Abstract
Background
BNT162b2 (Pfizer/BioNTech, Comirnaty) and mRNA-1273 (Moderna, Spikevax) are messenger RNA (mRNA) vaccines that elicit antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike receptor-binding domain (S-RBD) and have been approved by the US Food and Drug Administration to combat the coronavirus disease 2019 (COVID-19) pandemic. Because vaccine efficacy and antibody levels waned over time after the 2-shot primary series, the US Food and Drug Administration authorized a booster (third) dose for both mRNA vaccines to adults in the fall of 2021.
Objective
To evaluate the magnitude and durability of S-RBD immunoglobulin (Ig)G after the booster mRNA vaccine dose in comparison to the primary series. We also compared S-RBD IgG levels after BNT162b2 and mRNA-1273 boosters and explored effects of age and prior infection.
Methods
Surrounding receipt of the second and third homologous mRNA vaccine doses, adults in an employee-based cohort provided serum and completed questionnaires, including information about previous COVID-19 infection. The IgG to S-RBD was measured using an ImmunoCAP-based system. A subset of samples were assayed for IgG to SARS-CoV-2 nucleocapsid by commercial assay.
Results
There were 228 subjects who had samples collected between 7 and 150 days after their primary series vaccine and 117 subjects who had samples collected in the same time frame after their boost. Antibody levels from 7 to 31 days after the primary series and booster were similar, but S-RBD IgG was more durable over time after the boost, regardless of prior infection status. In addition, mRNA-1273 post-boost antibody levels exceeded BNT162b2 out to 5 months.
Conclusion
The COVID-19 mRNA vaccine boosters increase antibody durability, suggesting enhanced long-term clinical protection from SARS-CoV-2 infection compared with the 2-shot regimen.
Introduction
Immunoglobulin (Ig)G antibodies targeting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike receptor-binding domain (S-RBD) play an important role in host defense against the viral culprit of coronavirus disease 2019 (COVID-19).1 , 2 Accordingly, the S-RBD is the major antigen that has been targeted by commercially approved COVID-19 vaccines. As vaccine-induced protection against SARS-CoV-2 waned and breakthrough infections increased after the primary series, in Fall 2021 the Food and Drug Administration (FDA) authorized third (“booster”) doses of 2 messenger RNA (mRNA) vaccines, BNT162b2 (Comirnaty, Pfizer [Manhattan, New York]/BioNTech [Mainz, Germany]) and mRNA-1273 (Spikevax, Moderna, Cambridge, Massachusetts).3, 4, 5 Although the third dose of each of these vaccines has been found to enhance protection against infection and severe disease, as compared with the primary 2-shot series, the durability of protection against SARS-CoV-2 infection over time remains an important question.6, 7, 8 Furthermore, although antibody levels are an imperfect surrogate of vaccine efficacy, it is clear that antibodies to S-RBD are an important component of a protective response.1 , 2 To date, there has been little data revealing the dynamics of the antibody response after booster vaccination in comparison to the initial primary series. In addition, there has been a lack of head-to-head studies comparing BNT162b2 and mRNA-1273 after booster vaccination. Here, we used a quantitative assay to evaluate the levels and durability of IgG to S-RBD elicited by booster doses of both mRNA vaccines in an employee cohort. This work builds on prior investigations of the same cohort in which we found that antibodies elicited by BNT162b2 decayed more rapidly after the primary vaccine series as compared with mRNA-1273.9 , 10 These studies also revealed that BNT162b2 elicited lower levels of antibodies in older adults (age ≥ 50 years) as compared with younger adults, an effect that was not found with mRNA-1273. Here, we sought to address the following hypotheses about BNT162b2 and mRNA-1273 booster vaccines: (1) IgG to S-RBD would reach a higher peak level after the booster vaccination as compared with the primary vaccine series; (2) IgG to S-RBD levels would be more durable after booster vaccination; and (3) the differences in IgG levels elicited by BNT162b2 and mRNA-1273 observed after the primary series would persist after booster vaccination. We also investigated the effects of prior infection and age on IgG levels after these 2 vaccines.
Methods
Study Design and Populations
This cohort study was approved by the University of Virginia (UVA) institutional review board, and all participants provided verbal and written consent. Adults affiliated with UVA were recruited from December 2020 to August 2021 by flyer and e-mail announcements to participate in a study investigating antibody responses surrounding the initial vaccine series, as previously reported.9 , 10 In Fall 2021, the study was modified and opened to adults in the greater Charlottesville community. Most enrollees in this study were health care workers employed by the UVA Health System. The current analysis includes participants who received 2 primary series doses and those who received an additional homologous boost dose of the BNT162b2 (30 µg) or mRNA-1273 (50 µg) vaccines. For inclusion, participants must have had a blood sample collected between 7 and 150 days after the second or third vaccine. There were no exclusion criteria relating to preexisting comorbidities. No samples were included in this analysis from subjects who received additional vaccine doses, heterologous booster doses, alternative dosing regimens (ie, 3 doses of 100 µg mRNA-1273), or other COVID-19 vaccines. Blood samples were processed, and serum was isolated and banked at −30°C before being assayed. We screened subjects for symptomatic COVID-19 infection at each visit by asking participants to self-report positive COVID-19 antigen or polymerase chain reaction test results and/or symptoms suggestive of COVID-19 infection.
Antibody Assays
The IgG to S-RBD was measured in serum using a quantitative ImmunoCAP-based system with a Phadia 250 (Thermo Fisher/Phadia, Waltham, Massachusetts), as previously described.11 In brief, S-RBD (RayBiotech, Peachtree Corners, Georgia) was biotinylated and conjugated to streptavidin-coated ImmunoCAPs (Thermo Fisher/Phadia, Waltham, Massachusetts). Background signal was accounted for by subtracting the signal of an unconjugated streptavidin ImmunoCAP, which was run in parallel with each sample. The IgG antibodies to the nucleocapsid protein were measured in a subset of the cohort—specifically subjects who had paired “early” (days 7-31) and “late” (days 90-150) post-booster samples available—using the Abbott SARS-CoV-2 Nucleocapsid Protein IgG assay (Architect i2000, Abbott, Abbott Park, Illinois). To optimize sensitivity of this assay for detecting positive cases, we used an index threshold of 0.26 as an indicator of previous infection (as compared with the standard index of 1.4), as previously reported.12 A subject was considered to have been infected before their booster vaccine if antinucleocapsid IgG was present in their early sample. A subject was considered to have been infected after their booster vaccine if antinucleocapsid IgG was not present in the early sample but was present in the late sample.
Statistical Analysis
Antibody levels were expressed by geometric mean (GM) with 95% confidence intervals (CIs). Continuous data were compared using Student's t test, Mann-Whitney U test, and analysis of variance, as appropriate. Categorical data were compared using χ2 test. Regression modeling was performed using log-transformed antibody levels. Only subjects who had paired early and late samples from the primary series or booster series were used in the longitudinal linear regression model. For subjects with multiple samples collected between days 7 and 31, the sample with the maximum antibody level was used as their early time point in this model, and all draws between days 32 and 150 were included as continuous data. Statistical analysis was performed with GraphPad Prism 8 (GraphPad Software, San Diego, California) and R software, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Vaccine Cohort
There were 228 subjects who provided at least 1 blood sample between 7 and 150 days after their second dose of BNT162b2 or mRNA-1273 (primary series group) and 117 subjects who provided at least 1 blood sample in the same time window after their third dose of BNT162b2 or mRNA-1273 (booster group). Most subjects in the booster group participated in the initial primary series study and thus have data included in both the primary and booster series analysis [n = 106 (91%)].9 The booster group tended to be older than the primary series group, with median age of 44 (interquartile range [IQR], 34-57) years vs 41 (IQR, 32-54) years, respectively, but this difference was not significant (P = .06) (Table 1 ). Both groups were predominantly of female sex, with women representing 75% of the participants in the primary series group and 77% of the participants in the booster group. Race was similarly distributed among the participants in the primary series group and the booster group. More subjects in the booster group self-reported infection (13%) compared with the primary series group (4%) (P = .001). There was a similar distribution of BNT162b2 and mRNA-1273 recipients in the primary series group (50% and 50%) and the booster group (51% and 49%). Most subjects received their primary series vaccinations in the winter of 2020 to 2021 and their booster vaccination in the fall/winter of 2021 to 2022 during the Delta and Omicron surges of infections (Fig 1 ).13 Most infections reported in the cohort occurred during the winter of 2021 to 2022, which coincided with the emergence of Omicron and a dramatic increase in COVID-19 cases in the state of Virginia (Fig 1).13 , 14
Table 1.
Demographics and Characteristics of COVID-19 Vaccine Cohort
| Characteristics | Primary series(n = 228) | Booster vaccine(n = 117) | P value | |
|---|---|---|---|---|
|
Age |
Median (range) | 41 (19-85) | 44 (19-87) | .06a |
| <50 y, n (%) | 152 (67%) | 68 (58%) | .12b | |
| ≥50 y, n (%) | 76 (33%) | 49 (42%) | ||
| Sex | Female, n (%) | 171 (75%) | 90 (77%) | .69b |
| Male, n (%) | 57 (25%) | 27 (23%) | ||
| Race | White, n (%) | 179 (79%) | 96 (82%) | .64b |
| Black, n (%) | 17 (8%) | 6 (5%) | .46b | |
| Asian, n (%) | 24 (11%) | 10 (9%) | .71b | |
| Other, n (%) | 8 (4%) | 5 (4%) | .59b | |
| Vaccine received | BNT162b2, n (%) | 114 (50%) | 60 (51%) | .82b |
| mRNA-1273, n (%) | 114 (50%) | 57 (49%) | ||
| 2 or more chronic comorbidities,c n (%) | 6 (3%) | 4 (3%) | .68b | |
| On immunosuppressing medications,d n (%) | 8 (4%) | 4 (3%) | .97b | |
| Self-report of previous COVID-19 infection, n (%) | 8 (4%) | 15 (13%) | .001b | |
| Baseline samples, n (%) | 41 (18%) | 34 (29%) | .02b | |
| S-RBD IgG, GM µg/mL (95% CI) | 0.3 (0.3-0.5) | 4.4 (3.0-6.3) | <.001e | |
| Early samples (D7-31 postvaccine), n (%) | 169 (74%) | 62 (48%) | <.001b | |
| Days, median (IQR) | 21 (18-24) | 17.5 (13-24) | .008a | |
| S-RBD IgG, GM µg/mL (95% CI) | 55.3 (49.8-61.3) | 55.3 (46.5-65.6) | .49e | |
| Late samples (D90-150 postvaccine), n (%) | 98 (43%) | 98 (76%) | <.001b | |
| Days, median (IQR) | 133 (112.5-139) | 126 (121-133) | .98a | |
| S-RBD IgG, GM µg/mL (95% CI) | 9.5 (8.1-11.1) | 29.1 (24.3-34.9) | <.001e | |
| Total number of samples per subject, mean (range) | 1.79 (1-7) | 2.27 (1-8) | .003a | |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; d, day; IgG, immunoglobulin G; IQR, interquartile range; GM, geometric mean; S-RBD, spike receptor-binding domain.
Unpaired t test.
χ2 test.
Defined as hypertension, heart disease, diabetes, COPD, chronic kidney disease, and/or asthma.
Defined by self-report of treatment with immunomodulatory or immunosuppressant medication, including oral corticosteroids.
Mann-Whitney U test.
Figure 1.
Timeline of vaccines, blood draws, and COVID-19 infections in the vaccine cohort. The VDH-confirmed COVID-19 infections and genotyped SARS-CoV-2 variants reported by the CDC in HHS region 3 are also illustrated.13,14 CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; HHS, Health and Human Services; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VDH, Virginia Department of Health.
IgG to SARS-CoV-2 Spike RBD Following Primary Series and Booster Vaccination
We first compared S-RBD IgG levels elicited by the primary vaccine series and the booster vaccine using all samples collected within the following 3 time intervals: (1) 0 to 7 days pre-vaccine (baseline), (2) 7 to 31 days postvaccine (early), and (3) 90 to 150 days postvaccine (late). As expected, baseline antibody levels were higher in subjects from the booster group (GM, 4.4 µg/mL [95% CI, 3.0-6.3]) compared with the primary series (GM, 0.3 µg/mL [95% CI, 0.3-0.5]) (P < .001), as the latter subjects had not been previously vaccinated (Fig 2 A). Early after vaccination, there was no difference in antibody levels measured after the primary series (GM, 55.3 µg/mL [95% CI, 49.8-61.3]) or homologous booster vaccination (GM, 55.3 µg/mL [95% CI, 46.5-65.6]). By contrast, in the late time interval, IgG levels after the booster mRNA vaccine were approximately 3-fold higher (GM, 29.1 µg/mL [95% CI, 24.3-34.9]) compared with the primary series vaccination (GM, 9.5 µg/mL [95% CI, 8.1-11.1]) (P < .001) (Fig 2A). We then confirmed that there was a difference in trajectories between the booster and primary series by evaluating subjects who had paired early and late samples available. Linear regression indicated a slower IgG decay after the booster compared with the primary series vaccination, with respective slopes of −0.0024 (95% CI, −0.0037 to −0.0012) vs −0.0074 (95% CI, −0.0083 to −0.0065) (P < .001) (Fig 2B). A sensitivity analysis restricted to 30 subjects who had paired samples available after both the primary series and booster vaccination also revealed more persistent IgG after the booster vaccination (data not shown).
Figure 2.
SARS-CoV-2 S-RBD IgG trajectory after 2-dose (primary series) and 3-dose (booster) COVID-19 mRNA vaccine series. (A) IgG levels in the pre-vaccine, early and late windows. (B) Regression analysis of longitudinal paired samples. Bold lines indicate regression slopes and shaded area 95% confidence intervals. COVID-19, coronavirus disease 2019; d, day; IgG, immunoglobulin G; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S-RBD, spike receptor-binding domain.
Effect of Coronavirus Disease 2019 Infection on Booster Vaccination Immunoglobulin G Levels
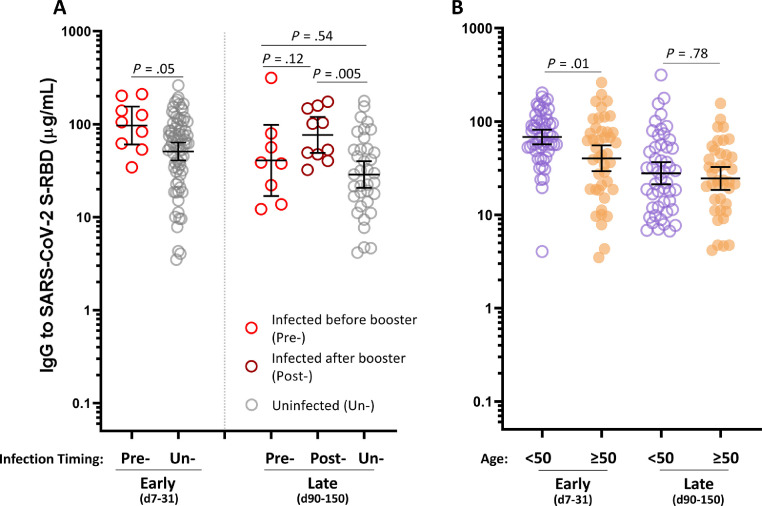
Because the booster arm of the study coincided with the wave of infections associated with the Omicron variant in the winter of 2021 to 2022, we sought to determine whether prior or intercurrent COVID-19 infection affected IgG levels in the recipients of the booster vaccines. Positive infection status was determined by either self-report or nucleocapsid IgG testing (among the subjects who had paired early and late samples available). In the early time window, S-RBD IgG levels were higher in those who were infected before the booster vaccine dose (GM, 96.7 µg/mL [95% CI, 60.4-154.8]) as compared with those who were not infected before the booster vaccine dose (GM, 50.9 µg/mL [95% CI, 40.8-63.5]) (P = .05) (Fig 3 A). By the late time window, this difference in antibody levels between those who were infected before the booster vaccine dose (GM, 40.7 µg/mL [95% CI, 16.9-98.2]) and those who were uninfected (GM, 28.7 µg/mL [95% CI, 20.6-40.0]) was no longer present (P = .54). However, those who were infected after their booster vaccine (GM, 76.5 µg/mL [95% CI, 48.9-119.7]) had significantly greater IgG levels than the uninfected subjects in the late time window (P = .005). Of note, to be considered as uninfected in this analysis required a combination of negative self-report and negative nucleocapsid IgG testing result.
Figure 3.
The effects of infection and age on SARS-CoV-2 S-RBD IgG levels. (A) IgG levels in uninfected (Un-) as compared with those infected before (Pre-) or after (Post-) booster vaccination. (B) S-RBD IgG levels stratified by age (excluding participants with prior self-reported COVID-19 infection). COVID-19, coronavirus disease 2019; d, day; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S-RBD, spike receptor-binding domain.
Effect of Age on Booster Vaccination Immunoglobulin G Levels
We next sought to investigate the effect of age on IgG levels after booster immunization. Building off our previous reports, we stratified the subjects into older (≥50 years) and younger (<50 years) age groups.9 , 10 To account for the effect of natural infection on IgG levels, this analysis excluded subjects who reported prior COVID-19 infection. Antibody levels were significantly higher in the younger subjects (GM, 68.1 µg/mL [95% CI, 56.8-81.6]) compared with the older subjects (GM, 40.2 µg/mL [95% CI, 29.2-55.3]) in the early time window (P = .01). Interestingly, this difference did not persist in the late time window (P = .78) (Fig 3B).
Comparison of Immunoglobulin G to Severe Acute Respiratory Syndrome Coronavirus 2 Spike Receptor-Binding Domain After Booster Vaccination With BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna)
We then compared S-RBD IgG levels in those who received BNT162b2 and mRNA-1273 booster vaccines, again excluding subjects who reported prior COVID-19 infection. Before the boost, recipients of 2 doses of mRNA-1273 (GM, 4.5 µg/mL [95% CI, 3.3-6.1]) had higher antibody levels than recipients of 2 doses of BNT162b2 (GM, 2.1 µg/mL [95% CI, 1.5-3.0]) (P = .004). In the early post-boost time window, antibody levels were higher in those subjects who received 3 doses of mRNA-1273 (GM, 65.1 µg/mL [95% CI, 52.8-80.3]) vs those who received 3 doses of BNT162b2 (GM, 42.1 µg/mL [95% CI, 31.2-56.8]) (P = .01). Moreover, during the late time window, subjects who received mRNA-1273 retained nearly twice the level of antispike RBD IgG (GM, 36.8 µg/mL [95% CI, 28.6-47.4]) as those who received BNT162b2 (GM, 18.6 µg/mL [95% CI, 14.2-24.4]) (P < .001) (Fig 4 A).
Figure 4.
Comparison SARS-CoV-2 S-RBD IgG levels by vaccine and interaction of vaccine and age. (A) S-RBD IgG stratified by BNT162b2/Pfizer-BioNTech (P) or mRNA-1273/Moderna (M) vaccine. (B) S-RBD IgG stratified by age and vaccine. (C) Linear regression of S-RBD levels in relation to age. d, day; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S-RBD, spike receptor-binding domain.
We further stratified the subjects by both age and vaccine to investigate the combined effects of these variables on IgG magnitude and persistence. Consistent with our prior findings during the primary series, antibody levels in the early time window were significantly lower in the older subjects who received BNT162b2 (GM, 25.2 µg/mL [95% CI, 15.7-40.2]) compared with the younger subjects who received BNT162b2 (GM, 70.5 µg/mL [95% CI, 55.8-89.1]) (P = .001).9 For mRNA-1273, there was no age effect and older subjects who received mRNA-1273 had significantly higher antibody levels (GM, 62.9 µg/mL [95% CI, 43.4-91.1]) compared with age-similar subjects who received BNT162b2 (P = .004). In the late time window, antibody levels were similar among older (GM, 16.5 µg/mL [95% CI, 9.7-28.2]) and younger (GM, 19.8 µg/mL [95% CI, 14.3-27.5]) recipients of BNT162b2 and older (GM, 31.1 µg/mL [95% CI, 22.7-42.5]) and younger (GM, 45.6 µg/mL [95% CI, 30.0-69.5]) recipients of mRNA-1273, respectively. Taken together, in the late window, S-RBD levels were higher in the recipients of mRNA-1273 vs BNT162b2 regardless of age (Fig 4B). To further explore the effect of age, we carried out linear regression comparing S-RBD IgG vs age in the early post-boost window (using peak antibody levels for subjects who had more than 1 sample available). Antibody levels elicited by BNT162b2 significantly decreased with increasing age (P = .003), whereas antibody levels elicited by mRNA-1273 remained stable (P = .78) (Fig 4C).
Discussion
Building on our prior investigations of COVID-19 mRNA vaccine immunogenicity after the primary vaccination series, here we have evaluated the magnitude and trajectory of S-RBD IgG antibodies elicited by BNT162b2 and mRNA-1273 up to 5 months after the booster regimen.9 , 10 We also investigated the effects of infection and age on antibody durability after the booster vaccine. Our results revealed that a third dose of BNT162b2 or mRNA-1273 did not result in a higher peak of S-RBD IgG levels compared with a 2-dose mRNA vaccine series. This contrasts with some reports, which have found higher peak IgG levels after a third mRNA vaccine compared with the primary series.15, 16, 17, 18 The explanation for the discrepancy is not clear, but the window in which the samples were collected could be relevant given that antibody levels are dynamic and there are differences in trajectory between the 2 mRNA vaccines. The discrepancy could also relate to variability in assays that have been used to measure IgG to S-RBD. For example, differences in the capacity of the solid phase could affect assay dynamic ranges.
Our finding that antibody durability after a third mRNA dose was enhanced compared with the primary series is supported by a few reports, but there has been little published data comparing antibody levels as far as 5 months postvaccination.19, 20, 21 Although there is no specific antibody level that has been found to definitively correlate with protective immunity against SARS-CoV-2, antibody magnitude and persistence have been found to confer greater protection against infection and disease.22, 23, 24, 25 Accordingly, understanding the durability of antibodies after the third vaccination could be relevant to guiding the timing of any additional follow-up vaccinations.
When investigating the relevance of prior COVID-19 infection on antibody levels and durability, we observed that infection before the boost was associated with significantly higher antibody levels in the early time window, but the difference did not persist at 3 to 5 months post-booster. These findings indicate that the enhanced antibody durability observed after the booster vaccination was not explained by hybrid immunity and raise questions about the durability of immune protection resulting from natural infection. Of note, there have been mixed findings reported on the antibody response stemming from hybrid vs vaccine-elicited immunity.17 , 26 , 27 In a separate finding, we observed that subjects who were boosted and subsequently infected had the highest levels of antibodies after 4 months. We surmise that vaccine-induced antibodies likely peaked and began to decline in these subjects before a natural infection further enhancing the IgG levels, though we lacked sufficiently granular sampling to demonstrate this.
Our comparison of the IgG post-boost response between the 2 FDA-authorized COVID-19 mRNA vaccines revealed that mRNA-1273 had enhanced immunogenicity vs BNT162b2. Antibody levels elicited by mRNA-1273 were higher than BNT162b2 when assessed during the early and the late post-boost time window. To our knowledge, this is a novel finding, but it is not unexpected as we and others have previously found that mRNA-1273 elicits greater levels of S-RBD IgG than BNT162 after the primary vaccination.9 , 10 , 28 , 29 Moreover, an enhanced antibody response to mRNA-1273 is biologically plausible as it incorporates a greater amount of mRNA than BNT162b2 both during the initial priming series (100 µg in mRNA-1273 vs 30 µg in BNT162b2) and the boost (50 µg in mRNA-1273 vs 30 µg in BNT162b2).
Our finding that S-RBD IgG levels were lower in older vs younger subjects early after a third mRNA vaccine was accounted for by lower antibody levels in older subjects who received 3 doses of the BNT162b2 vaccine. Between 7 and 31 days after a third vaccine, there was no difference in antibody levels between older and younger subjects who received mRNA-1273. In the early time window, older subjects who received BNT162b2 had not only lower antibody levels than their younger counterparts but also lower antibody levels than the older subjects who received mRNA-1273. In contrast to the early findings, by 4 months post-boost, we did not observe differences in older vs younger recipients of BNT162b2. However, we found that mRNA-1273 antibody levels were significantly higher than BNT162b2 antibody levels between 3 and 5 months after booster vaccination regardless of age. These data reinforce the finding that mRNA-1273 elicits more durable antibodies than BNT162b2 and parallel what has previously been reported regarding S-RBD IgG levels after the primary series.9 , 10 , 28 , 29
There are several limitations to consider. We did not measure binding antibodies to new SARS-CoV-2 variants, such as Omicron and its subvariants. Moreover, the emergence of these variants could nullify the effects of enhanced antibody durability to the native S-RBD.8 , 30 , 31 In addition, we did not measure neutralizing antibody titers in this cohort. We and others, however, have previously revealed that neutralizing antibodies correlate moderately to strongly with binding IgG levels.9 , 16 , 32 Another limitation is that we had a relatively small sample size for some of the vaccine and age comparisons, particularly after excluding subjects with history of prior COVID-19 infection. We also did not measure nucleocapsid IgG in all samples, which could contribute to an underestimate of COVID-19 infection in this cohort.
In conclusion, we found that a booster dose of an mRNA vaccine elicits greater S-RBD IgG durability compared with a primary 2-dose regimen, regardless of previous infection status. This finding suggests that a third dose of an mRNA vaccine provides more persistent protection from COVID-19 than a 2-dose regimen. Our data also indicate that 3 doses of mRNA-1273 elicit a stronger antibody response than 3 doses of BNT162b2, regardless of age. Whether mRNA-1273 confers superior protection against circulating strains compared with BNT162b2 remains an open question. Although vaccine-elicited protection against SARS-CoV-2 infection wanes over time, we would highlight that both FDA-authorized mRNA vaccines were found to have valuable protection against severe disease and death from COVID-19.6 , 16 , 33 , 34
Acknowledgments
We are grateful to the University of Virginia staff and Charlottesville community members who participated in the study, Dr Jaimin Patel for helping with patient enrollment, Jacob Goldstein-Greenwood for statistical counseling, and Panwichit Tongvichit, Kelly Dinwiddie, and Judy Hundley with the University of Virginia Clinical Laboratory.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Funding: Dr Wilson reports receiving funding from the University of Virginia Manning COVID-19 Research Fund and salary support from the American Academy of Allergy, Asthma & Immunology Faculty Development Award. Dr Platts-Mills reports receiving funding from the National Institutes of Health R37-AI20565.
References
- 1.Scourfield D, Reed S, Quastel M, Alderson J, Bart VMT, Crespo AT, et al. The role and uses of antibodies in COVID-19 infections: a living review. Oxf Open Immunol. 2021;2(1) doi: 10.1093/oxfimm/iqab003. iqab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molodtsov I, Kegeles E, Mitin A, Mityaeva O, Musatova OE, Panova A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T cells and antibodies in coronavirus disease 2019 (COVID-19) protection: a prospective study. Clin Infect Dis. 2022;75(1):e1–e9. doi: 10.1093/cid/ciac278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA expands eligibility for COVID-19 vaccine boosters. 2021. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters. Accessed July 15, 2022.
- 4.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mrna BNT162B2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, et al. Covid-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022;22(7):1002–1010. doi: 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butt AA, Talisa VB, Yan P, Shaikh OS, Omer SB, Mayr FB. Vaccine effectiveness of 3 versus 2 doses of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) mRNA vaccines in a high-risk national population. Clin Infect Dis. 2022;75(1):e579–e584. doi: 10.1093/cid/ciac178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patalon T, Saciuk Y, Peretz A, Perez G, Lurie Y, Maor Y, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun. 2022;13(1):3203. doi: 10.1038/s41467-022-30884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keshavarz B, Richards NE, Workman LJ, Patel J, Muehling LM, Canderan G, et al. Trajectory of IgG to SARS-COV-2 after vaccination with BNT162B2 or mRNA-1273 in an employee cohort and comparison with natural infection. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.850987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards NE, Keshavarz B, Workman LJ, Nelson MR, Platts-Mills TA, Wilson JM. Comparison of SARS-COV-2 antibody response by age among recipients of the BNT162B2 vs the mRNA-1273 vaccine. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keshavarz B, Wiencek JR, Workman LJ, Straesser MD, Muehling LM, Canderan G, et al. Quantitative measurement of IgG to severe acute respiratory syndrome coronavirus-2 proteins using immunoCAP. Int Arch Allergy Immunol. 2021;182(5):417–424. doi: 10.1159/000514203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohanraj D, Bicknell K, Bhole M, Webber C, Taylor L, Whitelegg A. Antibody responses to SARS-COV-2 infection-comparative determination of seroprevalence in two high-throughput assays versus a sensitive spike protein ELISA. Vaccines (Basel) 2021;9(11):1310. doi: 10.3390/vaccines9111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virginia Open Data Portal. VDH-COVID-19-PublicUseDataset-EventDate. 2022. Available at: https://data.virginia.gov/Government/VDH-COVID-19-PublicUseDataset-EventDate/9d6i-p8gz/data. Accessed August 26, 2022.
- 14.Centers for Disease Control and Prevention. SARS-CoV-2 variant proportions. 2022. Available at: https://data.cdc.gov/Laboratory-Surveillance/SARS-CoV-2-Variant-Proportions/jr58-6ysp. Accessed August 26, 2022.
- 15.Chu L, Vrbicky K, Montefiori D, Huang W, Nestorova B, Chang Y, et al. Immune response to SARS-COV-2 after a booster of mRNA-1273: an open-label phase 2 trial. Nat Med. 2022;28(5):1042–1049. doi: 10.1038/s41591-022-01739-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lustig Y, Gonen T, Meltzer L, Gilboa M, Indenbaum V, Cohen C, et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat Immunol. 2022;23(6):940–946. doi: 10.1038/s41590-022-01212-3. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Ibarguengoitia M, Rivera-Salinas D, Hernández-Ruíz Y, Armendariz-Vazquez AG, Gonzalez-Cantu A, Barco-Flores IA, et al. Effect of the third dose of BNT162b2 vaccine on quantitative SARS-CoV-2 spike 1-2 IgG antibody titers in healthcare personnel. PLoS One. 2022;17(3) doi: 10.1371/journal.pone.0263942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belik M, Jalkanen P, Lundberg R, Reinholm A, Laine L, Vaisanen E, et al. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat Commun. 2022;13(1):2476. doi: 10.1038/s41467-022-30162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilboa M, Regev-Yochay G, Mandelboim M, Indenbaum V, Asraf K, Fluss R, et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 omicron infection. JAMA Netw Open. 2022;5(9) doi: 10.1001/jamanetworkopen.2022.31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzberg J, Fischer B, Becher H, Becker AK, Honarpisheh H, Guraya SY, et al. Short-term drop in antibody titer after the third dose of SARS-CoV-2 BNT162b2 vaccine in adults. Vaccines (Basel) 2022;10(5):805. doi: 10.3390/vaccines10050805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roeder AJ, Koehler MA, Jasbi P, McKechnie D, Vanderhoof J, Edwards BA, et al. Longitudinal comparison of neutralizing antibody responses to COVID-19 mRNA vaccines after second and third doses. Vaccines. 2022;10(9):1459. doi: 10.3390/vaccines10091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earle K, Ambrosino D, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry J, Osman S, Wright J, Richard-Greenblatt M, Buchan SA, Sadarangani M, et al. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0266852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montez-Rath M, Garcia P, Han J, Cadden L, Hunsader P, Morgan C, et al. SARS-CoV-2 infection during the omicron surge among patients receiving dialysis: the role of circulating receptor-binding domain antibodies and vaccine doses [e-pub ahead of print] J Am Soc Nephrol. 2022;33(10):1832–1839. doi: 10.1681/ASN.2022040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng S, Phillips D, White T, Sayal H, Aley P, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kontopoulou K, Nakas C, Papazisis G. Significant increase in antibody titers after the 3rd booster dose of the Pfizer–BioNTech mRNA COVID-19 vaccine in healthcare workers in Greece. Vaccines (Basel) 2022;10(6):876. doi: 10.3390/vaccines10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Tong P, Whiteman N, Moghaddam AS, Zarghami N, Zuiani A, et al. Immune recall improves antibody durability and breadth to SARS-COV-2 variants [e-pub ahead of print] Sci Immunol. 2022 doi: 10.1126/sciimmunol.abp8328. accessed July 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montoya J, Adams A, Bonetti V, Deng S, Link NA, Pertsch S, et al. Differences in IgG antibody responses following BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines. Microbiol Spectr. 2021;9(3) doi: 10.1128/Spectrum.01162-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Accorsi E, Britton A, Fleming-Dutra K, Smith ZR, Shang N, Derado G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu P, Faraone J, Evans J, Zou X, Zheng YM, Carlin C, et al. Neutralization of the SARS-CoV-2 omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med. 2022;386(26):2526–2528. doi: 10.1056/NEJMc2206725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda K, Amano M, Uemura Y, Tsuchiya K, Matsushima T, Noda K, et al. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. Sci Rep. 2021;11(1):22848. doi: 10.1038/s41598-021-01930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abu-Raddad L, Chemaitelly H, Bertollini R. National Study Group for COVID-19 Vaccination. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N Engl J Med. 2022;386(8):799–800. doi: 10.1056/NEJMc2117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenforde M, Self W, Naioti E, Ginde A, Douin D, Olson S, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults-United States, March-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1156–1162. doi: 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]