Abstract
Background
Low-grade serous ovarian and peritoneal cancer (LGSC) is a rare disease and few data on the clinical and genomic landscape have been published.
Methods
A retrospective analysis of patients diagnosed with LGSC between 1996 and 2019 was conducted in MITO centers. Objective Response Rate (ORR) to treatments, progression-free survival (PFS) and overall survival (OS) were assessed. Additionally, the tumor molecular profile of 56 patients was evaluated using the Next Generation Sequencing (NGS) FoundationOne CDX (Foundation Medicine®).
Results
A total of 128 patients with complete clinical data and pathologically confirmed diagnosis of LGSC were identified. ORR to first and subsequent therapies were 23.7% and 33.7%, respectively. PFS was 43.9 months (95% CI:32.4–53.1) and OS was 105.4 months (95% CI: 82.7–not reached). The most common gene alterations were: KRAS (n = 12, 21%), CDKN2A/B (n = 11, 20%), NRAS (n = 8, 14%), FANCA (n = 8, 14%), NF1 (n = 7, 13%) and BRAF (n = 6, 11%). Unexpectedly, pathogenetic BRCA1 (n = 2, 4%), BRCA2 (n = 1, 2%) and PALB2 (n = 1, 2%) mutations were found.
Conclusions
MITO 22 suggests that LGSC is an heterogenous disease for both its clinical behavior in response to standard therapies and its molecular alterations. Future prospective studies should test treatments according to biological and molecular tumor’s characteristics.
Clinical trial registration
This study is registered under NCT02408536 on ClinicalTrials.gov.
Subject terms: Ovarian cancer, Ribosome
Introduction
Low-Grade Serous Ovarian Cancer (LGSOC) represents a rare entity accounting for 2% of all epithelial ovarian cancers (OC) and for 4.7% of serous ovarian carcinomas [1]. Due to its rarity, LGSOC has been less studied with respect to high-grade serous ovarian cancer (HGSOC) counterpart, and many clinical and molecular aspects remain unknown. It is characterized by an indolent clinical course, with a lower biologic aggressiveness and a longer 5-year overall survival compared to HGSOC [2]. Patients with LGSOCs are often treated with cytoreductive surgery followed by platinum/paclitaxel chemotherapy. However, due to its low proliferative activity LGSOC is a relatively chemo-resistant disease and data coming from retrospective single institution studies have shown an objective response rate (ORR) to both platinum and non-platinum-based chemotherapies of only 24% and 4% in first line and recurrent setting, respectively [3, 4]. Furthermore, recently reported conflicting results about chemotherapy responses suggest that LGSOC includes patients with different characteristics [5]. Because of the clinical heterogeneity of LGSOC and the lack of prospective data indicating the most effective therapeutic strategy in first line as well as in the recurrent setting, in the last few years several studies have been directed towards a better knowledge of the intrinsic disease characteristics, including genomic alterations and biomarkers expression, with the aim to identify novel molecular drivers that might be predictive of clinical behavior and/or guide the use of targeted therapies.
Molecular and genomic studies have shown that LGSOC appears to have a very low prevalence of TP53 mutations (<8%) [6, 7], loss of CDKN2A/B (15–53%) [8, 9] and often harbors activating mutations of genes involved in the mitogen-activated protein kinase (MAPK) pathway, such as KRAS (20–40%), BRAF (7–26%), ERBB2 (0–30%), and NRAS (5–33%) [10–13].
In this context, the identification of oncogenic mutations affecting MAPK genes (RAS/RAF/MEK/ERK) led to the evaluation of targeted therapy, such as MEK inhibitors (MEKi) in clinical trials, that have shown a promising activity with a better ORR and a statistically significant improvement in PFS in persistent and recurrent LGSOC when compared to the standard of care [14–16]. Additionally, hormone receptors are frequently overexpressed in LGSOC with respect to HGSOC, with reported rates of ER expression of 96% and PR expression of 58%, respectively [17, 18]. In this respect, endocrine therapies like tamoxifen and aromatase inhibitors are currently adopted options in recurrent disease and first line maintenance setting based on relatively large retrospective series and prospective clinical trials supporting this therapeutic strategy [19–21].
Finally, recent published data, in a small cohort of Asian patients, showed an “unexpected” mutational landscape of LGSOC reporting in some cases mutations in Homologous Recombination Repair (HRR) DNA pathway genes such us BRCA 1 / 2, BARD 1, ATR, BRIP1, and CHECK2 [22].
These findings suggest a heterogeneous molecular profile of this rare disease and the need to identify actionable mutations and biomarkers that might predict tumor response.
With the aim of better define clinical and molecular characteristics of LGSOCs we conducted this descriptive retrospective analysis of the data from the Multicenter Italian Trials in Ovarian Cancer (MITO) group.
Materials and methods
MITO 22 is an Italian, multicenter, observational, and retrospective analysis involving 8 MITO centers and coordinated by National Cancer Institute of Naples. The study was approved by the ethical committees of each participating institution and written consent was required.
Between 1996 and 2019, 171 patients diagnosed with de novo low-grade serous carcinoma of the ovary and peritoneum (LGSC) or recurrence of LGSC after surgical resection of borderline serous ovarian carcinoma were identified and enrolled in this study and demographic, clinical, surgical, and molecular data were collected. Pathologic diagnosis was based upon the two-tiered classification system for grading serous ovarian cancers published in 2004 [23], and gynecologic pathologists at the participating MITO centers with significant expertise in this grading system reviewed all cases. In addition, immunohistochemical staining for p53 and WT1 was performed to confirm the diagnosis. Given that several cases were diagnosed before 2014, the 1988 FIGO criteria were used to classify these patients [24]. Patients were included in this analysis if they had pathologically confirmed early or advanced- stage LGSC.
Patients’ data were collected into a centralized database recording demographic and clinical information, including age at diagnosis, stage according to International Federation of Gynecology and Obstetrics (FIGO) criteria, body mass index (BMI), serum CA 125 levels, type of cytoreductive surgery, residual disease status after cytoreduction, treatment details at the time of diagnosis and at the time of subsequent relapses, response rate during primary and subsequent treatments, and date of last contact or death. Cytoreductive surgery was considered complete in case of Residual Tumor (RT) = 0, optimal if RT was <1 cm, and suboptimal if residual disease was ≥1 cm diameter. Eastern Cooperative Oncology Group (ECOG) definitions were used to define performance status.
Follow up data were collected until January 2021. Descriptive statistics were used to characterize the patient’s population. The follow-up was assessed from the start of first line chemotherapy to the date of the last contact or date of death. Clinical response was determined using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, assessed separately in each of the 8 MITO centers, and stable disease (SD) was reported for those patients who met RECIST v1.1 for a minimum of 12 weeks. Clinical Benefit Rate (CBR) is defined as the percentage of patients who achieved complete response, partial response, and stable disease. Progression-free survival (PFS) was defined as the time between the start of first line chemotherapy and first observation of recurrence or the date of last follow-up or death for any cause. Overall survival (OS) was defined as the time between the start of first line chemotherapy and the date of last follow-up or death for any cause. Median follow up was estimated by means of reverse Kaplan–Meier curve. PFS and OS curves were described according to the reverse Kaplan–Meier method. Univariate and multivariate Cox’s regression model was used to analyze the prognostic role of clinical factors on survival. Schoenfeld residuals test was used to judge the proportional-hazards assumption. Differences were considered statistically significant at p value < 0.05. All the analyses were performed with STATA 14 MP (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Molecular analysis
For the genomic data, samples of histologically confirmed LGSC cases that had a tumor block available and sufficient tumor fraction were test with the NGS platform FoundationOne CDX (Foundation Medicine®).
Of 128 LGSCs included in our analysis, 79 tumor specimens were considered adequate for the genomic evaluation. Formalin-fixed paraffin-embedded (FFPE) samples were collected, centralized at MITO coordinating center, and stored according to Italian guidelines [25]. A 5 μm section was cut from each FFPE block, stained with hematoxylin and eosin (H&E), and reviewed by a gynecologic pathologist with significant expertise at the National Cancer Institute of Naples.
Results from the Foundation Medicine® reports were collected, stored in the centralized datasheet, with the data points collected related to patient demographics, their cancer diagnosis and treatments, and analyzed.
The principal endpoint was to describe the mutations discovered.
Results
Between 1996 and 2019, 128 patients with diagnosis of pathologically confirmed LGSC or invasive recurrence after surgical resection of borderline serous ovarian carcinoma were treated and followed up within the 8 MITO centers in Italy and enrolled in this analysis. Of the 171 identified patients, 43 were excluded from this analysis due to incomplete clinical data and follow-up information (n = 36) or pathologically unconfirmed diagnosis of LGSC (n = 7) (Supplementary Appendix 1).
Demographics and clinical characteristics at baseline of the included patients (N = 128) are summarized in Table 1. Median age at the time of diagnosis was 53.2 years (Interquartile range: 42.3–63.1). The majority of LGSCs were diagnosed at an advanced stage (FIGO III and IV), with 68.8% women (n = 88) at stage III and 7.8% (n = 10) at stage IV. Twenty patients (15.6%) had stage I and ten (7.8%) had stage II. Two patients with invasive recurrence after surgical resection of borderline serous ovarian cancer were classified as FIGO stage III.
Table 1.
Patients’ characteristics at diagnosis.
| (N = 128) | |
|---|---|
| Age, median (IQR) | 53.2 (42.3–63.1) |
| BMI, median (IQR) | 23.4 (21.2–26.0) |
| Stage FIGO, n (%) | |
| I | 20 (15.6) |
| II | 10 (7.8) |
| III | 88 (68.8) |
| IV | 10 (7.8) |
| ECOG PS, n (%) | |
| 0 | 80 (62.5) |
| 1 | 4 (3.1) |
| Missing | 44 (34.4) |
| CA 125 value, n (%) | |
| <35 U/ml | 11 (8.6) |
| >35 U/ml | 63 (49.2) |
| Missing | 54 (42.2) |
| Primary debulking surgery, n (%) | |
| Yes | 115 (89.8) |
| No | 13 (10.2) |
| Interval debulking surgery, n (%) | |
| Yes | 11 (8.6) |
| No | 117 (91.4) |
| Residual Tumor, n (%) | |
| 0 | 88 (68.7) |
| <1 cm | 13 (10.2) |
| ≥1 cm | 25 (19.5) |
| Missing | 2 (1.6) |
BMI body mass index, ECOG PS Eastern Cooperative Oncology Group Performance Status, FIGO International Federation of Gynecology and Obstetrics, IQR Interquantile Range.
Primary debulking surgery was performed in 115 patients (89.8%) while 11 patients (8.6%) underwent neoadjuvant chemotherapy (NACT) x 3–6 cycles followed by interval debulking surgery. Among these patients receiving upfront or interval cytoreductive surgery, 68.7% were completely debulked (RT = 0), while 10.2% underwent suboptimal cytoreduction (RT < 1 cm) and 19.5% received incompletely debulking (RT ≥ 1 cm).
No fertility-sparing surgery with comprehensive staging was performed, and all patients with FIGO stage I (n = 20) underwent radical surgical staging.
114 patients received first line chemotherapy (Supplementary Appendix 2). Within the chemotherapy group, 103 patients (90.3%) received carboplatin intravenously every 3 weeks plus paclitaxel, 10 patients (8.8%) were treated with carboplatin monotherapy and only one patient, who refused hair losing therapies, was treated with carboplatin plus pegylated doxorubicin liposomal (PDL).
Thirty patients (26.3%) received bevacizumab in combination with first line chemotherapy and then as maintenance, and only 4 patients received hormone therapy as maintenance after first line platinum-based chemotherapy. Of these, 2 patients received tamoxifen and 2 women received letrozole.
Overall, 76 patients (66.7%) recurred and 19 (25%) underwent secondary cytoreductive surgery at the time of the first relapse, with 8 women (42.1%) completely debulked (Supplementary Appendix 3). Of the 76 patients with relapsed disease, treatment details are summarized in Supplementary Appendix 3: most of them (64.5%) received platinum-based chemotherapy at the time of first recurrence, followed by PDL monotherapy (18.4%) and topotecan (5.3%), according to platinum free interval (PFI) and based upon the treating physician’s preference. Bevacizumab was administered in 16 patients (21.0%) in combination with second line platinum-based chemotherapy and as maintenance. Hormone therapy was administered in 23 patients (30.3%): 6 patients received it as active treatment and in 17 women hormone therapy was administered as maintenance treatment after second-line chemotherapy. Aromatase inhibitors were the most common drugs administered, with 11 patients (14.5%) receiving letrozole, 6 patients (8.6%) anastrozole, and 1 patient treated with exemestane.
Data on second recurrence and subsequent treatment details were available for 37 patients (Supplementary Appendix 4): most of patients (35.1%) received platinum-based chemotherapy, followed by weekly paclitaxel (18.9%) and PLD (10.8%). Hormone therapy was administered in 11 patients and 6 of them received letrozole.
Regarding the response evaluation using RECIST v1.1 criteria, in line with previous reported data [1], the objective response rate (ORR) to first line chemotherapy was of 23.7%, with 14 (12.3%) complete responses (CR) and 13 (11.4%) partial responses (PR). Stable disease (SD) was achieved in 4 patients (3.5%), with a CBR of 27.2%. Of the 30 patients receiving Bevacizumab in first line in combination with platinum-based chemotherapy and as maintenance, 22 underwent complete debulking surgery and 8 were evaluable for response, with 4 patients that achieved complete responses and 4 women that obtained partial responses.
Unexpectedly, a higher ORR of 33.7% was achieved in subsequent chemotherapy lines, with 11 (10.3%) complete responses and 25 (23.4%) partial responses. CBR was 59.9%, and 28 patients (26.2%) achieved a stable disease. Furthermore, in this disease setting we found a high rate of complete and partial responses among the 16 patients receiving Bevacizumab at the time of first recurrence. Thirtheen (81.2%) women were evaluable for response, and we found 5 (31.2%) complete responses, 7 (43.7%) partial responses and 1 (6.2%) stable disease.
Response rate (RR) to hormone therapy was 8.5%, with only 2 complete responses achieved, instead CBR was 82.6% (Supplementary Appendix 5).
The survival analysis was performed on 114 patients. With a median follow up of 70.5 months (95% CI: 55.0–87.5), PFS was 43.9 months (95% CI: 32.4–53.1) (Fig. 1) and OS was 105.4 months (95% CI: 82.7–not reached) (Fig. 2).
Fig. 1. Progression-Free Survival (PFS) of the overall population.
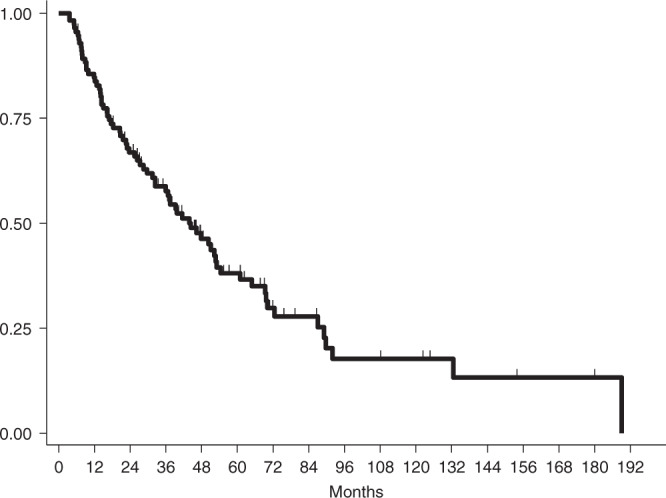
The median PFS was 43.9 months (95% CI: 32.4–53.1).
Fig. 2. Overall Survival (OS) of the overall population.

The median OS was 105.4 months (95% CI: 82.7– not reached).
In the multivariate Cox model (Supplementary Appendix 6) only residual tumor at the time of upfront surgery was confirmed as an independent prognostic factor for PFS, while age >50 years, primary debulking surgery and residual tumor were prognostic factors for OS.
Considering these findings, we evaluated separately the median PFS in patients completely debulked and in group of patients underwent optimal and suboptimal cytoreductive surgery, and we found a median PFS of 69.3 months (95% CI: 51.2–89.7) in the first group and a median PFS of 13.6 months (95% CI: 8.8–20.7), respectively. In addition, we assessed the PFS and OS in our patients after excluding the stage I women, and we found a median PFS of 39.2 months (95% CI: 28.5–52.3) and a median OS of 103.2 (95% CI: 81.3-not reached).
Molecular analysis
Seventy-nine of the 128 patients provided FFPE tumor: of these, 56 were considered suitable for FoundationOne CDX (Foundation Medicine®) tissue-testing and 23 failed at the mutational analysis. The reason given by Foundation Medicine® for sample failure were as follows: two specimens did not have adequate nucleated cellularity, three did not yield sufficient DNA and eighteen did not meet the minimum performance specifications in preparations for sequencing. Of the 56 patients who had the mutation analyses done, 53 (94.6%) were tissues deriving from primary tumor and 3 (5.4%) were tissues from recurrent tumor obtained at the time of secondary surgery. In this report we have focused on 45/56 patients harboring mutation of genes involved in HRR pathway, in MAPK pathway and in Endocrine-Resistance pathway.
A summary of mutations detected are listed in Table 2 and other details are summarized in Table 3.
Table 2.
Frequency of mutations in HRR-pathway, MAPK-pathway and Endocrine-Resistance pathway detected in 56 patients underwent molecular profile.
| Type of mutations | N (%) | Pathogenetic, n | VUS, n |
|---|---|---|---|
| HRR- pathway | |||
| ATR | 3 (5) | 0 | 3 |
| BARD1 | 1 (2) | 0 | 1 |
| BRCA1 | 2 (4) | 2 | 0 |
| BRCA2 | 5 (9) | 1 | 4 |
| BRIP1 | 1 (2) | 0 | 1 |
| CHEK2 | 2 (4) | 0 | 2 |
| FANCA | 8 (14) | 0 | 8 |
| FANCG | 1 (2) | 0 | 1 |
| PALB2 | 4 (7) | 1 | 3 |
| RAD21 | 1 (2) | 0 | 1 |
| RAD51B | 2 (4) | 0 | 2 |
| RAD52 | 1 (2) | 0 | 1 |
| MAPK- pathway | |||
| BRAF | 6 (11) | 6 | 0 |
| ERBB2 | 3 (5) | 1 | 2 |
| KRAS | 12 (21) | 12 | 0 |
| NF1 | 7 (13) | 4 | 3 |
| NF2 | 2 (4) | 1 | 1 |
| NRAS | 8 (14) | 8 | 0 |
| Endocrine Resistance-pathway | |||
| AKT1 | 1 (2) | 1 | 0 |
| CDKN2A/B | 11 (20) | 11 | 0 |
| ESR1 | 2 (4) | 0 | 2 |
| PIK3CA | 3 (5) | 2 | 1 |
Table 3.
Molecular details of 45/56 patients with molecular profiling.
| ID | Age (Years) | Stage | Key variants | Type of alteration | MSI | TMB (Mutation/Mb) |
|---|---|---|---|---|---|---|
| LGSOC 1 | 69 | III | NF1 | Loss exons 13–29 | MSS | 3 |
| LGSOC 2 | 65 | III | KRAS; NF1a | G12V P678T | MSS | 1 |
| LGSOC 3 | 36 | III | KRAS | G12D | MSS | 0 |
| LGSOC 4 | 59 | I | KRAS | G12V | MSS | 1 |
| LGSOC 5 | 28 | III | ERBB2 | A775_G776insYVM A | MSS | 1 |
| LGSOC 6 | 73 | I | CHEK2a; BRAF | L363F V600E | MSS | 0 |
| LGSOC 7 | 58 | I | RAD52a; KRAS | E288K G12D | MSS | 1 |
| LGSOC 8 | 26 | IV | KRAS | Q61K | MSS | 1 |
| LGSOC 9 | 55 | III | FANCAa; PALB2 | L1138V H762fs*8 | N/A | N/A |
| LGSOC 10 | 58 | III | RAD51Ba | Amplification | MSS | 1 |
| LGSOC 11 | 53 | III | FANCAa; ERBB2a; NF2; CDKN2A/B | A746S amplification R57a loss | MSS | 1 |
| LGSOC 12 | 36 | III | BRAF | V600E | MSS | 4 |
| LGSOC 13 | 71 | III | NF1 | Loss exons 21–47 | MSS | 0 |
| LGSOC | 58 | III | FANCGa; | N167S | MSS | 0 |
| 14 | RAD21a; | AMPLIFICATION | ||||
| ERBB2a | Q57R | |||||
| LGSOC 15 | 69 | III | NRAS; CDKN2A/B | Q61R loss | MSS | 0 |
| LGSOC 16 | 54 | III | NRAS | Q61R | MSS | 6 |
| LGSOC 17 | 32 | III | BRAF | MKRN1-BRAF fusion | MSS | 3 |
| LGSOC 18 | 45 | III | NFI | F443fs*30 | N/A | N/A |
| LGSOC 19 | 66 | III | RAD51Ba; KRAS | R159H G12D | MSS | 3 |
| LGSOC 20 | 43 | III | BRCA1; BRAF CDKN2A/B | Loss exons 2–21 G596R loss | MSS | 0 |
| LGSOC 21 | 61 | III | ATRa; NRAS | R515H Q61R | MSS | 1 |
| LGSOC 22 | 59 | III | BRIP1a; KRAS | P47A G12V | N/A | N/A |
| LGSOC 23 | 33 | I | ATRa; BRAF | E665K L485S | MSS | 1 |
| LGSOC 24 | 52 | III | AKT1; ESR1a | E114Q A59V | N/A | N/A |
| LGSOC 25 | 53 | III | BARD1a; BRCA2a; PIK3CAa | Q730P E3002D amplification | MSS | 6 |
| LGSOC 26 | 65 | III | NF1a; CDKN2A/B | N45S CDKN2A/B p16INK4a loss and p14ARF loss exons 2–3 | MSS | 1 |
| LGSOC 27 | 52 | III | FANCAa; PALB2a; NRAS | L1143V L939W Q61R | MSS | 0 |
| LGSOC 28 | 68 | III | NRAS | Q61R | MSS | 1 |
| LGSOC 29 | 67 | III | NF1 | S557fs*11 | MSS | 3 |
| LGSOC 30 | 58 | III | KRAS | Q61R | MSS | 0 |
| LGSOC 31 | 63 | I | FANCAa; | L1143V | MSS | 1 |
| LGSOC 32 | 39 | III | CHEK2a; PALB2a; NRAS | L183F K957Q Q61R | MSS | 1 |
| LGSOC 33 | 60 | II | NRAS; PIK3CA | Q61K N345K | MSS | 8 |
| LGSOC 34 | 26 | III | CDKN2A/B | Loss | MSS | 1 |
| LGSOC 35 | 40 | III | BRCA2; NF1a | Splice site 632–2A>G V253del | MSS | 1 |
| LGSOC 36 | 68 | III | FANCAa; KRAS; CDKN2A/B | R1011C G12R loss | MSS | N/A |
| LGSOC 37 | 50 | III | BRCA2a; KRAS; CDKN2A/B | I505T G12V p16INK4a loss and p14ARF loss exon 23 | MSS | 0 |
| LGSOC 38 | 48 | I | BRAF | V600E | MSS | 0 |
| ID | Age | Stage | Key Variants | Type of Alteration | MSI | TMB |
| (Years) | (Mutation/Mb) | |||||
| LGSOC 39 | 75 | III | PALB2a; NF2a; CDKN2A/B | Rearrangement I495V p16INK4a loss and p14ARF loss exon 23 | MSS | 0 |
| LGSOC 40 | 33 | I | KRAS | G12V | MSS | 4 |
| LGSOC 41 | 41 | I | BRCA1; BRCA2a FANCAa KRAS CDK2A/B PIK3CA | Rearrangement exon 10 G1771D H1000Q A146T Loss N345I M278I | MSS | 0 |
| LGSOC 42 | 80 | III | NRAS CDKN2A | Q61R rearrangement intron 1 | MSS | 1 |
| LGSOC 43 | 45 | III | FANCAa; | L1138V | N/A | 6 |
| LGSOC 44 | 37 | III | ATRa; CDKN2A/B | V1267I loss | MSS | 1 |
| LGSOC 45 | 25 | III | BRCA2a; FANCAa; ESR1a | K3326 T126R H6Y | MSS | 0 |
MSS Microsatellite stable, MSI Microsatellite instable, TMB tumor mutational burden.
aVariant of Unknown Significance (VUS).
The most common Foundation Medicine® mutations identified (including pathogenetic and variant of unknown significance) were: KRAS (n = 12, 21%), CDKN2A/B (n = 11, 20%), NRAS (n = 8, 14%), FANCA (n = 8, 14%), NF1 (n = 7, 13%), and BRAF (n = 6, 11%). Unexpectedly, pathogenetic BRCA1 (n = 2), BRCA2 (n = 1) and PALB2 (n = 1) mutations were found in some patients. Furthermore, about the genes involved in endocrine-resistance pathway, pathogenetic CDKN2A/B (n = 11), PIK3CA (n = 2) and AKT1 (n = 1) mutations were identified.
Among the 23 patients harboring mutation of genes involved in HRR-pathway, 18 women were evaluable for response: among them 3 CR (16.7%), 2 PR (11.1%) and 1 SD (5.6%) to the first line platinum-based chemotherapy were reported. Similarly, among 36 women with mutated genes of the MAPK- pathway, 30 women underwent first-line chemotherapy, of which 3 CR (10%), 5 PR (16.7%) and 1 SD (3.3%) were observed. In the group of 14 patients harboring mutation of genes involved in endocrine-resistance pathway, 1 patient had FIGO stage I and 13 women received first line chemotherapy: 3 PR (23.1%) and 2 SD (15.4%) were identified; no complete responses were recorded in this group.
Additionally, we evaluated response rate to subsequent chemotherapy lines for each group: in the HRR altered pathway group we observed 21.4% PR and 35% SD, in the MAPK altered pathway group 5% CR, 30% PR and 35% SD, and finally in endocrine-resistance altered pathway group 12.5% PR and 16.7% SD.
Survival analysis was performed in each of these three groups. Since HRD is a widely recognized biomarker of platinum sensitivity, we evaluated survival outcomes in terms of PFS and OS, in the HRR-deficient group and HRR-proficient group, finding a trend of survival advantage in HRD-positive patients compared to HRD-negative patients. Specifically, the median PFS was 47.9 months (95% CI: 27.3-not reached) and 31.5 months (95% CI: 14.4–39.7) in HRD-positive and HRD-negative groups, respectively; the median OS was 74.2 months (95% CI: 66.2-not reached) in HRD-positive patients and 72.1 months (95% CI: 33.4-.) in HRD-negative group. Moreover, the median PFS was 47.8 months (95% CI: 20.7–70.1) in MAPK-pathway group, and 31.5 months (95% CI: 14.5–50.4) in endocrine-resistance pathway group and the median OS was 82.7 months (95% CI: 56.3-not reached) in MAPK-pathway group and 72.1 months (95% CI: 42.5-not reached) in endocrine-resistance pathway group.
In addition, we evaluated response to hormone-therapy when administered in first, second and third treatment line in patients with mutated genes of the endocrine-resistance pathway. Of 14 patients harboring these mutations, only 7 received hormone-therapy and all showed SD as best response.
Discussion
LGSC is a rare histological subtype of epithelial ovarian and peritoneal cancer. To date, few available data have highlighted the clinical aspects and the prognosis of this disease, and the most appropriate therapeutic approach has not yet been defined. In our series we describe the clinical outcome and the molecular aspects of a large cohort of LGSCs.
LGSCs are characterized by indolent course and published data showed better outcome of this disease with respect to HGSOCs. In this context, in their comparative analysis, Gockley et al. demonstrated a median OS of 90.8 months (95% CI = 78.7–106.3) and 40.7 months (95% CI = 40.08–41.5) in low-grade and high-grade ovarian cancers, respectively [2]. According to literature data demonstrating a longer OS in these patients [26], in our series we found a median OS of 105.3 months (95% CI = 82.7–not reached). On the other hand, in our population we found a longer PFS of 43.9 months (95% CI: 32.4–53.0) than that described in the literature, highlighting this finding a meaningful role of complete cytoreductive surgery on the survival outcome.
Interestingly, we found a higher ORR to standard chemotherapy than that reported in previous studies in the recurrent setting. Specifically, response rate to cytotoxic agents at the time of relapse (including second and third subsequent therapy lines) was 33%. These data are interesting and need to be interpreted with caution. Over the past decade, a series of publications have indicated that LGSC is less chemo sensitive than HGSOC. Gershenson et al., reported an ORR of only 3.7% in a cohort of 58 patients with relapsed disease treated with conventional chemotherapy [4]. Additionally, in a study of 48 women with low-grade primary peritoneal cancer receiving chemotherapy, 66.7% of patients were noted to have persistent or progressive disease [27]. Furthermore, Schmeler et al., identified 25 women who received neoadjuvant chemotherapy, with ORR of only 4% [28]. Given these reports, some authors suggested that LGSCs are resistant to standard cytotoxic agents, and conventional chemotherapies should be abandoned, as result. In our view, this perspective is premature. Although LGSOC is less chemotherapy sensitive respect to HGSOC, it is not completely chemotherapy resistant. In the MILO study, the responses to chemotherapy were greater than in previous reported single institution retrospective series, with an ORR of 13% and a CBR of 73% [15]. On the other hand, we noted that in our analysis the majority of recurrent LGSOCs that achieved complete or partial response were platinum sensitive patients and received platinum-based chemotherapy plus bevacizumab at the time of relapse. Given the high rate of complete and partial responses that we found in the group of patients treated with Bevacizumab, these findings may justify the higher ORR reported in our series. Indeed, some authors suggested that bevacizumab have an antitumor activity in recurrent LGSC setting. Recently, Dalton et al., reported an ORR of 47.5% in a cohort of forty patients with recurrent low-grade serous disease treated with bevacizumab [29]. The same results were showed previously by Grisham et al., that reported an ORR of 45% among 17 serous borderline and LGSCs treated with bevacizumab alone or in conjunction with chemotherapy at the time or recurrence [30].
Another important issue to investigate is response rate to hormone therapy. The benefit of hormone therapy in LGSOC have been widely demonstrated and our findings reinforce this issue. Gershenson et al., evaluated the activity of hormone therapy in recurrent LGSOC showing an ORR of 9% and a CBR of 82.7% [19]. Overlapping results were found in our cohort of patients with an ORR of 8% and a CBR of 82.6%. Data on immunohistochemical evaluation of ER and PG receptors are no available in our report and may be a focus for future research.
Regarding the genomic analysis, it is known that precision medicine is growing enormously in the management of many types of cancers. The detection of “oncogenic driver” mutations and the use of targeted therapy has dramatically improved prognosis of some tumors. Due to relatively reported chemoresistance of LGSC, in the last few years growing attention has been dedicated to its molecular landscape, and published data showed that these patients often carry mutations of genes involved in MAPK-pathway [31]. These findings led to evaluate the activity of MEK inhibitors in several prospective clinical trials with conflicting results reported. In the MILO trial, binimetinib was evaluated in persistent and recurrent LGSCs and no significant difference in the primary endpoint of PFS versus physician’s choice chemotherapy was achieved [5]. Contrary, the prospective randomized trial GOG 281 demonstrated statistically significant superiority of trametinib when compared to standard of care in terms of PFS. In addition, a post hoc analyses of the two clinical trials demonstrated that the presence of the MAPK-pathway mutation appears to be a predictive biomarker [31]. Indeed, patients with recurrent LGSOC harboring a KRAS mutation and treated with binimetinib displayed a longer PFS (median 17.7 vs 10.8 months) and were 3.4 times more likely to have a complete or partial radiographic response to treatment when compared to those that were KRAS wild type [32]. A similar trend was found for patients treated with binimetinib harboring any MAPK mutation (ORR of 41% in those with binimetinib versus 13% in those without) [32].
In GOG 281 trial, the same treatment effect was observed. Patients harboring activating mutations in KRAS, BRAF, or NRAS (HR 0.55 [95% CI 0.28–1.07]) respect to mutation-negative patients (0.64 [0.39–1.03]) [14] had a PFS benefit with trametinib treatment.
On the contrary, in a phase II prospective trial, Farley et al., demonstrated that Selumetinib is a well-tolerated and an active treatment in recurrent LGSOCs with PFS > 6 months achieved in 63% of patients but response to this MEK inhibitor did not appear to be related with RAS/RAF mutational status [16]. However, these results should be interpreted with caution, because the analysis was carried out on a small sample and despite the molecular assessment in most patients (82%) was made on the primary tissue tumor, the concordance of BRAF or KRAS mutational activation between primary and recurrent/metastatic disease has not been adequately studied [16].
These published results are appealing suggesting that molecular subtyping of LGSOCs still remains an open field for research.
In our series, further to the more typical mutation related to LGSOC, we found mutations of genes involved in HRR- pathway (ATR, BARD1, BRCA 1 /2, BRIP1, CHEK2, FANCA, FANCG, PLAB2, RAD21, RAD51B and RAD52) with two cases of BRCA1, one case of BRCA2, and one case of PALB2 pathogenetic mutations. In a recently published retrospective analysis evaluating the mutational spectra of 17 LGSOC, several variants in multiple HR pathway genes were observed, including five BRCA2 variants, with two being confirmed to be of germline origin [22]. These findings could suggest that heterogeneity is strong in patients with LGSOC identified on a morphological base, and that some mutations identify patients that could benefit from chemo or PARP inhibitors; this hypothesis should be tested in prospective trials.
Besides, in our series we found that 20% of patients (n = 11) harbored CDKN2A/B mutations and that patients with mutations of genes involving in hormone resistance pathway (including CDKN2A/B, AKT1, ESR1 and PIK3CA) showed a shorter PFS of 31.5 months (95% CI; 4.5; 50.4) and a shorter OS of 72.1 months (95% CI 42.5-not reached) compared to patients harboring mutations of genes involved in the other two pathways evaluated. This finding highlights the previous reported data suggesting that CDKN2A/B aberrations are enriched in OC cases with shorter survival [33] and potential drug target of CDKN2A could represent a promising avenue for therapeutic intervention to improve outcomes for these patients.
In our study, due to the small numbers, we failed to correlate the mutational landscape with the response to chemo and hormone therapy. Of course, the study suffers of the intrinsic limitation related to the retrospective nature of the data collection. However, to our knowledge, this is the largest genomic study of this rare disease reported, analyzing 324 genes. International collaboration should be undertaken to verify if a better molecular classification of LGSOC might help in guiding patients toward chemotherapy, hormone therapy of other target-based drugs.
Supplementary information
Acknowledgements
The authors are grateful to Gelsomina Iovane, Margherita Tambaro and Angela Maria Trujillo for data management.
Author contributions
SP conceived the study; LM, DC and SP contributed to methodology; All authors collected the data; DC, GFZ, NSL were responsible for the assessment of the primary FFPE tumor tissue, including the estimation of tumor cellularity; LM and LA interpreted and analyzed the data; LM wrote the manuscript; LA, LM, DC and SP played a key role in interpreting the results and revised the article critically for important intellectual content; SP supervised the project and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; All authors reviewed and approved the final version of the manuscript.
Funding
A specific funding was received for this work. The research leading to these results has received funding from AIRC under IG 2016 – ID. 18921 and IG 2021 – ID. 25932 projects – P.I. SP and CO-2018–12367051 (Ministero della Salute) P.I SP; Ricerca Corrente grant M2/7 from Ministero della Salute to DC, Ricerca Corrente from Ministero della Salute to SP.
Data availability
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Competing interests
DL reports research funding from Clovis, GSK and MSD, personal interests with AstraZeneca, Clovis Oncology, GSK, Pharmamar, MSD and financial interests with Clovis, Genmab, GSK, MSD. Board of Directors, GCIG (Gynecologic Cancer Inter Group). FR reports honoraria from GSK, Pharmamar, Clovis, MSD and Roche. VS reports honoraria from GSK, PharmaMar, Roche, MSD, EISAI, Clovis, Oncology, AstraZeneca. FP reports honoraria for educational and advisory activity from Incyte, GSK, Eli Lilly, Ipsen, Astellas, AstraZeneca, Roche, BMS, Bayer, Clovis, Pierre Fabre and grants for clinical trials to his institution from Roche, Astra Zeneca, Pfizer, MSD, Bayer, Incyte Taiho, Janssen, Exelixis, Ailenor, Daiichi Sankyo. GS reports research support from MSD and honoraria from Clovis Oncology. Consultant for Tesaro and Johnson & Johnson. SP reports honoraria from AstraZeneca, MSD, Roche, Pfizer, Clovis, GSK, Pharmamar and research funding from MSD, Roche, Astrazeneca and Pfizer. LM, DC, MB, LA, NSL, GC, SG, GV, CP, AS, DR, MDS, VC, FF, GFZ, VL, VG, CC, VT, MD, VDV, SS and DP have nothing to disclose.
Ethics approval and consent to participate
This study was approved by the ethics committee of the National Cancer Institute of Naples (22/14 OSS) and the ethics committees of the participating centers, and it was conducted in accordance with the Declaration of Helsinki. The study participants gave written informed consent.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01897-1.
References
- 1.Slomovitz B, Gourley C, Carey MS, Malpica A, Shih IM, Huntsman D, et al. Low-grade serous ovarian cancer: state of the science. Gynecol Oncol. 2020;156:715–25. doi: 10.1016/j.ygyno.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 2.Gockley A, Melamed A, Bregar AJ, Clemmer JT, Birrer M, Schorge JO, et al. Outcomes of women with high-grade and low-grade advanced-stage serous epithelial ovarian cancer. Obstet Gynecol. 2017;129:439–47. doi: 10.1097/AOG.0000000000001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowski JP, Harter P, Heitz F, Pujade-Lauraine E, Reuss A, Kristensen G, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol. 2016;140:457–62. doi: 10.1016/j.ygyno.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Gershenson DM, Sun CC, Bodurka D, Coleman RL, Lu KH, Sood AK, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114:48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Monk BJ, Grisham RN, Banerjee S, Kalbacher E, Mirza MR, Romero I, et al. MILO/ENGOT-ov11: binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J Clin Oncol. 2020;38:3753–62. doi: 10.1200/JCO.20.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer G, Stöhr R, Cope L, Dehari R, Hartmann A, Cao DF, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29:218–24. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 7.Van Nieuwenhuysen E, Busschaert P, Laenen A, Moerman P, Han SN, Neven P, et al. Loss of 1p36.33 frequent in low-grade serous ovarian cancer. Neoplasia. 2019;21:582–90.. doi: 10.1016/j.neo.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter SM, Anglesio MS, Ryland GL, Sharma R, Chiew YE, Rowley SM, et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget. 2015;6:37663–77. doi: 10.18632/oncotarget.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rambau PF, Vierkant RA, Intermaggio MP, Kelemen LE, Goodman MT, Herpel E, et al. Association of p16 expression with prognosis varies across ovarian carcinoma histotypes: an Ovarian Tumor Tissue Analysis Consortium study. J Pathol Clin Res. 2018;4:250–61.. doi: 10.1002/cjp2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer G, Oldt R, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 11.Wong KK, Tsang YTM, Deavers MT, Mok SC, Zu Z, Sun C, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177:1611–7. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmanuel C, Chiew Y-E, George J, Etemadmoghadam D, Anglesio MS, Sharma R, et al. Genomic classification of serous ovarian cancer with adjacent borderline differentiates RAS pathway and TP53-mutant tumors and identifies NRAS as an oncogenic driver. Clin Cancer Res. 2014;20:6618–30. doi: 10.1158/1078-0432.CCR-14-1292. [DOI] [PubMed] [Google Scholar]
- 13.Cheasley D, Nigam A, Zethoven M, Hunter S, Etemadmoghadam D, Semple T, et al. Genomic analysis of low-grade serous ovarian carcinoma to identify key drivers and therapeutic vulnerabilities. J Pathol. 2021;253:41–54. doi: 10.1002/path.5545. [DOI] [PubMed] [Google Scholar]
- 14.Gershenson DM, Miller A, Brady WE, Paul J, Carty K, Rodgers W, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399(Feb):541–53. doi: 10.1016/S0140-6736(21)02175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monk BJ, Grisham RN, Banerjee S, Kalbacher E, Mirza MR, Romero I, et al. MILO/ENGOT-ov11: binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J Clin Oncol. 2020;38(Nov):3753–62. doi: 10.1200/JCO.20.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farley J, Brady WE, Vathipadiekal V, Lankes HA, Coleman R, Morgan MA, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14:134–40. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobar J, Klimowicz AC, Dean M, Chu P, Nation JG, Nelson GS, et al. Quantification of ER/PR expression in ovarian low-grade serous carcinoma. Gynecol Oncol. 2013;128:371–6. doi: 10.1016/j.ygyno.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Buttarelli M, Mascilini F, Zannoni GF, Ciucci A, Martinelli E, Filippetti F, et al. Hormone receptor expression profile of low-grade serous ovarian cancers. Gynecol Oncol. 2017;145:352–60.. doi: 10.1016/j.ygyno.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Gershenson DM, Sun CC, Iyer RB, Malpica AL, Kavanagh JJ, Bodurka DC, et al. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2012;125:661–6. doi: 10.1016/j.ygyno.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershenson DM, Bodurka DC, Coleman RL, Lu KH, Malpica A, Sun CC. Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum. J Clin Oncol. 2017;35:1103–11. doi: 10.1200/JCO.2016.71.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang M, O’Connell RL, Amant F, Beale P, McNally O, Sjoquist KM, et al. PARAGON: a Phase II study of anastrozole in patients with estrogen receptor-positive recurrent/metastatic low-grade ovarian cancers and serous borderline ovarian tumors. Gynecol Oncol. 2019;154:531–8. doi: 10.1016/j.ygyno.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Devins K, Ko EM, Reyes MC, Simpkins F, Drapkin R, et al. Mutational spectrum in clinically aggressive low-grade serous carcinoma/serous borderline tumors of the ovary-Clinical significance of BRCA2 gene variants in genomically stable tumors. Gynecol Oncol. 2021;161:762–8. doi: 10.1016/j.ygyno.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Prat J, FIGO Committee on Gynecologic Oncology Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Calzolari A, Napolitano M, Bravo E. Review of the Italian current legislation on research biobanking activities on the eve of the participation of national biobanks’ network in the legal consortium BBMRI-ERIC. Biopreserv Biobank. 2013;11:124–8. doi: 10.1089/bio.2012.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gershenson DM, Sun CC, Wong KK. Impact of mutational status on survival in low-grade serous carcinoma of the ovary or peritoneum. Br J Cancer. 2015;113:1254–8. doi: 10.1038/bjc.2015.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmeler KM, Sun CC, Malpica A, Deavers MT, Bodurka DC, Gershenson DM. Low-grade serous primary peritoneal carcinoma. Gynecol Oncol. 2011;121:482–6. doi: 10.1016/j.ygyno.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Schmeler KM, Sun CC, Bodurka DC, Deavers MT, Malpica A, Coleman RL, et al. Neoadjuvant chemotherapy for low-grade `or peritoneum. Gynecol Oncol. 2008;108:510–4. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Dalton HJ, Fleming ND, Sun CC, Bhosale P, Schmeler KM, Gershenson DM. Activity of bevacizumab-containing regimens in recurrent low-grade serous ovarian or peritoneal cancer: a single institution experience. Gynecol Oncol. 2017;145:37–40. doi: 10.1016/j.ygyno.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grisham RN, Iyer G, Sala E, Zhou Q, Iasonos A, DeLair D, et al. Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int J Gynecol Cancer. 2014;24:1010–4. doi: 10.1097/IGC.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaldawy A, Segev Y, Lavie O, Auslender R, Sopik V, Narod SA. Low-grade serous ovarian cancer: a review. Gynecol Oncol. 2016;143:433–8. doi: 10.1016/j.ygyno.2016.08.320. [DOI] [PubMed] [Google Scholar]
- 32.Grisham RN, Vergote I, Banerjee SN, Drill EN, Fabbro M, Mirza MR, et al. Molecular results and potential biomarkers identified from MILO/ENGOT-ov11 phase 3 study of binimetinib versus physicians’ choice of chemotherapy (PCC) in recurrent low-grade serous ovarian cancer (LGSOC) J Clin Oncol. 2021;39:5519–5519. doi: 10.1200/JCO.2021.39.15_suppl.5519. [DOI] [Google Scholar]
- 33.Dong Y, Walsh MD, McGuckin MA, Gabrielli BG, Cummings MC, Wright RG, et al. Increased expression of cyclin-dependent kinase inhibitor 2 (CDKN2A) gene product P16INK4A in ovarian cancer is associated with progression and unfavorable prognosis. Int J Cancer. 1997;74:57–63. doi: 10.1002/(SICI)1097-0215(19970220)74:1<57::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.


