Abstract
Background
It has been considered that activation of peripheral μ-opioid receptors (MORs) induces side effects of opioids. In this study, we investigated the possible improvement of the immune system in tumour-bearing mice by systemic administration of the peripheral MOR antagonist naldemedine.
Methods
The inhibitory effect of naldemedine on MOR-mediated signalling was tested by cAMP inhibition and β-arrestin recruitment assays using cultured cells. We assessed possible changes in tumour progression and the number of splenic lymphocytes in tumour-bearing mice under the repeated oral administration of naldemedine.
Results
Treatment with naldemedine produced a dose-dependent inhibition of both the decrease in the cAMP level and the increase in β-arrestin recruitment induced by the MOR agonists. Repeated treatment with naldemedine at a dose that reversed the morphine-induced inhibition of gastrointestinal transport, but not antinociception, significantly decreased tumour volume and prolonged survival in tumour-transplanted mice. Naldemedine administration significantly decreased the increased expression of immune checkpoint-related genes and recovered the decreased level of toll-like receptor 4 in splenic lymphocytes in tumour-bearing mice.
Conclusions
The blockade of peripheral MOR may induce an anti-tumour effect through the recovery of T-cell exhaustion and promotion of the tumour-killing system.

Subject terms: Pharmacodynamics, Cancer immunotherapy
Background
Although opioid analgesics, such as morphine, have very strong analgesic effects and are widely used to manage moderate to severe pain, they cause various side effects [1]. These opioid analgesics have the highest affinity for μ-opioid receptors (MORs). The MOR is expressed in not only the brain and spinal cord but also a wide range of peripheral sites, including the lungs, gastrointestinal tract, and immune cells [2, 3]. The central MOR is highly associated with analgesia, whereas the peripheral MOR is responsible for side effects such as constipation and nausea. In particular, MOR is highly expressed throughout the gastrointestinal tract, and binding of opioids reduces intestinal peristalsis, resulting in opioid-induced constipation (OIC) [4].
Recently, antagonism of peripheral MORs has been reported to reduce opioid-related side effects, analgesic tolerance and opioid-induced hyperalgesia [5]. On the other hand, basic and clinical studies have proposed that MORs may play a role in cancer progression. The blockade of peripheral MORs has been shown to suppress tumour growth in mice [6]. Furthermore, treatment with the peripheral MOR antagonist methylnaltrexone significantly prolongs survival compared with placebo in patients with advanced cancer [7]. These findings strongly support the idea that the blockade of peripheral MORs may be beneficial for cancer patients. However, little is known about the role of peripheral MORs in the function of immune cells under a cancer state.
Naldemedine has recently been approved for the treatment of OIC in Japan. Naldemedine is an amide derivative of the opioid receptor antagonist naltrexone, which acts only in the periphery by limiting passage through the blood-brain barrier by steric hindrance caused by carbamoyl groups [8, 9]. The use of naldemedine is believed to have no influence on the analgesic effects of opioids in cancer patients using opioids and is expected to improve OIC. In this study, we sought to investigate the effect of antagonising peripheral MORs by the administration of naldemedine on tumour growth and the possible immune mechanism of this event.
Materials and methods
Drugs
The drugs used in this study were morphine hydrochloride (Daiichi-Sankyo Co., Ltd., Tokyo, Japan), fentanyl citrate (Daiichi-Sankyo Co., Ltd.), methylnaltrexone bromide (Merck KGaA), loperamide hydrochloride (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) and naldemedine tosylate. Naldemedine tosylate was synthesised by us. Almost no impurities were observed in the synthesized naldemedine, as shown in the proton and carbon NMR spectra. All drugs were dissolved in saline, except that naldemedine was dissolved in dimethyl sulfoxide (DMSO) or methyl cellulose solution. All drugs were administered in a volume of 0.1 ml/10 g body weight.
cAMP assay
To measure the inhibition of the intracellular cAMP level by activation of MORs, GloSensorTM cAMP Assay (Promega Corp., Madison, WI, USA) was performed according to the manufacturer’s protocol. Briefly, HEK293 cells stably expressing Halo-tagged human MOR and GloSensor-22F cAMP were used. Following pre-incubation with naldemedine for 10 min, each well was treated with morphine or fentanyl for 10 min. Subsequently, 3 µM forskolin was added to each well, and then incubated for 50 min. The intracellular cAMP level, as reflected by chemiluminescence, was measured for each well by a microplate reader (GloMax®, Promega Corp.).
β-Arrestin-2 recruitment assay
β-Arrestin-2 recruitment after activation of MORs was analysed to determine the agonist/antagonist activity of ligands for MORs using a PathHunter® eXpress OPRM1 CHO-K1 β-arrestin GPCR Assay (Eurofins DiscoverX products, Fremont, CA, USA) according to the manufacturer’s protocol. CHO-K1 cells stably expressing human MORs were used. Briefly, 30 min after treatment with naldemedine, morphine or fentanyl was treated and incubated for 90 min. β-galactosidase activity, as reflected by chemiluminescence, was measured by a microplate reader (GloMax®, Promega Corp.).
Animals
Male ICR mice (20–25 g, Tokyo Laboratory Animals Science Co. Ltd, Tokyo, Japan) were used for the hot-plate test followed by the gastrointestinal transit test (n = 81 mice) and locomotor assay (n = 45 mice). C57BL/6J mice at age 7–8 weeks (Jackson Laboratory, Bar Harbor, ME, USA) were used for graft tumour growth assay (n = 96 mice). Mice were housed in a room maintained at 24 ± 1 °C with a 12 h light–dark cycle (light on 8:00 a.m. to 8:00 p.m.). Food and water were available ad libitum in their home cages. Power calculation for sample sizes was not performed. Mice were randomly selected for each group by cage prior to baseline testing. No formal blinding was used for in vivo experiments, but various steps in key studies were performed by different investigators, who were each independent of the steps performed by the other investigator. The present study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals at Hoshi University, as adopted by the Committee on Animal Research of Hoshi University. Every effort was made to minimise the numbers and any suffering of animals used in the following experiments.
Hot-plate test
The nociceptive response on a hot plate (55 ± 0.5 °C; Muromachi Kikai Co., Ltd., Tokyo, Japan) was evaluated by recording the latency to paw-licking or -tapping as described previously [10]. To avoid tissue damage, a cut-off time of 30 s was selected. Antinociceptive effects were measured at the peak time after the administration of morphine (1–20 mg/kg, s.c.). Naldemedine (1, 3 mg/kg, p.o.) was administered 15 min before treatment with morphine. Antinociception was calculated as a percentage of the maximum possible effect (% antinociception) according to the following formula:
The antinociceptive response represents the mean ± SEM of the % antinociception.
Gastrointestinal transit
Gastrointestinal transit was determined based on a previous method [10]. Briefly, at 15 min after the administration of naldemedine (1, 3 mg/kg, p.o.), each animal was injected with morphine (1–20 mg/kg, s.c.). At the peak time of morphine, ink (0.3 ml/mouse; Pilot Co. Ltd., Tokyo, Japan) was administered orally. The movement of ink within the small intestine for 30 min was measured. The percentage inhibition of gastrointestinal transit was calculated as follows:
Locomotor assay
As described previously [11], the locomotor activity of mice was measured by an ambulometer (Three-point Meter, O’Hara & Co., Ltd., Tokyo, Japan) using ICR mice. After a 60-min habituation period, the total activity counts in each 5-min window were automatically recorded for 180 min after the administration of morphine (1–10 mg/kg, s.c.). Naldemedine (1, 3 mg/kg, p.o.) was administered 15 min before treatment with morphine.
Cell culture
The murine Lewis lung carcinoma (LLC) cells and B16 melanoma cells were cultured in α-MEM (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% foetal bovine serum (Thermo Fisher Scientific, Inc.) containing 1% penicillin–streptomycin (Thermo Fisher Scientific, Inc.). Cells were maintained in a water-humidified incubator at 37 °C with 5% CO2.
Graft tumour growth assay
LLC cells were resuspended at a concentration of 2 × 106 cells/0.5 mL in a mixture of extracellular matrix gel (Merck KGaA) and Hank’s balanced salt solution (HBSS; Thermo Fisher Scientific, Inc.) (3:1). To evaluate tumour growth, suspended LLC cells in a volume of 0.5 mL were subcutaneously transplanted into the right lower back of C57BL/6J mice under isoflurane anaesthesia (3%; FUJIFILM Wako Pure Chemical Corp.). Tumour volume was monitored for 14 or 21 days after transplantation using a calliper. Tumour volume was calculated as (L × W2)/2, (L: length and W: width).
Naldemedine (p.o.) and loperamide (s.c.) were administered once and twice a day, respectively, based on their half-lives. Methylnaltrexone was continuously administered by the subcutaneous implantation of an ALZET mini pump (0.25 µL/h, DURECT Corp., Cupertino, CA, USA).
Fluorescence-activated cell sorting (FACS)
Twenty-one days after the transplantation of LLC cells, the spleen was isolated under isoflurane anaesthesia (3%, inhalation), and then homogenised with PBS by pipetting. To remove cell aggregate, the homogenised suspension was segregated using a 100-μm cell strainer and a nylon mesh. Subsequently, ammonium chloride was applied to a single-cell suspension for haemolysis, and the cell suspension was fractionated at 6 × 106 cells/tube. For blocking, cells were treated with an anti-CD16/32 antibody (BD Biosciences, Inc., Franklin Lakes, NJ, USA). Immune cells were labelled with antibodies (Supplementary Table 1), whereas dead cells were stained with propidium iodide (PI). Immune cells were sorted using a BD FACS AriaTM II Cell Sorter (BD Biosciences, Inc.) and then analysed.
Quantitative reverse-transcription polymerase chain reaction (RT-qPCR)
Total RNAs were extracted using the mirVanaTM miRNA Isolation Kit (Thermo Fisher Scientific, Inc.) from immune cells sorted by FACS, and then cDNAs were synthesised using the SuperScript® VILOTM cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.). RT-qPCR was performed using specific primer pairs and Fast SYBR® Green Master Mix (Thermo Fisher Scientific, Inc.). Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as an internal control. Supplementary Table 2 lists all primers used in this study.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Key experiments were performed at least twice. Normal data distribution was confirmed by Shapiro–Wilk test. Data were evaluated by the unpaired t test, one-way ANOVA followed by Tukey’s post hoc test or two-way repeated measures ANOVA followed by Bonferroni’s post hoc test. To compare the survival distributions of two groups, a log-rank test was used. Prism software (ver 8.0; GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis, and differences were considered significant at P < 0.05.
Results
Inhibitory effect of naldemedine on MOR-mediated signalling
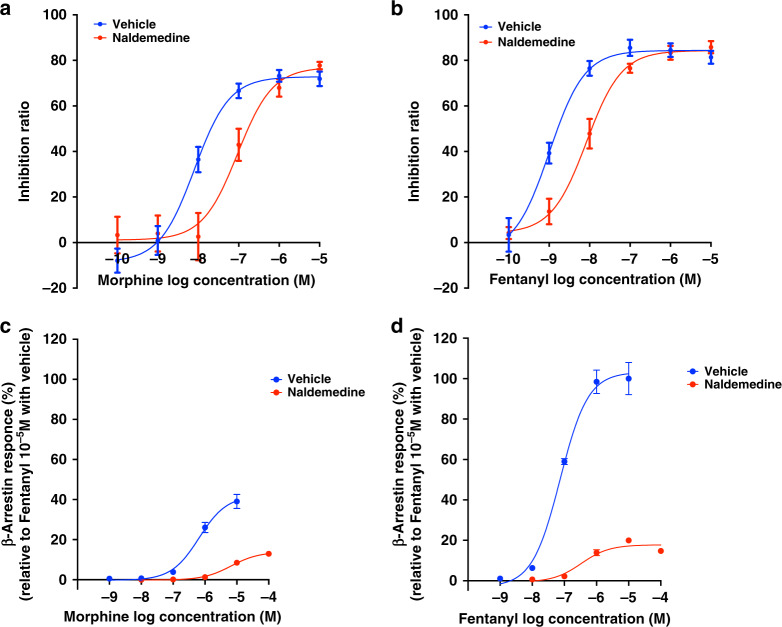
To confirm the potential of naldemedine as a MOR antagonist, we examined whether naldemedine could inhibit the MOR-mediated signalling induced by MOR agonists. In the cAMP assay, naldemedine suppressed morphine- or fentanyl-induced cAMP inhibition, and the concentration-response curves were dramatically (11.7- and 8.2-fold) shifted to the right, respectively (Fig. 1a, b, EC50 value; vehicle-morphine: 8.00 nM [95% CI: 4.71–13.61 nM], naldemedine-morphine: 93.8 nM [95% CI: 38.6–227.9 nM], vehicle-fentanyl: 1.02 nM [95% CI: 0.59–1.76 nM], naldemedine-fentanyl: 8.39 nM [95% CI: 5.17–13.61 nM]). In the β-arrestin recruitment assay, treatment with naldemedine dramatically suppressed β-arrestin recruitment for MOR induced by morphine (Fig. 1c) or fentanyl (Fig. 1d). Notably, maximal responses of β-arrestin recruitment for MOR induced by the treatment of either morphine or fentanyl were significantly suppressed in the presence of naldemedine (Fig. 1c, d, Emax of naldemedine-morphine: 13.9%, Emax of naldemedine-fentanyl: 17.8%).
Fig. 1. Evaluation of the antagonistic activity of naldemedine for µ-opioid receptors.
a–d Cell-based assays assessing cAMP inhibition and β-arrestin-2 recruitment for µ-opioid receptors. a, b Effects of naldemedine (10 nM) on the inhibition of cAMP accumulation induced by treatment with morphine (a, 100 pM–10 µM) or fentanyl (b, 100 pM–10 µM). Each point represents the mean ± SEM. n = 6 experiments. c, d Effects of morphine (c, 1 nM–100 µM) or fentanyl (d, 1 nM–100 µM) on β-arrestin-2 recruitment in the presence of naldemedine (10 nM). Each point represents the mean ± SEM n = 3 experiments.
Inhibitory effects of naldemedine on the suppression of gastrointestinal transit, but not antinociception and hyperlocomotion, induced by morphine
Morphine dose-dependently induced antinociceptive effects in a hot-plate test. Oral administration of naldemedine at 1 and 3 mg/kg did not affect the dose–response curve of morphine for antinociceptive effects in mice (Fig. 2a). In the absence of naldemedine, the ED50 value for the antinociceptive effect of morphine was 8.42 mg/kg, whereas the ED50 values for morphine-induced antinociception were 9.71 mg/kg and 10.3 mg/kg in the presence of naldemedine at 1 mg/kg and 3 mg/kg, respectively. The shifting ratio by naldemedine at 1 or 3 mg/kg for the antinociceptive effects of morphine was 1.15 or 1.22, respectively.
Fig. 2. Evaluation of the pharmacological profiles of the peripheral µ-opioid receptor antagonist naldemedine.
a, b Effects of naldemedine (1, 3 mg/kg, p.o.) on antinociceptive effects (a) and the delay of gastrointestinal transit (b) induced by morphine (1–20 mg/kg, s.c.). Vehicle: morphine 1 mg/kg, n = 7; 3 mg/kg, n = 7; 5 mg/kg, n = 7; 10 mg/kg, n = 10; 20 mg/kg, n = 10. Naldemedine 1 mg/kg: morphine 3 mg/kg, n = 5; 5 mg/kg, n = 5; 10 mg/kg, n = 5; 20 mg/kg, n = 5. Naldemedine 3 mg/kg: morphine 3 mg/kg, n = 5; 5 mg/kg, n = 5; 10 mg/kg, n = 5; 20 mg/kg, n = 5. c Effect of naldemedine on morphine-induced hyperlocomotion in mice. Total activity was counted after treatment with morphine (1–10 mg/kg, s.c.) for 180 min in the presence of naldemedine (1, 3 mg/kg, p.o.). n = 5 per data point. The data represent the mean ± SEM of 5–10 animals.
We next investigated the effects of naldemedine on the suppression of gastrointestinal transit induced by morphine in mice using a gastrointestinal transit test. Oral administration of naldemedine potently shifted the dose–response curve for the suppression of gastrointestinal transit induced by morphine to the right (Fig. 2b). The ED50 value of morphine for suppressing gastrointestinal transit without naldemedine was 7.73 mg/kg, whereas this value with naldemedine was 15.1 mg/kg at 1 mg/kg or 16.1 mg/kg at 3 mg/kg. The shifting ratio to reflect the antagonistic effect of naldemedine at 1 mg/kg and 3 mg/kg on the inhibition of gastrointestinal transit by morphine was 1.95 and 2.08, respectively.
To confirm that naldemedine at these doses does not have antagonistic activity on MORs in the central nervous system (CNS), we investigated the effect of naldemedine on the morphine-induced hyperlocomotion of mice. As a result, oral administration of naldemedine at 1 mg/kg and 3 mg/kg failed to suppress the hyperlocomotion produced by morphine (Fig. 2c).
Inhibitory effects of naldemedine on tumour growth
We investigated whether repeated oral administration of naldemedine could produce an anti-tumour effect in tumour-bearing mice, in which LLC cells had been subcutaneously transplanted into the right lower back, by the daily oral administration of naldemedine for 3 weeks (Fig. 3a). Tumour volume gradually increased in a time-dependent manner after the transplantation of LLC cells. Under the present condition, daily oral treatment with naldemedine (1 mg/kg) significantly decreased tumour volume compared to that in the vehicle-treated group (Fig. 3b). Furthermore, repeated oral administration of naldemedine significantly prolonged survival in LLC cell-transplanted mice compared to that in vehicle-treated mice (Fig. 3c). To evaluate whether this inhibitory effect of naldemedine on tumour progression could be observed in another tumour-bearing model, we tested the effect using a model generated by the transplantation of B16 melanoma cells. Consistent with the results in the LLC model, tumour volume was significantly decreased by the repeated oral administration of naldemedine in the B16 melanoma-bearing model (Fig. 3d). To further confirm the effects of naldemedine on tumour progression, the effects of an antagonist or an agonist of peripheral MORs were also examined. The repeated administration of methylnaltrexone, which is a peripheral MOR antagonist, significantly suppressed tumour growth compared to that in the vehicle-treated group (Fig. 3e). On the other hand, repeated administration of loperamide, which is a peripheral MOR agonist, significantly increased tumour volume compared to that in the vehicle-treated group (Fig. 3f). To verify whether the stimulation of MORs could directly regulate the growth of cancer cells, we evaluated the viability of LLC cells under treatment with agonist or antagonists of MORs was evaluated. As a result, treatment with naldemedine, methylnaltrexone or morphine had no effect on the viability of LLC cells (Supplementary Fig. S1).
Fig. 3. Changes in tumour growth by the repeated treatment with peripheral µ-opioid receptor agonists and antagonists.
a–d Changes in tumour progression by the repeated oral administration of naldemedine. a Experimental timeline. b Quantitative analysis of tumour volume between vehicle- and naldemedine (1 mg/kg) -treated mice that are LLC-bearing (vehicle, n = 23. Naldemedine, n = 24, two-way ANOVA test with Bonferroni’s post hoc test: ***P < 0.001 vs. vehicle group). c Survival curve of mice after transplantation of LLC cells under treatment with naldemedine for 21 days (vehicle, n = 9. Naldemedine, n = 10, Log-rank test: P = 0.042 vs. vehicle group). d Quantitative analysis of tumour volume between vehicle- and naldemedine (1 mg/kg) -treated mice that are B16-bearing (n = 6, two-way ANOVA test with Bonferroni’s post hoc test: *P < 0.05 vs. vehicle group). e, f Changes in tumour volume by the repeated administration of methylnaltrexone (e, 10 mg/kg/day by infusion, vehicle, n = 4. Methylnaltrexone, n = 5, two-way ANOVA test with Bonferroni’s post hoc test: *P < 0.05, ***P < 0.001 vs. vehicle group), and loperamide (f, 5 mg/kg, twice a day, vehicle, n = 4. Loperamide, n = 5, two-way ANOVA test with Bonferroni’s post hoc test: *P < 0.05 vs. vehicle group) in mice that are LLC-bearing.
Effects of naldemedine on the innate immune system
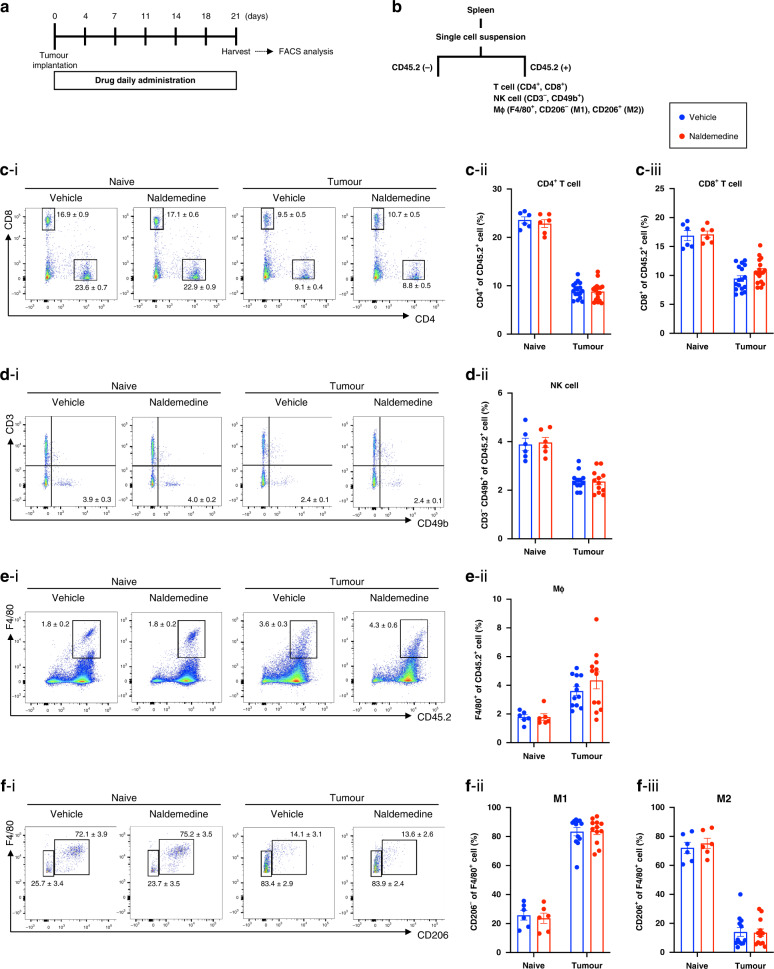
To clarify the mechanisms of the anti-tumour effects of naldemedine, we focused on the changes in the systemic immune system under the administration of naldemedine. First, changes in the number of lymphocytes from the spleen of mice treated with repeated oral-naldemedine were evaluated using FACS (Fig. 4a, b). Repeated administration of naldemedine failed to change the number of CD4+ T cells, CD8+ T cells, NK cells or macrophages in the spleen of mice without tumour implantation compared to those in vehicle-treated mice at 21 days after transplantation (Fig. 4c–f).
Fig. 4. Changes in the number of spleen-derived immune cells by the repeated administration of naldemedine in LLC cell-transplanted mice.
a Experimental timeline. b Schematic workflow of the isolation of splenic leucocytes using FACS. c–f Representative flow cytometric plots of CD4+ T and CD8+ T cells (c–i), NK cells (d-i), total macrophages (e-i), M1-, and M2-type macrophages (f-i) derived from the spleen of naldemedine-treated mice bearing LLC. Quantitative evaluation of the number of CD4+ T cells (c-ii), CD8+ T cells (c-iii), NK cells (d-ii), total macrophages (e-ii), M1-type macrophages (f-ii) and M2-type macrophages (f-iii) derived from the spleen of naive or tumour-bearing mice treated repeatedly with naldemedine (1 mg/kg). Each value represents the mean ± SEM n = 6–17.
To further assess the effect of naldemedine on the function of cytotoxic lymphocytes, which can eradicate cancer cells by recognising tumour-associated antigens, we next investigated the possible changes in the expression of immune checkpoint-related genes in CD8+ T cells in the mouse spleen by the administration of naldemedine. Under the condition of LLC cell transplantation into mice, mRNA levels of Pdcd1, Havcr2/Tim3, Lag3, Pvrig (CD112R) and Tigit, but not Ctla4 or Cd96, were significantly increased in CD8+ T cells in the spleen compared to those in mice without tumour transplantation (Fig. 5a–g). Among them, the increased mRNA levels of Havcr2/Tim3 and Lag3 in the spleen of mice with LLC cell transplantation were recovered by repeated oral administration of naldemedine (Fig. 5c, d). These results suggest that the repeated administration of naldemedine may relieve the exhaustion of splenic CD8+ T cells under tumour transplantation.
Fig. 5. Changes in mRNA expression of immune checkpoint receptors and cytotoxicity-related genes in CD8+ T cells by the repeated administration of naldemedine in LLC cell-transplanted mice.
a–j Changes in mRNA expression of Pdcd1 (a), Ctla4 (b), Havcr2/Tim3 (c), Lag3 (d), Cd96 (e), Pvrig (f), Tigit (g), GzmB (h), Prf1 (i) and Ifng (j) in CD8+ T cells sorted from the spleen of LLC-bearing mice by repeated oral administration of naldemedine (1 mg/kg). Each data point represents the mean ± SEM n = 6 in naive mice, n = 10 in LLC-bearing mice. One-way ANOVA test with Tukey’s post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle-treated naive group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. vehicle-treated LLC-bearing group.
We next examined the possible changes in the expression of cytotoxic factors in CD8+ T cells in the spleen of mice after repeated oral administration of naldemedine. As a result, mRNA levels of GzmB and Ifng were significantly increased by repeated oral administration of naldemedine in CD8+ T cells of LLC cell-transplanted mice compared to those in the vehicle-treated group with LLC cell transplantation (Fig. 5h–j).
We also investigated possible changes in the expression level of opioid receptor subtypes (MOR, DOR, KOR) in CD4+ T cells, CD8+ T cells and NK cells in the mouse spleen under the repeated oral administration of naldemedine. As a result, there were no changes in mRNA expression of MOR, DOR or KOR in CD4+ T cells, CD8+ T cells or NK cells under a condition of tumour transplantation with repeated oral administration of naldemedine (Fig. 6a–c).
Fig. 6. Changes in mRNA expression of opioid receptors and pattern recognition receptors in lymphocytes by the repeated administration of naldemedine in LLC cell-transplanted mice.
a–c Changes in mRNA expression of MOR, DOR and KOR in CD4+ T cells (a), CD8+ T cells (b) and NK cells (c) sorted from the spleen of LLC-bearing mice by repeated oral administration of naldemedine. d, e Changes in mRNA expression of TLR4 in CD8+ T cells (d) and NK cells (e) sorted from the spleen of LLC-bearing mice by repeated oral administration of naldemedine (1 mg/kg). Each data point represents the mean ± SEM. n = 6 in naive mice, n = 15 (d) and n = 10 (e) in LLC-bearing mice. One-way ANOVA test with Tukey’s post hoc test: ***P < 0.001 vs. vehicle-treated naive group, ##P < 0.01 vs. vehicle-treated LLC-bearing group. f Relationship between tumour volume and the expression level of TLR4 mRNA in CD8+ T cells (r2 = 0.153, P = 0.032, n = 30) derived from the spleen of naldemedine-treated LLC-bearing mice. g, h There was a significant correlation between the expression level of TLR4 mRNA and the expression levels of Lag3 (g, r2 = 0.237, P = 0.030, n = 20) and Tim3 (h, r2 = 0.233, P = 0.031, n = 20) in CD8+ T cells in the spleen of LLC cell-transplanted mice with repeated oral administration of naldemedine (1 mg/kg). The data were subjected to a comparative analysis by testing the null hypothesis for the Pearson product moment correlation.
It has been documented that MOR agonists bind to toll-like receptor 4 (TLR4) as well as MOR [12]. As shown in Fig. 6d, e, the decrease in the mRNA level of TLR4 in both CD8+ T cells and NK cells in the spleen of mice with LLC cell transplantation was significantly recovered by repeated oral administration of naldemedine. Furthermore, the recovery of the mRNA level of TLR4 in CD8+ T cells was significantly correlated with changes in tumour volume by repeated oral administration of naldemedine under the condition of LLC transplantation (Fig. 6f). Interestingly, the mRNA level of TLR4 was negatively correlated with the mRNA expression level of either Lag3 or Tim3 in CD8+ T cells in the spleen of LLC cell-transplanted mice with repeated oral administration of naldemedine (Fig. 6g, h).
Discussion
The peripheral MOR antagonist naldemedine has been widely used to treat OIC. To confirm the pharmacological properties of naldemedine, we first evaluated its MOR antagonistic properties. Activation of MORs is known to induce a Gi protein-mediated reduction in intracellular cAMP levels and the translocation of β-arrestin to the plasma membrane [13]. In this study, we confirmed that treatment with naldemedine reversed morphine- and fentanyl-induced reductions in cAMP levels. Interestingly, treatment with naldemedine significantly and dramatically inhibited the translocation of β-arrestin even at high concentrations of morphine and fentanyl, suggesting that naldemedine may strongly inhibit the translocation of β-arrestin to the plasma membrane induced by MOR activation.
Next, we investigated the effect of naldemedine on the analgesic effect of morphine and the inhibition of gastrointestinal transport induced by morphine. The results showed that morphine-induced inhibition of gastrointestinal transport was reversed by concomitant administration of naldemedine (1, 3 mg/kg) at doses that did not affect the analgesic effect of morphine. In addition, administration of naldemedine at the same doses failed to inhibit morphine-induced hyperlocomotion. These results confirm that naldemedine at adequate doses has very low central penetration and can improve OIC without affecting the analgesic effect of MOR agonists.
To date, basic and clinical studies have suggested that antagonizing peripheral MOR by treatment with methylnaltrexone inhibits tumour progression [6, 7]. By contrast, no basic research has been conducted on the effect of naldemedine under a condition of cancer. In this study, we demonstrated using LLC-bearing mice that repeated administration of naldemedine significantly inhibited tumour enlargement and prolonged survival. We confirmed under the same condition that the repeated administration of methylnaltrexone produced an anti-tumour effect, whereas daily treatment with the peripheral MOR agonist loperamide significantly promoted tumour growth. These findings provide further evidence that inhibition of peripheral MORs may be a useful approach for suppressing tumour progression.
The distribution of MOR expression has been documented to occur in cells of the gastrointestinal tract and immune system as well as in the CNS [2, 3]. The type of tumour cells expressing high levels of MOR has also been identified, and interestingly, MOR expression has been shown to correlate with patient prognosis [14, 15]. However, since we found no change in tumour cell proliferation or survival after in vitro treatment with naldemedine, we propose that the development of the anti-tumour effect of naldemedine may not result from a direct effect on tumour cells.
Moreover, we investigated the effect of repeated administration of naldemedine on the immune system. Cancer cells have shown to induce the expression of immune checkpoints against immune cells to escape attack from the immune system, causing immune cell exhaustion and suppression of function [16, 17]. The present analysis of changes in the expression of immunocheckpoint receptors in splenic CD8+ T cells showed that repeated administration of naldemedine induced a significant reduction in the increased expression of Tim3 and Lag3 in the tumour transplant state. Under these conditions, repeated administration of naldemedine resulted in the increased expression of granzyme B and IFNγ in splenic CD8+ T cells. Granzyme B and IFNγ have been considered to have important cytotoxic effects against tumour cells [18]. These findings suggest that the anti-tumour effect of naldemedine may partly result from the functional recovery of CD8+ T cells from the state of exhaustion and the improvement in cytotoxicity. However, it remains unclear whether these changes associated with the repeated administration of naldemedine are due to direct or indirect effects on immune cells.
Opioid receptors expressed in various immune cells, including T cells, B cells and macrophages, have been shown to play a role in the modulation of both innate and adaptive immunity [3]. Recent pharmacological and pharmacogenic research has provided evidence that activation of peripheral MORs mediates immunosuppressive effects in the peripheral immune system [19]. In this study, we investigated the expression of three opioid receptors, MOR, DOR, and KOR, in CD8+ T cells, CD4+ T cells, and NK cells isolated from the spleen. Unexpectedly, relatively lower levels of the expression of opioid receptors were observed in these immune cells. No changes in the expression of these three opioid receptors were observed in the cancer state under the repeated administration of naldemedine.
It has been well-documented that opioid receptors and TLR4, which is a lipopolysaccharide (LPS) receptor and plays a key role in the innate immune response, are co-expressed in immune cells [12, 20]. MOR agonists have been shown to bind to TLR4, as well as MORs, in a manner parallel to LPS, resulting in the activation of TLR4 signalling. This reaction may in turn lead to the expression of nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) expression and the production of tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 [12]. These findings suggest that a TLR4 signalling pathway may be tightly linked to MOR agonist-induced immunosuppression. Interestingly, it has been reported that treatment of MOR/TLR4-expressing cells with the standard MOR antagonist naltrexone increases TLR4 levels, indicating that the endogenous MOR system plays a crucial role in TLR4-mediated responses [21]. In the present study, we found that repeated administration of naldemedine resulted in the recovery of the dramatic decrease in the expression level of TLR4 in both splenic CD8+ T cells and NK cells under the tumour transplant state. Furthermore, the recovery level of TLR4 expression in CD8+ T cells was correlated with the suppression of tumour growth along with decreased levels of immune checkpoint receptors in splenic CD8+ T cells obtained from tumour-transplanted mice. It has been reported that immune checkpoint receptor Tim3 may be a negative regulator of TLR-mediated immune responses in sepsis [22]. Furthermore, endogenous TLR4 ligand has been shown to induce type I IFN production in conventional dendritic cells and precipitates disease flares in systemic lupus erythematosus [23]. Although further investigations are needed, we propose that an increase in cytotoxic molecules to enhance the immune system against tumour progression due to the recovery of a decrease in the expression of TLR4 via downregulation of the expression of immune checkpoint receptors, especially Tim3 and Lag3, in splenic immune cells under a cancer state may partly contribute to the anti-tumour effect of naldemedine.
In conclusion, we demonstrated for the first time that repeated oral administration of the peripheral MOR antagonist naldemedine, which has been widely used in a clinical setting to treat OIC, had an anti-tumour effect through the activation of peripheral immune mechanisms due to the recovery of T-cell exhaustion and promotion of the tumour-killing system. These findings suggest that modulation of the endogenous immune system under a cancer state may be one of the mechanisms underlying the unique profile of naldemedine, and such modulation may be responsible for the anti-tumour effect of naldemedine.
Supplementary information
Acknowledgements
The authors thank Ms. Kana Morita, Ms. Arisa Gina, Ms. Anna Hori, Mr. Shoki Matsumura, Mr. Takuya Kurimura, Ms. Natsumi Kawamura and Ms. Mayu Takagi for their help with the experiments.
Author contributions
EG, YH, TM and Minoru N wrote the manuscript. NK and Minoru N edited the manuscript. Michiko N, YS, KM and NK conducted in vitro studies. YH, YI, AS, DS, MT, SY, KT and KY conducted in vivo studies. HT provided technical support. HA, TY and KH synthesised naldemedine. EG, KA, MI and EI provided medical comments. NK and Minoru N supervised the overall project. All of the authors discussed the results and commented on the manuscript.
Funding
This work was supported in part by grants from the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018, S1411019. This research was also supported by Hoshi University.
Data availability
All data presented within the article are available upon request from the corresponding author.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal studies were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals at Hoshi University, as adopted by the Committee on Animal Research of Hoshi University.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Eizoh Gondoh, Yusuke Hamada.
Contributor Information
Naoko Kuzumaki, Email: n-kuzumaki@hoshi.ac.jp.
Minoru Narita, Email: narita@hoshi.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01928-x.
References
- 1.Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and poppies: the good, the bad, and the ugly of opioid analgesics. J Neurosci. 2015;35:13879–88. doi: 10.1523/JNEUROSCI.2711-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012;124:223–8. doi: 10.1016/j.drugalcdep.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machelska H, Celik MO. Opioid Receptors in immune and glial cells-implications for pain control. Front Immunol. 2020;11:300. doi: 10.3389/fimmu.2020.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, Mawe GM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014;26:1386–95. doi: 10.1111/nmo.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, et al. Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med. 2017;23:164–73. doi: 10.1038/nm.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112:558–67. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janku F, Johnson LK, Karp DD, Atkins JT, Singleton PA, Moss J. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol. 2018;29:1076. doi: 10.1093/annonc/mdx776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanemasa T, Koike K, Arai T, Ono H, Horita N, Chiba H, et al. Pharmacologic effects of naldemedine, a peripherally acting mu-opioid receptor antagonist, in in vitro and in vivo models of opioid-induced constipation. Neurogastroenterol Motil. 2019;31:e13563. doi: 10.1111/nmo.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watari R, Matsuda A, Ohnishi S, Hasegawa H. Minimal contribution of P-gp on the low brain distribution of naldemedine, a peripherally acting mu-opioid receptor antagonist. Drug Metab Pharmacokinet. 2019;34:126–33. doi: 10.1016/j.dmpk.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Doi, S, Mori, T, Uzawa, N, Arima, T, Takahashi, T, Uchida, M et al. Characterization of methadone as a beta-arrestin-biased mu-opioid receptor agonist. Mol Pain 2016;12:1744806916654146. [DOI] [PMC free article] [PubMed]
- 11.Suda Y, Kuzumaki N, Sone T, Narita M, Tanaka K, Hamada Y, et al. Down-regulation of ghrelin receptors on dopaminergic neurons in the substantia nigra contributes to Parkinson’s disease-like motor dysfunction. Mol Brain. 2018;11:6. doi: 10.1186/s13041-018-0349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins LR, Wang X, Mustafa S, Hutchinson MR. In vivo veritas: (+)-Naltrexone’s actions define translational importance: a letter in response to Skolnick et al. ‘Translational potential of naloxone and naltrexone as TLR4 antagonists’. Trends Pharm Sci. 2014;35:432–3. doi: 10.1016/j.tips.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Chan HCS, McCarthy D, Li J, Palczewski K, Yuan S. Designing safer analgesics via mu-opioid receptor pathways. Trends Pharm Sci. 2017;38:1016–37. doi: 10.1016/j.tips.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zylla D, Gourley BL, Vang D, Jackson S, Boatman S, Lindgren B, et al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119:4103–10. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Sun M, Zhou D, Gorur A, Sun Z, Zeng W, et al. Increased mu-opioid receptor expression is associated with reduced disease-free and overall survival in laryngeal squamous cell carcinoma. Br J Anaesth. 2020;125:722–9. doi: 10.1016/j.bja.2020.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–8. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 17.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–47. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 19.Rossaint J, Zarbock A. Perioperative inflammation and its modulation by anesthetics. Anesth Analg. 2018;126:1058–67. doi: 10.1213/ANE.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol. 2020;11:1455. doi: 10.3389/fimmu.2020.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchi S, Moretti S, Castelli M, Lattuada D, Scavullo C, Panerai AE, et al. Mu opioid receptor activation modulates Toll like receptor 4 in murine macrophages. Brain Behav Immun. 2012;26:480–8. doi: 10.1016/j.bbi.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, et al. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. J Immunol. 2013;190:2068–79. doi: 10.4049/jimmunol.1202661. [DOI] [PubMed] [Google Scholar]
- 23.Souza-Fonseca-Guimaraes F, Parlato M, Philippart F, Misset B, Cavaillon JM, Adib-Conquy M, et al. Toll-like receptors expression and interferon-gamma production by NK cells in human sepsis. Crit Care. 2012;16:R206. doi: 10.1186/cc11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented within the article are available upon request from the corresponding author.