Abstract
The present cross-sectional study aims to understand the fungal community composition of the nasopharyngeal region of SARS-CoV-2 infected individuals and how the infection influences the mycobiome therein. The infection significantly (p < 0.05) influenced the alpha diversity. Interestingly, a higher abundance of Cladosporium and Alternaria was noted in the infected individuals and inter-individual variation in mycobiome composition was well supported by beta dispersion analysis (p < 0.05). Moreover, decrease in Aspergillus abundance was observed in infected patients across the four age groups. This study provides insight into the alteration in mycobiome during the viral disease progression and demands continuous investigation to monitor fungal infections.
Keywords: Mycobiome, SARS-CoV-2, Nasopharyngeal, COVID-19, Pandemic
Coronavirus disease caused by the novel severe acute respiratory syndrome coronavirus (SARS-CoV-2) which predominantly affects the respiratory system has been widely studied from a panoramic perspective owing to its pandemic nature that has overwhelmed the global healthcare system since the end of 2019 [1]. Of the notable features of SARS-CoV-2 such as high transmissibility and rapid mutational capacity; the wide spectrum of clinical manifestations patients’ exhibit ranging from mild to severe and requiring brief to prolonged hospitalization has further challenged disease prognosis and treatment [2]. Recently, several studies have tried to answer the question as to why certain infected individuals exhibit a mixed set of symptoms with a different magnitude of severity while a majority remain asymptomatic [3]. Although the heterogenous immune status among the individuals and their response to infections remain at the center of this argument at large, plausible interaction between the host, microbiome, and disease severity/progression has added a layer to this understanding [1]. Despite the fact that fungi have a significant contribution in human respiratory and chronic infections; this group of organisms has received shallow attention in human microbiome studies [1,[4], [5], [6], [7]]. In the light of COVID- 19 pandemic, most of the microbiome studies have focused on understanding the role of bacteria in SARS-CoV-2, neglecting the importance of fungi [[8], [9], [10]]. Considering the fact that COVID-19 involve a dysregulated immune response with cytokine storm and impaired T cell response during severe illness [11,12] and the role of fungi to shape immunological responses and T cell action has been previously reported [13]. Hence, it is important to perform fungal profiling of SARS-CoV-2 infected individuals because very few studies have been performed to understand the alteration of fungal populations during COVID-19 [1,7,[14], [15], [16], [17]]. These studies have reported an increase in the abundance of Candida sp. along with the decrease in species diversity and richness in COVID-19 patients. It has been observed that dominating fungal species are highly variable among patients even within the groups [16]. There are reports where several fungal taxa have been depleted in critically ill patients [[15], [16], [17]] and acute respiratory distress syndrome in COVID-19 was characterized by lung dysbiosis and decreased fungal diversity [7]. Since nasal cavity is one of the main entry points for the SARS-CoV-2 infection, it would be interesting to have a better understanding of SARS-CoV-2 infection on autochthonous mycobiome composition in nasopharynx of COVID-19 patients. The recent spike in the COVID-19-associated mucormycosis (an invasive fungal infection) cases in India provides an opportunity to consider the importance of mycobiome in future viral pandemics [18]. The current study is designed to assess the effect of SARS-CoV-2 infection on the composition of nasopharyngeal mycobiome in COVID-19 patients and to further understand the association of these changes with host conditions. This work is in continuation to our previous study where we assessed the prevalence of opportunistic bacterial pathogens in SARS-CoV-2 infected individuals [19].
1. Materials and methods
A total of 89 nasopharyngeal swabs previously collected from patients of SARS-CoV-2 infection were used for the mycobiome analysis [19]. Details of the recruited subjects, clinical characteristics, and real-time PCR testing for COVID-19 as per the ICMR guidelines were described in Ref. [19]. Sample collection was performed as per the standard Indian Council of Medical Research (ICMR), Government of India, guidelines. Swab samples were immediately put in Viral Transport Medium (VTM) and was transported in cold chain conditions and triple packaging to the laboratory of B J Government Medical College, Pune for COVID-19 real-time Polymerase Chain Reaction (RT-PCR). Out of the 89 nasopharyngeal samples, DNA from 80 samples yielded amplification of ITS1 region using primer set (ITS1F and ITS2R) [20]. These 80 samples were used for further downstream processing and demographic characteristics are presented in Table S1. The resultant amplicons were processed for library preparation, the barcoded libraries were pooled in equimolar concentration and sequenced on the Illumina MiSeq platform using 2 × 250 bp v2 chemistry. The PCR negative control was also sequenced to remove contaminants from the main datasets. The obtained raw reads were quality checked using FastQC [21]. The reads were pre-processed and analyzed using DADA2 package v1.6.0 [22] in R 3.6.0. Non-chimeric, error free reads were used for taxonomic assignment using UNITE database [23]. Decontam package was used to remove contaminants from the datasets using prevalence-based method [24]. Phyloseq v3.4.2 R package [25]. was used to generate alpha and beta diversity matrices. Pairwise Wilcoxon test was used to compare the changes in the alpha diversity parameters in the infected and non-infected individuals. Principal Co-ordinate Analysis (PCoA) was performed with Bray–Curtis matrix using phyloseq package. Permutational multivariate analysis of variance (PERMANOVA) was performed between the study groups using Bray–Curtis dissimilarity matrix to assess the difference in beta diversity. A permutation-based test of multivariate homogeneity of group dispersions (PERMDISP) was conducted using betadisper function of vegan package. Linear discriminant analysis Effect Size (LEfSe) was performed to find out the differentially enriched taxa between groups. The raw ITS1 gene amplicon sequencing data generated in this study was submitted to NCBI SRA database and it is available under the BioProject ID: PRJNA707350.
2. Results
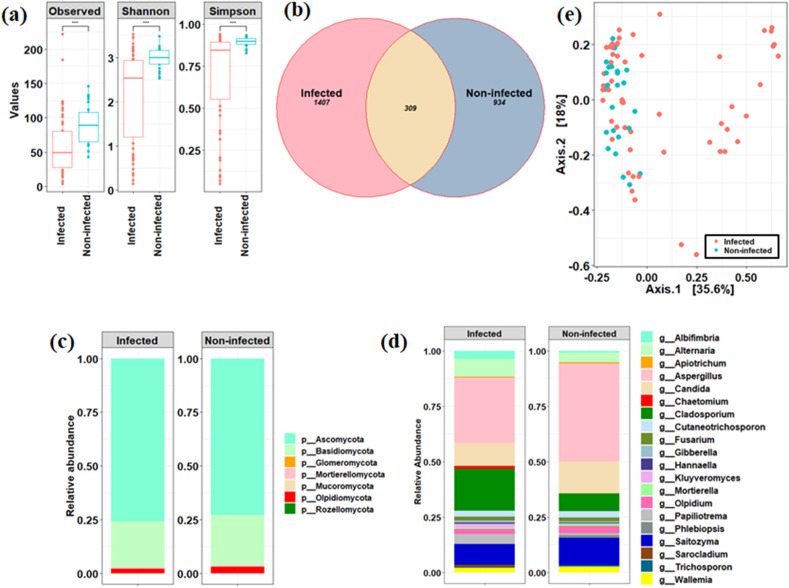
Using ITS1 region, fungal community composition of the nasopharyngeal region of the SARS-CoV-2 infected individuals showed significant decrease (p < 0.05) in the number and richness of fungal taxa than the non-infected individuals (Fig. 1 a). Out of the total detected ASVs, only 309 ASVs were found to be shared between the two cohorts (Fig. 1b). The ratio of Basidiomycota to Ascomycota was not significantly differed between these two groups (p > 0.05) as depicted in Fig. 1c. Increased average relative abundance of Alternaria and Cladosporium together with decreased count of Aspergillus, Candida, Olpidium, Saitozyma, Mortierella, and Wallemia was observed in the infected individuals (Fig. 1d). However, an inter-individual mycobiome variation was observed in the infected individuals with dominance of a few fungal taxa such as Albifimbria, Cutaneotrichosporon, Sarocladium, Hannaella, Chaetomium, and Kluyveromyces (Fig. S1). LefSe-based analysis found 10 differentially abundant fungal ASVs affiliated to Cladosporium, Aspergillus, Wallemi a, Candida, and Olpidium between the infected and non-infected individuals at FDR-adjusted p < 0.1. Furthermore, PCoA was performed to assess the overall difference in the mycobiome community composition between infected and non-infected individuals. PERMANOVA analysis displayed difference (p < 0.007) in the overall mycobiome community structure between infected and non-infected individuals (Fig. 1e). However, beta-dispersion analysis described the higher inter-individual variation in infected subjects than non-infected ones (PERMDISP, p < 0.0008).
Fig. 1.
Compositional differences in nasopharyngeal mycobiome between patients infected with SARS-CoV-2 and non-infected subjects. Alpha diversity measures between infected and non-infected individuals (a). Venn diagram-based identification of core and distinct ASVs between the cohorts (b). Relative abundance of major taxa at phylum (c) and genus level (d). PCoA based analysis to assess the difference in fungal community composition between the infected and non-infected individuals (e).
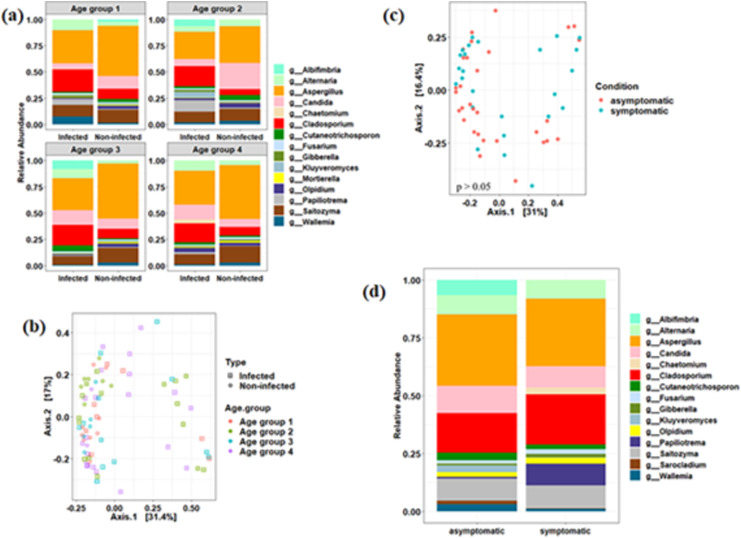
We further investigated the association of mycobiome with host age as SARS-CoV-2 was found to have more pronounced effect on older age group. We segregated our subjects into four distinct age groups (age group 1: 0–15 years; age group 2: 16–30 years; age group 3: 31–45 and age group 4: 46 and above) and found that alpha diversity decreased significantly (p < 0.05) in infected individuals across all the age groups (Fig. S2). Abundance of Aspergillus and Saitozyma was found to be decreased in all the age groups of infected individuals as compared to non-infected ones (Fig. 2 a). Interestingly, the relative abundance of Candida was found to be decreased in infected individuals within age group 1 and 2 and vice-versa for age group 3 and 4 (Fig. 2a). Cladosporium read count was enhanced in all the age groups of infected individuals. Similar trend in the abundance pattern of Alternaria was also observed, except for age group 2 (Fig. 2a). Notably, few taxa were enhanced in specific age groups such as Papiliotrema in age group 1, Kluyveromyces in age group 2, and Wallemia in age group 1 (Fig. 2a). No significant differences were observed between the categorical age groups using PCoA (Fig. 2b) and Pairwise PERMANOVA (p > 0.05, FDR corrected) (Table S2).
Fig. 2.
Association between mycobiome and host types (age and conditions). Mycobiome profile of major genera in SARS-CoV-2 infected and non-infected individuals across different age groups (a). PCoA based analysis to assess the difference in fungal community composition across different age groups (b). PCoA based analysis to assess the difference in fungal community composition between asymptomatic and symptomatic SARS-CoV-2 infected individuals (c). PERMANOVA analysis did not yield significant difference (p > 0.05). Relative abundance of major genera between asymptomatic and symptomatic SARS-CoV-2 infected individuals (d). Number of individuals belonged to each age category: [Infected ones: Age group 1: 8; Age group 2: 16; Age group 3: 12; Age group 4: 20] and [Non-Infected ones: Age group 1: 9; Age group 2: 7; Age group 3: 5; Age group 4: 3].
We further asked to understand the relationship between fungal composition and asymptomatic and/or symptomatic conditions of infected individuals. No significant difference was observed in the alpha diversity parameters between asymptomatic and symptomatic infected individuals. Additionally, beta diversity was not affected significantly in these two conditions (PERMANOVA, p > 0.05) (Fig. 2c). We did not find very significant changes in the relative abundance of the taxa, however, few genera such as Albifimbria, Wallemia, Sarocladium, Kluyveromyces, etc. were found to be abundant in the asymptomatic individuals (Fig. 2d). However, inter-individual variation in fungal composition was clearly observed across the asymptomatic and symptomatic infected subjects (Fig. S3). For example, Cladosporium and Papiliotrema constituted the major proportions of the fungal constituents in few of the symptomatic subjects.
3. Discussion
The upper respiratory system is consistently exposed to air and forms a unique microbiota and mycobiota [26]. Even though the abundance or biomass of latter is found to be very low in comparison to its bacterial counterpart, the shift in its composition is well observed in immunocompromised patients with respiratory or chronic diseases [4,27,28]. The present study is aimed to understand the impact of SARS-CoV-2 infection on nasopharyngeal mycobiome of the infected individuals. Our results showed the disruption and diminution in the fungal species richness in the nasopharyngeal region. Similar observation was reported by Lv et al. [15] in gut mycobiome of COVID-19 and healthy controls. Furthermore, reduction in fungal diversity in Bronchoalveolar lavage (BAL) samples from patients with COVID-19 with Candida spp. colonization in comparison to uncolonized ones was reported [7]. On contrary, Soffritti et al. [14] reported an increase in species richness in oral mycobiome of COVID-19 patients. Such changes clearly indicate that SARS-CoV-2 infection has pronounced effect on the mycobiome composition and is site-specific.
Interestingly, even though we have not found significant changes in the major taxa, increased abundance of two known opportunistic pathogens and decreased in Aspergillus, Wallemia, Candida, etc. in our study highlighted the influence of SARS-CoV-2 infection on fungal composition [Fig. 1]. In the recent years, Cladosporium is becoming increasingly important opportunistic pathogen, and known to cause superficial and invasive infections in human [4]. Similarly, Alternaria spp. were detected in asthmatic patients and also been reported from allergic bronchopulmonary mycosis, hypersensitivity pneumonitis, and allergic sinusitis and rhinitis [29,30]. Increment in such taxa in COVID-19 patients is of great concern, hence it is imperative to investigate the underlying pathogenesis in SARS-CoV-2 like infections. On contrary to previous reports, our data describe the decrease in Candida populations (which form the major portion of the human mycobiome and have been associated with various respiratory diseases) in the infected individuals [7,12]. However, it has been reported that Candida spp. colonization was significantly higher in BAL samples from COVID-19 patients, while patients which were not colonized by Candida showed the distinct mycobiome profile with higher abundance of unclassified fungi from the Ascomycota phylum [7]. In line with this, decrease in Candida members in our study has promoted the preponderance of opportunistic pathogens (Cladopsorium and Alternaria) in COVID-19 patients. Recently, Lv et al. [15] has shown the association between various metabolic markers and fungal groups in COVID-19 and H1N1 infected patients, which might be responsible for increased viral load, hypersensitivity, and secondary infections. Our study further tried to identify the unique fungal taxonomic markers associated with a particular age group. As a result, we have found the association of few fungal taxa which were either decreased or increased in particular age groups. These changes might be the results of COVID-19 or impaired host mechanisms. For example, Aspergillus populations was found to be decreased in all the age groups, while abundance of Candida was found to be more prominent in patients with older age group, this might be due to their higher susceptibility to Candida infection or impaired host defense mechanisms. Conversely, our study did not find significant variation in the fungal mycobiome profiling of the infected asymptomatic versus symptomatic patients. Inter-individual variations were well evident between these two conditions; hence we can hypothesize that inter-individual variation might be one of the factors responsible for symptomatic and asymptomatic nature of the disease. To further understand the inter-kingdom association between fungus and bacterial populations in infected patients as compared to non-infected individuals, we compared the fungus taxonomic profile with our pervious study on these recruited samples [19]. We have reported the increment of Pseudomonas in the nasopharynx of COVID-19 infected individuals; antagonistic association between Pseudomonas aeruginosa and Candida albicans has been reported by Ref. [26]. Overall decrease in abundance of Candida in the infected patients might be due to the negative effect of Pseudomonas on its growth [26]. It has been documented that symbiotic gut fungi can promote local and systemic immunity by providing complementary microbial stimulation and decrease host susceptibility to colitis and H1N1 virus infection [31]. Therefore, in the present study, depletion of commensal fungi in COVID-19 patients might lead to the loss of their beneficial functions. The main limitation of our study is the low number of the recruited individuals which did not enable us to ascertain the fungal composition with robust statistical analysis, especially in developing effective prevention strategies based on mycobiome profile. Therefore, longitudinal studies with higher number of subjects along with detailed immunological profiling would certainly define the biomarkers and open unique therapeutic opportunities to prevent the development of severe symptoms and combat SARS and other viral infections.
Ethical clearance
The study was approved by the Institutional Ethical Committees of both National Centre for Cell Science, Pune, India and BJ Medical College, Pune, India.
Funding
This work was supported by the DBT/Wellcome Trust India Alliance under the project grant (IA/E/17/1/503700). Sequencing was performed at Centre for Excellence, National Centre for Microbial Resource (NCMR-NCCS), Pune.
Declaration of competing interest
Authors declare no competing interest.
Acknowledgement
Authors are thankful to BJ medical staff for helping in sample collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micinf.2022.105059.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zuo T., Zhang F., Lui G.C., Yeoh Y.K., Li A.Y., Zhan H., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020 Sep 1;159(3):944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021 Mar;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolhe N.V., Fluck R.J., Selby N.M., Taal M.W. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med. 2020 Oct 30;17(10) doi: 10.1371/journal.pmed.1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen L.D., Viscogliosi E., Delhaes L. The lung mycobiome: an emerging field of the human respiratory microbiome. Front Microbiol. 2015 Feb 13;6:89. doi: 10.3389/fmicb.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L., Zhan H., Xu W., Yan S., Ng S.C. The role of gut mycobiome in health and diseases. Therapeut Adv Gastroenterol. 2021 Sep;14 doi: 10.1177/17562848211047130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto S., Saito M., Tamura A., Prawisuda D., Mizutani T., Yotsuyanagi H. The human microbiome and COVID-19: a systematic review. PLoS One. 2021 Jun 23;16(6) doi: 10.1371/journal.pone.0253293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viciani E., Gaibani P., Castagnetti A., Liberatore A., Bartoletti M., Viale P., et al. Critically ill patients with COVID-19 show lung fungal dysbiosis with reduced microbial diversity in patients colonized with Candida spp. Int J Infect Dis. 2022 Apr 1;117:233–240. doi: 10.1016/j.ijid.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rueca M., Fontana A., Bartolini B., Piselli P., Mazzarelli A., Copetti M., et al. Investigation of nasal/oropharyngeal microbial community of COVID-19 patients by 16S rDNA sequencing. Int J Environ Res Publ Health. 2021 Jan;18(4):2174. doi: 10.3390/ijerph18042174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nardelli C., Gentile I., Setaro M., Di Domenico C., Pinchera B., Buonomo A.R., et al. Nasopharyngeal microbiome signature in COVID-19 positive patients: can we definitively get a role to fusobacterium periodonticum? Front Cell Infect Microbiol. 2021 Feb 15;11:18. doi: 10.3389/fcimb.2021.625581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021 Apr 1;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coperchini F., Chiovato L., Ricci G., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev. 2021 Apr 1;58:82–91. doi: 10.1016/j.cytogfr.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheffold A., Bacher P., LeibundGut-Landmann S. T cell immunity to commensal fungi. Curr Opin Microbiol. 2020 Dec 1;58:116–123. doi: 10.1016/j.mib.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022 Feb;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 14.Soffritti I., D'Accolti M., Fabbri C., Passaro A., Manfredini R., Zuliani G., et al. Oral microbiome dysbiosis is associated with symptoms severity and local immune/inflammatory response in COVID-19 patients: a cross-sectional study. Front Microbiol. 2021;12:1397. doi: 10.3389/fmicb.2021.687513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv L., Gu S., Jiang H., Yan R., Chen Y., Chen Y., et al. Gut mycobiota alterations in patients with COVID-19 and H1N1 infections and their associations with clinical features. Commun Biol. 2021 Apr 13;4(1) doi: 10.1038/s42003-021-02036-x. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinold J., Farahpour F., Schoerding A.K., Fehring C., Dolff S., Konik M., et al. The fungal gut microbiome exhibits reduced diversity and increased relative abundance of Ascomycota in severe COVID-19 illness and distinct interconnected communities in SARS-CoV-2 positive patients. Front Cell Infect Microbiol. 2022:466. doi: 10.3389/fcimb.2022.848650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A., Bhanushali S., Sanap A., Shekatkar M., Kharat A., Raut C., et al. Oral dysbiosis and its linkage with SARS-CoV-2 infection. Microbiol Res. 2022 Aug 1;261 doi: 10.1016/j.micres.2022.127055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aranjani J.M., Manuel A., Abdul Razack H.I., Mathew S.T. COVID-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic's second wave in India. PLoS Neglected Trop Dis. 2021 Nov 18;15(11) doi: 10.1371/journal.pntd.0009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A., Karyakarte R., Joshi S., Das R., Jani K., Shouche Y., et al. Nasopharyngeal microbiome reveals the prevalence of opportunistic pathogens in SARS-CoV-2 infected individuals and their association with host types. Microb Infect. 2022 Feb 1;24(1) doi: 10.1016/j.micinf.2021.104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellemain E., Carlsen T., Brochmann C., Coissac E., Taberlet P., Kauserud H. ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol. 2010 Dec;10(1):1–9. doi: 10.1186/1471-2180-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews S., Babraham Bioinformatics 2010 FastQC: a quality control tool for high throughput sequence data. Manual. 2017 http://www.bioinformatics.babraham.ac.uk/projects/fastqc [Google Scholar]
- 22.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016 Jul;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson R.H., Larsson K.H., Taylor A.F., Bengtsson-Palme J., Jeppesen T.S., Schigel D., et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019 Jan 8;47(D1):D259–D264. doi: 10.1093/nar/gky1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis N.M., Proctor D.M., Holmes S.P., Relman D.A., Callahan B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 2018 Dec;6(1):1–4. doi: 10.1186/s40168-018-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013 Apr 22;8(4) doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krüger W., Vielreicher S., Kapitan M., Jacobsen I.D., Niemiec M.J. Fungal-bacterial interactions in health and disease. Pathogens. 2019 Jun;8(2):70. doi: 10.3390/pathogens8020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver D., Gago S., Bromley M., Bowyer P. The human lung mycobiome in chronic respiratory disease: limitations of methods and our current understanding. Curr Fungal Infect Rep. 2019 Sep;13(3):109–119. [Google Scholar]
- 28.Martinsen E.M., Eagan T.M., Leiten E.O., Haaland I., Husebø G.R., Knudsen K.S., et al. The pulmonary mycobiome—a study of subjects with and without chronic obstructive pulmonary disease. PLoS One. 2021 Apr 7;16(4) doi: 10.1371/journal.pone.0248967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh B., Denning D.W. Allergic bronchopulmonary mycosis due to Alternaria: case report and review. Med Mycol Case Rep. 2012 Jan 1;1(1):20–23. doi: 10.1016/j.mmcr.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denning D.W., Pashley C., Hartl D., Wardlaw A., Godet C., Del Giacco S., et al. Fungal allergy in asthma–state of the art and research needs. Clin Transl Allergy. 2014 Dec;4(1):1–23. doi: 10.1186/2045-7022-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang T.T., Shao T.Y., Ang W.G., Kinder J.M., Turner L.H., Pham G., et al. Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell Host Microbe. 2017 Dec 13;22(6):809–816. doi: 10.1016/j.chom.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.