥ 45–< 100 kg. Except for paracetamol/acetaminophen or ibuprofen, use of over-the-counter medications (including vitamins) 7 days before screening, or prescription medications and herbal remedies 30 days before screening, was not permitted until the end of treatment.
The phase II study was a randomized, double-blind, placebo-controlled trial in which adults (18–85 years of age) with computed tomography-confirmed non-cystic fibrosis bronchiectasis and two or more documented exacerbations in the previous 12 months were randomized 1:1:1 to receive brensocatib 10 mg, brensocatib 25 mg, or placebo once daily (QD) for 24 weeks. Intensive blood samples for PK analysis were collected from 20 active participants pre-dose on day 1 and at week 4 and post-dose at 1, 2, 3, 4, 6, and 8 h. Trough samples were to be collected pre-dose at weeks 2, 12, 24, and 28 from 138 participants. Detailed methods and results from the WILLOW study were previously published [11].
Plasma PK Assay
Brensocatib was extracted from plasma samples treated with an anticoagulant (di-potassium ethylenediaminetetraacetic acid) and quantified using a validated method of tandem liquid chromatography–mass spectrometry with a quantitation range of 0.25–150 ng/mL.
PPK Analysis Software and Data Handling
All data management activities were performed using SAS version 9.4 software. The PPK analysis was conducted using NONMEM version 7.4.3 (ICON Development Solutions, Ellicott City, MD, USA), implementing first-order conditional estimation with η–ε interaction, as facilitated by Perl-Speaks-NONMEM version 4.7.0 and Pirana version 2.9.9 (Certara). Covariate analysis was conducted using the automated stepwise covariate modeling algorithm implemented in Perl-Speaks-NONMEM. Creatinine clearance (CLcr) was calculated from baseline serum creatinine, age, and weight (kg) using the Cockcroft–Gault equation [14] and then normalized to body surface area (BSA). All other analyses, including calculation of post-hoc PK parameters and Monte Carlo simulations, were performed using the R statistical software package version 3.6.
Demographic information (age, weight, height, BSA, and sex) and clinical characteristics (serum albumin and serum creatinine) were used to characterize the analysis population and evaluate the interindividual variability for selected PK parameters.
Because of the difficulties associated with fitting a model to the pooled first-dose and steady-state data across the two studies, first-dose PK data were excluded. Brensocatib plasma concentrations were also excluded from the PPK analyses if the dates and times of the previous dose or sample collection or any covariate information (demographics or laboratory data) were missing. Concentrations below the lower limit of quantification (LLOQ) were set to missing.
PPK Model Development
Models were selected based on a comparison of the minimum value of objective function (MVOF) for nested models, evaluation of individual- and population-predicted accuracy, graphic examination of goodness-of-fit plots, reduction in both intraindividual variability (σ2) and interindividual variability (ω2), Akaike’s information criterion and/or the Bayesian information criterion for non-nested models [15], and biological plausibility. Throughout model development, normalized prediction distribution errors (NPDEs) were computed as an additional model diagnostic to assess bias, with a resemblance to a standard normal distribution [N(0, 1)] expected for a model without bias. The condition number (derived as the ratio of largest to smallest eigenvalue) was also assessed for possible overparameterization; a model with a condition number < 1000 was considered not overparameterized [16, 17].
A base structural model was developed using PK data from the healthy participants to evaluate the number of distributional compartments (1–3), oral absorption kinetics, and error models. Once an adequate model was developed, PK data were pooled with the data from patients with non-cystic fibrosis bronchiectasis in the phase II study to test the adequacy of the structural model. Following confirmation of the structural model, a formal covariate analysis was conducted using stepwise forward inclusion (p < 0.05; ΔMVOF ≥ 3.84 U) and backward elimination (p < 0.001; ΔMVOF ≥ 10.8 U). Covariates evaluated include age, weight, height, BSA, sex, race, serum albumin, and CLcr. The final PPK model was evaluated using sampling-importance resampling to assess the uncertainty distribution of each of the predicted parameters [18, 19]. Prediction-corrected visual predictive checks (pc-VPCs) were used to determine the accuracy of model-predicted concentration values [20].
The final PPK model was used to predict the individual concentration profiles and PK parameters—including apparent oral clearance (CL/F), apparent oral volume of distribution of the central compartment (Vc/F), terminal elimination half-life (t½), maximum plasma concentration (Cmax), and area under the plasma concentration–time curve over the dosing interval (AUCτ)—in both healthy participants and patients with non-cystic fibrosis bronchiectasis.
PK/PD Analyses of Brensocatib in Patients With Bronchiectasis
A total of 241 patients from the phase II study were included in the PD population for the PK/PD analyses: 157 brensocatib-treated patients (n = 75, brensocatib 10 mg; n = 82, brensocatib 25 mg) and 84 placebo-treated patients.
Efficacy and Safety Measurements
Pulmonary exacerbations were defined as the presence of three or more of the following symptoms for 48 h that resulted in a physician’s decision to prescribe antibiotic treatment: hemoptysis, fatigue, malaise, change in sputum consistency, decreased exercise tolerance, and increased cough, sputum volume, sputum purulence, or breathlessness [21]. Sputum samples for determination of neutrophil elastase activity were collected at screening (days −42 to −1); baseline (day 1); weeks 2, 4, 12, and 24; and follow-up (week 28). Neutrophil elastase activity was measured by ProteaseTag Active Neutrophil Elastase Immunoassay (ProAxsis Ltd, Belfast, UK). Safety data included adverse events of special interest (AESIs)—such as periodontal disease, hyperkeratosis, and infections other than pulmonary infections—that were selected based on the mechanism of action of brensocatib.
PK/PD Analyses
Exploratory analyses were conducted to determine any potential PK/PD relationships between the independent PK exposure measures (AUCτ, Cmax, and minimum plasma concentration [Cmin]), derived from the fit of the PPK model, and the dependent variables (pulmonary exacerbations, sputum neutrophil elastase, and AESIs). Age (< 65 and ≥ 65 years; < 75 and ≥ 75 years), baseline sputum neutrophil elastase concentration below the limit of quantification (BLQ), use of rescue medications, use of macrolides for prevention of pulmonary exacerbations, and sputum culture positive for Pseudomonas aeruginosa were identified as potential covariates to ensure that any PK/PD relationships associated with brensocatib exposure were independent of potential predictors. Exacerbations were evaluated as dichotomous (yes or no for occurrence within the 24-week treatment period) and by using time-to-event models for time to occurrence within 24 weeks.
Sputum neutrophil elastase was evaluated as both a dichotomous variable (for attaining one or more post-baseline concentration below the LLOQ) and a continuous variable (absolute values or change over time and maximum change from baseline). When sputum neutrophil elastase was evaluated as a continuous variable, BLQ values were imputed as one-half of the reported LLOQ for that observation (LLOQ could vary among samples based on the extent of dilution necessary for that sample). When sputum neutrophil elastase was evaluated as a categorical variable, values below the LLOQ were retained in the data set. Post-baseline values below the LLOQ that were achieved after the occurrence of a pulmonary exacerbation were excluded because the administration of antibiotics for an exacerbation has the potential to suppress sputum neutrophil elastase activity [22].
Brensocatib exposures were evaluated as continuous variables in exposure quartiles; patients who received placebo were assigned a quartile of 0. Results from the exploratory PK/PD analyses were graphically displayed to examine potential relationships between exposure and the dependent variables. Continuous independent variables were evaluated both as continuous and categorical independent variables. Categorical transformations of the independent variables were made based on a recursive partitioning algorithm designed to identify thresholds above and below which substantial differences in the dependent variables were apparent. For this analysis, recursive partitioning was accomplished using classification-and-regression tree (CART) algorithms as implemented in the R statistical software package. If potential PK/PD relationships were identified for the dichotomous endpoints, univariate evaluations were conducted using contingency tables (using χ2 or Fisher exact test) for the dichotomous independent variables, and logistic regression was used for continuous independent variables. Univariable relationships for continuous endpoints (e.g., maximum change in sputum neutrophil elastase) were evaluated using correlation coefficients (Pearson χ2 and Spearman rank correlation coefficient) and linear regression. Relationships for time-to-event endpoints (e.g., occurrence of pulmonary exacerbation) were examined using log-rank tests for categorical independent variables and Cox proportional hazards regression for continuous independent variables.
PK/PD Model-Based Simulations
Clinical outcomes, including inhibition of sputum neutrophil elastase and reduction in the occurrence of pulmonary exacerbations, were simulated based on the PK–efficacy relationships to support the selection of brensocatib doses for future clinical studies. A population of 1000 patients with non-cystic fibrosis bronchiectasis was generated by resampling body weight and CLcr from the phase II study population (n = 255). Each patient was assigned a value for CL/F based on the PPK model using the resampled median for the CLcr effect on CL/F and the estimated interindividual variability (IIV) in CL/F from the final population PK model per the following equation:
Steady-state drug exposure within a dosing interval (AUCτ) was then predicted from CL/F for each simulated patient for brensocatib doses of 2.5, 5, 10, 25, and 40 mg QD according to the following equation:
A bootstrapping approach was used to simulate likely outcomes in terms of sputum neutrophil elastase and pulmonary exacerbation by replicating the simulation process 500 times. For each replicate, the probability of achieving post-baseline sputum neutrophil elastase BLQ and the probability of the occurrence of a pulmonary exacerbation was recalculated by sampling the observed data with replacement. In this manner, it was possible to obtain a distribution of predicted outcomes at each brensocatib dose that more robustly accounted for the variability in the observed outcomes and the relatively small sample sizes.
Results
PPK Analysis and Model Development
Participants and Plasma PK Data
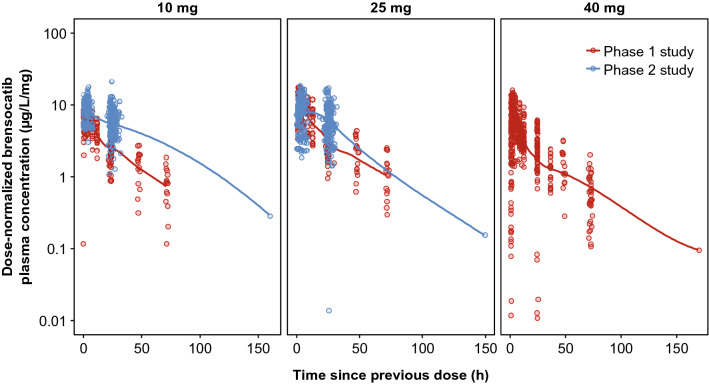
Participants in the phase I study were predominantly male, with a mean age of 36.2 years; the number of Japanese and White participants was equal (Table 1). Patients with non-cystic fibrosis bronchiectasis from the phase II WILLOW study were mostly female and White, with a mean age of 64.1 years (Table 1). Other than the slightly lower brensocatib concentrations observed in the 10-mg group in the phase I study, dose-normalized brensocatib plasma concentrations were consistent across doses and between studies (Fig. 1).
Table 1.
Demographics and baseline clinical characteristics
| Phase I (n = 68) | Phase II (n = 157) | Total (N = 225) | |
|---|---|---|---|
| Age, years | |||
| Mean (CV%) | 36.2 (22.7) | 64.1 (19.8) | 55.7 (30.9) |
| Median (range) | 36.0 (20.0–49.0) | 66.0 (22.0–83.0) | 60.0 (20.0–83.0) |
| Sex, n (%) | |||
| Male | 38 (55.9) | 45 (28.7) | 83 (36.9) |
| Female | 30 (44.1) | 112 (71.3) | 142 (63.1) |
| Race, n (%) | |||
| White | 34 (50.0) | 142 (90.4) | 176 (78.2) |
| Black | 2 (1.27) | 2 (0.889) | |
| Asian | 34 (50.0) | 10 (6.37) | 44 (19.6) |
| Native Hawaiian or other Pacific Islander | 2 (1.27) | 2 (0.889) | |
| Other | 1 (0.637) | 1 (0.444) | |
| Height, cm | |||
| Mean (CV%) | 168 (5.06) | 166 (5.19) | 166 (5.17) |
| Median (range) | 168 (151–183) | 165 (145–190) | 166 (145–190) |
| Weight, kg | |||
| Mean (CV%) | 68.3 (16.7) | 71.0 (23.9) | 70.2 (22.1) |
| Median (range) | 64.9 (49.5–95.9) | 68.0 (40.4–155) | 67.5 (40.4–155) |
| BMI, kg/m2 | |||
| Mean (CV%) | 24.2 (12.8) | 25.8 (20.5) | 25.3 (18.9) |
| Median (range) | 24.5 (18.4–29.9) | 25.0 (18.3–53.8) | 24.7 (18.3–53.8) |
| BSA, m2 | |||
| Mean (CV%) | 1.77 (9.73) | 1.78 (12.2) | 1.78 (11.5) |
| Median (range) | 1.75 (1.46–2.16) | 1.75 (1.28–2.54) | 1.75 (1.28–2.54) |
| CLcr, mL/min/1.73 m2 | |||
| Mean (CV%) | 98.3 (16.7) | 67.6 (28.6) | 76.9 (30.2) |
| Median (range) | 99.1 (65.4–149) | 65.0 (26.7–129) | 75.6 (26.7–149) |
BMI body mass index, BSA body surface area, CLcr creatinine clearance, CV coefficient of variation
Fig. 1.
Steady-state brensocatib plasma concentrations. Open circles are observed concentrations, normalized based on dose administered. Solid lines are LOESS smoothers through the data. When normalized by dose, brensocatib plasma concentrations were consistent across doses and between the two studies, except for slightly lower concentrations observed in the 10-mg group in the phase 1 study. LOESS locally weighted scatterplot smoothing
PPK Model Development and Qualification
Using data from the healthy participants in the phase I study, several potential models to describe brensocatib absorption and elimination kinetics were attempted. For the absorption model, simple first-order models and more complex transit-chain models were attempted. For elimination, 1-, 2-, and 3-compartment models with linear elimination were attempted; nonlinear elimination models were not explored because they were not applicable based on the observed data. Brensocatib PK profiles were best described with two disposition compartments and a chain of six transit compartments with separate absorption rate constants (ktr) for fed and fasted states (Supplementary Fig. 1). This base model was then applied to the pooled PPK data set, and goodness-of-fit plots indicated that the model provided a robust fit to the observed concentrations, with minimal bias based on the distribution of NPDEs. PPK parameters for the model were well estimated, with acceptable interindividual and residual variabilities. The condition number was low (23.3), indicating that the model was not overparameterized.
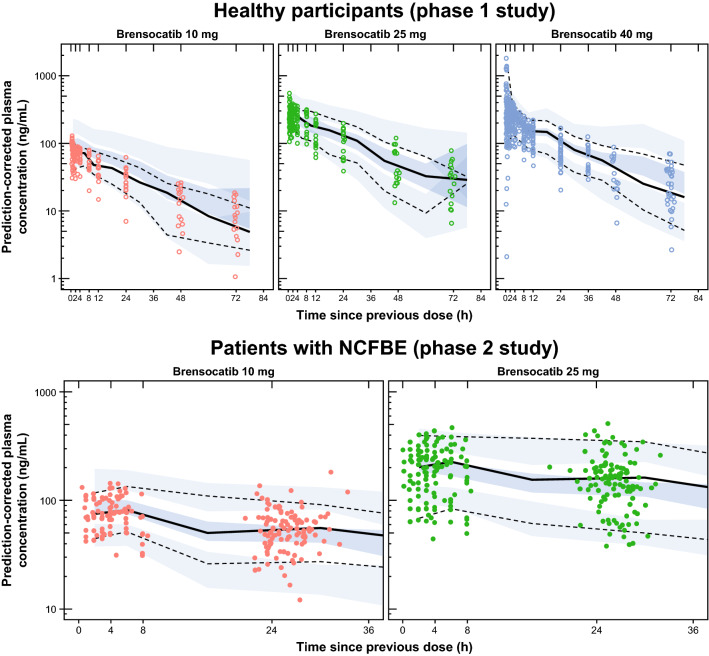
Using the forward inclusion/backward elimination procedure described in the methods, two statistically significant covariate relationships were identified: CLcr on CL/F (CL/F × [CLcr/75.2]0.276) and age on Vc/F (Vc/F × [age/60]0.396). Since all parameters were dependent on body weight using allometric principles [23], the additional covariate relationships were significant after accounting for the effect of body weight on PK. Other covariates tested (sex, race, body weight, and serum albumin levels) did not have a significant relationship with the variability in PPK model parameters. PPK parameters from the fit of the final model, along with the summary statistics from the resampled parameters, are provided in Table 2. Goodness-of-fit plots for the final model indicated a lack of systematic bias and good precision, with r2 = 0.62 for population predictions versus observations and r2 = 0.96 for individual predictions versus observations (Supplementary Fig. 2, see ESM). The NPDE plots also did not indicate bias (Supplementary Fig. 3, see ESM). Qualification of the final model using simulation-based diagnostics (pc-VPC) plots indicate that the final model adequately described both the central tendency and variability in observed brensocatib concentrations over time for both the healthy participants in the phase I study and patients with non-cystic fibrosis bronchiectasis in the phase II WILLOW study (Fig. 2).
Table 2.
Final PPK model parameter estimates compared with the resampled PPK parameter estimates (pooled phase I and phase II data)
| Parameter | Final model | Resample statistics (N = 1150) | ||||
|---|---|---|---|---|---|---|
| Estimate | %SEM | Mean | Median | %SEM | 95% CI | |
| CL/F, L/h/70 kg | 6.23 | 0.208 | 6.168 | 6.169 | 0.656 | 6.085–6.247 |
| Vc/F, L/70 kg | 196 | 0.208 | 201.832 | 201.823 | 0.906 | 198.301–205.429 |
| CLd/F, L/h | 6.26 | 0.202 | 6.349 | 6.351 | 0.587 | 6.277–6.423 |
| Vp/F, L/70 kg | 80.6 | 0.266 | 80.914 | 80.917 | 1.034 | 79.238–82.518 |
| ktr,fasted, 1/h | 11.7 | 0.292 | 11.664 | 11.659 | 0.978 | 11.454–11.898 |
| ktr,fed, 1/h | 9.96 | 0.577 | 10.048 | 10.059 | 1.865 | 9.647–10.406 |
| Vc/F: age, power | 0.396 | 13.1 | 0.544 | 0.540 | 14.614 | 0.393–0.719 |
| CL/F: CLcr, power | 0.276 | 12.0 | 0.399 | 0.394 | 16.253 | 0.281–0.540 |
| IIV_CL/F | 0.139 | 5.38 | 0.148 | 0.147 | 8.642 | 0.126–0.174 |
| IIV_Vc/F | 0.193 | 1.16 | 0.182 | 0.181 | 3.927 | 0.170–0.198 |
| IIV_ktr | 0.0921 | 8.97 | 0.159 | 0.160 | 7.696 | 0.134–0.181 |
| Covariance, CV% | ||||||
| IIV_CL/F, IIV_VC/F | 0.0464 | 14.3 | 0.072 | 0.073 | 17.348 | 0.0459–0.0925 |
Shrinkage estimates (%) for CL/F, Vc/F, ktr,fasted, and proportional RV for the final model were < 1.0, 36.6, 31.2, and 5.17, respectively
CI confidence interval, CLcr creatinine clearance, CLd/F apparent oral distributional clearance, CL/F apparent oral clearance, CV coefficient of variation, IIV interindividual variability, ktr,fasted rate constant between transit compartments in the fasted state, ktr,fed rate constant between transit compartments in the fed state, PPK population pharmacokinetics, RV residual variability, SEM standard error of the mean, Vc/F apparent oral volume of distribution of the central compartment, Vp/F apparent oral volume of distribution of the peripheral compartment
Fig. 2.
Final pooled pharmacokinetic model compared with observed data. The simulation-based diagnostics (pc-VPC plots) indicate that the final model adequately described both the central tendency and the variability in observed brensocatib concentrations over time in healthy participants (top) and in patients with NCFBE (bottom). Dots indicate observed concentrations; solid black lines indicate median observed concentrations; dashed black lines indicate 5th and 95th percentiles of the observed concentrations; blue-shaded regions indicate 90% CIs for the median and 5th and 95th percentiles from the simulations. NCFBE non-cystic fibrosis bronchiectasis, pc-VPC prediction-corrected visual predictive check
Predicted Individual PK Parameters in Patients with Non-cystic Fibrosis Bronchiectasis
The mean estimated steady-state AUCτ from the final PPK model was 1760 in patients treated with brensocatib 10 mg and 4540 ng·h/mL in patients treated with brensocatib 25 mg compared with 1110 and 3660 ng·h/mL, respectively, in healthy participants (Table 3). In the brensocatib 10- and 25-mg groups, the mean t½ values were 38.5 h and 39.1 h, respectively, in patients with non-cystic fibrosis bronchiectasis compared with 24.3 h and 26.0 h in healthy participants (Table 3). The PPK-predicted parameters were comparable to those determined using a noncompartmental analysis method based on observed PK concentrations [10]. Estimates for steady-state AUCτ, Cmax, and Cmin by age (Supplementary Fig. 4), CLcr (Supplementary Fig. 5), and body weight (Supplementary Fig. 6, see ESM) in patients in the phase II study were consistent across doses, indicating that dose adjustments are unlikely to be warranted in patient subpopulations.
Table 3.
Predicted steady-state brensocatib PPK parameters
| Parameter, mean (CV%) |
Phase I | Phase II | |||
|---|---|---|---|---|---|
| 10 mg (n = 16) | 25 mg (n = 17) | 40 mg (n = 35) | 10 mg (n = 75) | 25 mg (n = 82) | |
| AUCτ, ng·h/mL | 1110 (27.1) | 3660 (35.3) | 5420 (37.1) | 1760 (34.0) | 4540 (42.3) |
| Cmax, ng/mL | 76.2 (21.1) | 257 (33.0) | 401 (35.7) | 101 (30.3) | 260 (37.0) |
| Tmax, h | 1.13 (32.9) | 1.26 (22.8) | 1.27 (37.5) | 1.27 (6.55) | 1.27 (7.20) |
| Cmin, ng/mL | 27.7 (40.5) | 93.1 (43.3) | 131 (49.1) | 54.4 (40.7) | 140 (51.0) |
| CL/F, L/h | 9.05 (27.1) | 6.83 (35.3) | 7.38 (37.1) | 5.68 (34.0) | 5.51 (42.4) |
| Vc/F, L | 197 (24.1) | 141 (34.5) | 139 (43.4) | 207 (31.2) | 203 (34.8) |
| t½,α, h | 5.20 (15.3) | 4.58 (18.1) | 4.54 (24.7) | 5.83 (22.3) | 5.74 (22.1) |
| t½,β, h | 24.3 (23.6) | 26.0 (20.3) | 25.3 (26.5) | 38.5 (26.7) | 39.1 (34.2) |
AUCτ area under the plasma concentration–time curve from time 0 to 24 h, Cmax maximum plasma concentration, Cmin minimum plasma concentration, CL/F apparent oral clearance, CV coefficient of variation, PPK population pharmacokinetics, t½,α half-life of the first distributional phase, t½,β half-life of the second distributional phase, Tmax time at maximum concentration, Vc/F apparent oral volume of distribution of the central compartment
PK/PD Analyses of Brensocatib in Patients with Bronchiectasis
Steady-state AUCτ, Cmax, and Cmin were found to be highly correlated (Supplementary Fig. 7 and Supplementary Fig. 8, see ESM). Therefore, AUCτ was used as the independent variable for brensocatib exposure for the PK/PD analyses.
Sputum Neutrophil Elastase and Brensocatib Exposure
A greater percentage of patients treated with brensocatib had sputum neutrophil elastase levels BLQ compared with patients treated with placebo (76.8% vs 50.0%). The incidence of neutrophil elastase BLQ was the greatest (86.8%) in patients whose brensocatib AUCτ was in the highest quartile (4845–9466 ng·h/mL) compared with 77.5% in the lowest AUCτ quartile (624–1758 ng·h/mL) and 50.0% in the placebo group (Table 4). Additionally, two AUCτ thresholds (1150 and 3811 ng·h/mL) were identified via CART for attaining neutrophil elastase BLQ: 50% with AUCτ <1150 ng·h/mL, 72.7% with AUCτ 1150–3810, and 86.4% with AUCτ > 3810 ng·h/mL.
Table 4.
Incidence of pulmonary exacerbations and post-baseline neutrophil elastase BLQ by brensocatib exposure
| n/N (%) | Placebo | AUCτ quartile range | |||
|---|---|---|---|---|---|
| Q1 624–1758 ng·h/mL |
Q2 1813–2753 ng·h/mL |
Q3 2840–4824 ng·h/mL |
Q4 4845–10,562 ng·h/mL |
||
| Pulmonary exacerbations | 42/84 (50.0) | 12/40 (30.0) | 14/39 (35.9) | 16/39 (41.0) | 12/39 (30.8) |
| 624–1758 ng·h/mL | 1813–2753 ng·h/mL | 2840–4824 ng·h/mL | 4845–9466 ng·h/mL | ||
| Sputum neutrophil elastase < BLQ | 41/82 (50.0) | 31/40 (77.5) | 28/39 (71.8) | 27/38 (71.1) | 33/38 (86.8) |
AUCτ area under the plasma concentration-time curve from time 0 to 24 h, BLQ below the level of quantification, Q quartile
Pulmonary Exacerbations and Brensocatib Exposure
The incidence of pulmonary exacerbations was lower in patients in the pooled brensocatib group than in patients in the placebo group (34.4% vs 50.0%), with the incidence ranging from 30.0% in those in the first brensocatib AUCτ quartile (624–1758 ng·h/mL) to 41.0% in the third quartile (2840–4824 ng·h/mL) (Table 4). The time to pulmonary exacerbation was reduced in each AUCτ quartile relative to placebo (Supplementary Fig. 9, see ESM); however, no clear relationship between higher exposure and longer time to pulmonary exacerbation was evident.
Exploratory analyses of sputum neutrophil elastase as a continuous variable revealed substantial within-patient variability such that the absolute values or change over time did not predict outcome. When evaluated as a dichotomous variable, sputum neutrophil elastase was found to be a predictor of pulmonary exacerbations. Patients who achieved neutrophil elastase BLQ post-baseline experienced significantly fewer exacerbations and a prolonged time to first exacerbation compared with those with persistently detectable neutrophil elastase. The hazard ratios (95% CI) were 0.32 (0.21–0.48; p < 0.0001) for all patients (Fig. 3a) and 0.29 (0.17–0.51; p < 0.0001) for brensocatib-treated patients (Fig. 3b).
Fig. 3.
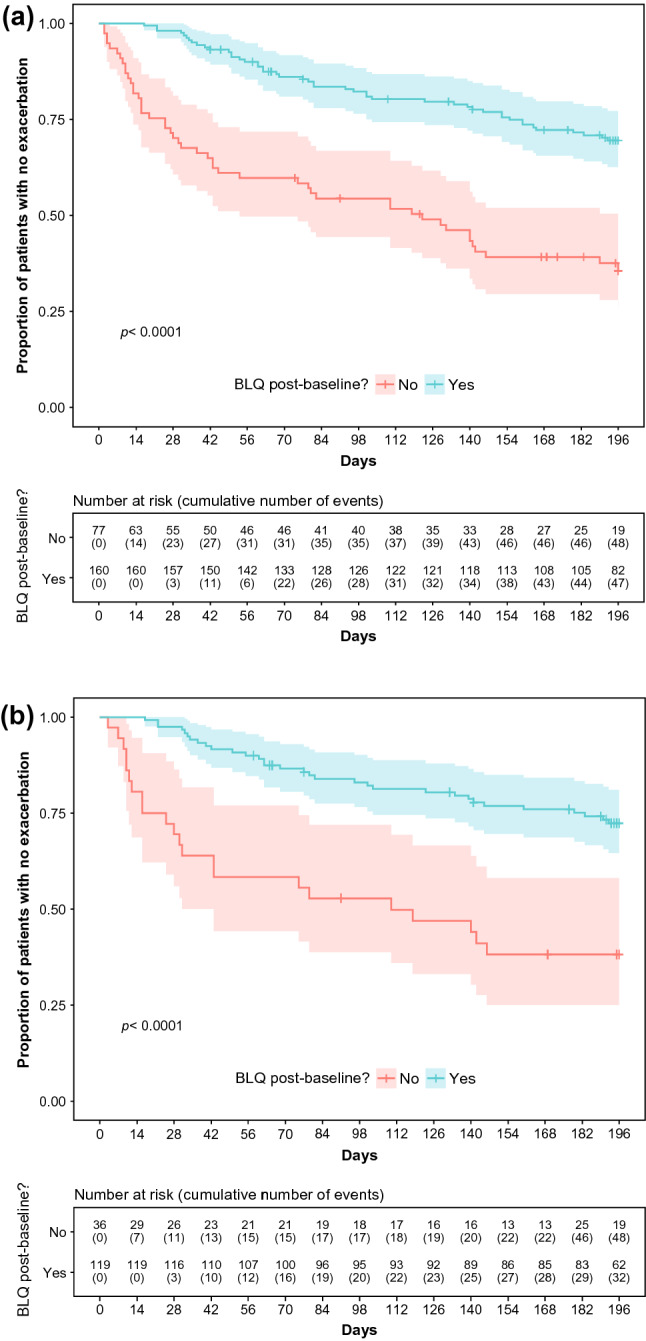
Time to pulmonary exacerbation by post-baseline sputum neutrophil elastase concentration. All patients (a) and brensocatib-treated patients (b) with neutrophil elastase BLQ post-baseline experienced significantly fewer exacerbations than those with detectable neutrophil elastase. BLQ below the level of quantification
PK–Safety Analyses
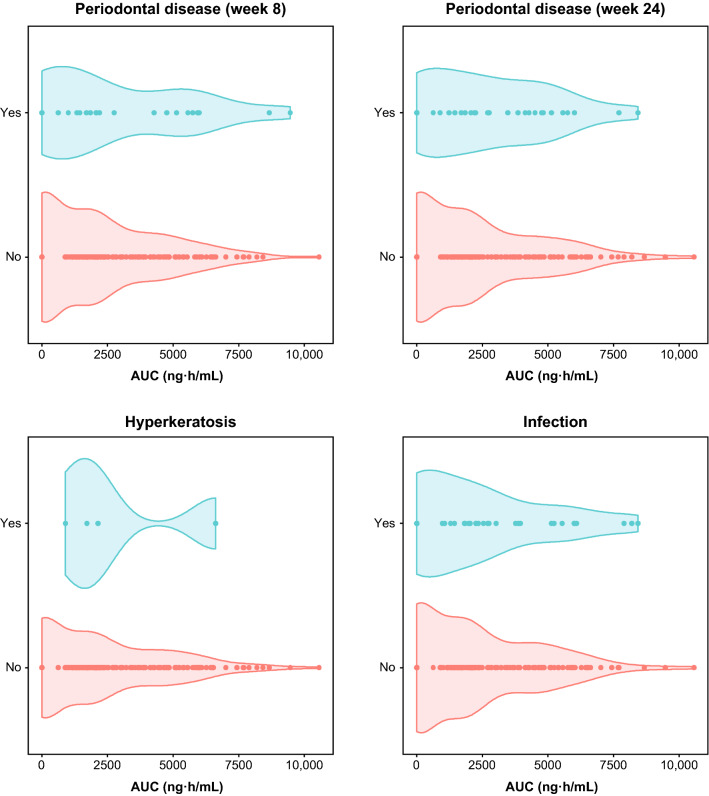
The PK/PD analyses for safety did not reveal any clinically relevant relationships between brensocatib exposure and the incidence of AESIs (Fig. 4; Supplementary Table 1, see ESM). Although a possible relationship between steady-state AUCτ and the occurrence of periodontal disease at week 8 was suggested by objective criteria, the relationship was not statistically significant.
Fig. 4.
Distribution of steady-state AUC by incidence of AESIs. No significant relationships between brensocatib exposure and the incidence of AESIs were evident. AESI adverse event of special interest, AUC area under the concentration-time curve
PK/PD Model-Based Simulations
Simulated PK exposures using a range of brensocatib dose regimens showed that, at doses of 5 mg QD or lower, brensocatib steady-state AUCτ values were expected to be below the target minimum AUCτ threshold of 1150 ng·h/mL in the majority (78%) of patients with non-cystic fibrosis bronchiectasis. At 10 mg or higher, most patients (> 77%) were expected to achieve the desired AUCτ levels. In the brensocatib 2.5-mg and 5-mg QD groups, the predicted clinical outcomes for both sputum neutrophil elastase BLQ and pulmonary exacerbations were similar to those observed in the placebo group, whereas better clinical outcomes were predicted at 10 mg QD or higher; a clear separation from placebo was projected at brensocatib 25-mg and 40-mg QD dose levels. At 40 mg QD, further improvement in the clinical outcomes appeared to be minimal compared with the 25-mg QD dose.
Discussion
The objectives of these analyses were to (i) develop a PPK model to characterize the disposition of brensocatib in plasma using data pooled from a phase I study in healthy volunteers and a phase II study in patients with non-cystic fibrosis bronchiectasis; (ii) use PK exposure estimates from the PPK model to analyze potential PK/PD relationships between brensocatib exposure and efficacy and safety measures; and (iii) use model-based simulations to inform dose-selection decisions for future clinical studies.
A robust PPK model that describes brensocatib PK in both healthy participants and patients with non-cystic fibrosis bronchiectasis was successfully developed. The model consisted of two distributional compartments with first-order absorption kinetics and linear clearance. Two significant PK covariates were identified: age for Vc/F and CLcr for CL/F. However, these relationships were weak and did not warrant dose adjustments based on age or the presence of mild or moderate renal impairment.
Using the final model, the estimated steady-state AUCτ in patients treated with brensocatib 10 mg and 25 mg was 1760 and 4540 ng·h/mL, respectively, compared with 1110 and 3660 ng·h/mL in healthy participants. A direct relationship between brensocatib exposure and reduction in pulmonary exacerbation incidence was not detected. However, a threshold AUC effect for attaining sputum neutrophil elastase levels BLQ was detected, as was a strong relationship between sputum neutrophil elastase BLQ and a reduction in pulmonary exacerbations. Given the association between elevated neutrophil elastase and the incidence of exacerbations and disease sequelae, these findings are an important step in our understanding of the vicious cycle of airway destruction, abnormal mucociliary clearance, bacterial infection, and inflammation that defines bronchiectasis [24]. Previous studies have suggested a relationship between elevated NSPs and exacerbation frequency but could not prove a causal relationship [25, 26]. The demonstration of reduced exacerbations upon suppression of NSP activity is the strongest evidence to date that NSP activity directly contributes to exacerbations. Furthermore, no significant relationships between brensocatib exposure and the occurrence of AESIs (periodontal disease, hyperkeratosis, and infections other than pulmonary infections) were detected. These results support the favorable safety profile of brensocatib that was observed in the phase II study and indicate that DPP-1 inhibition is a potentially safe and effective method for the treatment of inflammatory diseases involving NSPs.
Model-based simulations using the PPK model and the PK/PD relationships for efficacy showed that 10 mg and 25 mg QD are the optimal brensocatib doses for the desired clinical outcomes in patients with non-cystic fibrosis bronchiectasis. Based on the predicted likelihood of clinical outcomes for sputum neutrophil elastase BLQ and pulmonary exacerbations, brensocatib doses of 10 mg and 25 mg QD were selected for a phase III clinical trial in patients with non-cystic fibrosis bronchiectasis (NCT04594369).
Study limitations included the relatively small sample size of patients with non-cystic fibrosis bronchiectasis for each dose and the relatively short (24 weeks) treatment phase in which to examine potential AESIs that could emerge with longer drug exposures. Substantial variability in sputum neutrophil elastase activity was observed both among and within individuals. While some variability is expected, the method of sample collection is likely to be important in explaining this variability [27]. Indeed, sputum samples are often inconsistent in terms of quantity and the depth from which the sample originates within the lung. Such inherent variability likely contributed to our inability to discover PK/PD relationships when treating sputum neutrophil elastase activity as a continuous variable.
Conclusions
We developed a PPK model that accurately predicted the observed brensocatib concentrations after a single dose and at steady state. Statistically significant and clinically relevant PK/PD relationships were identified between brensocatib exposure and neutrophil elastase levels BLQ and between neutrophil elastase levels and the occurrence of pulmonary exacerbations. The data presented here support the assertion that model-based simulations are an important tool in drug development, predicting both the central tendency and variability in expected drug exposures at various doses. While these simulations are not predictive of efficacy in phase III studies, they provide a strong basis for comparing likely outcomes for various doses to inform the design of clinical trials. Furthermore, these data indicate a threshold AUC effect for attaining sputum neutrophil elastase levels BLQ and the strong relationship between attaining sputum neutrophil elastase BLQ and reduction in pulmonary exacerbations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Kristen A. Andersen, PhD, of Meditech Media, Ltd, provided medical writing assistance; editorial and creative support were provided by MediTech Media, Ltd. Insmed Incorporated provided funding to MediTech Media, Ltd for these services.
Declarations
Funding
The phase I study, phase II WILLOW study, and PK/PD analysis presented in this publication were funded by Insmed Incorporated, Bridgewater, NJ, USA. The Open Access fee for the published article also was supported by Insmed Incorporated.
Conflicts of interest
Institute for Clinical Pharmacodynamics (ICPD), Inc. received funding from Insmed Incorporated to conduct the analyses and provide general consulting to Insmed Incorporated. Helen Usansky, Carlos Fernandez, Ariel Teper, Jun Zou, and Kevin C. Mange are employees of and shareholders in Insmed Incorporated. Christopher M. Rubino is an employee of ICPD, Inc. James D. Chalmers has received grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Zambon, and Insmed Incorporated; a grant from Gilead; and personal fees from Novartis and Chiesi.
Availability of data and materials
Individual anonymized participant data will not be made available.
Ethics approval
Both the phase I and WILLOW studies were performed in accordance with the ethical principles of the Declaration of Helsinki, the Good Clinical Practice guidelines of the International Council for Harmonisation, and applicable regulatory requirements.
Consent to participate
All study participants provided written informed consent prior to any study procedures.
Consent for publication
All authors approved and provided consent for publication.
Author contributions
All authors contributed to study conception, data visualization, and the review and editing of the manuscript. JDC conducted research investigations. HU and CMR curated and analyzed data, conducted research investigations, developed methods, administered projects, supplied resources, developed and programmed software, supervised research activities, validated experiments, and wrote the original draft of the manuscript. AT and CF analyzed data, developed methods, and administered projects. JZ analyzed data, developed methods, developed and programmed software, and validated experiments. KCM analyzed data, developed methods, and supervised research activities.
Code availability
Not applicable.
References
- 1.Chalmers JD, Chang AB, Chotirmall SH, Dhar R, McShane PJ. Bronchiectasis. Nat Rev Dis Primers. 2018;4(1):45. doi: 10.1038/s41572-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 2.Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 3.Goeminne P, Dupont L. Non-cystic fibrosis bronchiectasis: diagnosis and management in 21st century. Postgrad Med J. 2010;86(1018):493–501. doi: 10.1136/pgmj.2009.091041. [DOI] [PubMed] [Google Scholar]
- 4.Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, et al. Inflammatory responses, spirometry, and quality of life in subjects with bronchiectasis exacerbations. Respir Care. 2015;60(8):1180–1189. doi: 10.4187/respcare.04004. [DOI] [PubMed] [Google Scholar]
- 5.Hagner M, Frey DL, Guerra M, Dittrich AS, Halls VS, Wege S, et al. New method for rapid and dynamic quantification of elastase activity on sputum neutrophils from patients with cystic fibrosis using flow cytometry. Eur Respir J. 2020;55(4):1902355. doi: 10.1183/13993003.02355-2019. [DOI] [PubMed] [Google Scholar]
- 6.Benarafa C. Regulation of neutrophil serine proteases by intracellular serpins. In: Geiger M, Wahlmèuller F, Furtmèuller M, editors. The serpin family: proteins with multiple functions in health and disease. Cham: Springer; 2015. pp. 59–76. [Google Scholar]
- 7.Dubois AV, Gauthier A, Brea D, Varaigne F, Diot P, Gauthier F, et al. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am J Respir Cell Mol Biol. 2012;47(1):80–86. doi: 10.1165/rcmb.2011-0380OC. [DOI] [PubMed] [Google Scholar]
- 8.Sibila O, Perea L, Canto E, Shoemark A, Cassidy D, Smith AH, et al. Antimicrobial peptides, disease severity and exacerbations in bronchiectasis. Thorax. 2019;74(9):835–842. doi: 10.1136/thoraxjnl-2018-212895. [DOI] [PubMed] [Google Scholar]
- 9.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392(10150):880–890. doi: 10.1016/S0140-6736(18)31767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer R, Maenpaa J, Jauhiainen A, Larsson B, Mo J, Russell M, et al. Dipeptidyl peptidase 1 inhibitor AZD7986 induces a sustained, exposure-dependent reduction in neutrophil elastase activity in healthy subjects. Clin Pharmacol Ther. 2018;104(6):1155–1164. doi: 10.1002/cpt.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalmers JD, Haworth CS, Metersky ML, Loebinger MR, Blasi F, Sibila O, et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med. 2020;383(22):2127–2137. doi: 10.1056/NEJMoa2021713. [DOI] [PubMed] [Google Scholar]
- 12.Usansky H, et al. Safety, tolerability, and pharmacokinetic evaluation of single and multiple doses of the dipeptidyl peptidase 1 (DPP1) inhibitor brensocatib in healthy Japanese and White adults. In: Poster presented at virtual ATS 2021 conference. [DOI] [PMC free article] [PubMed]
- 13.Usansky H, et al. Effects of food intake on the pharmacokinetics, safety, and tolerability of a single dose of the dipeptidyl peptidase 1 (DPP1) inhibitor brensocatib in healthy Japanese and White adults. In: Poster presented at virtual ATS 2021 conference.
- 14.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 15.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 16.Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90(2):154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery DC, Peck EA, Vining GG. Introduction to linear regression analysis. 5. New York: Wiley; 2012. [Google Scholar]
- 18.Dosne AG, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43(6):583–596. doi: 10.1007/s10928-016-9487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dosne AG, Bergstrand M, Karlsson MO. An automated sampling importance resampling procedure for estimating parameter uncertainty. J Pharmacokinet Pharmacodyn. 2017;44(6):509–520. doi: 10.1007/s10928-017-9542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J. 2017;49(6):1700051. doi: 10.1183/13993003.00051-2017. [DOI] [PubMed] [Google Scholar]
- 22.Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186(7):657–665. doi: 10.1164/rccm.201203-0487OC. [DOI] [PubMed] [Google Scholar]
- 23.Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 24.Oriano M, Gramegna A, Terranova L, Sotgiu G, Sulaiman I, Ruggiero L, et al. Sputum neutrophil elastase associates with microbiota and Pseudomonas aeruginosa in bronchiectasis. Eur Respir J. 2020;56(4):2000769. doi: 10.1183/13993003.00769-2020. [DOI] [PubMed] [Google Scholar]
- 25.Chalmers JD, Moffitt KL, Suarez-Cuartin G, Sibila O, Finch S, Furrie E, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med. 2017;195(10):1384–1393. doi: 10.1164/rccm.201605-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoemark A, Cant E, Carreto L, Smith A, Oriano M, Keir HR, et al. A point-of-care neutrophil elastase activity assay identifies bronchiectasis severity, airway infection and risk of exacerbation. Eur Respir J. 2019;53(6):1900303. doi: 10.1183/13993003.00303-2019. [DOI] [PubMed] [Google Scholar]
- 27.Keir HR, Fong CJ, Crichton ML, Barth P, Chevalier E, Brady G, et al. Personalised anti-inflammatory therapy for bronchiectasis and cystic fibrosis: selecting patients for controlled trials of neutrophil elastase inhibition. ERJ Open Res. 2019;5(1):00252-2018. doi: 10.1183/23120541.00252-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual anonymized participant data will not be made available.