Does the Brain Regulate the Immune System?
While long considered to be separate entities, increasing evidence has demonstrated that the brain and the peripheral immune system interact through bidirectional feedback [1]. The brain hosts a vibrant immune environment (e.g. microglia and perivascular macrophages) that is responsible for maintaining the integrity of the central nervous system [2]. In addition, the brain is the central regulator of the rest of the body and its homeostasis. Recently, this notion has been extended with new information that suggests the brain helps synchronize the immune system with a multitude of physiological parameters across different organs and tissues (such as sensory stimuli, blood flow, heart rate, blood pressure, oxygen level etc.) [3]. Equally important is the brain’s role in predicting an incoming threat based on a priori experience (e.g. immune conditioning) and coordinating a faster immune response through well-established pathways not readily available to the immune system, i.e. endocrine, autonomic, sensory, and meningeal lymphatic systems [3]. For example, triggering the brain’s reward system (activated in anticipation of a positive experience) can enhance the antibacterial and anti-tumoral immune response [3]. Several brain regions play a central role in supervising and orchestrating the body’s response to stressful and offending stimuli, including the hypothalamus/pituitary, the amygdala, the vagal nuclei, and more recently, the insula.
What is the Role of the Insula in Immunomodulation?
Evidence on insular involvement with immunity was reported more than two decades ago, when Ramirez-Amaya demonstrated that insular damage in rats is associated with disrupted acquisition of conditioned immunosuppression [4]. Later studies found increased insular activity and altered functional connectivity of the insula in response to pro-inflammatory conditions (i.e. vaccine administration) or disease, such as rheumatoid arthritis, inflammatory bowel disease, asthma, chronic hepatitis C, and bipolar disorder. Recently in Cell, using versatile genetic manipulations, Koren et al. elegantly illustrated increased neuronal activity (defined as elevated tdTomato expression) in the insular cortex during colitis and peritonitis in targeted-recombination- inactive-populations mouse models [5]. Furthermore, reactivation of neuronal ensembles using synthetic ligand activation caused increased immune activity in the colon, as evidenced by sequestration of mucosal leukocytes, as well as increased peritoneal monocytes, granulocytes, TLR-4+ cells, and peritoneal cytokine levels. In contrast, nonspecific insular activation did not elicit an apparent cellular immune response in the colon. Interestingly, this immune effect was only partially attenuated following administration of acetaminophen, thereby supporting that it was not pain mediated. Lastly, inhibition of the insular cortex impaired the cellular immune response and associated inflammatory clinical parameters (colon length and spleen weight).
The author’s findings beautifully extend previous studies that have shown an increase in insular activity in fMRI studies under inflammatory model conditions [6] as well as increased insular grey matter volume [7]. This insular activation has also been found to be associated with the physical and psychological manifestations of the systemic inflammatory response, including malaise, pain, and anxiety. Whether this gross change is the result of alterations in insular microstructure is a field of ongoing investigation (e.g. increased magnetization exchange from free (water) to macromolecular-bound protons based on quantitative magnetization transfer imaging) [8].
Finally, the influence of insular laterality on the immune response remains to be determined. While the right anterior insula plays a central role in the interoceptive model of Craig et al, findings are mixed with regard to insular subregions that are activated in stressful/inflammatory states: some research teams have found that the right anterior insula projects to the orbitofrontal and anterior cingulate gyrus, whereas others have noted that the left anterior insula communicates with the basal ganglia. Of note, the anterior/rostral insula and the anterior cingulate are unique areas of the brain in containing the large bipolar Von Economo neurons, which may be responsible for initiating fast adaptive responses to change, such as in acute inflammation [9].
How Does the Insula Specifically Interact with Immune Cells?
The role of the insula in interoception (the integrative perception of internal stimuli and bodily state) was first described by Craig in 2002 [10]. According to that model, sympathetic and parasympathetic afferents as well as somatosensory fibers from peripheral organs and tissues travel through the vagus nerve and the lamina I spinothalamic tract to the nucleus of the solitary tract, the thalamus (ventroposteromedial and ventromedial nuclei) and subsequently to the insula. Specific information about viscero-sensation is transmitted to the mid/posterior insula and then the anterior insula, which is responsible for the orchestration of viscero-motor action [8]. With regard to connectivity, research has demonstrated increased connectivity with the anterior cingulate gyrus (and to a lesser extent with the medial subregion), the prefrontal cortex, the inferior parietal lobule, and the basal ganglia (particularly the putamen), as well as decreased connectivity with the postcentral gyrus.
The literature to date has primarily provided indirect evidence on the association between the insular cortex and the immune cell populations. For example, changes in the regional cerebral blood flow of the insula based on PET studies have been found to correlate with the number of natural killer and CD4+ T-cells in circulation. The study by Koren et al. was among the first to apply polysynaptic retrograde tracing using a pseudorabies virus in order to identify anatomical pathways between the insular cortex and the colon. Moreover, the authors revealed involvement of the dorsal motor nucleus of the vagus, the vagal control center, and the rostral ventrolateral medulla through anterograde and retrograde transport mechanisms.
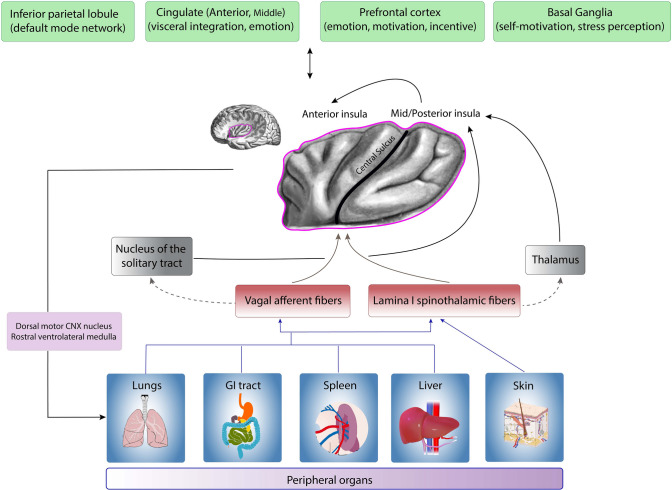
Compiling all these findings, we constructed a model regarding the role of the insula in immune system regulation (afferent fibers, interactions with other brain regions, and efferent projections) (Fig. 1).
Fig. 1.
Summary of our proposed model of the interaction between the insula and the immune system based on available evidence. Information flows from peripheral organs through vagal and spinothalamic afferent fibers to the nucleus of the solitary tract, the thalamus, and the posterior insula. The posterior insula projects information to the anterior insula. In addition, the insula has bidirectional connections with the prefrontal/orbitofrontal cortex, the cingulate gyrus (primarily the anterior and to a lesser extent the medial subregion), the inferior parietal lobe, andds the basal ganglia. In this way, the insula integrates inputs from the peripheral immune system as well as brain regions involved in emotion, motivation, incentive, and visceral sensation. The anterior insula then projects back to the dorsal motor nucleus of the vagus nerve and the rostral ventrolateral medulla, and possibly directly to the immune cells in the periphery, stimulating the inflammatory response, e.g., monocytes, granulocytes, and natural killer cells. (part of this figure is
modified from an open-source image, under the Creative Commons License, Attribution-ShareAlike 2.1 Japan, CC BY-SA 2.1 JP, https://creativecommons.org/licenses/by-sa/2.1/jp/deed.en)
What Are the Implications of the Findings in the Work by Koren et al.?
The authors’ findings support new avenues of research on the role of insula and other brain regions in modulating the immune response (Table 1). First, we need a more complete characterization of the molecular profile of the immune cells and the neurotransmitters/neuropeptides involved in insular activation. Second, can similar findings be obtained in disease states affecting other organ systems such as the skin and lungs? Third, can electrical stimulation of the insula modify the peripheral immune response? And if so, how do the stimulation parameters affect immunity? For example, does high-frequency stimulation (e.g. in the gamma range [40–50 Hz and above]) activate immune cells while low-frequency stimulation (e.g. 1 Hz) inhibits them, or vice versa? Fourth, given the ongoing investigations on the efficacy of vagus nerve stimulation for suppressing the immune response in inflammatory conditions (rheumatoid arthritis and inflammatory bowel disease), it would be highly informative to determine how the insula is affected. In humans, prolonged extra-operative invasive electroencephalography and permanently-implanted sensing devices offer the opportunity to further study insular physiology as it relates to immune responses.
Table 1.
Questions for future research regarding the implication of insula in immunity
| Questions |
|---|
| What is the molecular profile of immune cells activated/inhibited by insular activation? |
| Which neurotransmitters and neuropeptides are involved in this process? |
| Whether and how electrical stimulation of the insula affects peripheral immunity? |
| How does vagus nerve stimulation affect the insula? |
| What is the neuronal circuit in the insula that specifically controls immunity in different organs? |
References
- 1.Reardon C, Murray K, Lomax AE. Neuroimmune communication in health and disease. Physiol Rev. 2018;98:2287–2316. doi: 10.1152/physrev.00035.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eyo UB, Wu LJ. Microglia: Lifelong patrolling immune cells of the brain. Prog Neurobiol. 2019;179:101614. doi: 10.1016/j.pneurobio.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller M, Ben-Shaanan TL, Rolls A. Neuronal regulation of immunity: Why, how and where? Nat Rev Immunol. 2021;21:20–36. doi: 10.1038/s41577-020-0387-1. [DOI] [PubMed] [Google Scholar]
- 4.Ramírez-Amaya V, Alvarez-Borda B, Ormsby CE, Martínez RD, Pérez-Montfort R, Bermúdez-Rattoni F. Insular cortex lesions impair the acquisition of conditioned immunosuppression. Brain Behav Immun. 1996;10:103–114. doi: 10.1006/brbi.1996.0011. [DOI] [PubMed] [Google Scholar]
- 5.Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184:5902–5915.e17. doi: 10.1016/j.cell.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Kraynak TE, Marsland AL, Wager TD, Gianaros PJ. Functional neuroanatomy of peripheral inflammatory physiology: a meta-analysis of human neuroimaging studies. Neurosci Biobehav Rev. 2018;94:76–92. doi: 10.1016/j.neubiorev.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Månsson KNT, Lasselin J, Karshikoff B, Axelsson J, Engler H, Schedlowski M, et al. Anterior Insula morphology and vulnerability to psychopathology-related symptoms in response to acute inflammation. Brain Behav Immun. 2022;99:9–16. doi: 10.1016/j.bbi.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, et al. Quantitative magnetization transfer imaging as a biomarker for effects of systemic inflammation on the brain. Biol Psychiatry. 2015;78:49–57. doi: 10.1016/j.biopsych.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, et al. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- 10.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]