Abstract
Background
In Europe, IBS is commonly treated with musculotropic spasmolytics (eg, otilonium bromide, OB). In tertiary care, a low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet provides significant improvement. Yet, dietary treatment remains to be explored in primary care. We evaluated the effect of a smartphone FODMAP-lowering diet application versus OB on symptoms in primary care IBS.
Methods
IBS patients, recruited by primary care physicians, were randomised to 8 weeks of OB (40 mg three times a day) or diet and followed for 24 weeks. We compared IBS Symptom Severity Score and the proportion of responders (improvement ≥50 points) in all patients and the subgroup fulfilling Rome IV criteria (Rome+). We also evaluated treatment efficacy, quality of life, anxiety, depression, somatic symptom severity (Patient Health Questionnaire (PHQ15, PHQ9)) and treatment adherence and analysed predictors of response.
Results
459 primary care IBS patients (41±15 years, 76% female, 70% Rome+) were randomised. The responder rate after 8 weeks was significantly higher with diet compared with OB (71% (155/218) vs 61% (133/217), p=0.03) and more pronounced in Rome+ (77% (118/153) vs 62% (98/158), p=0.004). Patients allocated to diet (199/212) were 94% adherent compared with 73% with OB (148/202) (p<0.001). The significantly higher response rate with diet was already observed after 4 weeks (62% (132/213) vs 51% (110/215), p=0.02) and a high symptom response persisted during follow-up. Predictors of response were female gender (OR=2.08, p=0.04) for diet and PHQ15 (OR=1.10, p=0.02) for OB.
Conclusion
In primary care IBS patients, a FODMAP-lowering diet application was superior to a spasmolytic agent in improving IBS symptoms. A FODMAP-lowering diet should be considered the first-line treatment for IBS in primary care.
Trial registration number
Keywords: IRRITABLE BOWEL SYNDROME, QUALITY OF LIFE, PRIMARY CARE
Significance of this study.
What is already known on this subject?
The low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet is efficacious for the treatment of IBS, as shown in tertiary care patients.
The complexity of the diet precludes its application in primary care IBS patients.
In primary care, pharmacological agents such as spasmolytics are most frequently used as first-line therapy.
What are the new findings?
In a controlled trial enrolling 470 newly diagnosed primary care IBS patients, a self-management FODMAP-lowering smartphone application was superior to standard medical therapy in alleviating IBS symptoms.
The superiority of the diet app was already present at 4 weeks and persisted at 8 and 16 weeks.
The diet app had a high acceptability and adherence rate.
How might it impact on clinical practice in the foreseeable future?
In primary care IBS patients, a self-management FODMAP-lowering smartphone application is the most effective initial therapeutic approach.
Introduction
IBS, defined by the Rome IV criteria as recurrent abdominal pain associated with a change in stool frequency or form and/or related to defecation, affects 4.1% of the adult population.1 2 Based on the dominant stool pattern, IBS can be subdivided into diarrhoea-predominant IBS (IBS-D), constipation-predominant IBS, mixed subtype IBS and unclassified IBS. Besides abdominal pain, patients also report bloating, abdominal distension and flatulence.2 3 Patients with IBS have a decreased quality of life, and the disorder has a high socioeconomic impact.2 4 5
Multiple treatment options have been proposed for IBS, most of them yielding only limited therapeutic gain. In Europe, musculotropic spasmolytics are the most frequently prescribed pharmacological treatment for IBS and are considered as standard medical therapy.3 6 7 Otilonium bromide (OB), an L-type calcium channel blocking agent, improves abdominal pain and bloating in placebo-controlled trials, with significant response rates occurring after 4 or more weeks of treatment.8 9 Over the last decade, a dietary intervention has emerged as an effective treatment option. In tertiary care, a diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) improves symptoms after 6–8 weeks.10 However, due to the complexity of the intervention, this approach requires several visits with an experienced dietitian and is therefore less suitable for primary care.10 11 Moreover, the low FOMAP diet has been associated with decreased caloric intake, some nutrient inadequacies, as well as with potentially unfavourable effects on gut microbiota composition.11 12 More recently, less stringent FODMAP-lowering dietary interventions improve symptoms in tertiary care IBS patients.11 13 However, clinical trials evaluating the effect of a FODMAP-lowering diet in primary care are lacking.
Therefore, the present study aimed to evaluate the efficacy of a self-management approach guided by a smartphone application, aimed at lowering FODMAP intake besides NICE/BDA dietary lifestyle recommendations for IBS, to provide symptom relief in primary care IBS patients compared with the standard of care with OB.
Materials and methods
Trial design
This was a pragmatic randomised open-label parallel group trial in primary care IBS patients comparing the effect of a FODMAP-lowering diet application to OB (see online supplemental figure 1). The trial design and coordination was supervised by a trial board, which included gastroenterologists, dieticians, general practitioners, a patient representative and clinical trial experts. Patients newly treated for IBS were eligible for the trial. IBS was clinically diagnosed by primary care physicians (PCPs, n=105) involved as investigators in the trial. During the screening visit, signed informed consent was obtained prior to any study procedures. Inclusion and exclusion criteria were reviewed and demographic data were collected. Within 14 days after the screening visit, consenting patients were randomised to either a spasmolytic agent (OB, 40 mg three times a day) or a custom-made software application providing FODMAP-lowering dietary instructions. Patients completed questionnaires at baseline, 4 weeks and 8 weeks. At 8 weeks or thereafter, depending on symptom improvement, PCPs were allowed to alter the original treatment. However, starting a FODMAP-lowering diet was not allowed for those randomised to OB. Patients were followed up for an additional 16 weeks with visits and questionnaires at 8-week intervals.
gutjnl-2021-325821supp001.pdf (75.4KB, pdf)
Participants
Patients newly treated for IBS were eligible for the trial. Exclusion criteria included concurrent GI disease or a history of major abdominal surgery, diabetes or uncontrolled coexisting diseases such as thyroid dysfunction, active malignancy, symptomatic endometriosis, a major psychiatric disorder or dosage alteration of antidepressants in the last 3 months. Women with active pregnancy plans in the upcoming 6 months as well as women of childbearing potential not using contraception were excluded.
Patients could not have a history of treatment with OB for more than 3 consecutive weeks in the past and/or any intake within the last 3 months. Additionally, patients were excluded if they had previously followed a FODMAP-lowering diet or if on any elimination diet. The use of medications for IBS during the last 3 weeks prior to and during the treatment phase was prohibited.
Interventions
Patients randomised to medication received a prescription for 3 boxes of 60 tablets OB 40 mg (Spasmomen, Menarini, Zaventem, Belgium), to be taken three times a day. Patients randomised to diet were instructed to download an application for smartphone and/or tablet (developer EverywhereIM, Amsterdam, the Netherlands). Subjects without access to suitable electronic devices received the diet instructions as a booklet (2%). The dietary intervention was set up as a mobile application with instructions written in French and Dutch. The design of the mobile application was based on the self-determination theory to stimulate a change in dietary behaviour. No dietitian was involved in this process. The diet was not a strict low FODMAP diet, but rather designed as a FODMAP-lowering diet in combination with dietary recommendations from the NICE/BDA guidelines for IBS (online supplemental data). This means that small amount of FODMAPs were allowed and only some were advised to be avoided. To achieve this, the mobile application provided patients with general dietary advice and with instructions to the food items that needed to be avoided or decreased with suggested alternatives. In addition, 105 recipes for breakfast, lunch, snacks and dinner were provided. Additionally, the application included interactive tools allowing patients to create a weekly menu and shopping list.14 In addition, information about sleep and physical activity was provided to both groups.
Randomisation
Patients were allocated 1:1 to each treatment arm. The allocation sequence was generated by a random sequence generating programme in web-based software (eCRF). The programme generated a randomisation sequence per PCP. To minimise site influences, a block randomisation schedule was used, based on variable block sizes.
Questionnaire assessments
All questionnaires were completed on an online platform prior or up to 3 days after each study visit.
At baseline, patients filled out the Rome IV IBS diagnostic questionnaire to determine if patients fulfilled the Rome criteria (Rome+). At each study visit, patients completed the IBS Symptom Severity Scale (IBS-SSS).14 Additionally, patients completed validated questionnaires to rate quality of life (IBS-QoL), anxiety (GAD), depression (PHQ9) and levels of (extraintestinal) somatic symptoms (PHQ15).
At each visit, patients also reported treatment adherence by indicating the number of times they forgot to take the medication or to follow the diet from never (zero) to several/most of the times or constantly (six) (Likert scale). Patients who reported having forgotten this at least 2 days per week were considered as non-adherent. In addition, treatment satisfaction was scored as an improvement in IBS symptoms compared with baseline and ranging from ‘a lot worse’ (zero) to ‘extremely better’ (seven).
Safety
All (serious) adverse events were recorded by the PCP in the patient’s medical record in agreement with standard clinical practice measures. Serious adverse reactions, whether reported by the patient or noted by the PCP, were recorded in the online platform and reported to the Ethical Committee.
The body weight of the participants was monitored at every visit and compared at baseline between both treatment groups as well as changes in body weight during the trial.
Statistical analysis
Baseline qualitative (categorical) measures were compared using the Pearson χ2 test, while quantitative measures were compared using the Mann-Whitney test. All continuous variables are reported as mean and SD and with 95% CI.
Power analysis
The number of subjects per arm to obtain 85% power at the 0.05 significance level (two-sided) for a contrast of 50% success for OB and 65% for dietary intervention is 200.9 11 13 15 The sample size was increased to 235 per arm (470 patients in total) to compensate for drop-outs.
Primary endpoint
The analysis was conducted on an intention-to-treat basis. A responder was defined as a patient who improved ≥50 points on IBS-SSS compared with baseline. The proportion of responders after 8 weeks was considered the primary endpoint and compared between treatment arms using the Pearson χ2 test.
Secondary endpoint
Change in IBS-SSS was compared between treatment groups using all responses available at each visit in a mixed model where the interaction between treatment group and time was used to evaluate differences in change from baseline. All time points were included in a single model in which the interaction coefficients were parameterized to represent specific contrasts of the change between baseline and a given trial stage between medication and diet groups. Formal statistical inference employed the non-parametric bootstrap (2000 replications) due to non-normal distribution of the outcome measures. A similar approach was used for the exploratory analysis of the subgroup fulfilling the Rome IV criteria (Rome+ subgroup, see below) and the scores on anxiety, depression, PHQ15 and IBS quality of life. Treatment satisfaction and adherence were evaluated in both treatment groups. The difference in distribution of satisfaction and adherence ratings between medication and diet was tested by Pearson χ2 test. Only the contrast of response rates between study groups at the end of therapy is specified in the primary study hypothesis, all other analyses are considered secondary. For this reason, adjustment for multiple comparisons was not undertaken.
Predictors of response
Parameters for prediction of response (age, body mass index, initial psychological status (as defined by PHQ), quality of life, IBS-SSS and stool pattern subtype) were analysed in both arms (logistic regression) with effect sizes reported as ORs.
Subgroup analysis
A prespecified subgroup analyses comprised analysis of the outcome in Rome+ patients. The percentage of responders was also compared across treatment groups in Rome+.
Missing data handling
Missing data on an entire measure were handled using maximum likelihood estimation in mixed models. The electronic case report form almost eliminated missing data.
Statistical software
SAS software (University Edition; SAS Institute, Cary, North Carolina, USA) and Stata (Stata Statistical Software: Release 16; StataCorp, College Station, Texas, USA) have been used to conduct all statistical analyses.
Results
Patient population
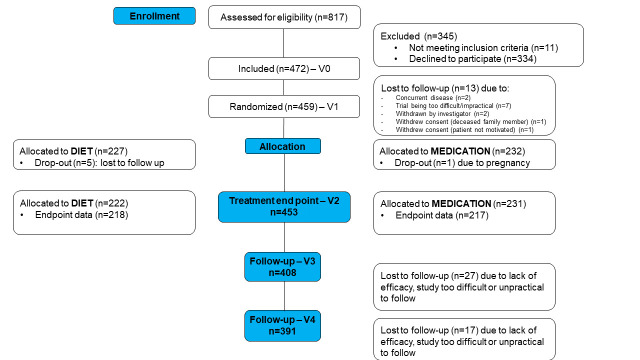
A total of 472 newly treated patients with IBS (70% Rome+) were recruited by 69 PCPs (CONSORT (Consolidated Standards of Reporting Trials) diagram, figure 1) between July 2018 and December 2019. Average age was 41±15 years and the majority (76%) were women. After inclusion, 459 patients were randomised to diet or OB (figure 1). Both groups were well matched (table 1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Table 1.
Baseline characteristics of medication arm (n=232) and diet arm (n=227)
| Medication (n=232) | Diet (n=227) | P value | |
| Age (years old), mean±SD | 41.2±15 | 40.6±15 | 0.61 |
| Gender (% females) | 76 (n=175/231) | 76 (n=168/222) | 0.98 |
| BMI (kg/m2), mean±SD | 24.9±5 | 24.3±4 | 0.16 |
| Stool type (%) IBS-C IBS-D IBS-M IBS-U |
21 (n=48/231) 27 (n=63/231) 42 (n=97/231) 10 (n=23/231) |
20 (n=44/222) 28 (n=62/222) 38 (n=85/222) 14 (n=31/222) |
0.60 |
| IBS-SSS, mean±SD | 267±100 | 267±96 | 0.87 |
| IBS-QoL, mean±SD | 32.1±17.7 | 31.6±16.3 | 0.99 |
| Anxiety, mean±SD | 5.9±3.7 | 6.1±3.6 | 0.47 |
| Depression, mean±SD | 6.8±4.9 | 7.0±5.0 | 0.62 |
| PHQ15, mean±SD | 10.0±3.9 | 9.8±4.1 | 0.47 |
BMI, body mass index; IBS-C, constipation-predominant IBS; IBS-D, diarrhoea-predominant IBS; IBS-M, mixed subtype IBS; IBS-QoL, IBS quality of life; IBS-SSS, IBS Symptom Severity Scale; IBS-U, unclassified IBS.
Primary endpoint and IBS symptom severity score
In the diet group, 71% (155/218) (95% CI: 65 to 77) of patients were responders at 8 weeks, which was significantly higher than 61% (133/217) (95% CI: 54 to 68) in the OB arm (p=0.03, figure 2). A significant difference in responder rate was already present after 4 weeks (diet 62% (132/213) (95% CI: 55 to 68) versus OB 51% (n=110/215) (95% CI: 44 to 57), p=0.02). The difference in responder rates was also significant in an intention-to-treat analysis after 4 (58% (132/227) vs 47% (110/232), p=0.02) and 8 weeks of treatment (68% (155/227) vs 57% (133/232), p=0.02).
Figure 2.
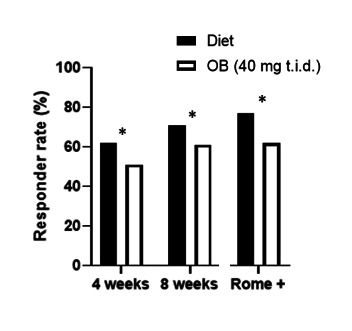
Responder rate was significantly higher in the diet group compared with medication after 4 (p=0.02) and 8 weeks (p=0.03) and more pronounced in Rome+ patients (p=0.004) based on Pearson χ2 test. OB, otilonium bromide; t.i.d., three times a day.
IBS-SSS improved significantly after 4 and 8 weeks compared with baseline in the diet group (respective change of −88±7.3 (p<0.001) and −97±7.4 (p<0.001)) and OB (respective change of −61±7.5 (p<0.001) and −77±7.4 (p<0.001)) (figure 3, table 2). The improvement was significantly higher in the diet group compared with OB (p=0.004 and p=0.02, respectively). In addition, all IBS-SSS subdomains improved significantly after 8 weeks in both the medication and diet arm, but a significantly higher improvement occurred in the diet group compared with medication for severity of abdominal distention and for number of days of abdominal pain (online supplemental data).
Figure 3.
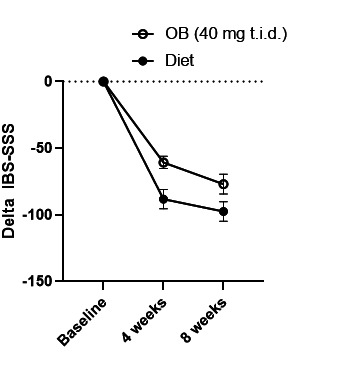
Change (±SE) in IBS-SSS at start, after 4 (p=0.007) and 8 weeks (p=0.049) of treatment based on mixed-model analysis. IBS-SSS, IBS Symptom Severity Scale; OB, otilonium bromide; t.i.d., three times a day.
Table 2.
Changes in IBS-SSS, quality of life, anxiety, depression and somatic symptoms (PHQ15) after 8, 16 and 24 weeks of treatment
| Changes after 8 weeks of treatment | ||||
| Medication (n=231) | Diet (n=222) | P value* | P value† | |
| IBS-SSS, mean±SD | −76.91±7.42 | −97.42±7.37 | 0.02 | 0.05 |
| IBS-QoL, mean±SD | −7.36±12.45 | −8.07±11.45 | 0.48 | 0.59 |
| Anxiety, mean±SD | −0.97±0.19 | −1.21±0.18 | 0.22 | 0.32 |
| Depression, mean±SD | −1.08±0.27 | −1.39±0.26 | 0.17 | 0.39 |
| Somatisation, mean±SD | −1.26±0.23 | −1.82±0.23 | 0.09 | 0.09 |
| Changes after 16 weeks of treatment | ||||
| Medication (n=206) | Diet (n=203) | P value* | P value† | |
| IBS-SSS, mean±SD | −78.50±8.04 | −104.98±7.66 | 0.005 | 0.02 |
| IBS-QoL, mean±SD | −8.69±14.07 | −9.35±12.23 | 0.29 | 0.62 |
| Anxiety, mean±SD | −1.03±0.20 | −1.31±0.18 | 0.22 | 0.30 |
| Depression, mean±SD | −1.19±0.28 | −1.63±0.25 | 0.25 | 0.23 |
| PHQ15, mean±SD | −1.48±0.24 | −1.81±0.22 | 0.70 | 0.33 |
| Changes after 24 weeks of treatment | ||||
| Medication (n=192) | Diet (n=196) | P value* | P value† | |
| IBS-SSS, mean±SD | −86.64±7.81 | −97.12±8.18 | 0.12 | 0.35 |
| IBS-QoL, mean±SD | −9.60±14.32 | −10.49±12.79 | 0.27 | 0.63 |
| Anxiety, mean±SD | −1.16±0.21 | −1.33±0.19 | 0.19 | 0.53 |
| Depression, mean±SD | −1.13±0.30 | −1.45±0.28 | 0.22 | 0.43 |
| PHQ15, mean±SD | −1.90±0.29 | −1.84±0.24 | 0.96 | 0.91 |
*Based on completers and Mann-Whitney test.
†Based on all available data and mixed models.
IBS-QoL, IBS quality of life; IBS-SSS, IBS Symptom Severity Scale.
In a mixed-model analysis, the difference between treatment groups in change from baseline was statistically significant after 4 weeks (p=0.01) and after 8 weeks (p=0.05).
Quality of life and psychosocial status
The effect of both treatments on quality of life and psychosocial parameters was evaluated over time. No significant differences were observed at the start of the study for quality of life, anxiety, depression and PHQ15 for diet or medication (table 1). After 8 weeks of treatment, quality of life, depression, anxiety and PHQ15 scores improved significantly in both groups, without significant difference between the two treatment arms (table 2). When analysing PHQ12, which eliminates three GI symptoms related questions, similar results were found.
Rome IV positive subgroup
Seventy per cent (n=309) of all patients fulfilled the Rome IV criteria of which 153 were randomised to diet and 158 to medication, with similar symptom severity (IBS-SSS: 296±83 vs 293±82, p=0.6). Responder rates to diet and medication were 77% (118/153) (95% CI: 70 to 84) and 62% (98/158) (95% CI: 54 to 70), respectively (p=0.004). The change in IBS-SSS from baseline to week 8 was significantly higher in the diet group than OB (−116±8.7 vs −82±8.1, p=0.003) which was confirmed in a mixed-model analysis (p=0.01).
Treatment adherence and satisfaction
Based on the treatment adherence questionnaire at 8 weeks, 94% of patients allocated to the diet (199/212) were defined as adherent compared with 73% in the medication group (148/202) (p<0.001). Treatment satisfaction was scored as at least slightly better compared with baseline by 67% (146/218) and 54% (117/217) of the diet and medication group, respectively, but no significant difference was observed between both treatments (p=0.20).
Predictors of response
Female gender (OR=2.08, 95% CI 1.08 to 4.03, p=0.04) was associated to response among diet-treated patients, whereas higher PHQ15 (OR=1.10 per point rise, 95% CI 1.02 to 1.19, p=0.02) to medication treatment response. Primary stool type was no response predictor for diet or medication (see online supplemental table 1 in the addendum).
Follow-up
Overall, 24 subjects (5.3%) changed treatment at some point during the follow-up period. At the 16-week follow-up, 99% (199/202) of diet and 92% (187/204) of medication group patients remained on their initial randomised treatment allocation (p=0.001), while at the 24-week follow-up these numbers were 98% (192/195) and 92% (175/190), respectively (p=0.003). The newly started treatments were heterogeneous and included probiotics, prebiotics or other IBS medications. The responder rate remained significantly higher in the diet group compared with medication after 16 (74% (149/202) (95% CI: 67 to 80) vs 57% (118/206)(95% CI: 50 to 64), p<0.0001), but no longer after 24 weeks (69% (134/195) (95% CI: 62 to 75) vs 70% (134/192) (95% CI: 63 to 76), p=0.82). The change in IBS-SSS after 16 weeks (diet −105±7.7 vs OB −78±8.0) differed significantly between both treatment arms (p=0.005) in the entire study population, as well as in the Rome+ subgroup (diet −119±9.1 vs OB −90±9.3, p=0.02). The change was no longer significant after 24 weeks in the entire group (diet −97±8.2 vs OB −87±7.8, p=0.12) or in Rome+ patients (diet −113±9.3 vs OB −96±9.0, p=0.09) (see online supplemental table 2 in the addendum). Quality of life, depression, anxiety and PHQ15 scores remained similar in both groups (table 2).
The mixed-model analysis showed a significant benefit for diet compared with OB after 16 weeks (p=0.02), but no longer after 24 weeks (p=0.35).
Safety
Two serious adverse events, but no serious adverse reactions, were recorded during the trial after randomisation to OB. One patient reported to be pregnant after inclusion, but before OB was prescribed. A second patient was hospitalised during the trial, but the reason for hospitalisation was not associated with the intake of OB.
Body weight
At baseline, body weight did not differ (p=0.30) between diet (69.9±14.1 kg) and medication (71.3±14.4 kg) groups. After 8 weeks of treatment, no difference was found compared with baseline in the diet (69.6±13.7 kg, p=0.78) or medication (71.3±14.2 kg, p=0.97) groups. Furthermore, body weight did not change in the follow-up of the diet (69.6±13.8 kg; 69.8±13.8 kg) and medication (71.4±14.5 kg; 70.9±14.3 kg) arms after 16 and 24 weeks, respectively.
Discussion
Although the efficacy of the low FODMAP diet is well established in tertiary care IBS,10 11 studies in primary care are lacking. To our knowledge, this is the first large-scale pragmatic trial of a diet application in primary care IBS compared with standard medical therapy.
While the low FODMAP diet requires intervention and extensive follow-up by a dietitian,16 we developed dietary instructions to optimise IBS self-management. In this study, patients showed a significantly higher response rate and a larger improvement of symptoms with a diet application compared with OB. The higher response rate was already observed at weeks 4 and 8, and persisted after 16 weeks, but no longer after 24 weeks. In addition, high treatment adherence rates were observed. The diet application, as well as the medication, led to improvement in quality of life and levels of psychological distress compared with baseline. In addition, the improvement in symptoms after following the diet responded in higher satisfaction scores. However, the difference in symptom control between both treatments was not as dominant to lead to a significant difference in quality of life and/or treatment satisfaction. Despite the fact that, based on these results, OB is considered safe and effective, lifestyle adjustments may be more attractive than long-term medication intake for a majority patients in primary care.
The patients enrolled in the study were newly treated primary care IBS patients, which is reflected in the demographic characteristics, the IBS severity scores and stool pattern subtypes.17 In this primary care cohort, 70% of the patients fulfilled the Rome IV criteria, which are well known to identify a more severe subset of clinically diagnosed IBS.18 This study showed that the comparative efficacy of the diet application over OB was higher in the Rome+ subset than in the entire patient cohort, confirming effectiveness of the diet application in patients with more severe symptoms.
In primary care, a simple approach with short-term symptom control and absence of major adverse effects are key goals for IBS management.17 Spasmolytic agents have an excellent safety profile and may provide symptomatic benefit after a few weeks of treatment, hence their frequent use in primary care.3 7–9 17 Surveys reveal that dietary adjustments are also often used by PCPs, but this is probably done in a non-standardised and less structured manner, and both efficacy and effectiveness data on dietary management in primary care are lacking.13 Standard dietary advice based on the NICE/BDA guideline showed a similar efficacy when compared with the dietitian-guided strict low FODMAP diet in tertiary care IBS, but was also not tested in a primary care setting.12 The strict low FODMAP diet is well-standardised and structured but is difficult to implement in primary care as it requires considerable effort from the patient for adherence and several visits with a dietitian.16 The diet application as used in the present study simplifies the dietary intervention and allows patients to independently manage and adapt it to their own needs (eg, food preferences or meal habits), allowing high adherence levels up to 24 weeks. Furthermore, dietary interventions are associated with a risk of major caloric intake restriction,11 12 but in the present study, the use of the diet application for up to 24 weeks was not associated with weight loss. In tertiary care, there is a tendency to select patients with IBS-D for the low FODMAP diet, based on the osmotic effect of FODMAPs that may contribute to diarrhoea. However, in the present study, stool subtype and IBS-SSS were not predictors of efficacy, indicating that the diet application is suitable for treating all stool pattern subtypes and a broad severity range of IBS. Hence, the diet application is an attractive, easy and safe candidate for a first-line therapeutic approach that can be proposed to a broad primary care IBS patient population.
Strengths of this study are the large number of patients recruited from primary care, the follow-up over 24 weeks and the use of a novel diet application. This pragmatic study closely followed usual clinical approaches in primary care IBS. The study has a number of limitations, in part due to its pragmatic nature. This includes the absence of daily symptom diaries, of food or diet intake tracking and the use of open-label treatment without placebo or sham intervention. Patients in the diet arm were ‘prescribed’ the use of the dietary application, but the study did not include any additional interventions, such as the use of food diaries allowing to quantify intake. Hence, no information could be collected regarding the exact amount of FODMAP intake in the diet arm. Also, patients allocated to the medication arm were instructed not to change their dietary habits during the trial but this was also not documented using food diaries. Future studies are needed to provide a more in-depth assessment of the impact on patients’ dietary intakes while using the app. Not all patients enrolled in this study fulfilled the Rome IV criteria, but this is in line with clinical reality and should not necessarily be considered a major limitation. While the 24-week follow-up is longer than standard IBS efficacy trials of 12 weeks, the effects of this diet application beyond 6 months would require additional long-term follow-up studies. In tertiary care, practical issues and social factors lead to a decrease in treatment adherence to the standard low FODMAP-diet, which is more complex and more demanding.19 20
In conclusion, in this large primary care IBS cohort, an 8-week usage of a diet application was superior to standard medical therapy. The dietary application was associated to long-lasting significantly higher responder rates and improvement of IBS-SSS. Higher efficacy of the app-guided diet compared with medication was also found in Rome+ patients. Thus, the use of a simple diet application should be considered a first-line approach to manage IBS in primary care.
Acknowledgments
This study was supported by the Belgian Health Care Knowledge Centre (KCE), in collaboration with Nelle Stocquart and Hilde Nevens and by the Rome Foundation Research Institute. LVO is a research professor funded by the KU Leuven Special Research Fund (Bijzonder Onderzoeksfonds, BOF). TV is supported by a senior clinical research fellowship of the Flanders Research Foundation (FWO Vlaanderen). This study was conducted in collaboration with CRI (Cera HealthCare, Zwijnaarde Belgium) for the collection of biological samples. The DOMINO application was developed by EverywhereIM (Amsterdam, The Netherlands). We thank and acknowledge the 105 Belgian primary care physicians for their good performance and commitment in this trial as well as the 472 participating patients. The list of recruited study investigators is attached in the addendum.
Footnotes
Collaborators: DOMINO study collaborators: Alain Goorden; Alegonda Snijkers; An Leys; Annemiek Roelofs; Bart Schoolmeesters; Bart Vander Putten; Benjamin Van den Broek; Birgitta Baade-Joret; Céline Huberlant; Christian Peetermans; David Van Humbeek; Dirk Van den Brande; Dirk Wyseyr; Els Lemmens; Ethel Brits; Guido Simons; Hans Baetens; Hendrika Van Overmeire; Hilde Tack; Ilse Cupers; Ive Talboom; Jeroen Stubbe; Jonas Docx; Judith Deseins; Julie Biot; Julie Vancaillie; Kara Vandeloo; Karlijn Louwies; Karolien De Ceulaer; Karolien Lemmens; Katrien Scheers; Leen Verleure; Lies De Sutter; Lies Plancke; Liesbet Bruyninckx; Liesbeth Vanzeir; Lieve Vandersmisse; Linde Wyseur; Lode Vermeersch; Lodewijk Pas; Lore De Greef; Luc Capiau; Luc Van Braeckel; Lut De Groote; Lydia Jones; Maria Groot; Marianne Busschots; Marie-Hélène Landenne; Marieke Monstrey; Marie-Magdalena Haemels; Marleen Snellings; Maura Sisk; Nathalie Van de Vyver; Nikea Sannen; Olivia Vandeput; Olivier Gernay; Philippe Thoné; Phouthalack Narongsack; Pierre Vrins; Pieterjan Geusens; Rik Sauwens; Rudy Van Boxstael; Sigrid Musch; Sigrid Nous; Sofie Mazereel; Sophie Maes; Sophie Van Steenbergen; Stéphanie Biot; Steven Ceulemans; Stijn Geeraert; Tine Caeyers; Vincent Vanbelle; Willem Raat.
Contributors: FC: trial design, study coordination, data collection, analysis, manuscript drafting and reviewing. KVdH: study coordination, data collection, analysis, manuscript drafting and reviewing. LB: data collection, study follow-up, manuscript review. CT: data analysis, manuscript review. JA, PC, HP, AV: trial organisation support, manuscript review. CM: trial design, trial organisation support, manuscript review. JB: trial design, manuscript review. LC, SM, CP, WR, JS, RVB, OV, SVS: data collection, manuscript review. LVO, TV: manuscript review. MJ: trial design, data analysis, manuscript review. JT: trial concept, design, organisation, data collection, analysis, manuscript drafting, manuscript review and guarantor of the overall content of the article.
Funding: The DOMINO study was funded through the Belgian Health Care Knowledge Centre (KCE) Trials Program (study ID KCE16001), a national public funding program of non-commercial trials. KCE provided feedback on the design and conduct of the study but was not involved in the collection, management, analysis or interpretation of the data. KCE provided comments on the drafted clinical study report and the manuscript for publication, but no publication restrictions apply. The Rome Foundation Research Institute provided diagnostic and patient-reported outcome questionnaires.
Competing interests: JT has given Scientific advice to Alfa Wassermann, Allergan, Christian Hansen, Danone, Grünenthal, Ironwood, Janssen, Kiowa Kirin, Menarini, Mylan, Neutec, Novartis, Noventure, Nutricia, Shionogi, Shire, Takeda, Theravance, Tramedico, Truvion, Tsumura, Zealand and Zeria Pharmaceuticals, has received research support from Shire, Sofar and Tsumura, and has served on the Speaker Bureau for Abbott, Allergan, AstraZeneca, Janssen, Kyowa Kirin, Menarini, Mylan, Novartis, Shire, Takeda, Truvion and Zeria. Funding was provided by a Methusalem grant from Leuven University to JT. HP has given scientific advice to Allergan, Danone, Menarini, Merck Serono, Shire and Zeria and has served on the speaker Bureau of Menarini, Merck Serono, Shire and Zeria. LVO has given scientific advice to Danone and received research support from Nestlé. TV has given scientific advice to VectivBio, Shire, Dr. Falk Pharma, Takeda and Baxter; has received research support from Danone, MyHealth and VectivBio; and has served on the Speaker Bureau for Abbott, Tramedico, Truvion, Will Pharma, My Health, Kyowa Kirin, Menarini, Biocodex, Remedus, Fresenius Kabi and Dr. Falk Pharma. CM has served on the Speaker Bureau for Coca-Cola and Zespri and received travel/conference grants from Danone, Nestlé Health Sciences, Fresenius Kabi. This study was supported by a research grant from the Belgian Health Care Knowledge Centre (KCE). Questionnaires in this trial were developed, translated and provided by the Rome Foundation Research Institute.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Domino Study Collaborators:
Alain Goorden, Alegonda Snijkers, An Leys, Annemiek Roelofs, Bart Schoolmeesters, Bart Vander Putten, Benjamin Van den Broek, Birgitta Baade-Joret, Céline Huberlant, Christian Peetermans, David Van Humbeek, Dirk Van den Brande, Dirk Wyseyr, Els Lemmens, Ethel Brits, Guido Simons, Hans Baetens, Hendrika Van Overmeire, Hilde Tack, Ilse Cupers, Ive Talboom, Jeroen Stubbe, Jonas Docx, Judith Deseins, Julie Biot, Julie Vancaillie, Kara Vandeloo, Karlijn Louwies, Karolien De Ceulaer, Karolien Lemmens, Katrien Scheers, Leen Verleure, Lies De Sutter, Lies Plancke, Liesbet Bruyninckx, Liesbeth Vanzeir, Lieve Vandersmisse, Linde Wyseur, Lode Vermeersch, Lodewijk Pas, Lore De Greef, Luc Capiau, Luc Van Braeckel, Lut De Groote, Lydia Jones, Maria Groot, Marianne Busschots, Marie-Hélène Landenne, Marieke Monstrey, Marie-Magdalena Haemels, Marleen Snellings, Maura Sisk, Nathalie Van de Vyver, Nikea Sannen, Olivia Vandeput, Olivier Gernay, Philippe Thoné, Phouthalack Narongsack, Pierre Vrins, Pieterjan Geusens, Rik Sauwens, Rudy Van Boxstael, Sigrid Musch, Sigrid Nous, Sofie Mazereel, Sophie Maes, Sophie Van Steenbergen, Stéphanie Biot, Steven Ceulemans, Stijn Geeraert, Tine Caeyers, Vincent Vanbelle, and Willem Raat
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Ethical Committee Research UZ/KU Leuven (S59482). Participants gave informed consent to participate in the study before taking part.
References
- 1. Drossman DA. Functional gastrointestinal disorders: what's new for Rome IV? Lancet Gastroenterol Hepatol 2016;1:6–8. 10.1016/S2468-1253(16)30022-X [DOI] [PubMed] [Google Scholar]
- 2. Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology 2021;160:99–114. 10.1053/j.gastro.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 3. Van den Houte K, Carbone F, Pannemans J, et al. Prevalence and impact of self-reported irritable bowel symptoms in the general population. United European Gastroenterol J 2019;7:307–15. 10.1177/2050640618821804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tack J, Stanghellini V, Mearin F, et al. Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries. BMC Gastroenterol 2019;19:69. 10.1186/s12876-019-0985-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther 2014;40:1023–34. 10.1111/apt.12938 [DOI] [PubMed] [Google Scholar]
- 6. Ford AC, Talley NJ, Spiegel BMR, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ 2008;337:a2313. 10.1136/bmj.a2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moayyedi P, Mearin F, Azpiroz F, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J 2017;5:773–88. 10.1177/2050640617731968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clavé P, Tack J. Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis. Therap Adv Gastroenterol 2017;10:311–22. 10.1177/1756283X16681708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glende M, Morselli-Labate AM, Battaglia G, et al. Extended analysis of a double-blind, placebo-controlled, 15-week study with otilonium bromide in irritable bowel syndrome. Eur J Gastroenterol Hepatol 2002;14:1331–8. 10.1097/00042737-200212000-00008 [DOI] [PubMed] [Google Scholar]
- 10. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014;146:67–75. 10.1053/j.gastro.2013.09.046 [DOI] [PubMed] [Google Scholar]
- 11. Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology 2015;149:1399–407. 10.1053/j.gastro.2015.07.054 [DOI] [PubMed] [Google Scholar]
- 12. Halmos EP, Christophersen CT, Bird AR, et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015;64:93–100. 10.1136/gutjnl-2014-307264 [DOI] [PubMed] [Google Scholar]
- 13. Eswaran SL, Chey WD, Han-Markey T, et al. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol 2016;111:1824–32. 10.1038/ajg.2016.434 [DOI] [PubMed] [Google Scholar]
- 14. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. 10.1046/j.1365-2036.1997.142318000.x [DOI] [PubMed] [Google Scholar]
- 15. Clavé P, Acalovschi M, Triantafillidis JK, et al. Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2011;34:432–42. 10.1111/j.1365-2036.2011.04730.x [DOI] [PubMed] [Google Scholar]
- 16. Whelan K, Martin LD, Staudacher HM, et al. The low FODMAP diet in the management of irritable bowel syndrome: an evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet 2018;31:239–55. 10.1111/jhn.12530 [DOI] [PubMed] [Google Scholar]
- 17. Hungin APS, Molloy-Bland M, Claes R, et al. Systematic review: the perceptions, diagnosis and management of irritable bowel syndrome in primary care--a Rome Foundation working team report. Aliment Pharmacol Ther 2014;40:1133–45. 10.1111/apt.12957 [DOI] [PubMed] [Google Scholar]
- 18. Aziz I, Törnblom H, Palsson OS, et al. How the change in IBS criteria from Rome III to Rome IV impacts on clinical characteristics and key pathophysiological factors. Am J Gastroenterol 2018;113:1017–25. 10.1038/s41395-018-0074-z [DOI] [PubMed] [Google Scholar]
- 19. Weynants A, Goossens L, Genetello M, et al. The long-term effect and adherence of a low fermentable oligosaccharides disaccharides monosaccharides and polyols (FODMAP) diet in patients with irritable bowel syndrome. J Hum Nutr Diet 2020;33:159–69. 10.1111/jhn.12706 [DOI] [PubMed] [Google Scholar]
- 20. O'Keeffe M, Jansen C, Martin L, et al. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol Motil 2018;30. 10.1111/nmo.13154. [Epub ahead of print: 14 07 2017]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2021-325821supp001.pdf (75.4KB, pdf)
Data Availability Statement
Data are available on reasonable request.