Abstract
Background
Recurrent head and neck squamous cell carcinoma (HNSCC) is associated with poor overall survival (OS). Prior studies suggested incorporation of nab-paclitaxel (A) may improve outcomes in recurrent HNSCC.
Methods
This Phase I study evaluated induction with carboplatin and A followed by concomitant FHX (infusional 5-fluorouracil, hydroxyurea and twice-daily radiation therapy administered every other week) plus A with cohort dose escalation ranging from 10–100 mg/m2 in recurrent HNSCC. The primary endpoint was maximally tolerated dose (MTD) and dose-limiting toxicity (DLT) of A when given in combination with FHX (AFHX).
Results
Forty-eight eligible pts started induction; 28 pts started AFHX and were evaluable for toxicity. Two DLTs occurred (both Grade 4 mucositis) at a dose level 20 mg/m2. No further DLTs were observed with subsequent dose escalation. The MTD and recommended Phase II dose (RP2D) of A was 100 mg/m2.
Conclusions
In this Phase I study, the RP2D of A with FHX is 100 mg/m2 (AFHX). The role of re-irradiation with immunotherapy warrants further investigation.
Clinical trial information
This clinical trial was registered with ClinicalTrials.gov identifier: NCT01847326.
Subject terms: Head and neck cancer, Cancer therapy
Background
The majority of patients diagnosed with head and neck squamous cell carcinoma (HNSCC) will present with locally or locoregionally advanced disease, and are generally treated with combined modality therapy, including surgery, radiotherapy, and systemic therapy with curative intent [1]. However, cure rates remain modest especially in human papillomavirus (HPV) negative HNSCC and locoregional recurrences remain a major cause of death [2]. Second primary cancers also occur in previously treated patients with HNSCC due to field carcinogenesis in the setting of long-standing exposure to alcohol and tobacco [3]. The standard of care in patients with recurrent HNSCC is surgical resection if feasible. However, based on the location or extent of the tumour, clear margins are not usually achieved, and only a minority of patients are ultimately cured [4]. Standard treatment for previously irradiated and unresectable recurrent HNSCC remains systemic therapy with palliative intent [5]. Treatment options in this setting generally include immune checkpoint blockade with pembrolizumab with or without concurrent chemotherapy, however, survival remains poor [6]. Re-irradiation with concurrent chemotherapy has been of interest as a potentially curative salvage treatment. Our institution has evaluated outcomes of re-irradiation with twice-daily radiation in 1.5 Gy fractions with concurrent paclitaxel, 5-fluorouracil (5-FU), and hydroxyurea (TFHX) in a week-on/week-off fashion, demonstrating long-term survival in 20–40% of patients which subsequently was evaluated in the RTOG 9610 multi-institutional re-irradiation trial [7–12]. nab-Paclitaxel is a cremophor-free, albumin-bound form of paclitaxel that avoids the toxicity of the paclitaxel solvent and may provide increased intratumoral concentration through albumin-mediated transport, with activity in multiple solid malignancies, including HNSCC. The substitution of paclitaxel with nab-paclitaxel would potentially make therapy more tolerable and further improve response rates. This led to the development of a Phase I dose escalation trial of nab-paclitaxel-based induction followed by nab-paclitaxel-based re-irradiation. Response to induction chemotherapy is a clinical predictor of outcome, and patients not responding to induction chemotherapy generally are unlikely to respond to subsequent chemoradiotherapy [13]. Therefore, for patients with tumour growth during re-induction chemotherapy, we sought to evaluate a course of hypofractionated stereotactic radiotherapy with nab-paclitaxel as a palliative approach. The primary endpoint was maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of nab-paclitaxel when given in combination with FHX (5-fluorouracil 600 mg/m2 and hydroxyurea 500 mg twice a day on days 0–5, and twice-daily radiation on days 1–5 of a 14-day cycle for five cycles), and to determine the role of re-induction chemotherapy in patient selection.
Methods
Patients
Patients were ≥18 years of age with ECOG Performance Status 0-1 and pathologically confirmed recurrent or second primary HNSCC with previously irradiated head and neck cancer without clinically measurable metastatic disease. Prior radiation therapy was completed ≥4 months, and/or chemotherapy completed ≥1 month before study entry with recovery from any adverse effects. Measurable disease prior to induction chemotherapy was required. Adequate organ/marrow function was required. All patients provided written informed consent prior to enrolment. Exclusion criteria included ≥Grade 2 pre-existing peripheral neuropathy, previously untreated patients with the locoregional-only disease, comorbidities interfering with therapy/survival, pregnancy or receiving other investigational therapies.
Treatment
Prior to entry, all patients were evaluated by surgical, medical, and radiation oncologists to determine optimal local treatment. All patients received two 21-day cycles of induction carboplatin (area under the curve = 6 on day 1) and nab-paclitaxel (100 mg/m2 on days 1 and 8) followed by clinical and radiographic evaluation and response assessment per RECIST criteria [14]. Patients with a good response to induction chemotherapy were evaluated by a head and neck surgeon for surgical resection prior to initiation of chemoradiation (partial or “debulking” surgery was not considered). Chemoradiotherapy was initiated approximately 2 weeks after the last dose of induction chemotherapy. If patients underwent surgical resection, they proceeded to chemoradiation within 4–6 weeks.
Patients with a response or no growth based on radiologic and clinical measurements during re-induction, or patients who underwent surgical resection with curative intent, proceeded to receive nab-paclitaxel in a cohort dose escalation Phase I design with 5-fluorouracil 500 mg/m2, hydroxyurea 500 mg twice a day, and twice-daily radiation on days 1–5 of 14-day cycles for five cycles, for a cumulative radiation dose of 66 to 75 Gy (or 60–66 Gy in patients undergoing surgery) (AFHX). If less than 3 days of radiation therapy (RT) was required during week 5, chemotherapy could be omitted. While on treatment, all acute toxicities were evaluated and scored using Common Terminology Criteria for Adverse Events. Dose-limiting toxicities (DLT) were defined during cycles one and two as ≥Grade 4 mucositis or esophagitis, ≥Grade 3 radiation dermatitis, ≥Grade 3 non-haematologic toxicity except transient nausea/vomiting and alopecia, or treatment-related toxicity causing a delay in initiation of treatment during the first four weeks of therapy. Patients who had tumour growth based on radiologic or clinical assessment during re-induction chemotherapy and not surgical candidates with curative intent were planned to receive nab-paclitaxel alone with 10 Gy of RT on day 1 of a 7-day cycle for 5 weeks for a cumulative radiation dose of 50 Gy (AX) with cohort Phase I dose escalation, however, this cohort closed due to poor accrual.
Patients were treated with volumetric modulated arc therapy or intensity-modulated RT (IMRT). Target delineation included pre-induction gross disease with an expansion of 1.5 cm to generate a planning target volume (PTV) 1 which was modified for normal tissue tolerance. In responding patients, PTV1 received 1.5 Gy twice daily to a total dose of 75 Gy over five cycles. The radiation oncologist had the option of adding a second volume (PTV2) as an expansion of PTV1 to include the first level of uninvolved lymph nodes in patients thought to be at high risk for microscopic disease. In resected patients, PTV1 was reconstructed from the pre-surgery scan, with PTV1 encompassing a 1.5-cm expansion of the gross disease prior to induction chemotherapy with adjustments for skin, cord, and lost tissue due to surgery to a dose of 60 to 66 Gy. The spinal cord was constrained to ≤54 Gy maximum life dose, no more than 20% of PTV1 received ≥110% of the prescribed dose, no more than 1% of any PTV1 or PTV2 received ≤93% of the prescribed dose, and no more than 1% or 1 cc of the tissue outside the PTVs received ≥110% of the prescribed dose to PTV1.
Treatment was continued until completion or until patients experienced disease progression, intercurrent illness preventing further treatment administration, unacceptable adverse events, patient decision to withdraw, or changes to patient’s condition rendering the patient unacceptable for further treatment in the judgement of the investigator. Four weeks following completion of treatment, all patients underwent radiographic evaluation. Patients were followed clinically until taken off-study, with recommended physician visits monthly for the first 3 months, then quarterly for the first 2 years, then bi-annually for the next 2 years, then annually thereafter.
Study design and statistical considerations
The primary objective was to establish the MTD, RP2D, safety of nab-paclitaxel when given in combination with 5-fluorouracil, hydroxyurea, and hyperfractionated radiation, and to explore the role of induction chemotherapy as a predictive tool for definitive head and neck cancer treatment for previously treated patients. The study design was a standard “3 + 3” Phase I study dose escalation design. Cohorts of 3–6 patients were enrolled, starting at nab-paclitaxel 20 mg/m2 with dose levels ranging from 10 to 100 mg/m2. DLTs were assessed at each dose level only in the first four weeks of chemoradiation, where two or more DLTs in 3 or 6 patients would be considered too toxic and the next lower dose level would be considered the MTD. If the MTD was not reached, the highest dose level assessed would be considered the RP2D. Secondary objectives included estimating progression-free survival (PFS) and overall survival (OS). The study was approved by the University of Chicago Institutional Review Board and registered on www.ClinicalTrials.gov (NCT01847326).
Kaplan–Meier analysis [15] was used to provide estimates and 95% confidence intervals of 5-year PFS and OS. Descriptive statistics, including medians, means and dispersion measures for continuous variables and frequency tables for categorical variables, were used to summarise the data. Multivariate survival analyses were performed using Cox proportional hazards regression. SAS version 9.4 (SAS Institute, Cary, NC) was used for statistical analysis.
Results
Patient characteristics
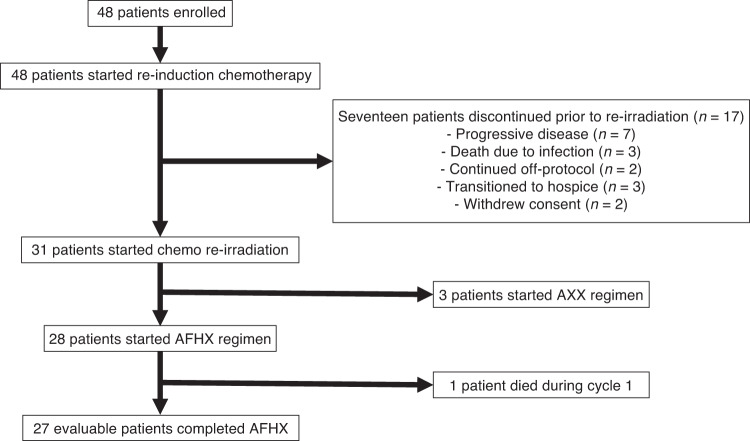
Patients were recruited between 2013 through 2020. A total of 48 patients were enrolled and started treatment from March 2013 through January 2020, with a median follow-up of 5.6 years. Of these 48 patients, 17 discontinued treatment prior to re-evaluation and initiation of concurrent chemotherapy and re-irradiation (Fig. 1). Seven patients experienced disease progression, three patients died due to infection, two patients transitioned to off-protocol therapy per treating physician, three patients transitioned to hospice, and two patients withdrew consent. Patient characteristics are summarised in Table 1. The median age was 60 years (range: 27–74) and was predominantly Caucasian (85.4%). The majority were male (70.8%), and approximately half were never smokers (50%). Recurrent disease was present in the vast majority (80%) of patients, while 15% were considered to have second primary cancers.
Fig. 1. Consort flow diagram.
Forty-eight patients were enrolled on the trial. All patients started re-induction chemotherapy. Seventeen patients discontinued therapy prior to concurrent chemotherapy and re-irradiation. Thirty-one patients started chemotherapy and re-irradiation. Three patients were treated on the AXX arm which subsequently closed due to poor accrual. Twenty-eight patients started AFHX. All twenty-eight patients completed re-irradiation with concurrent chemotherapy.
Table 1.
Patient characteristics.
| Characteristic | N = 48 |
|---|---|
| Age, median (range) | 60 (27–74) |
| Gender | |
| Male | 34 (70.8%) |
| Female | 14 (29.2%) |
| Race | |
| African–American | 5 (10.4%) |
| Caucasian | 41 (85.4%) |
| Other | 2 (4.2%) |
| BMI, median [IQR] | 25.4 (21.3–28.2) |
| Smoking history, pack years (PY) | |
| Never smoker | 23 (47.9%) |
| ≤10 PY | 7 (14.6%) |
| >10 PY | 17 (35.4%) |
| Unknown | 1 (2.1%) |
| Primary site | |
| Anterior tongue | 6 (12.5%) |
| Retromolar trigone | 2 (4.2%) |
| Lip | 1 (2.1%) |
| Oropharynx, tonsil and base of the tongue | 20 (41.7%) |
| Hypopharynx | 2 (4.2%) |
| Aryepiglottic fold | 1 (2.1%) |
| Larynx | 5 (10.4%) |
| Nasal cavities | 1 (2.1%) |
| Nasopharynx | 2 (4.2%) |
| Parotid gland | 3 (6.3%) |
| Unknown primary | 1 (2.1%) |
| Other | 4 (8.3%) |
| Disease state | |
| Recurrent | 40 (80.3%) |
| Primary | 7 (14.6%) |
| Metastatic | 1 (2.1%) |
| p16 + (for oropharynx) | 14 (29.2%) |
| ECOG PS | |
| 1 | 26 (54.2%) |
| 0 | 20 (40.7%) |
| Unknown | 2 (4.2%) |
Dosing
Patients who completed re-induction chemotherapy were subsequently treated with chemoradiation. Following re-induction, there were 28 patients who initiated treatment with re-irradiation with concurrent chemotherapy and AFHX per protocol, 11 of which underwent surgical resection prior per protocol. These 28 patients were enrolled across seven dose levels:7 patients at 10 mg/m2, 6 patients at 20 mg/m2, and 3 patients each at 40 mg/m2, 55 mg/m2, 70 mg/m2, 85 mg/m2 and 100 mg/m2. One patient on 10 mg/m2 dose level died due to progressive disease after one cycle and was not evaluable for DLT. Following the development of one DLT at dose level 20 mg/m2 in the first three-patient cohort, the decision was made to enrol six patients at dose level-1 (10 mg/m2), and an additional three patients at dose level 20 mg/m2, prior to proceeding with further dose escalation. There were three patients with measurable tumour growth based on radiologic or clinical evaluation following re-induction that were treated with AXX (nab-paclitaxel with 10 Gy of RT on day 1 of a 7-day cycle for 5 weeks for a total of 50 Gy) as designed, before this treatment arm was closed to accrual due to difficulty with enrolment. All 48 patients enrolled are included in the clinical outcomes data. Due to slow accrual following the approval of immunotherapy for recurrent HNSCC, the decision was made not to proceed with an expansion cohort as initially planned per protocol.
Safety
During re-induction chemotherapy, the most common Grade 3+ adverse events were anaemia (10%), and hypophosphatemia (6%). Table 2 summarises adverse events during re-induction chemotherapy. During AFHX re-irradiation with concurrent chemotherapy across seven dose levels, the most common Grade 3+ adverse events included anaemia (25%), mucositis (46%), and radiation dermatitis (21%). Table 3 summarises toxicities experienced during AFHX re-irradiation with concurrent chemotherapy. Grade 3 or greater toxicity was noted in 58% of patients overall and 57% during AFHX. Two patients on the 20 mg/m2 dose level developed a DLT of Grade 4 mucositis. The maximally tolerated dose and recommended Phase II dose of nab-paclitaxel with AFHX was determined to be 100 mg/m2.
Table 2.
Adverse events during re-induction chemotherapy (n = 48).
| Adverse event name | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Blood and lymphatic system | ||||||||||
| Anaemia | 16 | 33% | 11 | 23% | 5 | 10% | 0 | 0% | 0 | 0% |
| Blood and lymphatic system disorders (other) | 0 | 0% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Haemolytic uraemic syndrome | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| CD4 lymphocytes decreased | 11 | 23% | 7 | 15% | 1 | 2% | 1 | 2% | 0 | 0% |
| Neutrophil count decreased | 1 | 2% | 1 | 2% | 1 | 2% | 1 | 2% | 0 | 0% |
| Platelet count decreased | 10 | 21% | 3 | 6% | 2 | 4% | 0 | 0% | 0 | 0% |
| White blood cell decreased | 6 | 13% | 5 | 10% | 2 | 4% | 0 | 0% | 0 | 0% |
| Cardiac | ||||||||||
| Asystole | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2% |
| Ear and labyrinth | ||||||||||
| Ear pain | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Eye | ||||||||||
| Eye disorders (other) | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Gastrointestinal | ||||||||||
| Abdominal pain | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Constipation | 2 | 4% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Diarrhoea | 3 | 6% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dry mouth | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dyspepsia | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dysphagia | 2 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Gastroesophageal reflux disease | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Gastrointestinal disorders (other) | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Mucositis oral | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Nausea | 3 | 6% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Oral haemorrhage | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Oral pain | 3 | 6% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Vomiting | 2 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| General | ||||||||||
| Oedema face | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Fatigue | 6 | 13% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Fever | 2 | 4% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Gait disturbance | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Pain | 5 | 10% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Injury, poisoning and procedural complications | ||||||||||
| Bruising | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Tracheal haemorrhage | 0 | 0% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Hepatic | ||||||||||
| Alanine aminotransferase increased | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Aspartate aminotransferase increased | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Blood bilirubin increased | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Renal | ||||||||||
| Creatinine increased | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Metabolism and nutrition | ||||||||||
| Weight loss | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Anorexia | 4 | 8% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dehydration | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hypercalcemia | 5 | 10% | 1 | 2% | 2 | 4% | 1 | 2% | 0 | 0% |
| Hyperglycaemia | 13 | 27% | 2 | 4% | 1 | 2% | 0 | 0% | 0 | 0% |
| Hyperkalemia | 3 | 6% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Hypermagnesemia | 2 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hypernatremia | 3 | 6% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Hypoalbuminemia | 6 | 13% | 3 | 6% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hypocalcemia | 4 | 8% | 3 | 6% | 1 | 2% | 0 | 0% | 0 | 0% |
| Hypoglycemia | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hypokalemia | 4 | 8% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Hypomagnesemia | 2 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hyponatremia | 4 | 8% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Hypophosphatemia | 0 | 0% | 5 | 10% | 3 | 6% | 0 | 0% | 0 | 0% |
| Musculoskeletal and connective tissue | ||||||||||
| Arthralgia | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Neck pain | 3 | 6% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Nervous system | ||||||||||
| Dysgeusia | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Headache | 1 | 2% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Respiratory, thoracic and mediastinal | ||||||||||
| Cough | 2 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dyspnoea | 1 | 2% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Hoarseness | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Respiratory, thoracic and mediastinal (other) | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Sleep apnoea | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Sore throat | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Skin and subcutaneous tissue | ||||||||||
| Alopecia | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Pruritus | 1 | 2% | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Rash maculopapular | 0 | 0% | 0 | 0% | 1 | 2% | 0 | 0% | 0 | 0% |
| Vascular | ||||||||||
| Hypertension | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
Table 3.
Adverse events during AFHX (n = 28).
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Blood and lymphatic system | ||||||||||
| Anaemia | 10 | 36% | 7 | 25% | 7 | 25% | 0 | 0% | 0 | 0% |
| Blood and lymphatic system disorders (other) | 6 | 21% | 3 | 11% | 3 | 11% | 0 | 0% | 0 | 0% |
| Febrile neutropenia | 0 | 0% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Leukocytosis | 0 | 0% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Neutrophil count decreased | 1 | 4% | 1 | 4% | 2 | 7% | 0 | 0% | 0 | 0% |
| Platelet count decreased | 3 | 11% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| CD4 lymphocytes decreased | 5 | 18% | 4 | 14% | 1 | 4% | 0 | 0% | 0 | 0% |
| White blood cell decreased | 4 | 14% | 3 | 11% | 1 | 4% | 1 | 4% | 0 | 0% |
| Cardiac | ||||||||||
| Atrial fibrillation | 1 | 4% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Chest pain—cardiac | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Sinus tachycardia | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Ear and labyrinth | ||||||||||
| Ear and labyrinth disorders (other) | 3 | 11% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Ear pain | 4 | 14% | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hearing impaired | 2 | 7% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Tinnitus | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Endocrine | ||||||||||
| Hypothyroidism | 5 | 18% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Eye | ||||||||||
| Blurred vision | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Eye disorders (other) | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Watering eyes | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Gastrointestinal | ||||||||||
| Abdominal pain | 3 | 11% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Constipation | 22 | 79% | 1 | 4% | 1 | 4% | 0 | 0% | 0 | 0% |
| Diarrhoea | 10 | 36% | 3 | 11% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dry mouth | 13 | 46% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dysphagia | 20 | 71% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Gastroesophageal reflux disease | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Gastrointestinal disorders (other) | 5 | 18% | 3 | 11% | 1 | 4% | 0 | 0% | 0 | 0% |
| Haemorrhoids | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Mucositis oral | 18 | 64% | 18 | 64% | 9 | 32% | 4 | 14% | 0 | 0% |
| Nausea | 27 | 96% | 3 | 11% | 1 | 4% | 0 | 0% | 0 | 0% |
| Oral dysesthesia | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Oral pain | 10 | 36% | 5 | 18% | 1 | 4% | 0 | 0% | 0 | 0% |
| Salivary duct inflammation | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Upper gastrointestinal haemorrhage | 0 | 0% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Vomiting | 9 | 32% | 1 | 4% | 1 | 4% | 0 | 0% | 0 | 0% |
| General | ||||||||||
| Chills | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Oedema face | 4 | 14% | 1 | 4% | 2 | 7% | 0 | 0% | 0 | 0% |
| Oedema limbs | 6 | 21% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Facial pain | 4 | 14% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Fatigue | 26 | 93% | 4 | 14% | 0 | 0% | 0 | 0% | 0 | 0% |
| Fever | 5 | 18% | 1 | 4% | 1 | 4% | 0 | 0% | 0 | 0% |
| Localised oedema | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Neck oedema | 4 | 14% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Pain | 8 | 29% | 1 | 4% | 1 | 4% | 0 | 0% | 0 | 0% |
| Infections and infestations | ||||||||||
| Infections and infestations (other) | 1 | 4% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Laryngitis | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Pelvic infection | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Skin infection | 1 | 4% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Wound infection | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Injury, poising and procedural complications | ||||||||||
| Dermatitis radiation | 9 | 32% | 14 | 50% | 6 | 21% | 0 | 0% | 0 | 0% |
| Fall | 1 | 4% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Fracture | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hepatic | ||||||||||
| Alanine aminotransferase increased | 3 | 11% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Aspartate aminotransferase increased | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Metabolism and nutrition | ||||||||||
| Weight loss | 4 | 14% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Alkalosis | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Anorexia | 20 | 71% | 2 | 7% | 1 | 4% | 0 | 0% | 0 | 0% |
| Dehydration | 4 | 14% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Hypercalcemia | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hyperglycaemia | 7 | 25% | 3 | 11% | 1 | 4% | 0 | 0% | 0 | 0% |
| Hyperkalemia | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hypoalbuminemia | 3 | 11% | 3 | 11% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hypocalcemia | 1 | 4% | 2 | 7% | 1 | 4% | 0 | 0% | 0 | 0% |
| Hypokalemia | 2 | 7% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Hypomagnesemia | 3 | 11% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Hyponatremia | 2 | 7% | 0 | 0% | 2 | 7% | 0 | 0% | 0 | 0% |
| Hypophosphatemia | 1 | 4% | 6 | 21% | 1 | 4% | 0 | 0% | 0 | 0% |
| Metabolism and nutrition disorders (other) | 0 | 0% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Musculoskeletal | ||||||||||
| Back pain | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Muscle weakness upper limb | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Musculoskeletal and connective tissue disorder (other) | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Neck pain | 6 | 21% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Pain in extremity | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Trismus | 5 | 18% | 2 | 7% | 1 | 4% | 0 | 0% | 0 | 0% |
| Nervous system | ||||||||||
| Dizziness | 4 | 14% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dysarthria | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dysgeusia | 5 | 18% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Headache | 4 | 14% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Paraesthesia | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Peripheral sensory neuropathy | 5 | 18% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Tremor | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Psychiatric | ||||||||||
| Anxiety | 5 | 18% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Confusion | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Depression | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Insomnia | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Renal and urinary | ||||||||||
| Creatinine increased | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Renal and urinary disorders (other) | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Urine discolouration | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Respiratory | ||||||||||
| Aspiration | 2 | 7% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Cough | 7 | 25% | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% |
| Dyspnoea | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Nasal congestion | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Productive cough | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Pulmonary hypertension | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Respiratory, thoracic and mediastinal disorders (other) | 4 | 14% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Sleep apnoea | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Sore throat | 3 | 11% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Wheezing | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Skin and subcutaneous tissue | ||||||||||
| Alopecia | 6 | 21% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Erythema multiforme | 3 | 11% | 2 | 7% | 2 | 7% | 0 | 0% | 0 | 0% |
| Hyperhidrosis | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Palmar–plantar erythrodysesthesia syndrome | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Pruritus | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Rash acneiform | 1 | 4% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Rash maculopapular | 2 | 7% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Scalp pain | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Skin and subcutaneous tissue disorders (other) | 5 | 18% | 1 | 4% | 1 | 4% | 0 | 0% | 0 | 0% |
| Skin ulceration | 1 | 4% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Vascular | ||||||||||
| Hypotension | 2 | 7% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Thromboembolic event | 2 | 7% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
Outcomes
The median follow-up was 5.6 years. The estimated overall 60-month PFS rate is 23% (95% CI: 12–36%) (Fig. 2). The PFS was similar among primary and recurrent cancers at 29% and 22%, respectively. The median PFS estimate was 10.3 months (95% CI: 6–15 months). The OS rate at 60 months is 25% (95% CI: 13–40%) (Fig. 3). Responders following re-induction (CR or PR) versus non-responders (SD or PD) demonstrated an estimated 60-month PFS rate of 53% (95% CI: 18–80%) and 18% (95% CI: 7–33%), respectively (P = 0.04). Patients who were treated with re-irradiation with concurrent chemotherapy with AFHX demonstrated a 60-month PFS rate of 38% (95% CI: 20–55%), while the 60-month OS rate for AFHX-treated patients was 44% (95% CI: 24–62%) (Fig. 4).
Fig. 2. Progression-free survival.
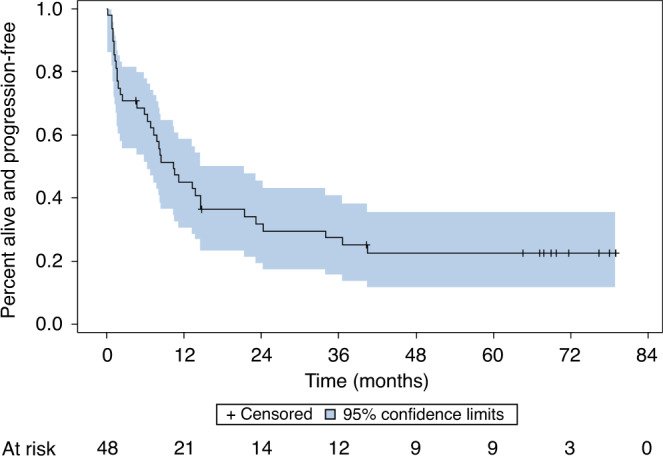
At a median follow-up of 67 months, the estimated 60-month progression-free survival for all patients that started treatment (n = 48) was 23% (95% CI: 12–36%).
Fig. 3. Overall survival.
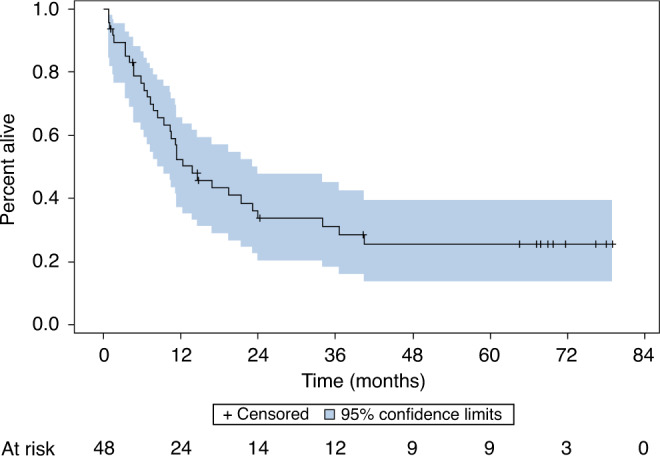
With a 67-month median follow-up, the estimated 60-month overall survival for all patients that started treatment (n = 48) was 25% (95% CI: 13–40%).
Fig. 4. Overall survival for AFHX-treated patients.
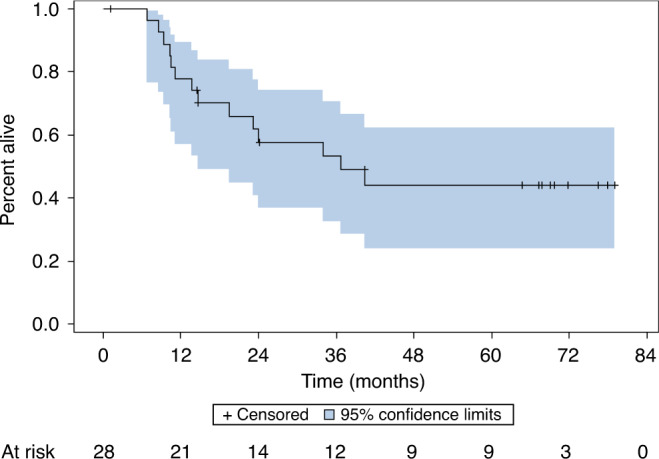
Twenty-eight patients proceeded to treatment with chemo-re-irradiation with AFHX following re-induction per protocol. The estimated 60-month overall survival was 44% (95% CI: 24–62%).
Late radiotherapy toxicities
Twelve patients were alive at the time of data analysis. Regarding late toxicities, eight (67%) were enteral feeding dependent, three (25%) had tracheostomies, five (42%) developed osteoradionecrosis and three (25%) developed trismus. Four patients (33%) were not enteral feeding tube dependent or tracheostomy dependent at the time of last follow-up.
Discussion
This Phase I trial in patients with recurrent HNSCC of induction carboplatin and nab-paclitaxel followed by nab-paclitaxel-based re-irradiation with concurrent chemotherapy demonstrated an acceptable toxicity rate with a recommended nab-paclitaxel dose of 100 mg/m2 with AFHX re-irradiation. In contrast to paclitaxel, nab-paclitaxel is not known to be associated with life-threatening hypersensitivity reactions and can be administered safely without steroid and antihistamine premedication. A number of studies have evaluated nab-paclitaxel-based induction and concurrent chemotherapeutic regimens in HNSCC, demonstrating favourable tolerability [16–19]. Furthermore, response rates in patients with HNSCC are similar between nab-paclitaxel and paclitaxel-based induction chemotherapy [20]. In our trial, Grade 3 or greater toxicity developed in 58% of patients with most common toxicities, including mucositis, radiation dermatitis, and anaemia, as has been reported previously in patients treated with 5-fluorouracil and hydroxyurea-based re-irradiation with concurrent chemotherapy [10]. Two patients developed DLTs both on the 20 mg/m2 dose level with Grade 4 mucositis, yet both patients completed AFHX with per-protocol dose reductions and no further DLTs were observed. Despite this tolerability profile, the estimated PFS rate at 60 months among all enrolled patients was 23%, which is similar to previous reports of re-irradiation with concurrent chemotherapy in this setting [10].
Re-irradiation in recurrent HNSCC remains an aggressive but potentially curative treatment option, yet its role in the context of the evolving treatment landscape in recurrent disease remains undefined. In the pre-intensity-modulated radiation therapy (IMRT) era, landmark re-irradiation trials such as Radiation Therapy Oncology Group (RTOG) 9610 with 5-FU, hydroxyurea and twice-daily radiation demonstrated a 5-year survival rate of 4%, albeit with lower 5-FU dose [12]. The RTOG 9911 trial evaluating cisplatin and paclitaxel with twice-daily radiation demonstrated a two-year survival rate of 26% [21]. In a pooled analysis of 115 patients treated with various 5-FU and hydroxyurea-based re-irradiation trials, three-year PFS and OS were 33% and 22%, respectively [10]. One randomised study of adjuvant chemotherapy and re-irradiation following surgical salvage was associated with an improvement in locoregional control, albeit without a survival benefit [22]. Recently, a multi-institution re-irradiation collaborative that evaluated over 400 HNSCC patients from seven institutions contributed to improving patient selection by characterising the time from initiation course of radiation, surgical resectability, and feeding tube or tracheostomy dependence, which can be used to stratify patients into risk categories in the setting of re-irradiation [23]. In contrast, in our study we did not observe a significant difference in survival between recurrent and second primary HNSCC, perhaps due to the higher proportion of recurrent disease and limited patients in this study. In addition to survival outcomes, previous reports of re-irradiation have identified late toxicities among long-term survivors, such as osteoradionecrosis, soft tissue necrosis, trismus, and dysphagia [10, 12, 21]. Similar to previous reports, our study identified high rates of enteral feeding dependency, tracheostomy dependency, osteoradionecrosis, and trismus. Significant toxicity burden for patients must be weighed when considering re-irradiation strategies for recurrent HNSCC. More recently, however, there has been a shift in the therapeutic landscape with the role of immune checkpoint blockade for the treatment of recurrent head and neck cancer [6, 24, 25]. The KEYNOTE-048 trial evaluated front-line recurrent/metastatic HNSCC and randomised patients to pembrolizumab alone, pembrolizumab plus chemotherapy, or cetuximab plus chemotherapy, including stratification based on programmed death ligand 1 (PD-L1) expression. Pembrolizumab alone demonstrated an improvement in survival among patients with PD-L1 combined positive score (CPS) of ≥20, and CPS ≥1 compared with cetuximab plus chemotherapy, but not in the overall population. Pembrolizumab plus chemotherapy demonstrated an improvement in survival among PD-L1 CPS of ≥20, CPS ≥1, and in the overall population with two-year survival of 29% [6]. This led to food and drug administration (FDA) approval of pembrolizumab monotherapy for front-line R/M HNSCC with PD-L1 CPS ≥1 and pembrolizumab plus chemotherapy regardless of PD-L1 expression. The long-term survival in our study is comparable to pembrolizumab plus chemotherapy in KEYNOTE-048.
This raises the question of the role of re-irradiation in the era of immune checkpoint blockade therapy alone or in combination with chemotherapy in locally recurrent disease. A subset analysis of KEYNOTE-048 demonstrated that the overall response rate among PD-L1-negative patients was only 4.5% with pembrolizumab monotherapy versus 42.2% with cetuximab plus chemotherapy [26]. This raises the question of whether there remains a potential role for re-irradiation in patients with low or negative PD-L1 expression. Furthermore, for patients with PD-L1 positive disease, the role of a combinatorial strategy of immune checkpoint blockade with re-irradiation remains undefined and should be explored further.
This study also evaluated the role of induction chemotherapy as a tool to select patients for re-irradiation. Among the 48 patients who enrolled and started induction therapy, 28 patients completed induction with a complete or partial response, no measurable growth, or surgical intervention, and subsequently started chemo-re-irradiation with AFHX. For these patients, the 5-year PFS and OS were 38 and 44%, respectively. This suggests a role for chemoselection as a dynamic predictive biomarker for subsequent response to chemo-re-irradiation, as has been suggested in previous studies [13, 16, 27]. Importantly, of the 48 patients enrolled, 17 patients (35%) discontinued treatment during induction and prior to re-irradiation with AFHX. This highlights the identification of a subset of recurrent HNSCC during systemic therapy with a particularly poor prognosis in whom an aggressive re-irradiation strategy would be unlikely to improve survival. Furthermore, this highlights the urgent need for improved therapeutic strategies in this challenging clinical scenario. Therefore, future re-irradiation studies should incorporate induction systemic therapy as a selection tool for patients most likely to benefit from an aggressive chemo-re-irradiation strategy, while avoiding toxicities in patients unlikely to benefit.
Limitations of our study include the prolonged period of enrolment, as the availability of immune checkpoint inhibitors subsequently affected the enrolment of this study and led to a decision to not enrol a planned expansion cohort. In addition, this was a single institutional trial, and as such, generalisability should be taken with caution. Despite these limitations, this study of re-induction therapy followed by re-irradiation therapy provides important insights into chemoselection for patient treated with chemo-re-irradiation in recurrent HNSCC.
Our study re-enforces the poor long-term prognosis for such patients and the critical need to incorporate novel systemic therapeutic strategies such as immunotherapy in the context of chemo-re-irradiation paradigms to improve survival and quality of life for patients with recurrent disease.
Supplementary information
Acknowledgements
We would like to acknowledge support from Celgene (Bristol Myers Squibb). This trial was supported, in part, by the University of Chicago Medicine Comprehensive Cancer Center Support Grant (#P30 CA14599) and support from the V. and R.W Svendsen Foundation. This study was presented in part at the ESMO Annual Meeting 2021.
Author contributions
Manuscript preparation: AJR and EEV. Study design: VMV, DJH and EEV. Patient data and study materials: AJR, ATP, JC, KR, VMV, TS, DJH and EEV. Data analysis and interpretation: AJR, JFC and EEV. Manuscript editing and review: all.
Funding
This study was funded by Celgene (Bristol Myers, Squibb).
Data availability
All data are available upon request.
Competing interests
AJR: consulting/advisory: EMD-Serono, Nanobiotix, Galectin Therapeutics, Privo Technologies. NA: stock options: Privo Technologies. ATP: consulting/advisory: Prelude, Abbvie, Elevar, Ayala; stock options: Privo Technologies; research funding: Abbvie, Kura Oncology. AJ: consulting: Isoray. Research funding: AstraZeneca. VMV: Stock: J&J. Research funding: Takeda. TS: research funding: BMS, AstraZeneca, Genentech, Nanobiotix, Merck. Honoraria: Merck, Nanobiotix. Consulting/advisory: Innate, Regeneron, Vir, Merck, Nanobiotix, BostonGene EEV: consulting/advisory: AstraZeneca, BeiGene, BioNTech, Eli Lilly, EMD-Serono, Genentech, GlaxoSmithKline, Novartis. The remaining authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the University of Chicago institutional review board. Furthermore, written consent was obtained from all participants.
Consent to publish
Not relevant.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01941-0.
References
- 1.Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398:P2289–99. doi: 10.1016/S0140-6736(21)01550-6. [DOI] [PubMed] [Google Scholar]
- 2.Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15:1179–86. doi: 10.1093/annonc/mdh308. [DOI] [PubMed] [Google Scholar]
- 3.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N. Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 4.Guo T, Qualliotine JR, Ha PK, Califano JA, Kim Y, Saunders JR, et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer. 2015;121:1977–84. doi: 10.1002/cncr.29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Head and Neck Cancers (Version 2.2020). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 6.Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 7.Vokes EE, Panje WR, Schilsky RL, Mick R, Awan AM, Moran WJ, et al. Hydroxyurea, fluorouracil, and concomitant radiotherapy in poor-prognosis head and neck cancer: a phase I-II study. J Clin Oncol. 1989;7:761–8. doi: 10.1200/JCO.1989.7.6.761. [DOI] [PubMed] [Google Scholar]
- 8.Haraf DJ, Weichselbaum RR, Vokes EE. Re-irradiation with concomitant chemotherapy of unresectable recurrent head and neck cancer: a potentially curable disease. Ann Oncol. 1996;7:913–8. doi: 10.1093/oxfordjournals.annonc.a010793. [DOI] [PubMed] [Google Scholar]
- 9.Vokes EE, Haraf DJ, Mick R, McEvilly JM, Weichselbaum RR. Intensified concomitant chemoradiotherapy with and without filgrastim for poor-prognosis head and neck cancer. J Clin Oncol. 1994;12:2351–9. doi: 10.1200/JCO.1994.12.11.2351. [DOI] [PubMed] [Google Scholar]
- 10.Salama JK, Vokes EE, Chmura SJ, Milano MT, Kao J, Stenson KM, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:382–91. doi: 10.1016/j.ijrobp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Spencer SA, Harris J, Wheeler RH, Machtay M, Schultz C, Spanos W, et al. RTOG 96-10: reirradiation with concurrent hydroxyurea and 5-fluorouracil in patients with squamous cell cancer of the head and neck. Int J Radiat Oncol Biol Phys. 2001;51:1299–304. doi: 10.1016/S0360-3016(01)01745-X. [DOI] [PubMed] [Google Scholar]
- 12.Spencer SA, Harris J, Wheeler RH, Machtay M, Schultz C, Spanos W, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008;30:281–8. doi: 10.1002/hed.20697. [DOI] [PubMed] [Google Scholar]
- 13.Villaflor VM, Melotek JM, Karrison TG, Brisson RJ, Blair EA, Portugal L, et al. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol. 2016;27:908–13. doi: 10.1093/annonc/mdw051. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 16.Seiwert TY, Foster CC, Blair EA, Karrison TG, Agrawal N, Melotek JM, et al. OPTIMA: a phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann Oncol. 2019;30:1673. doi: 10.1093/annonc/mdz171. [DOI] [PubMed] [Google Scholar]
- 17.Oppelt P, Ley J, Daly M, Rich J, Paniello R, Jackson RS, et al. nab-Paclitaxel and cisplatin followed by cisplatin and radiation (Arm 1) and nab-paclitaxel followed by cetuximab and radiation (Arm 2) for locally advanced head and neck squamous-cell carcinoma: a multicenter, non-randomized phase 2 trial. Med Oncol. 2021;38:35. doi: 10.1007/s12032-021-01479-w. [DOI] [PubMed] [Google Scholar]
- 18.Weiss J, Gilbert J, Deal AM, Weissler M, Hilliard C, Chera B, et al. Induction chemotherapy with carboplatin, nab-paclitaxel and cetuximab for at least N2b nodal status or surgically unresectable squamous cell carcinoma of the head and neck. Oral Oncol. 2018;84:46–51. doi: 10.1016/j.oraloncology.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Adkins D, Ley J, Oppelt P, Wildes TM, Gay HA, Daly M, et al. nab-Paclitaxel-based induction chemotherapy with or without cetuximab for locally advanced head and neck squamous cell carcinoma. Oral Oncol. 2017;72:26–31. doi: 10.1016/j.oraloncology.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg AJ, Agrawal N, Pearson A, Gooi Z, Blair E, Cursio J, et al. Risk and response adapted de-intensified treatment for HPV-associated oropharyngeal cancer: Optima paradigm expanded experience. Oral Oncol. 2021;122:105566. doi: 10.1016/j.oraloncology.2021.105566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer CJ, Harris J, Horwitz EM, Nicolaou N, Kies M, Curran W, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25:4800–5. doi: 10.1200/JCO.2006.07.9194. [DOI] [PubMed] [Google Scholar]
- 22.Janot F, de Raucourt D, Benhamou E, Ferron C, Dolivet G, Bensadoun RJ, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26:5518–23. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 23.Ward MC, Riaz N, Caudell JJ, Dunlap NE, Isrow D, Zakem SJ, et al. Refining patient selection for reirradiation of head and neck squamous carcinoma in the IMRT era: a multi-institution cohort study by the MIRI collaborative. Int J Radiat Oncol Biol Phys. 2018;100:586–94. doi: 10.1016/j.ijrobp.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–67. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 26.Burtness B, Rischin D, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol. 2022:JCO2102198. [DOI] [PMC free article] [PubMed]
- 27.Seiwert TY, Melotek JM, Blair EA, Stenson KM, Salama JK, Witt ME, et al. Final results of a randomized phase 2 trial investigating the addition of cetuximab to induction chemotherapy and accelerated or hyperfractionated chemoradiation for locoregionally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2016;96:21–9. doi: 10.1016/j.ijrobp.2016.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon request.