Abstract
Tumor Treating Fields (TTFields) is a physical therapy that uses moderate frequency (100–300 kHz) and low-intensity (1–3 V/cm) alternating electric fields to inhibit tumors. Currently, the Food and Drug Administration approves TTFields for treating recurrent or newly diagnosed glioblastoma (GBM) and malignant pleural mesothelioma (MPM). The classical mechanism of TTFields is mitotic inhibition by hindering the formation of tubulin and spindle. In addition, TTFields inhibits cell proliferation, invasion, migration and induces cell death, such as apoptosis, autophagy, pyroptosis, and cell cycle arrest. Meanwhile, it regulates immune function and changes the permeability of the nuclear membrane, cell membrane, and blood-brain barrier. Based on the current researches on TTFields in various tumors, this review comprehensively summarizes the in-vitro effects, changes in pathways and molecules corresponding to relevant parameters of TTFields (frequency, intensity, and duration). In addition, radiotherapy and chemotherapy are common tumor treatments. Thus, we also pay attention to the sequence and dose when TTFields combined with radiotherapy or chemotherapy. TTFields has inhibitory effects in a variety of tumors. The study of TTFields mechanism is conducive to subsequent research. How to combine common tumor therapy such as radiotherapy and chemotherapy to obtain the maximum benefit is also a problem that’s worthy of our attention.
Subject terms: Cancer therapy, CNS cancer, Non-small-cell lung cancer, Autophagy, Apoptosis
Facts
TTFields inhibits the growth of various tumors, such as GBM, lung cancer, malignant pleural mesothelioma, liver cancer, ovarian cancer, and pancreatic cancer.
The inhibition of cell proliferation, migration, and invasion by TTFields depends on frequency, intensity, duration, and direction.
TTFields causes multiple death modes, such as apoptosis, autophagy, immunogenic cell death, and pyroptosis.
TTFields combined with radiotherapy or chemotherapy generally exerts a synergistic effect.
TTFields alone or combined with radiotherapy and chemotherapy affects the Fanconi Anemia-BRCA, cGAS-STING, NF-κB, MAPK, and PI3K/AKT signaling pathways.
Open questions
Could TTFields lead to a new mode of cell death?
Which regimen can cause maximum tumor suppression when TTFields alone or combined with radiotherapy and chemotherapy?
Can bioinformatics analysis such as single-cell sequence reveal more mechanisms for TTFields?
What other signaling pathways can TTFields affect?
Introduction
TTFields is a physical tumor therapy that bases on medium frequency (100-300 kHz) and low-intensity (1–3 V/cm) alternating electric fields. In vitro/vivo experiments and clinical trials have shown that TTFields inhibits the growth of various tumors (such as GBM [1–8], lung cancer, malignant pleural mesothelioma [9–13], liver cancer [14, 15], ovarian cancer [16, 17] and pancreatic cancer [18, 19]), and prolongs survival. Furthermore, combined with radiotherapy [20–25], chemotherapy [22, 26–38], and other treatments, TTFields obtains better therapeutic effects. As a non-invasive physical therapy, TTFields has mild adverse reactions, mostly grade 1–2 cutaneous adverse reactions such as mild to moderate rash under the electrodes [3, 6], erythema, dermatitis, pruritus [2, 4, 9, 10, 14, 15, 19, 39, 40], erosions [24, 41, 42], with no or minimal grade 3 skin adverse events [2, 10, 14]. Reassuringly, these symptoms improve with steroid treatment, electrode replacement, or temporary cessation of TTFields [2, 9, 10, 19].
The classic mechanism of TTFields is to interfere with the mitosis of tumor cells, but it has little effect on normal cells [43]. Moreover, follow-up studies have demonstrated that TTFields induces various functions such as cell death, changes in cell membrane permeability, and immune regulation. Although previous researches have summarized the effects of TTFields on gliomas, GBM, and other tumors, this review focuses on in-vitro studies of various tumors. It comprehensively lists the experimental parameters, making it more convenient and clearer to update the research status of TTFields. In addition, we firstly summarized the comprehensive changes in molecular pathways after TTFields.
The parameters of TTFields: inhibit proliferation, migration, and invasion of tumor cell
The inhibition of proliferation, migration, and invasion by TTFields depends on frequency [41, 44, 45], intensity [1, 20, 23, 28, 30, 31, 45, 46], duration [23, 47–49], direction [41, 50, 51], and cell volume [49].
The commonly used frequency of TTFields on tumor cells is 100-200 kHz. In contrast, a few tumor cells (such as MZ-54, DAOY, and some primary cells) are out of the range [41, 44, 45]. Non-small cell lung cancer, cervical cancer, breast cancer, pancreatic cancer, and osteosarcoma have an optimal frequency of 150 kHz while ovarian cancer, glioma, GBM, or GBM-like stem cells are general at 200 kHz [49]. Malignant pleural mesothelioma is mostly inhibited at 150 or 200 kHz [28, 48]. However, the inappropriate frequency may promote cell growth. The higher frequency could weaken inhibitory effect, but the mechanism has not been studied yet [20, 23, 28, 45, 52]. Meanwhile, tumor cell growth is favored at fragile intensity and non-optimal frequency of TTFields [50]. Giladi et al. [48] report that the optimal inhibitory frequency is related to the doubling time of tumor cells. In addition, the optimal frequency remains consistent in different intensities [46].
The inhibitory effect of TTFields is time-dependent. Generally, 48–72 hours [23, 47–49], and the duration in a part of studies is ≤24 hours [1, 20, 31, 41, 51, 53] or >100 hours [35, 36, 48, 54–56]. The dependence of duration on tumor cell suppression is significantly reduced when the duration exceeds 6 hours/day. Cytostatic effect appears indistinguishable for the same duration, no matter TTFields administered continuously or dividedly [57].
Commonly applied intensity ranges 1–2 V/cm, mostly 1.75 V/cm, with a few studies in relatively low or high intensity (0.6 V/cm or >4 V/cm) [36, 37, 51]. Generally, the inhibition of TTFields is intensity-dependent [1, 20, 23, 28, 30, 31, 45, 46]. TTFields have a certain intensity threshold for tumor inhibition. When the intensity is <0.7 V/cm, no significant reduction in tumor volume is observed [34, 35].
Different TTFields directions have different inhibition effects. Parallel or perpendicular application of TTFields significantly reduces scratching speed, migration distance and direction, and cell polarization. Moreover, compared with the parallel application of TTFields, the vertical one has a more significant effect on the migration velocity [51]. However, some studies show that TTFields functionates when its direction is parallel to the spindle [50]. Increasing TTFields directions enhances inhibitory efficiency [41]. The inhibitory effect of TTFields on cells is also related to cell size [49].
Researches at present mainly focus on tumor cell lines, with a few studies on primary GBM cells [36, 41, 54]. Few studies have focused on drug-resistant strains: cell lines of pancreatic cancer [29, 50], breast cancer [27, 33] and GBM-like stem cells [54], ovarian cancer [27]. Their sensitivity frequencies are consistent with standard tumor cell lines. In addition, a few studies have reported the effect of TTFields on animal cell lines [1, 27, 34, 35, 53, 58]. The TTFields parameters of tumor inhibition are shown in Table 1.
Table 1.
Overview of the frequency, intensity, duration, and effect of TTFields alone on various tumor cells.
| Cancer type | Cell | Species | Frequency (kHz) | Intensity (V/cm) | Time and effect | Reference | Device | Note |
|---|---|---|---|---|---|---|---|---|
| Breast cancer | MCF-7, MCF-7/Mx, MDA-MB-231, MDA-MB-231/Dox | Human | 150 | 1.75 | 72 h: wild-type and ABC transporters-expressing resistant cells proliferation ↓ | [27] | The inovitroTM system | MCF-7/Mx has ABC transporter |
| MCF-7, MDA-MB-231 | 150 | 0.63,1.1,1.75,4 | 24 h or 72 h: proliferation and clonal formation ↓ , Intensity dependent | [1, 31, 36, 48] | The inovitroTM system | Doubling time :29.3 h | ||
| Cervical cancer | HeLa | Human | 150 | 1.75 | Apoptosis ↑ | [38, 48] | The inovitroTM system | Doubling time :24 h |
| Colon cancer | HCT116 | Human | 150 | 1 | 24 h:TP53 dependence, apoptosis↑ | [66] | – | – |
| CT-26 | Mouse | 200 | 1.75 | 24h-72h: apoptosis ↑ | [52] | – | – | |
| Ependymoma | DKFZ-EPN1, BXD-1425EPN | Human | 100, 200 | 1.75 | 72 h: Cell count↓ | [45] | The inovitroTM system | – |
| Glioblastoma | Primary cells | Human | 150-220 | 1–2.2 | 24 h: cell count = , 48 h: cell count ↓ . Intensity dependency, TEFT-random ≥ TEFT-fixed | [41] | TEFTS, CL-301A | – |
| GaMG, U-343 MG, U-138 MG, KNS42, GIN-31, LN-229, LN-18 | 200 | 0.6, 1.7,1.75 | 24 h: Invasion↓ or 72 h: proliferation↓ | [1, 45, 49, 51] | The inovitroTM system | – | ||
| MZ-54 | 250 | 1.48 | 72 h: cell count↓ | [44] | The inovitroTM system | – | ||
| Primary cells GBM2, GBM39 | 200 | 4 | 100 h: GBM39 proliferation ↓ , 150-200 h: GBM2 proliferation↓ | [36] | The inovitroTM system | – | ||
| U251 | 200 | 1.48 | – | [44] | The inovitroTM system | – | ||
| U-87 MG, U-118 MG, A-172 | 200 | 0.6,1.7,1.75 | 24 h: migration and invasion↓ or 72 h: proliferation and clonal formation↓ | [48, 49, 51, 58] | The inovitroTM system | Doubling time :34 h | ||
| U87-MG, U-373 MG, 528NS, 83NS | 150 | 0.9 | 48 h or 72 h: proliferation, clonal formation, migration, invasion and EMT-associated protein expression ↓ , apoptosis ↑ | [47, 55, 56] | Self-made | – | ||
| U87-MG | 200 | 4 | 24 h: apoptosis = , 240 h: proliferation↓ | [36, 63] | The inovitroTM system | – | ||
| patient-derived GBM stem-like cells (GSCs): TMZ resistant/sensitive | 200 | 1 | – | [54] | The inovitroTM system | – | ||
| Glioma | U-118, U-87, LN-18, LN-229, T-325, ZH-161 | Human | 100 | 1.1,2 | 24 h: proliferation, invasion and migration↓ | [1, 20] | – | – |
| 48–72 h: caspase-independence apoptosis ↑ | ||||||||
| U373 | 150 | 1.2 | <24 hours, with time goes by, tumor cell apoptosis↑ but not in normal cell | [57] | – | – | ||
| F98 | Rat | 200 | 1.1, 1.7, 1.75 | 24 h: Cell count↓ or 72 h: proliferation and clonal formation↓ | [1, 57] | The inovitroTM system | – | |
| Liver cancer | HEPG2, Huh7 | Human | 150 | 1.75 | 24h-72h: Apoptosis ↑ | [38, 52] | – | – |
| MPM | MSTO-211H, NCI-H2052 | Human | 150, 200 | 1–1.5,1.75 | 72 h: Proliferation and clonal formation ↓ . apoptosis ↑ | [28, 48, 58] | The inovitroTM system | Doubling time :18.9 h |
| Medulloblastoma | DAOY, UW228-3 | Human | 300, 100 | 1.75 | 72 h: Cell count↓ | [45] | The inovitroTM system | – |
| Melanoma | B16F10 | Mouse | 100 | 1.1 v/cm or peak Voltage:30 v | 24 h: Cell count ↓ . Peak voltage-dependent manner | [1, 53] | Self-made or the inovitroTM system | – |
| Lung cancer | H157, H4006, A549, NCI-H1299, H1650, HTB-182, HCC827 (NSCLC) | Human | 100, 150, 150/200, 100, 100 | 1.75 | 72 h: Proliferation and clonal formation↓ | [25, 34, 48] | The inovitroTM system | Doubling time :23.8 h |
| LLC1, KLN205 | Mouse | 150 | 1.75 | 72 h: cell count↓ | [34, 58] | The inovitroTM system | – | |
| H520(Squamous cell lung cancer) | Human | 150 | 1.75 | 24h-72h: apoptosis ↑ | [52] | – | – | |
| Osteosarcoma | U2OS, KHOS/NP | Human | 150 | 1.5 | 48 h: cell count, migration and invasion↓ | [81] | – | – |
| Ovarian cancer | A2780, OVCAR3, CAOV-3 | Human | 200 | 1.7,1.75, 4.6 | 72 h: proliferation ↓ | [37, 46, 48] | The inovitroTM system | Doubling Time :18.7 h |
| MOSE-L | Mouse | 200 | 1.75 | 24–72h: apoptosis ↑ | [52] | – | – | |
| EmtR1 | Hamster | 150 | 1.75 | 72 h: wild-type and ABC transporters-expressing resistant cells ↓ | [27] | The inovitroTM system | EmtR1 cells ATP dependent MDR1 type drug resistance | |
| Pancreatic cancer | CFPAC-1, HPAF-11, AsPC-1(Human), Pc-1.0 (hamster) | Human, hamster | 150 | 1.75, 1.2, 2.9 ± 0.2 | 48 h or 72 h: proliferation and clonal formation ↓ | [23, 35, 48, 58] | The inovitroTM system or Self-made | Doubling Time :54 h |
| BxPC–3, BxPC-3 cells BxGem cell, AsPC-1, non-malignant human hTERT-HPNE immortalized pancreatic duct cell line CRL-4032 | Human | – | 96 h: BxPC-3, BxGem, AsPC-1 cell proliferation ↓ , CRL-4032:no effect. | [50] | Self-made | 150 kHz is the optimal frequency of BxPC-3 or BxGem AsPC-1, inhibiting cell proliferation and having no effect on CRL-4032 | ||
| 144 h: apoptosis and necrosis= |
↑ up-regulate, ↓ down-regulate, = unchanged.
ABC transporters ATP-binding cassette transporters, EMT epithelial-mesenchymal transition, TMZ temozolomide, GBM glioblastoma, MDA-MB-231/Dox cells doxorubicin resistant MDA-MB-231 cells, EmtR1 cells AA8 cells- Emetine-resistant sub-lines, MCF-7/Mx MCF-7 cells Mitoxantrone-resistant sub-lines, BxGem cell gemcitabine-resistant BxPC-3 cells.
The different effects of TTFields on tumor cells and normal cells
The most classical mechanism of different effects of TTFields on tumor cells and normal cells bases on the difference in the biological behavior of two cells. Characterized by maintaining proliferative signals, evading growth inhibition, tumor cells have shorter doubling time and more vigorous mitosis than normal cells [59]. Meanwhile, inhibition by TTFields negatively correlates with doubling time of cells [48]. The different effects of TTFields on normal cells and tumor cells reflected in the following four aspects:
Cell proliferation and death. TTFields inhibits the proliferation of neural stem cells but not astrocytes [45]. Similarly, TTFields significantly suppresses tumor proliferation and induces apoptosis when applied to the skin or abdomen [50, 60] while the normal cells are unaffected [38, 50, 60, 61]. However, normal cells HaCaT proliferated slightly after TTFields [62].
DNA damage repair and cell cycle arrest. TTFields inhibits tumor cells and causes DNA damage [62] but does not cause DNA double-strand breaks and cell cycle arrest in normal cells [57, 61]. However, studies show that TTFields leads to G2/M arrest in IEC6 normal cells and tumor cells, but the increased degree varies with the duration of TTFields (0–24 hours/day) [57].
Duration. Within 12 hours treatment of TTFields, there is no significant change in IEC6 normal cells. Apoptosis cells slightly rise at 24 hours, but are far less than that of tumor cells [57]. When TTFields treats for 3–12 hours/day, the inhibition on normal cells and tumor cells is quite different. However, when the duration is longer than 24 hours/day, the degree of differential inhibition decreases [45].
Cell membrane permeability. TTFields increases the number and diameter of membrane pores in tumor cells but does not affect normal cell membranes [63].
Apoptosis
TTFields alone or combined with hyperthermia or drugs such as Paclitaxel, Sorafenib, and MPS1-IN-3 (spindle assembly checkpoint inhibitor) increase apoptosis in glioma or GBM [26, 32, 45, 49, 55, 57, 64, 65]. In general, the portion of apoptosis cells varies among cell lines, is positively related to the intensity [20]. However, some studies indicate that TTFields does not increase apoptosis at higher field intensity and optimal inhibition frequency [20, 63]. Inhibition of autophagy leads to increased apoptosis and cell death [58].
TTFields alone or in combination with drugs (5-Fluorouracil, Paclitaxel) on various tumors such as ovarian cancer [37, 48], colon cancer [30, 52, 66], melanoma [53, 61], MPM [28], and breast cancer [33] also induces apoptosis. However, TTFields combined with the drugs does not necessarily and synergistically increase apoptosis. Thymidine attenuates TTFields-induced apoptosis in glioma cells [62]. TTFields restraints Osimertinib-induced apoptosis in lung adenocarcinoma cells and reduces the efficacy of Osimertinib [29].
Autophagy
Aberrant mitosis, aneuploidy, and increased cellular granularity often induce prominent autophagy [67]. Time-lapse microscopy monitoring of mitotic index, mitotic duration, and intracellular autophagosome formation during TTFields demonstrates that TTFields induces autophagy due to abnormal mitosis and endoplasmic reticulum stress [58]. TTFields induces autophagy in gliomas or GBM [21, 55], which usually manifest as elevated autophagosomes and autophagic flux, mitochondrial matrix swelling or endoplasmic reticulum expansion, increasing expression of LC3, Atg5, Beclin1, and other autophagy-related genes [20, 26, 55]. Kim et al. demonstrate that TTFields induces autophagy in GBM via the AKT2/miR-29b axis [55]. However, autophagy may be a protective mechanism for tumor cells against TTFields [58]. Knockdown of AMPK or ATG7 inhibits TTFields-induced autophagy and results in cell death, suggesting that TTFields-induced autophagy depends on AMPK activation [58]. Colon cancer treated with TTFields alone or in combination with 5-Fluorouracil, or pancreatic cancer treated with TTFields combined with hyperthermia induces autophagy obviously [30, 60].
Cell cycle arrest
By bioinformatics analysis, TTFields affects mitosis-related processes such as DNA replication and cell cycle [55, 60]. TTFields functionates in the anaphase of mitosis. TTFields prevents cell division by producing heterogeneous intensity at the cleavage furrow of dividing cells, resulting in apocyte [66]. Giladi et al. [35] demonstrate that cell proliferation is inhibited with prolonged exposure to TTFields, and the cells become significantly larger [41]. The rate of metaphase plate formation maintains whether or not TTFields is applied. Meanwhile, with the sustention of mitosis, DNA content heightens after TTFields exposure, which demonstrates that TTFields acts in the anaphase of mitosis [66].
The effect of TTFields depends on the cell cycle. The application of TTFields in the G1 phase does not affect the portion of G1 phase; similarly, the same as in the M phase [66]. For the G1/S phase-blockade cell, TTFields could not induce cell death, apoptosis, and DNA damage [62], indicating that entering into the G1/S phase is necessary for TTFields to inhibit tumors.
The effect of TTFields results in different cell cycle arrest. G2/M arrest often occurs in glioma cells [26, 55, 56]. The changes in the G2/M phase may be related to the duration and frequency of TTFields. Jo et al. [57] explodes the time gradient of TTFields (0, 3, 6, 12, 24 hours/day). With the prolongation of duration, normal cells with G2/M arrest slightly increase, while the increase of glioma cells is significant. However, there is a contradiction in other studies. No difference in the G2 phase is found when TTFields treats for 5 days, while some studies report that the G2 phase increases when TTFields treats at the optimal frequency for 72 hours [45]. G1 and S phases show different trends in various studies [20, 26, 45, 65]. TTFields has no apparent cycle-blocking regularity in other tumors [25, 28, 35, 37, 48].
TTFields leads to apoptosis, the formation of specific-size DNA fragments, which is reflected in the appearance of Sub G1 peak in cell cycle. However, the timing of Sub G1 peak appears inconsistent among cell lines [20, 21, 25]. Lee et al. [68] detect the changes of cycle-related genes after TTFields treatment in cells with different TP53 statuses, which provided research data to elucidate the mechanism.
However, some studies indicate TTFields induces necrosis, immunogenic death, and necroptosis. TTFields does not increase apoptosis at higher intensity and optimal inhibition frequency [20, 63] but induces autophagy and necrosis [20]. Pancreatic cancer cells treated with TTFields for 144 hours show no apoptosis and necrosis but increased apoptosis after TTFields and radiotherapy [50]. TTFields induces ATP release by inducing autophagy, leading to immunogenic death [52]. In addition, the necroptosis induced by TTFields also leads to cell death [20].
In vitro and in vivo studies have shown that TTFields increase cell death through P53-dependent [57], reactive oxygen species elevation [30], caspase-dependent/independent pathways, and O6 -methylguanine-DNA methyltransferase (MGMT)-independent pathways [20, 41, 42].
Permeability (nuclear membrane, cell membrane, blood–brain barrier permeability, anti-angiogenesis) and drug infiltration
TTFields causes local rupture and perforation of the nuclear envelope, which are associated with the cell cycle. Entering into the S phase is required for TTFields to induce nuclear envelope disruption and micronucleus formation [69, 70]. Meanwhile, nuclear membrane disruption, micronuclei formation, and fragmented DNA release after TTFields activate Caspase1 to cleavage GSDMD, which induces pyroptosis and membrane disruption [69, 70].
TTFields enhances cell membrane permeability limitedly, and it is difficult for larger molecular weight substances to penetrate the cell membrane. TTFields causes significant morphological changes in the cytoplasm and membrane, including disruption of plasma membrane integrity and marked vacuolization, with increased membrane permeability [20, 55]. Previous studies have shown that exposure to TTFields at 4 V/cm and 200 kHz for 6-24 hours increases the membrane pores in GBM cells, and the membrane pore area approximately is doubled (240.6 ± 91.7 nm2 vs. 129.8 ± 31.9 nm2). TTFields only increases the absorption of relatively small molecular weight species such as 4–20 kDa dextran-FITC, 5-aminolevulinic acid, and ethidium D. However, no absorption is observed in relatively larger molecular weight species (≥50 kDa) [63].
The effect of TTFields on membrane permeability is reversible. Twenty-four hours after the termination of TTFields, the number and diameter of membrane pores decrease, and no accumulation of 7-Aminoactinomycin D in cells is observed, indicating that the integrity of cell membrane is repaired [71]. Gera et al. [66] demonstrate that TTFileds resulting in membrane rupture and vacuolization closely related to the timing of cell division, which usually occurs after the formation of mitotic plate.
The frequency of TTFields inducing the permeability of cell membrane or blood-brain barrier was not consistent with the optimal inhibition frequency. Different frequencies (50-500 kHz) of TTFields showed increased intracellular accumulation of 7-Aminoactinomycin D among various tumor cell lines. In all, 100 kHz TTFields changes the permeability of the blood–brain barrier in rats and increases Paclitaxel concentration in GBM. However, in current studies and clinical applications, the optimal frequency for treating GBM is 200 kHz [41, 42, 72].
Although TTFields increases permeability, few researches study the mechanism. TTFields induces the ion channel opening, such as Cav1.2, through cellular depolarization [65, 73]. However, the TTFields frequency of membrane pore opening is inconsistent with the opening of the ion channel. Meanwhile, the pore size that TTFields causes is different from the ion channel opening [63, 74]. Therefore, the opening of the ion channel appears to be secondary. Based on bio-electrorheological models, TTFields-induced changes in membrane shear stress, or electroporation-based models, TTFields-induced changes in the cell membrane and cytoskeleton may further clarify the mechanism of permeability changes [73].
TTFields also opens the blood–brain barrier reversibly, but the relationship with frequency is unclear. The blood-brain barrier is a vital structure to maintain the stability of the internal environment of brain. Chemotherapy for brain tumors usually lacks of effectiveness because most chemotherapeutic drugs are difficult to penetrate the blood-brain barrier [75]. In vivo experiments show that Evans Blue, TRITC-dextran, and magnetic resonance contrast agent Gd-DTPA increase in the brain [75–78]. Meanwhile, in vitro experiments showed that Claudin-5 and Occludin translocation in capillary endothelial cells [76, 79] points out that TTFields increases the permeability of the blood-brain barrier. The current studies have shown that 100 kHz is the best frequency for opening the blood–brain barrier in the rat [75–80]. Permeability is generally most pronounced 24 hours after TTFields exposure [80]. The blood-brain barrier recovery starts 48 hours after termination of TTFields and is fully recovered at 96 hours [75–80].
TTFields exerts antiangiogenic effects and enhances drug penetration. TTFields attenuates tube formation [26] and inhibits angiogenesis by down-regulating the expression of HIF1α, VEGF [47], and MMP2 [81]. In subcutaneous mouse model of melanoma, TTFields reduces the expression of CD34 and VEGF, possibly normalizing vascular and increasing blood flow in solid tumors [53]. Moreover, Kim et al. demonstrate that TTFields facilitates Trastuzumab penetrate to tumors [33].
Immune modulation
Effects of TTFields on immune cells in vitro. Similar to the pernicious effect of tumor cells, TTFields inhibits the proliferation of RAW264.7 and T cells. However, they also maintain functional activation status (morphological changes, molecular changes such as CD107a, PD-1, and secreted factors such as reactive oxygen species, NO, IL-1β, TNF-α, IFNγ) [82, 83].
Immune activation of TTFields. TTFields not only affects DNA but also alters mitochondrial and endoplasmic reticulum functions, including electron transport, metabolism, ion signaling, and protein folding [68]. After that, TTFields rises to a new mode of cell death. Voloshin et al. [52] demonstrate that TTFields induces immunogenic death of tumor cells (increasing expression of HMGB1, release of ATP, CRT). Hereafter, the product of immunogenic death activate dendritic cells (increased phagocytic index of bone marrow-derived dendritic cells, expression of co-stimulatory molecules such as MHCII, CD40, and CD80) and induce CD45 + leukocyte enrichment.
TTFields increases immune cell infiltration. Although no change is found in peripheral blood WBC, increased CD8 T cells are observed after TTFields treats for 14 days [41]. In the VX2 tumor model, TTFields inhibits the lung metastasis of melanoma. Meanwhile, tumor parenchyma and surrounding tissue are infiltrated with immune cells such as monocytes, CD4, CD8, and CD45 + T cells. Furthermore, among TILs, CD4 T cells are more prevalent than CD8 T cells [84]. Chen et al.’s [69] single-cell sequence results of GBM consistently demonstrate the immune modulation role of TTFields. TTFields increases total and activated DCs (CD80/CD86 + ), early (CD69+) or effector (CD44+/CD62L−) CD4+CD8+.
Possible targets of TTFields modulating immunity. TP53 may be a dependent target of TTFields regulating immune. Among TTFields-induced genes involved in immune and inflammatory responses, TP53-dependent/independent regulated genes were identified by bioinformatics analysis [68]. In addition, RhoA is a crucial factor in regulating leukocyte differentiation and function. Voloshin et al. [51] demonstrate that TTFields markedly and transiently activates RhoA signaling by regulating GEF-H1, resulting in cytoskeletal actin reorganization and focal adhesion formation in lung adenocarcinoma. However, no change in T cells and dendritic cells is observed.
TTFields leads to abnormal micronuclear clusters in GBM, lung adenocarcinoma, and pancreatic cancer cells, which recruits cGAS and AIM2 [69]. Finally, TTFields increases proinflammatory cytokines and type I interferon via the cGAS-STING pathway or the AIM2/caspase1 inflammasome release, resulting in activation of adaptive immunity [70]. Single-cell sequence results indicate that a higher proportion of pDC and T1IRG-expressing monocyte, XCL1/2 + KLRC1 + NK cells, are found in peripheral blood mononuclear cell after TTFields. TTFields promotes T cell activation, memory T cell formation, and peripheral T cell clonal expansion [69].
TTFields usually up-regulates immune checkpoints. Single-cell sequence show that the expression of PD-L1, CTLA-4, and TIGIT increase after TTFields, which provided a theoretical basis for immunotherapy [69]. In addition, TTFields significantly increases CLEC9, IRF8, SMPD3 in cDCs and pDCs, CD8A, IFNG, GZMB, PRF1, CXCR1, CCL4 in TILs [69]. Furthermore, the animal experiment has proven that TTFields combined with anti-PD-1 therapy effectively suppress tumors. However, the molecular mechanism remains unknown [52].
TTFields combined with radiotherapy
Radiation therapy (RT) causes DNA damage, leading to cell death through apoptosis, mitosis, autophagy, or growth arrest [85]. Regardless of the sequence in which ionizing radiation(IR) and TTFields is applied (TTFields [22, 23] or IR [20, 21, 24, 25] first), most studies show a combined effect. When combined with TTFields, relatively large dose like 4 Gy, 2 Gy are more effective than 2 Gy, 1 Gy [22]. Furthermore, proton therapy is more striking than X-ray [21]. NSCLC cells are more susceptible to radiation when they are exposed to TTFields before IR treatment [22]. The administration of TTFields after 1 h of RT is more pronounced than that after 4 h and 24 h of RT [24]. There are few studies on the combined application of IR and TTFields, mainly focusing on DNA damage and repair [25, 28, 56, 86, 87]. The parameters of TTFields combined with RT to inhibit tumors are shown in Table 2.
Table 2.
Overview of the frequency, intensity, duration, radiation dose, and dose rate, sequence, and the effect of TTFields combined radiation on various tumor cells.
| Cancer type | Cell | Dose and dose rate | TTFields parameters | Sequence | Time and effect | Ref. |
|---|---|---|---|---|---|---|
| Glioma | U-118 MG, LN-18 | 0–8 Gy (0.25 Gy/min) | 200 kHz,1.75 V/cm | RT then TTFields (RT 1 h, 4 h, 24 h then TTFields 72 h) | Radiation sensitization, cell proliferation ↓ | [24] |
| U-118 MG: γh2AX↑, DNA damage repair↓ | ||||||
| F98, U373 | 0-5 Gy X-ray or proton beam (3.45 Gy/min) | 150 kHz,0.9 V/cm | RT then TTFields (RT 48 h then TTFields 24 h,48 h) | Radiation sensitization: proton beam> X-rays. Proton beams+TTFields: apoptosis, autophagy↑, migration↓ | [21] | |
| LN-18, LN-229, T-325, ZH-161 | 3 Gy, 5 Gy | 2 V/cm | RT then TTFields | LN-18, T-325: radiation sensitization | [20] | |
| NSCLC | H157, H4006, A549, H1299, H1650 | 2 Gy, 4 Gy | 100–200 kHz | RT then TTFields (RT then TTFields 24 h, 48 h, 72 h) | Radiation sensitization. DNA damage repair↓ | [25] |
| 2 Gy+TTFields 24-72 h:CI:0.58–2.08, among which 53%>1 | ||||||
| 4 Gy+TTFields 24-72 h:CI:0.9–3.97, among which 86%>1. | ||||||
| H157, H4006, A549, H1299 | 2 Gy,4 Gy (3.47 Gy/min) | H157 (100 kHz), H4006(150 kHz),A549(200 kHz), H1299(100 kHz) | TTFields then RT (TTFields 48–72 h then RT) | Radiation sensitization: CI > 1.CI when TTFields first is relatively large | [22] | |
| Pancreatic cancer | CFPAC-I, HPAF-II | 5 Gy | 150 kHz,0.9 V/cm | TTFields then RT | Radiation sensitization. Clonal formation↓ | [23] |
| Apoptosis and PARP expression↑ |
↑ up-regulate, ↓ down-regulate, = unchanged
CI combination index, RT radiotherapy, PARP poly (ADP-ribose) polymerase, VS versus.
TTFields combined with drugs
Glioma or Glioblastoma. TTFields combined with drugs such as Paclitaxel, Mebendazole [45], Dacarbazine [31], MPS1-IN-3 [49], and Sorafenib [26, 32] significantly increase the sensitivity. MGMT status is often an indication of Temozolomide usage. Experiments on primary cells with different MGMT statuses have shown that Temozolomide and TTFields only have an additive effect (but another cell line with a sensitization phenomenon [20]). However, MGMT status does not affect TTFields efficacy [54]. Additionally, TTFields is synergistic with drugs only within a specific frequency range [36]. Dexamethasone is the most common corticosteroid used to treat edema in GBM patients. Linder et al. demonstrate that Dexamethasone limits radiotherapy efficacy but makes no difference in TTFields-induced GBM cell death. Furthermore, a retrospective analysis shows that Dexamethasone makes no impact in progression-free survival and overall survival when combined with TTFields therapy [44].
Breast cancer. TTFields combined with Doxorubicin, Paclitaxel, or Cyclophosphamide have synergistic effects, manifested as a decrease in half maximal inhibitory concentration (IC50) and dose reduction index (DRI), inhibition of cell proliferation and colony formation, and increased apoptosis [27, 31, 33, 36]. For drug-resistant tumor cells, TTFields combined with drug therapy improves drug resistance [33] and has similar effects on drug-resistant or drug-sensitive cells [27]. Continue propagation after 24 hours treatment of TTFields combined with drug shows that monotherapy group proliferated rapidly, suggesting that combination treatment may have a long-term effect [31].
Lung cancer. TTFields combined with chemotherapeutic drugs such as Cisplatin, Paclitaxel, or Pemetrexed, significantly inhibits proliferation and colony formation [34]. The function of epidermal growth factor receptor (EGRF) inhibitors in combination with TTFields is controversial. Giladi et al. [34] report that Erlotinib combined with TTFields inhibits tumor proliferation, but Li et al. [29] prove that TTFields attenuates the tumor-suppressive effect of Osimertinib. Karanam et al. [22] report that TTFields combined with Olaparib and IR is more inhibitory than the two-factor combination therapy.
Other tumors. MPM [28], abdominal tumors such as colon cancer [30], pancreatic cancer [35], liver cancer [38], ovarian cancer [37], and cervical cancer [38], have reported that TTFields improves the drug’s efficacy. In addition, the function of TTFields combined with hyperthermia is controversial [36, 60, 64]. The parameters of tumor inhibition by TTFields combined with drugs are shown in Table 3.
Table 3.
Summary of the frequency, intensity, duration, drug concentration, and the effect of TTFields combined drugs on various tumor cells.
| Cancer type | Cell | Drug | Concentration | TTFields parameters | Time and effect | Ref. |
|---|---|---|---|---|---|---|
| Ovarian cancer | A2780, OVCAR3, CAOV-3 | Paclitaxel | 0–100 nM | 200 kHz,2.7 V/cm | 72 h CI A2780:1.03, OVCAR3:0.81, CAOV-3:0.86. | [37] |
| MPM | MSTO-211H, NCI-H2052 | Cisplatin | 1–10,000 nM | 150 kHz, 1 V/cm | Pemetrexed+TTFields vs. Cisplatin+TTFields: additive vs. synergistical effect. | [28] |
| Pemetrexed | 1–100 nM | Cisplatin+TTFields: Apoptosis↑ | ||||
| Triple therapy (two drugs+TTFields): proliferation and clonal formation↓ | ||||||
| Lung cancer | Gefitinib-resistant PC-9GR, H1975 cells | Osimertinib | 0.5 μM | 1 V/cm | Proliferation↑, cell death, apoptosis↓ | [29] |
| TTFields attenuates the inhibitory effect of Osimertinib | ||||||
| HCC827 | Erlotinib | 0–20 nM | 150 kHz,1.75 V/cm | 72 h: Proliferation and clonal formation↓ | [34] | |
| H1299, LLC1, HTB-182, KLN205 | Pemetrexed | 0–0.1 nM | 150 kHz,1.75 V/cm | 72 h: Proliferation and clonal formation↓ | [34] | |
| Paclitaxel | 0–100 nM | |||||
| Cisplatin | 0–10 nM | |||||
| H157, H4006, A549, H1299 | Cisplatin | Cisplatin PIC25 (μM) : H157 1, H4006 0.75, A549 2, H1299 = 2. | 100-200 kHz | Olaparib+IR + TTFields:CI>1. | [22] | |
| Olaparib | Olaparib 0–40 μM | Olaparib+IR, Olaparib+TTFields:CI ≈ 1. | ||||
| Liver cancer | Huh7 | Doxorubicin | 0–10 µM | 120 kHz, 1 Vpp | 72 h: therapeutic effect↑ | [38] |
| Glioma | LN-18, LN-229, T-325, ZH-161 | TMZ | 5 μM-200 μM | 2 V/cm | 24 h: LN-229, ZH-161: sensitization | [20] |
| U118 | Dacarbazine | 0-100 mM | 150 kHz, 1.75 V/cm | 72 h: IC50: Dacarbazine 6.4 mM→0.023 mM, Paclitaxel 5 nM→0.005 nM, Doxorubicin 0.04 μM → 0.002 μM, Cyclophosphamide 6.6 mM→0.044 mM. | [31] | |
| DRI: Dacarbazine 175, Paclitaxel 316, Doxorubicin 23, Cyclophosphamide 152 | ||||||
| U373 | Thymidine | 2 mM | 200 kHz | For G1/S blocked cells, proliferation, clonal formation, DNA damage and apoptosis= | [62] | |
| U373, U87 | Sorafenib | 5 μM | 150 kHz, 0.9 V/cm | 48 h: proliferation ↓ , cell death ↑ . | [26, 32] | |
| STAT3 expression ↓ . Knocking down STAT3: TTFields effect↑ | ||||||
| 24 h: S phase, migration, invasion and angiogenesis↓ | ||||||
| 48 h: clonal formation ↓ , apoptosis, autophagy, ROS ↑ | ||||||
| U-87 MG, U-138 MG, U-343 MG | MPS1-IN-3 | 4 µM | 200 kHz, 1.7 V/cm | U-87 MG, GaMG: 72 h:proliferation ↓ . | [49] | |
| U-87 MG, 72 h: abnormal nuclei, G2/M, apoptosis ↑ . | ||||||
| MPS1-IN-3 prolongs TTFields effect | ||||||
| U87-MG, KNS42, SF188 | Paclitaxel | High vs Low concentration | 130 kHz, 10 V, 450 μs | Synergistically effect. | [45] | |
| Mebendazole | SubG0↑ | |||||
| GBM | KR158-luc | TMZ | 300 µM | 200 kHz | 72 h: adaptive immune= | [69] |
| Patient-derived glioblastoma stem cell-like cells (GSCs) | TMZ | 1.5 μM-160 μM | 200 kHz, 1 V/cm | 8 days: same inhibitory efficacy in two cell types (MGMT expression + /-). | [54] | |
| U251-MG, MZ-54 | Dexamethasone | 65 μM | 1.48–1.41 V/cm | 48 h: sensitization. | [44] | |
| Dexamethasone: decrease IR induced-effect but does not affect TTFields induced-effect | ||||||
| Dexamethasone+TTFields: PFS and OS = | ||||||
| U87-MG, GBM2, GBM39 | Withaferin A | 0–0.1uM | 200 kHz, 4 V/cm 2.5 V/cm | 50 kHz vs. 200 kHz, 500 kHz: no sensitization vs. sensitization. Intensity dependent | [36] | |
| Colonic cancer | HCT116 | 5-FU | 5 µmol/L | 0.9–1.2 V/cm | 48 h: sensitization. | [30] |
| Proliferation, clonal formation, migration, invasion↓ | ||||||
| Autophagy, apoptosis, organoid cell death↑ | ||||||
| Cervical cancer | HeLa | Doxorubicin | 0–10 µM | 120 kHz, 1 Vpp | 72 h: therapeutic effect↑ | [38] |
| Breast cancer | EmtR1 cells, MCF-7/Mx, MDA-MB-231/Dox cells | Doxorubicin | 0.04–0.6 μM, | 150 kHz, 1.75 V/cm | TTFields+chemotherapy 72 h: Same efficacy in WT cell and drug-resistant cell. DRI: Doxorubicin 105-250 vs. Paclitaxel 815- >10,000 | [27] |
| Paclitaxel | 5 nM–0.1 μM | |||||
| JIMT-1,BT-474 | Trastuzumab | 5 μM | – | 72 h: Synergistical effect, clonal formation ↓ , apoptosis↑ | [33] | |
| MDA-MB-231 | Doxorubicin | IC50 = 0.31 μM | 150 kHz, 4 V/cm | Synergistical effect | [36] | |
| MDA-MB-231 | Doxorubicin, Paclitaxel, Cyclophosphamide | 0–10 μM, 0–1000 nM, 0–100 mM | 150 kHz, 1.75 V/cm | 72 h IC50: Doxorubicin 0.04 μM → 0.002 μM, Paclitaxel 5.00 nM→0.005 nM, Cyclophosphamide 6.60 mM→0.044 mM. | [31] | |
| 24 h treatment then quit for 48 h: Control and the chemotherapy group vs. Combined group: cell proliferation recovered rapidly vs. did not recover. | ||||||
| Pancreatic cancer | PC-1.0 (hamster), AsPC-1 | Gemcitabine | – | 150 kHz, 2.9 ± 0.2 V/cm | Therapeutic effect↑ | [35] |
| Irinotecan | ||||||
| 5-FU | ||||||
| Paclitaxel | ||||||
| Liver cancer | Huh7 | Doxorubicin | 0–10 µM | 120 kHz, 1 Vpp | 72 h: therapeutic effect↑ | [38] |
↑ up-regulate,↓ down-regulate, = unchanged.
5-FU 5-Fluorouracil, WT wide type, DRI dose reduction index, IC50 half maximal inhibitory concentration, CI combination index, TMZ Temozolomide, STAT3 signal transducer and activator of transcription 3, MGMT O6 -methylguanine-DNA methyltransferase, OS overall survival, PFS progression-free survival, MDA-MB-231/Dox cells doxorubicin resistant MDA-MB-231 cells, EmtR1 cells AA8 cells- Emetine-resistant sub-lines, MCF-7/Mx MCF-7 cells Mitoxantrone-resistant sub-lines, Vs versus.
Molecular mechanism
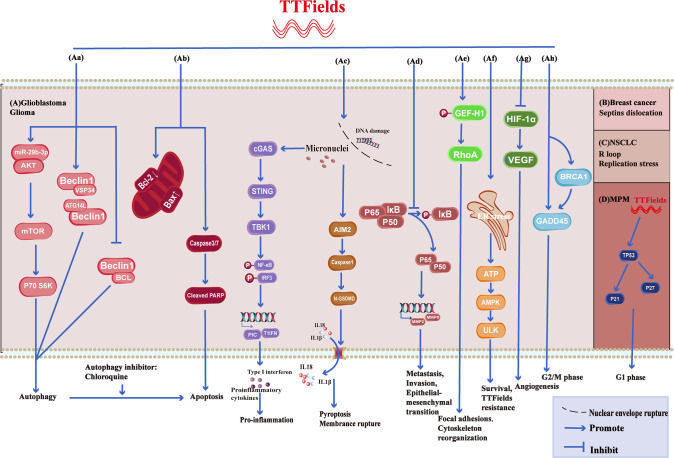
TTFields leads to abnormal Septins distribution and tubulin assembly blockage (Fig. 1B). Septins are integral components of the cytoskeleton, assembling into higher-order oligomers and filamentous polymers associated with actin filaments, microtubules, and cell membranes. Thus, abnormally expressed Septins may destabilize genomes [88]. Gera et al. [66] find that after TTFields treatment, the localization of Septins in the midline of the anaphase spindle and cell kinetic division grooves is significantly reduced and disorganized, resulting in abnormal progeny cells. Meanwhile, the mitotic spindle is abnormal in metaphase and telophase, resulting in the formation of abnormal cells, such as apocyte and abnormal chromosome, and the number of cells in interphase and telophase is reduced [35, 48, 56]. In addition, Voloshin, T et al. [51] demonstrate that despite blocking the assembly of tubulin proteins, TTFileds affects the directionality and cell polarity of tubulin. The small GTPase RhoA regulates stress fiber assembly and focal adhesion formation [89–91]. Disruption of tubulin after TTFields activates RhoA signaling by modulating GEF-H1 phosphorylation, leading to cytoskeletal actin reorganization and formation of focal adhesions (Fig. 1A, Ae) [48, 51].
Fig. 1. Molecular pathway changes caused by TTFields on glioma, GBM, MPM, NSCLC, and breast cancer Fig. 1A, Gliomas and glioblastomas.
A Aa After TTFields treatment, Beclin1 increases the binding of Atg14L and Vps34(the positively regulated autophagosome) and decreases Bcl-2(the negatively regulated autophagosome), leading to glioma cells and tumor stem cell autophagy. Meanwhile, activation of the AKT2/mTOR/p70S6K axis also leads to autophagy. A Ab TTFields up-regulates caspase3, caspase7 or increases BAX, down-regulates BCL-2 expression, and leads to apoptosis. A Ac TTFields destroys the nuclear membrane, generates micronuclei and double strand breaks, activate the cGAS-Sting signaling pathway to increase the expression of proinflammatory factors and type I interferon, and through the AIM2-Caspase1 inflammasome Cleavage of GSDMD and release of LDH leads to pyroptosis and immune activation ultimately. A Ad TTFields inhibits IκBα phosphorylation and NF-κB p65 translocation, the expression of MMP2 and MMP9, and ultimately inhibits cell invasion, metastasis, and EMT processes. A Ae TTFields promotes phosphorylation of GEF-H1, which further activates RhoA, ultimately leading to focal adhesions and cytoskeleton reorganization. A Af TTFields causes Endoplasmic Reticulum stress and releases ATP, which activates AMPK and ULK, leading to resistance to TTFields. A Ag TTFields attenuates tube formation and angiogenesis by down-regulating the expression of HIF1α and VEGF. A Ah Upregulation of BRCA1 and GADD45 results in G2/M phase arrest. B Breast cancer. Septins are abnormally distributed. C Non-small cell lung cancer. TTFields lead to R loop formation and replication stress. D MPM. Elevated TP53, P21, and P27 lead to G1 phase blockade.
Based on bioinformatics analysis, numerous gene expression and pathway changes are found, which is conducive to the in-depth study of TTFields. The PI3K-AKT, MAPK, DNA replication, cell cycle, and other pathways have been confirmed.
TTFields slows down replication forks and caused replication stress (Fig. 1C). After 72 hours of TTFields treatment, RPA increases and DNA fiber length decreases. TTFields induces replication stress with reducing genes expression of key regulators in mitotic and replication stress [22]. The nascent RNA binds to the template DNA strand during transcription, forming a unique RNA-DNA hybrid structure named the R-loop [92]. TTFields increased R-loop formation.
NF-κB, PI3K/AKT, and MAPK signaling pathways. TTFields inhibits IκBα phosphorylation and NF-κB p65 translocation, which suppresses MMP2 and MMP9 by downregulating NF-κB signaling [47] or inhibits GBM invasion and migration through epithelial-mesenchymal transition (EMT) (Fig. 1A, Ad) [47, 81]. The PI3K/AKT/mTOR signaling pathway is involved in the growth and survival of various tumors [93]. Targeting PI3K/AKT/mTOR-mediated autophagy is not only an essential strategy for treating tumors but also plays a vital role in improving the sensitivity of tumor cells to radiotherapy and chemotherapy. TTFields attenuates the efficacy of Osimertinib by activating p-AKT and p-FOXO3a and inhibiting the nuclear translocation of FOXO3a (Fig. 2A, Ad) [29]. However, other studies have shown that TTFields improves breast cancer sensitivity to Trastuzumab (Fig. 2B, Ba) and GBM radiosensitivity by downregulating p38, p-JNK, p-AKT, p-ERK, and p-HER2 (Fig. 2C, Ca) [21, 33]. In addition, TTFields activates RAW 264.7 cells by activating MAPK and NF-kB signaling pathways [83]. In addition, Shteingauz et al. [58] prove that TTFields induces autophagy by activating ULK1 in an AMPK-dependent manner, resulting in TTFields resistance (Fig. 1A. Af).
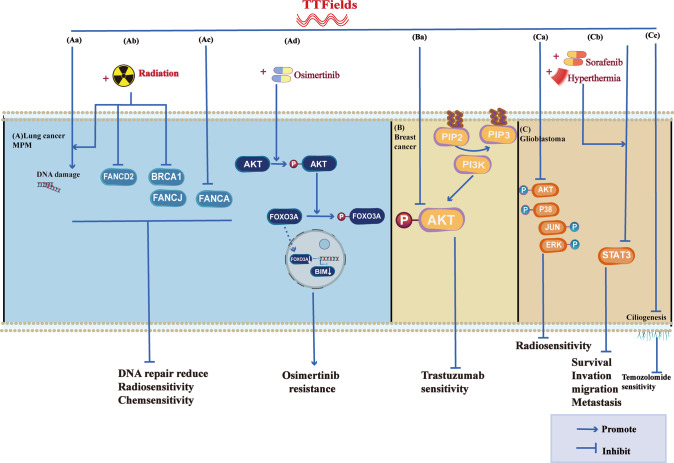
Fig. 2. Molecular pathway changes caused by TTFields combined with radiotherapy or drugs in GBM, MPM, NSCLC, and breast cancer.
A Lung cancer or MPM. A Aa–c TTFields combined with radiation causes DNA damage but reduces DNA damage repair by inhibiting the expression of FANCA, FANCD2, FANCJ, and BRCA. A Ad In addition, TTFields promotes the phosphorylation of AKT, which in turn promotes the phosphorylation of FOXO3A, reduces the nuclear entry of FOXO3A, and inhibits the expression of BIM, which ultimately leads to the weakening of the efficacy of Osimertinib. B Breast cancer. B Ba TTFields enhances breast cancer sensitivity to Trastuzumab by inhibiting AKT phosphorylation. C Glioblastoma. C Ca TTFields inhibits the phosphorylation of AKT, JUN, P38, and ERK, resulting in enhanced radiosensitivity while inhibiting ciliogenesis and enhancing the sensitivity of GBM to Temozolomide. C Cb In addition, TTFields combined with Sorafenib or hyperthermia resulted in cell death by inhibiting STAT3. C Cc TTFields inhibits ciliogenesis, thereby suppressing sensitivity to Temozolomide.
TTFields influences the expression of AKT2, a critical targets for regulating autophagy. Kim et al. [55] report that after TTFields, Beclin1-Atg14L/Vps34 complex increases and Beclin1-Bcl-2 complex decreases in glioma cells and tumor stem cell, leading to autophagy through AKT2/mTOR/p70S6K axis. Meanwhile, TTFields up-regulates miR-29b-3p, targeted binding AKT2, resulting in the decreased expression of AKT2 (Fig. 1A, Aa).
STAT3, a cytoplasmic transcription factor and a downstream molecule of mTOR, is activated in various cancers, including hematological malignancies and solid tumors, to induce proliferation, invasion, metastasis, and angiogenesis [94, 95]. TTFields alone, combined with Sorafenib or hyperthermia, downregulates STAT3 in GBM, resulting in enhanced efficacy (Fig. 2C, Cb) [32, 64].
TTFields induces type I interferon and proinflammatory cytokines via the cGAS-STING pathway, which may lead to immune activation. The cGAS-STING pathway is involved in pyroptosis. In case of infection, cellular stress, and tissue damage, the cGAS-STING signaling pathway senses DNA damage and regulates infection, inflammatory diseases, and tumor immunity [96–98]. TTFields alone or in combination with radiotherapy destroy the nuclear membrane, and generate micronuclei and double strand breaks of DNA, which activates the cGAS-STING signaling pathway to increase the expression of proinflammatory factors and type I interferon [28]. Meanwhile, TTFields leads to pyroptosis and immune activation via the AIM2-Caspase1 inflammasome which slices GSDMD and releases LDH (Fig. 1A, Ac) [69].
TTFields regulates DNA damage repair, radiation and drug resistance via the Fanconi Anemia-BRCA pathway. Genomic instability is often associated with tumorigenesis, and the Fanconi Anemia-BRCA pathway is involved in the repair of interstrand crosslinks and double-strand DNA breaks by homologous recombination [86, 87]. The effect of TTFields on BCRA1 expression is controversial. Jeong et al. [56] show that TTFields increases the expression of BRCA1, GADD45, TP53, and FOXO3A, and decreased protein expression of CDC2 and Cyclin B1, respectively, confirming the occurrence of G2/M phase arrest (Fig. 1A, Ah). However, TTFields combined with IR down-regulates BRCA1 expression and reduces DNA double-strand breaks repair, resulting in sensitivity to ionizing radiation (Fig. 2A, Ab) [25]. Likewise, TTFields down-regulates the Fanconi Anemia-BRCA pathway, promoting chemosensitivity in malignant pleural mesothelioma (Fig. 2A, Ac).
In addition, p21 and p27 are elevated after TTFields, which activates the cell cycle checkpoint (Fig. 1D) [28, 56]. TTFields also inhibits angiogenesis by suppressing HIF1α and VEGF (Fig. 1A, Ag) [47]. TTFields down-regulates BCL2, up-regulates cleaved PARP and BAX [33], and induces apoptosis in breast cancer, ovarian cancer, and glioma cells through a caspase-dependent pathway (Fig. 1A, Ab) [48].
Other mechanisms show that TTFields activates the Cav1.2 ion channel resulting in permeability [65], and inhibits ciliogenesis thereby enhancing Temozolomide toxicity (Fig. 2C. Cc) [99]. The molecular pathway changes of TTFields alone or in combination with other treatments are shown in Figs. 1 and 2.
Conclusion and future perspectives
TTFields is a non-invasive tumor therapy. In vitro/vivo experiments and clinical trials have demonstrated the therapeutic effects of TTFields alone or in combination with radiotherapy and chemotherapy in various tumors [8, 9, 15, 17, 19, 24, 31]. For the first time, we summarize the relevant parameters of TTFields used in the current studies, such as frequency, intensity, duration. TTFields changes the effect of radiotherapy [20–25] and chemotherapy [26–38]. Therefore, we summarize the combined regimen of current researches, but it is difficult to make clear the best combination scheme due to the lack of adequate research. Last but not least, we firstly sum up the pathway and molecular alternation of TTFields.
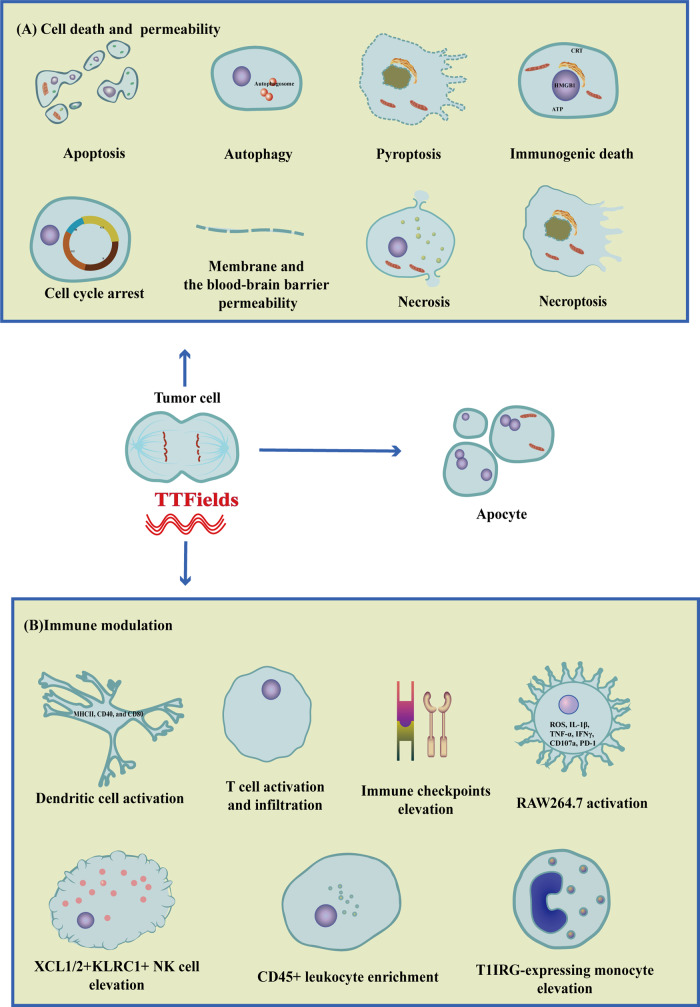
TTFields studies are relatively limited, but the future is bright. First, we should consider parameters related to TTFields. More studies should focus on combining TTFields and radiotherapy or chemotherapy, making clear the best-combined formula [20–38]. In addition, TTFields promotes various mode of cell death (Fig. 3A). TTFields induces the immunogenic death of tumor cells, releases proinflammatory factors, activates immune cells, and adaptive immunity [69]. As a non-invasive physical therapy, TTFields plays an essential role in regulating immune function (Fig. 3B). Its combination with anti-PD-1 significantly inhibits tumors [52], which attracts us to pay more attention to the combination of TTFields and immunotherapy.
Fig. 3. TTFields induces cell death, permeability and immune modulation.
The classical effect of TTFields is mitosis inhibitions and formation of apocyte. A TTFields induces various mode of tumor cell death, including apoptosis, autophagy, pyroptosis, immunogenic death, necrosis, necroptosis, and cell cycle arrest. Meanwhile, TTFields affects the integrity of membrane and the blood-brain barrier, increasing permeability of tumor cell. B TTFields induces activation of dendritic cell, RAW264.7. In addition, TTFields leads to T cell infiltration and CD45 + leukocyte enrichment. Meanwhile, T1IRG-expressing monocyte, NK cell and immune checkpoints are elevated after TTFields treatment.
At present, although a small part of fundamental researches study on various tumors, such as lung cancer [25, 34, 48, 52, 58], breast cancer [1, 27, 31, 36, 48], and pancreatic cancer [50], it mainly focuses on glioma [1, 20, 57] and GBM [1, 36, 44, 45, 47–49, 51, 54–56, 63]. In addition, with the emergence of drug or radiotherapy resistance, the role of TTFields is not yet conclusive. We look forward to applying TTFields in other tumor cells or drug-/radio-resistant cell lines and clarifying its mechanism and changes in molecular pathways.
The anti-mitotic effect of TTFields is first discovered to inhibit tumor cells’ division and proliferation. And the current researches have undoubtedly proven that TTFields induces apoptosis [26, 32, 45, 49, 55, 57, 64, 65] and autophagy [20, 21, 26, 30, 55, 58, 60] in cells, leads to cell membrane permeability [20, 55, 63, 66, 69–71], immune regulation [41, 51, 52, 69, 70, 82, 83], resulting in tumor cell killing. In molecular pathway research, TTFields inhibits tubulin assembly and direction, and changes the distribution of Septins [51, 66]. Thus, DNA replication stress and damage increases, activating DNA damage-related pathways, such as the cGAS-STING pathway [69] and Fanconi anemia-BRCA pathway [25, 56]. In addition, TTFields inhibits cell proliferation and promotes sensitivity to radiation or drugs through NF-κB, MAPK, and PI3K/AKT signaling pathways [21, 29, 33, 47, 55, 58, 81, 83, 93]. We look forward to bioinformatics analysis such as single-cell sequence to discover new molecular mechanisms.
Acknowledgements
We thank the team members for their valuable and constructive comments. Also, we thank members of Hunan Antai Kangcheng Biotechnology Co., Ltd. for their help. This study was supported by the Natural Science Foundation of Hunan (2022JJ30992 to RRZ).
Author contributions
GT contributed to the manuscript. LC collected and revised the tables and figures. GX, WS, HP, and DC modified the grammar and polished the paper. All authors reviewed and approved the final manuscript.
Data availability
Data openly available in a public repository.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirson ED, Dbaly V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA. 2007;104:10152–7. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song A, Bar-Ad V, Martinez N, Glass J, Andrews DW, Judy K, et al. Initial experience with scalp sparing radiation with concurrent temozolomide and tumor treatment fields (SPARE) for patients with newly diagnosed glioblastoma. J Neurooncol. 2020;147:653–61.. doi: 10.1007/s11060-020-03466-z. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J-J, O’Donnell RT, Goldlust S, Ram Z. CTNI-77. EF-19, a post-approval registry study of Tumor Treating Fields (TTfields) in recurrent glioblastoma (rGBM) Neuro Oncol. 2020;22:ii60–ii. doi: 10.1093/neuonc/noaa215.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanner AA, Wong ET, Villano JL, Ram Z, Investigators EF. Post Hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A system versus best physician’s choice chemotherapy. Semin Oncol. 2014;41:S25–34. doi: 10.1053/j.seminoncol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–16. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballo MT, Urman N, Lavy-Shahaf G, Grewal J, Bomzon Z, Toms S. Correlation of tumor treating fields dosimetry to survival outcomes in newly diagnosed glioblastoma: a large-scale numerical simulation-based analysis of data from the phase 3 EF-14 randomized trial. Int J Radiat Oncol Biol Phys. 2019;104:1106–13.. doi: 10.1016/j.ijrobp.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Taphoorn MJB, Dirven L, Kanner AA, Lavy-Shahaf G, Weinberg U, Taillibert S, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:495–504. doi: 10.1001/jamaoncol.2017.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pless M, Droege C, von Moos R, Salzberg M, Betticher D. A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer. 2013;81:445–50.. doi: 10.1016/j.lungcan.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Ceresoli GL, Aerts JG, Dziadziuszko R, Ramlau R, Cedres S, van Meerbeeck JP, et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 2019;20:1702–9. doi: 10.1016/S1470-2045(19)30532-7. [DOI] [PubMed] [Google Scholar]
- 11.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–14.. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 12.Scagliotti GV, Gaafar R, Nowak AK, Nakano T, van Meerbeeck J, Popat S, et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2019;7:569–80.. doi: 10.1016/S2213-2600(19)30139-0. [DOI] [PubMed] [Google Scholar]
- 13.Grosso F, Steele N, Novello S, Nowak AK, Popat S, Greillier L, et al. Nintedanib plus pemetrexed/cisplatin in patients with malignant pleural mesothelioma: phase II results from the randomized, placebo-controlled LUME-meso trial. J Clin Oncol. 2017;35:3591–600.. doi: 10.1200/JCO.2017.72.9012. [DOI] [PubMed] [Google Scholar]
- 14.Gkika E, Grosu AL, Macarulla Mercade T, Cubillo Gracian A, Brunner TB, Schultheiss M, et al. Tumor treating fields concomitant with sorafenib in advanced hepatocellular cancer: Results of the HEPANOVA Phase II Study. Cancers. 2022;14:1568. [DOI] [PMC free article] [PubMed]
- 15.Gkika E, Brunner T, Thimme R, Grosu A-L. Abstract CT186: HEPANOVA: interim safety analysis from a phase 2 study of Tumor Treating Fields (TTFields, 150 kHz) concomitant with sorafenib in advanced hepatocellular carcinoma (HCC) Cancer Res. 2020;80:CT186–CT. doi: 10.1158/1538-7445.AM2020-CT186. [DOI] [Google Scholar]
- 16.Vergote I, Moos RV, Manso L, Sessa C. INNOVATE: a phase II study of TTFields (200 kHz) concomitant with weekly paclitaxel for recurrent ovarian cancer—Updated safety and efficacy results. J Clin Oncol. 2017;35:5580. doi: 10.1200/JCO.2017.35.15_suppl.5580. [DOI] [Google Scholar]
- 17.Vergote I, von Moos R, Manso L, Van Nieuwenhuysen E, Concin N, Sessa C. Tumor Treating Fields in combination with paclitaxel in recurrent ovarian carcinoma: results of the INNOVATE pilot study. Gynecol Oncol. 2018;150:471–7. doi: 10.1016/j.ygyno.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Rivera F, Gallego J, Guillen C, Benavides M, Lopez-Martin JA, Betticher DC, et al. PANOVA: a pilot study of TTFields concomitant with gemcitabine for front-line therapy in patients with advanced pancreatic adenocarcinoma. J Clin Oncol. 2016;34:269. doi: 10.1200/jco.2016.34.4_suppl.269. [DOI] [Google Scholar]
- 19.Rivera F, Benavides M, Gallego J, Guillen-Ponce C, Lopez-Martin J, Kung M. Tumor treating fields in combination with gemcitabine or gemcitabine plus nab-paclitaxel in pancreatic cancer: results of the PANOVA phase 2 study. Pancreatology. 2019;19:64–72. doi: 10.1016/j.pan.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Silginer M, Weller M, Stupp R, Roth P. Biological activity of tumor-treating fields in preclinical glioma models. Cell Death Dis. 2017;8:e2753. doi: 10.1038/cddis.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WS, Seo SJ, Chung HK, Park JW, Kim JK, Kim EH. Tumor-treating fields as a proton beam-sensitizer for glioblastoma therapy. Am J Cancer Res. 2021;11:4582–94. [PMC free article] [PubMed] [Google Scholar]
- 22.Karanam NK, Ding L, Aroumougame A, Story MD. Tumor treating fields cause replication stress and interfere with DNA replication fork maintenance: Implications for cancer therapy. Transl Res. 2020;217:33–46. doi: 10.1016/j.trsl.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Jo Y, Oh G, Gi Y, Sung H, Joo EB, Lee S, et al. Tumor treating fields (TTF) treatment enhances radiation-induced apoptosis in pancreatic cancer cells. Int J Radiat Biol. 2020;96:1528–33.. doi: 10.1080/09553002.2020.1838658. [DOI] [PubMed] [Google Scholar]
- 24.Giladi M, Munster M, Schneiderman RS, Voloshin T, Porat Y, Blat R, et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017;12:206. doi: 10.1186/s13014-017-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karanam NK, Srinivasan K, Ding L, Sishc B, Saha D, Story MD. Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 2017;8:e2711. doi: 10.1038/cddis.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo Y, Kim EH, Sai S, Kim JS, Cho JM, Kim H, et al. Functional biological activity of sorafenib as a tumor-treating field sensitizer for glioblastoma therapy. Int J Mol Sci. 2018;19:3684. [DOI] [PMC free article] [PubMed]
- 27.Schneiderman RS, Shmueli E, Kirson ED, Palti Y. TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer. 2010;10:229. doi: 10.1186/1471-2407-10-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mumblat H, Martinez-Conde A, Braten O, Munster M, Dor-On E, Schneiderman RS, et al. Tumor Treating Fields (TTFields) downregulate the Fanconi Anemia-BRCA pathway and increase the efficacy of chemotherapy in malignant pleural mesothelioma preclinical models. Lung Cancer. 2021;160:99–110. doi: 10.1016/j.lungcan.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Hu C, Lu C, Zhang K, Han R, Lin C, et al. Applied electric fields suppress osimertinib-induced cytotoxicity via inhibiting FOXO3a nuclear translocation through AKT activation. Carcinogenesis. 2020;41:600–10.. doi: 10.1093/carcin/bgz150. [DOI] [PubMed] [Google Scholar]
- 30.Lee YJ, Cho JM, Sai S, Oh JY, Park JA, Oh SJ, et al. 5-fluorouracil as a tumor-treating field-sensitizer in colon cancer therapy. Cancers. 2019;11:1999. [DOI] [PMC free article] [PubMed]
- 31.Kirson ED, Schneiderman RS, Dbaly V, Tovarys F, Vymazal J, Itzhaki A, et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields) BMC Med Phys. 2009;9:1. doi: 10.1186/1756-6649-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Jo Y, Oh HK, Kim EH. Sorafenib increases tumor treating fields-induced cell death in glioblastoma by inhibiting STAT3. Am J Cancer Res. 2020;10:3475–86.. [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JS, Cho JM, Kim H, Jeong YK, Kim JK, Kim EH. Tumor treating fields can effectively overcome trastuzumab resistant breast cancer multiplication. Am J Cancer Res. 2021;11:3935–45.. [PMC free article] [PubMed] [Google Scholar]
- 34.Giladi M, Weinberg U, Schneiderman RS, Porat Y, Munster M, Voloshin T, et al. Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin Oncol. 2014;41:S35–41. doi: 10.1053/j.seminoncol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Giladi M, Schneiderman RS, Porat Y, Munster M, Itzhaki A, Mordechovich D, et al. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology. 2014;14:54–63. doi: 10.1016/j.pan.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Chang E, Pohling C, Beygui N, Patel CB, Rosenberg J, Ha DH, et al. Synergistic inhibition of glioma cell proliferation by Withaferin A and tumor treating fields. J Neurooncol. 2017;134:259–68.. doi: 10.1007/s11060-017-2534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voloshin T, Munster M, Blatt R, Shteingauz A, Roberts PC, Schmelz EM, et al. Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. Int J Cancer. 2016;139:2850–8. doi: 10.1002/ijc.30406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei KF, Hsieh SC, Goh A, Kuo RL, Tsang NM. Proliferation arrest, selectivity, and chemosensitivity enhancement of cancer cells treated by a low-intensity alternating electric field. Biomed Microdevices. 2018;20:90. doi: 10.1007/s10544-018-0339-8. [DOI] [PubMed] [Google Scholar]
- 39.Stupp R, Kanner A, Engelhard H, Heidecke V, Taillibert S, Lieberman FS, et al. A prospective, randomized, open-label, phase III clinical trial of NovoTTF-100A versus best standard of care chemotherapy in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:LBA2007–LBA. doi: 10.1200/jco.2010.28.18_suppl.lba2007. [DOI] [Google Scholar]
- 40.Brozova H, Lucas A, Salmaggi A, Vymazal J. BMET-06COMET: a phase II randomized study of TTFields versus supportive care in non-small cell lung cancer patients with 1-5 brain metastases-initial safety results. Neuro Oncol. 2015;17:v46–v. doi: 10.1093/neuonc/nov208.06. [DOI] [Google Scholar]
- 41.Wu H, Yang L, Liu H, Zhou D, Chen D, Zheng X, et al. Exploring the efficacy of tumor electric field therapy against glioblastoma: an in vivo and in vitro study. CNS Neurosci Ther. 2021;27:1587–604.. doi: 10.1111/cns.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H, Wang C, Liu J, Zhou D, Chen D, Liu Z, et al. Evaluation of a tumor electric field treatment system in a rat model of glioma. CNS Neurosci Ther. 2020;26:1168–77.. doi: 10.1111/cns.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–95. doi: 10.1158/0008-5472.CAN-04-0083. [DOI] [PubMed] [Google Scholar]
- 44.Linder B, Schiesl A, Voss M, Rodel F, Hehlgans S, Gullulu O, et al. Dexamethasone treatment limits efficacy of radiation, but does not interfere with glioma cell death induced by tumor treating fields. Front Oncol. 2021;11:715031. doi: 10.3389/fonc.2021.715031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Branter J, Estevez-Cebrero M, Diksin M, Griffin M, Castellanos-Uribe M, May S, et al. Genome-wide expression and anti-proliferative effects of electric field therapy on pediatric and adult brain tumors. Int J Mol Sci. 2022;23:1982. [DOI] [PMC free article] [PubMed]
- 46.Porat Y, Giladi M, Schneiderman RS, Blat R, Shteingauz A, Zeevi E, et al. Determining the optimal inhibitory frequency for cancerous cells using Tumor Treating Fields (TTFields). J Vis Exp. 2017:55820. [DOI] [PMC free article] [PubMed]
- 47.Kim EH, Song HS, Yoo SH, Yoon M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget. 2016;7:65125–36.. doi: 10.18632/oncotarget.11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giladi M, Schneiderman RS, Voloshin T, Porat Y, Munster M, Blat R, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015;5:18046. doi: 10.1038/srep18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessler AF, Frombling GE, Gross F, Hahn M, Dzokou W, Ernestus RI, et al. Effects of tumor treating fields (TTFields) on glioblastoma cells are augmented by mitotic checkpoint inhibition. Cell Death Discov. 2018;4:12. doi: 10.1038/s41420-018-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfeifer T, Bai L, Gladkich J, Gross W, Liu L, Herr I, et al. Therapy of pancreatic cancer with alternating electric fields: limitations of the method. Bioelectrochemistry. 2021;141:107881. doi: 10.1016/j.bioelechem.2021.107881. [DOI] [PubMed] [Google Scholar]
- 51.Voloshin T, Schneiderman RS, Volodin A, Shamir RR, Kaynan N, Zeevi E, et al. Tumor Treating Fields (TTFields) hinder cancer cell motility through regulation of microtubule and acting dynamics. Cancers. 2020;12:3016. [DOI] [PMC free article] [PubMed]
- 52.Voloshin T, Kaynan N, Davidi S, Porat Y, Shteingauz A, Schneiderman RS, et al. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother. 2020;69:1191–204.. doi: 10.1007/s00262-020-02534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H, Liu R, Liu J, Tang J. Growth inhibition of malignant melanoma by intermediate frequency alternating electric fields, and the underlying mechanisms. J Int Med Res. 2012;40:85–94. doi: 10.1177/147323001204000109. [DOI] [PubMed] [Google Scholar]
- 54.Clark PA, Gaal JT, Strebe JK, Pasch CA, Deming DA, Kuo JS, et al. The effects of tumor treating fields and temozolomide in MGMT expressing and non-expressing patient-derived glioblastoma cells. J Clin Neurosci. 2017;36:120–4. doi: 10.1016/j.jocn.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim EH, Jo Y, Sai S, Park MJ, Kim JY, Kim JS, et al. Tumor-treating fields induce autophagy by blocking the Akt2/miR29b axis in glioblastoma cells. Oncogene. 2019;38:6630–46.. doi: 10.1038/s41388-019-0882-7. [DOI] [PubMed] [Google Scholar]
- 56.Jeong H, Sung J, Oh SI, Jeong S, Koh EK, Hong S, et al. Inhibition of brain tumor cell proliferation by alternating electric fields. Appl Phys Lett. 2014;105:5. doi: 10.1063/1.4902112. [DOI] [Google Scholar]
- 57.Jo Y, Sung J, Jeong H, Hong S, Jeong YK, Kim EH, et al. Effectiveness of a fractionated therapy scheme in tumor treating fields therapy. Technol Cancer Res Treat. 2019;18:1533033819845008. doi: 10.1177/1533033819845008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shteingauz A, Porat Y, Voloshin T, Schneiderman RS, Munster M, Zeevi E, et al. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields) Cell Death Dis. 2018;9:1074. doi: 10.1038/s41419-018-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Bai L, Pfeifer T, Gross W, De La Torre C, Zhao S, Liu L, et al. Establishment of tumor treating fields combined with mild hyperthermia as novel supporting therapy for pancreatic cancer. Front Oncol. 2021;11:738801. doi: 10.3389/fonc.2021.738801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jo Y, Hwang SG, Jin YB, Sung J, Jeong YK, Baek JH, et al. Selective toxicity of tumor treating fields to melanoma: an in vitro and in vivo study. Cell Death Discov. 2018;4:46. doi: 10.1038/s41420-018-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeong H, Jo Y, Yoon M, Hong S. Thymidine decreases the DNA damage and apoptosis caused by tumor-treating fields in cancer cell lines. Genes Genomics. 2021;43:995–1001. doi: 10.1007/s13258-021-01105-z. [DOI] [PubMed] [Google Scholar]
- 63.Chang E, Patel CB, Pohling C, Young C, Song J, Flores TA, et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018;4:113. doi: 10.1038/s41420-018-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jo Y, Han YI, Lee E, Seo J, Oh G, Sung H, et al. The combination of tumor treating fields and hyperthermia has synergistic therapeutic effects in glioblastoma cells by downregulating STAT3. Am J Cancer Res. 2022;12:1423–32. [PMC free article] [PubMed] [Google Scholar]
- 65.Neuhaus E, Zirjacks L, Ganser K, Klumpp L, Schuler U, Zips D, et al. Alternating Electric Fields (TTFields) activate Cav1.2 channels in human glioblastoma cells. Cancers. 2019;11:110. [DOI] [PMC free article] [PubMed]
- 66.Gera N, Yang A, Holtzman TS, Lee SX, Wong ET, Swanson KD. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS ONE. 2015;10:e0125269. doi: 10.1371/journal.pone.0125269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stingele S, Stoehr G, Storchova Z. Activation of autophagy in cells with abnormal karyotype. Autophagy. 2013;9:246–8. doi: 10.4161/auto.22558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee YJ, Seo HW, Baek JH, Lim SH, Hwang SG, Kim EH. Gene expression profiling of glioblastoma cell lines depending on TP53 status after tumor-treating fields (TTFields) treatment. Sci Rep. 2020;10:12272. doi: 10.1038/s41598-020-68473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen D, Le SB, Hutchinson TE, Calinescu AA, Sebastian M, Jin D, et al. Tumor Treating Fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J Clin Invest. 2022;132:e149258. [DOI] [PMC free article] [PubMed]
- 70.Lee J, Grabowski MM, Lathia JD. Tumor Treating Fields: killing two birds with one stone. J Clin Invest. 2022;132:e159073. [DOI] [PMC free article] [PubMed]
- 71.Voloshin T, Koltun B, Koren L, Porat Y, Volodin A, Kaynan N, et al. EXTH-74. increasing cancer cell membrane permeability through application of Tumor Treating Fields (TTfields) Neuro-Oncol. 2021;23:vi180–vi. doi: 10.1093/neuonc/noab196.713. [DOI] [Google Scholar]
- 72.Salvador E, Kessler AF, Giniunaite A, Burek M, Brami CT, Voloshin T, et al. Application of Tumor Treating Fields (TTFields) to open the blood brain barrier (BBB) as future CNS drug delivery strategy. Brain Spine. 2021;1:100536. doi: 10.1016/j.bas.2021.100536. [DOI] [Google Scholar]
- 73.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17:958–65. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aguilar AA, Ho MC, Chang E, Carlson KW, Natarajan A, Marciano T, et al. Permeabilizing cell membranes with electric fields. Cancers. 2021;13:2283. [DOI] [PMC free article] [PubMed]
- 75.Salvador E, Kessler AF, Hörmann J, Burek M, Brami CT, Sela TV, et al. Abstract 6251: Blood brain barrier opening by TTFields: a future CNS drug delivery strategy. Cancer Res. 2020;80:6251. doi: 10.1158/1538-7445.AM2020-6251. [DOI] [Google Scholar]
- 76.Brami CT, Salvador E, Kessler AF, Burek M, Voloshin T, Giladi M, et al. Transient opening of the blood brain barrier by Tumor Treating Fields (TTFields). In: Proceedings of the American Association for Cancer Research Annual Meeting 2021. Philadelphia (PA): AACR; Cancer Res 2021;81(13_Suppl):Abstract nr 279.
- 77.Salvador E, Kessler A, Hoermann J, Domroese D, Schaeffer C, Burek M, et al. Tumor treating fields effects on the blood-brain barrier in vitro and in vivo. J Clin Oncol. 2020;38:2551. doi: 10.1200/JCO.2020.38.15_suppl.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schulz E, Kessler AF, Salvador E, Domröse D, Burek M, Tempel Brami C, et al. EXTH-02. The blood brain barrier (BBB) permeability is altered by Tumor Treating Fields (TTFields) in vivo. Neuro-Oncol. 2019;21:vi82–vi. doi: 10.1093/neuonc/noz175.336. [DOI] [Google Scholar]
- 79.Kessler AF, Schaeffer CM, Burek M, Ruschig U, Tempel-Brami C, Voloshin T, et al. Abstract 252: Tumor treating fields (TTFields) affect blood brain barrier (BBB) integrity in vitro and in vivo. Cancer Res. 2019;79:252. doi: 10.1158/1538-7445.AM2019-252. [DOI] [Google Scholar]
- 80.Kessler AF, Schaeffer C, Burek M, Ruschig U, Ernestus R, Löhr M, et al. P04.33 Effects of Tumor Treating Fields (TTFields) on blood brain barrier (BBB) permeability. Neuro Oncol. 2018;20:iii286–iii. doi: 10.1093/neuonc/noy139.267. [DOI] [Google Scholar]
- 81.Oh JY, Lee YJ, Kim EH. Tumor-treating fields inhibit the metastatic potential of osteosarcoma cells. Technol Cancer Res Treat. 2020;19:1533033820947481. doi: 10.1177/1533033820947481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simchony H, Diamant D, Ram Z, Volovitz I. Evaluation of the compatibility of electric tumor treating fields with key anti-tumoral T-cell functions. Isr Med Assoc J. 2019;21:503. [PubMed] [Google Scholar]
- 83.Park JI, Song KH, Jung SY, Ahn J, Hwang SG, Kim J, et al. Tumor-Treating Fields induce RAW264.7 macrophage activation via NK-kappaB/MAPK signaling pathways. Technol Cancer Res Treat. 2019;18:1533033819868225. doi: 10.1177/1533033819868225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirson ED, Giladi M, Gurvich Z, Itzhaki A, Mordechovich D, Schneiderman RS, et al. Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin Exp Metastasis. 2009;26:633–40. doi: 10.1007/s10585-009-9262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thoms J, Bristow RG. DNA repair targeting and radiotherapy: a focus on the therapeutic ratio. Semin Radiat Oncol. 2010;20:217–22. doi: 10.1016/j.semradonc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 86.Stecklein SR, Jensen RA. Identifying and exploiting defects in the Fanconi anemia/BRCA pathway in oncology. Transl Res. 2012;160:178–97. doi: 10.1016/j.trsl.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 87.Kim H, D’Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26:1393–408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spiliotis ET, Nakos K. Cellular functions of actin- and microtubule-associated septins. Curr Biol. 2021;31:R651–R66.. doi: 10.1016/j.cub.2021.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Basant A, Glotzer M. Spatiotemporal regulation of RhoA during cytokinesis. Curr Biol. 2018;28:R570–R80.. doi: 10.1016/j.cub.2018.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Girouard MP, Pool M, Alchini R, Rambaldi I, Fournier AE. RhoA proteolysis regulates the actin cytoskeleton in response to oxidative stress. PLoS ONE. 2016;11:e0168641. doi: 10.1371/journal.pone.0168641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 2008;18:210–9. doi: 10.1016/j.tcb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Rinaldi C, Pizzul P, Longhese MP, Bonetti D. Sensing R-loop-associated DNA damage to safeguard genome stability. Front Cell Dev Biol. 2020;8:618157. doi: 10.3389/fcell.2020.618157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G, et al. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl Microbiol Biotechnol. 2020;104:575–87.. doi: 10.1007/s00253-019-10257-8. [DOI] [PubMed] [Google Scholar]
- 94.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136–54. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 95.Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wan D, Jiang W, Hao J. Research advances in how the cGAS-STING pathway controls the cellular inflammatory response. Front Immunol. 2020;11:615. doi: 10.3389/fimmu.2020.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang M, Chen P, Wang L, Li W, Chen B, Liu Y, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13:81. doi: 10.1186/s13045-020-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548–69.. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi P, Tian J, Ulm BS, Mallinger JC, Khoshbouei H, Deleyrolle LP, et al. Tumor treating fields suppression of ciliogenesis enhances temozolomide toxicity. Front Oncol. 2022;12:837589. doi: 10.3389/fonc.2022.837589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository.