Abstract
Crossmodal information processing in sensory cortices has been reported in sparsely distributed neurons under normal conditions and can undergo experience- or activity-induced plasticity. Given the potential role in brain function as indicated by previous reports, crossmodal connectivity in the sensory cortex needs to be further explored. Using perforated whole-cell recording in anesthetized adult rats, we found that almost all neurons recorded in the primary somatosensory, auditory, and visual cortices exhibited significant membrane-potential responses to crossmodal stimulation, as recorded when brain activity states were pharmacologically down-regulated in light anesthesia. These crossmodal cortical responses were excitatory and subthreshold, and further seemed to be relayed primarily by the sensory thalamus, but not the sensory cortex, of the stimulated modality. Our experiments indicate a sensory cortical presence of widespread excitatory crossmodal inputs, which might play roles in brain functions involving crossmodal information processing or plasticity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-022-00855-4.
Keywords: Crossmodal input, Crossmodal response, Crossmodal plasticity, Sensory cortex, Crossmodal task, Sensory loss, GABAergic transmission
Introduction
In addition to the basic function of unimodal information processing (i.e., receiving information of the corresponding sensory modality), the mammalian sensory system also receives crossmodal (multimodal) sensory information [1–4]. Crossmodal information transmission or processing in the sensory system has been extensively investigated at the cortical level and is thought to be accounted for by a sparsely distributed neuronal population [1–3]. In electrophysiological studies and brain imaging studies, it has been reported that a small number (<10%) of sensory cortical neurons respond to crossmodal stimulation, as shown by acoustic and tactile responses in the primary visual cortex (V1) [2, 5–7], visual and tactile responses in the auditory cortex (A1) [2, 8–11], and visual and acoustic responses in the somatosensory cortex (S1) [2, 12, 13]. In anatomical studies, crossmodal afferent inputs to sensory cortical areas have also been demonstrated [14–17], and have been suggested to arise from subcortical as well as cortical structures at both low and high hierarchical stages [10, 14, 16, 18, 19].
On the other hand, experience- or activity-induced plasticity has been found in sensory cortical representations of crossmodal information. A well-known phenomenon is the remodeling of representative cortical areas after the sensory input of a specific modality is lost (e.g., in blindness or deafness), which has been reported in sensory cortical areas of both the lost and intact modalities [20–23]. In addition, in the learning task to acquire the association of sensory information from different modalities, neuronal plasticity with the emergence of crossmodal sensory responsiveness or integration has been found in the visual cortex [10, 24–27], the auditory cortex [10, 24, 26–28], the somatosensory cortex [12, 13], as well as the olfactory and gustatory cortex [29, 30]. For example, single-unit recording in monkeys has demonstrated the emergence of non-tactile (visual or auditory) stimulus-evoked spike responses in a few S1 neurons after animals are trained to perform a crossmodal task in which the non-tactile stimulus is associated with a tactile stimulus (animal touching an object) [12, 13]. It has also been shown that crossmodal responsiveness of sensory cortical neurons is susceptible to modulation by brain state-associated activity, for example, with dependence on attention, GABAergic network activity, or the salience of sensory stimulation [31–35].
Previous reports on the sensory cortex have shown crossmodal information processing in sparsely distributed neurons as well as the induction of activity-dependent crossmodal plasticity, as found in perception, task learning, and neural circuit reorganization following sensory loss [1, 3, 26, 36]. Given the previous findings, an important issue that remains open is the topographic organization of crossmodal connectivity in sensory cortical areas. In the present study, we used perforated whole-cell recording in anesthetized adult rats to examine crossmodal synaptic responses that were evoked in sensory cortical neurons. We found that when the brain state was pharmacologically down-regulated by light anesthesia, nearly all neurons recorded from S1, A1, and V1 exhibited excitatory membrane potential (Vm) responses to crossmodal stimulation. Our findings indicate the existence of excitatory crossmodal inputs to a widespread population of sensory cortical neurons.
Materials and Methods
Ethical Approval
All procedures were approved by the Animal Care and Use Committee of East China Normal University (M20190210) and were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Sprague-Dawley rats (male, aged 10–14 weeks; for electrophysiological experiments) and C57BL/6 mice (male, aged 8–10 weeks; for tract-tracing experiments), were purchased from Slaccas Corp., Shanghai, China. Animals were fed ad libitum and housed under a 12/12-h light/dark cycle. After experiments, animals were euthanized by an extra dose of pentobarbital (2.5%; i.p; rats) or isoflurane (5%; inhaling; mice).
Animal Preparation and Surgery for Electrophysiological Recording
Rats were initially anesthetized with pentobarbital (80 mg/kg; i.p.) or urethane (1.2 g/kg; i.p.) to the surgical level (Event 1 in Table S1), which was sufficient to prevent reflex responses to noxious stimuli (including the following surgery and paw pinch). Tracheotomy was then performed. For the experiments to measure acoustic responses (in V1 or S1), the head was fixed by screwing a customized metal plate attached on the skull (with Vetbond tissue adhesive and dental cement) to a head holder, with the ears unobstructed. For experiments to record from A1 neurons (responses to visual/tactile stimuli), the same methods of head fixation were used, but with the eardrum ruptured and the external auditory canal plugged to avoid any possible acoustic stimulation when crossmodal (particularly, tactile) stimulation was delivered. In other groups of experiments (for measuring visual/tactile responses in V1 or S1), the head was restrained in a stereotaxic apparatus (David Kopf Instruments, California, USA) using rupture ear bars. A customized heating blanket maintained the body temperature at 37.3–37.8°C.
Perforated Whole-cell Recording in the Cortex
The procedures of our in vivo whole-cell recording were as described previously in detail [37, 38]. A 1–1.5 mm craniotomy was made above the right hemisphere (from bregma: 5.8–8 mm posterior and 3–4 mm lateral for V1 recordings; 1.2–3.4 mm posterior and 5–6 mm lateral for S1 recordings, which were targeted to the barrel cortex; 3.5–5.5 mm posterior, together with reference to vascular patterns [39, 40], for A1 recordings). A small piece of dura mater was then carefully removed. Internal solution containing (in mmol/L) 136.5 K-gluconate, 17.5 KCl, 9.0 NaCl, 1.0 MgCl2, 10.0 HEPES, 0.2 EGTA, and amphotericin B (0.5 mg/mL); small amounts (0.5–0.8 mg/mL) of glass beads (5–15 µm in diameter; Polysciences, Inc., Pennsylvania, USA) were included for the availability of precipitate-free solution in the pipette tip, as described [37, 38]. The pH of the internal solution was adjusted to 7.3. Patch pipettes with a tip opening of 2.5–3.0 µm were pulled from borosilicate glass tubing (Kimble Glass Inc., Illinois, USA), with a resistance of 1.6–2.0 MΩ (this increased to 3.0–4.5 MΩ when glass beads were pushed to pipette tip [37, 38]). The pipette was advanced with a motor-driven micromanipulator (Siskiyou MMX7630, Siskiyou Corp., Oregon, USA) at 15–30 µm/s, during which positive pressure (250–350 mbar) was applied to the pipette interior. Recordings were made at a cortical depth of 0.3–1 mm (in layers II/III and IV and superficial layer V). Signals were acquired with a patch-clamp amplifier (Axopatch 200B, Axon Instruments, California, USA) and sampled at 5 kHz by a data acquisition card (Digidata 1440, Axon Instruments), with 2 or 5 kHz low-pass filtering. Cortical neurons were randomly sampled in our “blind” whole-cell recording method. The cell type was not determined, and both principal neurons and interneurons were included together for the subsequent data analysis.
Extracellular Unit Recording in the Ventral Posterior Medial Nucleus (VPM)
Glass pipettes (filled with saline) with a tip opening of 5–8 µm were used for extracellular unit recording in the VPM (from bregma: 3.2–3.8 mm posterior and 2.7–2.9 mm lateral; 5.3–6.0 mm beneath the cortical surface). Signals were acquired with a patch-clamp amplifier (Axopatch 200B, Axon Instruments) and sampled at 10 kHz.
Light Anesthesia and Pharmacologically Down-regulated Brain Activity States for Recording
After initial anesthesia for animal preparation and surgery (Event 1 in Table S1), the anesthesia was allowed to gradually return to light levels. In this study, light anesthesia was defined as a depth that was just below the threshold of body movements consisting of spontaneous licking and scratching, as well as reflex responses to noxious stimuli (e.g., the paw pinch). For pentobarbital (but not urethane) anesthesia, the light level was maintained by constant injection of a low dose of pentobarbital (16–20 mg/kg/h; i.p.), which was usually started 2–4 h after initial anesthesia. Recording under light anesthesia (with pentobarbital or urethane) was then achieved. During the experiment, the anesthesia was regularly checked and carefully managed to the level at which the animal showed no spontaneous licking and scratching as well as no reflex responses to noxious stimuli.
The experimental procedures for recordings during a brain activity state down-regulated by different drug treatments are summarized in Table S1, and example recordings of the significantly reduced spontaneous Vm oscillations in down-regulated brain activity states are displayed in Fig. S1. For the experiments named Down p/p (Table S1), a rapid down-regulating injection of additional pentobarbital (60%–80% of the dose for initial anesthesia to the surgical level, i.p.; Event 2 in Table S1) was conducted on the basis of light pentobarbital anesthesia. In this study, two different experimental procedures were used for recording during Down p/p, including a control response measurement under light anesthesia (i.e., before down-regulating injection, as shown in Table S1 for Down p/p (Exp. I); data in Figs. S1A, 1, 5, 7, and 8) or not [Down p/p (Exp. II) in Table S1; data in Figs. 3 and 4]. In Down p/p (Exp. II), but not Down p/p (Exp. I), due to the long period of recording after the down-regulating injection (up to 7 h, as presented in Table S1 for Event 4), the down-regulated brain activity states were maintained by constant injection of a low dose of pentobarbital (16–20 mg/kg, per h, i.p.; Event 3 in Table S1), and spontaneous licking and scratching as well as reflex responses to noxious stimuli were regularly checked for the management (adequacy) of anesthesia.
Fig. 1.
Flash-evoked Vm responses in S1 neurons in Down p/p. A Schematic for visual stimulation and S1 recording. B Two representative S1 cells showing the absence of Vm responses to a flash stimulus under light pentobarbital anesthesia (left), as well as excitatory Vm responses in Down p/p (right), with experimental procedures for Down p/p (Exp. I) shown in Table S1. Traces are averages of 100 stimulation trials (shadows, ± SEM; inter-stimulus-interval, 6 s). Dashed line, baseline Vm in average traces; arrow, stimulus onset. C For the two cells in B, time course plots for response amplitudes. Dots with bars represent the mean ± SEM of 20 consecutive trials; time 0, down-regulating injection of pentobarbital (Pento). In the recordings under light anesthesia, during which no responses were detected, the amplitudes of Vm changes were measured according to the peak time of responses in Down p/p. D, E Summary for all experiments as displayed in B and C (n = 18 from 14 animals), with Vm responses in Down p/p normalized to their peak amplitudes in individual cells and indicated by the color scale (D; 0 and 1 on the color scale represent the baseline and peak amplitude in the average Vm responses, respectively; Time 0, stimulus onset), as well as the time courses of response amplitudes (E; as in C).
Fig. 5.
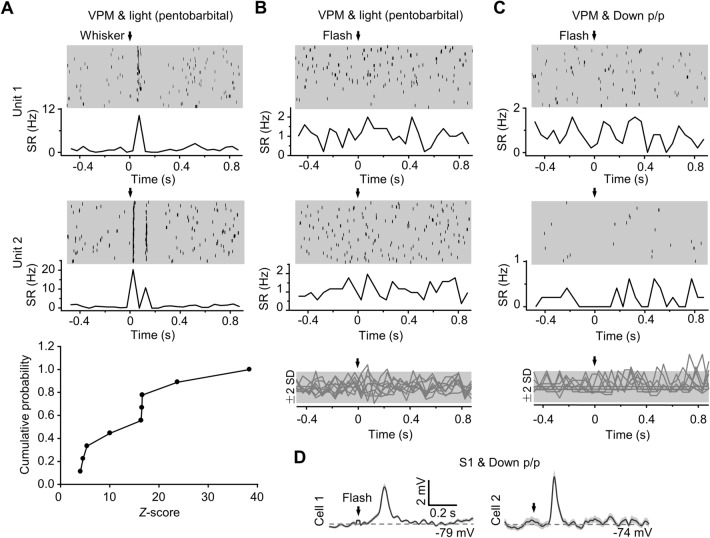
No flash-evoked spike responses in VPM neurons in Down p/p. A For extracellular unit recordings in the VPM during light pentobarbital anesthesia, two example data (Units 1 and 2; upper and middle) and summarized data (lower; n = 9 from 5 animals) showing spike-rate (SR) responses to whisker stimuli. For each recording, raster plots and peristimulus time histograms (PSTHs, with SRs calculated using a bin width of 50 ms) are displayed (time 0 and arrow, stimulation onset). The summary is plotted with cumulative distributions of Z-scores of SR responses (with reference to SD values of baseline noise). B, C For the example unit recordings and all unit recordings displayed in A, no SR responses to flash stimuli were recorded under light pentobarbital anesthesia (B) or in Down p/p (C). For the summary (lower), Z-scored SRs (in PSTHs) are shown (each trace representing each recording; gray areas, range of ±2-fold-SD in baseline noise). D In Down p/p, flash-evoked Vm responses in two example S1 cells that are simultaneously recorded with VPM unit recordings.
Fig. 7.
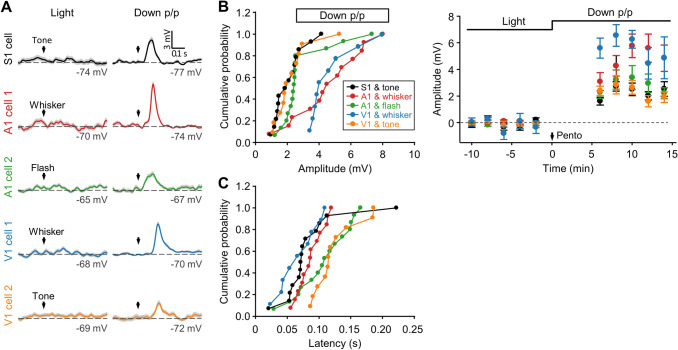
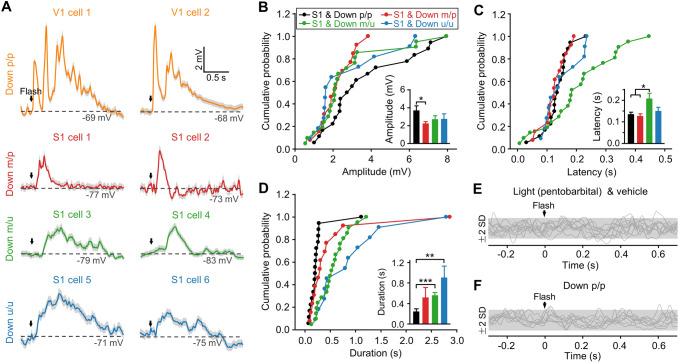
Crossmodal responses of S1, A1, and V1 neurons by stimulating different sensory modalities. A Example cells in S1, A1, and V1 showing Vm responses to tone, whisker, or flash stimuli that were first measured during light pentobarbital anesthesia and then in Down p/p. In each cortical area, different cells were recorded when different stimuli were used. B, C Summary of response amplitudes (B, with cumulative distributions and time courses shown at left and right, respectively) and onset latencies (C) for all experiments as displayed in A (S1 and tone, n = 14 from 8 animals; A1 and whisker, n = 13 from 9 animals; A1 and flash, n = 15 from 10 animals; V1 and whisker, n = 9 from 5 animals; V1 and tone, n = 11 from 9 animals). Data are plotted as in Figs. 1E and 2B, C.
Fig. 8.
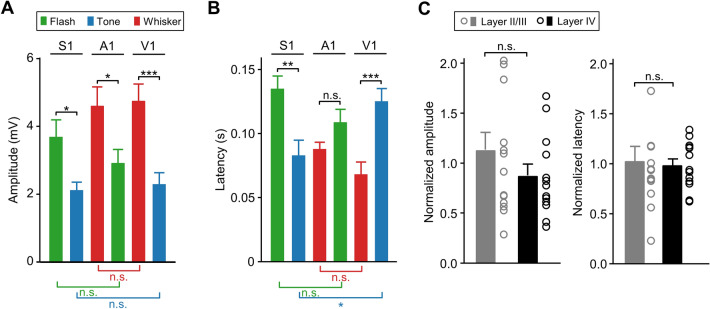
Comparison of different groups of crossmodal responses in Down p/p. A, B Amplitudes (A) and onset latencies (B) for different groups of crossmodal responses in Down p/p evoked by flash, tone, or whisker stimuli in S1, A1, or V1 (experiments in Figs. 1 and 7). C Amplitudes and onset latencies of flash- and tone-evoked S1 responses (in the experiments summarized in A and B) measured at cortical depths of 195–400 µm (337 ± 55 µm; assumed to be layers II/III; n = 10 and 4 from flash- and tone- stimulation groups, respectively) and 410–620 µm (482 ± 58 µm; assumed to be layer IV; n = 7 and 6 from flash- and tone- stimulation groups, respectively). In each stimulation group, data are normalized by the corresponding averaged values as presented in A and B (for S1 and tone and S1 and flash). Dots and histograms, individual normalized data, and mean ± SEM, respectively. *P <0.05; **P <0.01; ***P <0.001; n.s., not significant; unpaired Student’s t-test.
Fig. 3.
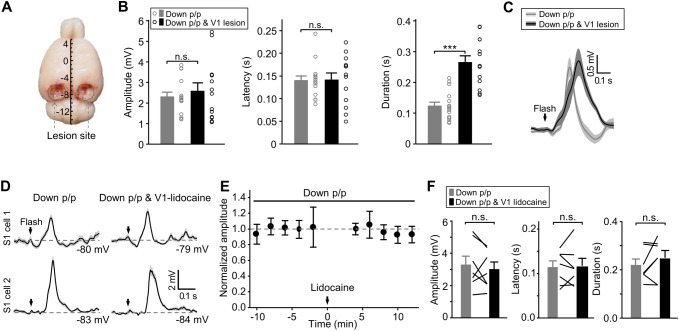
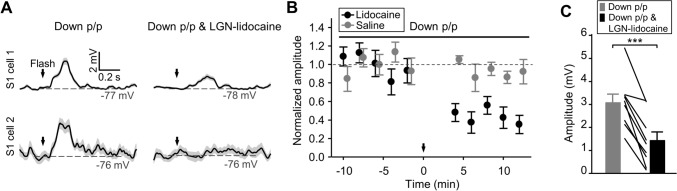
Effects of V1 lesion and V1-lidocaine infusion on flash-evoked S1 responses in Down p/p. A, B With mechanical lesion to the entire V1 regions, as illustrated in A (scale in mm showing the coordinates from Bregma), flash-elicited Vm responses recorded in Down p/p in S1 neurons (n = 13 from 9 animals) exhibit similar amplitudes and onset latencies, but increased durations, compared with control recordings in Down p/p (i.e., without lesion; n = 15 from 12 animals). The experimental procedures of these recordings (for both the control and lesion groups) are shown in Table S1 for Down p/p (Exp. II). Dots and histograms, individual data and mean ± SEM, respectively. C For data as in B, Vm responses averaged across population data in reference to stimulus onset (shadows, ± SEM). D Vm responses of two example S1 cells before and after lidocaine infusion into V1. E, F Summary of all the recordings as shown in D (n = 7 from 6 animals), with time course plots of the response amplitudes (E; each dot represents the average of 20 consecutive trials, which was further normalized to the value before lidocaine infusion; time 0, lidocaine infusion; error bars, SEM), as well as the amplitudes, onset latencies, and durations before and after V1-lidocaine infusion (F; data from the same cells connected by lines). ***P <0.001; n.s., not significant; unpaired Student’s t-test for B and paired t-test for F.
Fig. 4.
Effects of LGN-lidocaine infusion on flash-evoked S1 responses in Down p/p. A With lidocaine infusion into the LGN, two S1 cells are recorded in Down p/p showing reduced Vm responses (Cell 1) and no detectable responses (Cell 2) [experimental procedures shown in Table S1 for Down p/p (Exp. II)]. B, C Summary of the reduction of S1 responses by LGN-lidocaine infusion (n = 9 from 4 animals), including a saline control shown in B (n = 8 from 8 animals). Data are displayed as in Fig. 3E, F. Time 0 (arrow), lidocaine/saline infusion. ***P <0.001; paired Student’s t-test.
For the experiments named Down m/p (Table S1), a down-regulating injection of muscimol into the ventricle (2.9 µg/1.5 µL) was made bilaterally on the basis of light pentobarbital anesthesia, which was also followed by constant injection of a low dose of pentobarbital for maintaining the down-regulated brain activity state, as in Down p/p (Exp. II). Recordings were then made within 6 h after down-regulating injection.
For the experiments named Down m/u (Table S1), the down-regulating injection of muscimol was the same as that for Down m/p, but on the basis of light urethane anesthesia. Recordings were made within 6 h after down-regulating injection.
For the experiments named Down u/u (Table S1), down-regulating injection of urethane (60%–80% of the dose for initial anesthesia to the surgical level, i.p.) was conducted on the basis of light urethane anesthesia. Recordings were made within 3 h after down-regulating injection.
Visual, Acoustic, and Tactile Stimulation, as Well as Response Measurement
For visual stimulation, each eyeball was fixed to a metal ring to prevent closure of the eyelid and possible movement of the eyeball and irrigated with saline [38, 41],. An LED screen (7 × 7 cm2) was placed at a distance of ~2 cm from each eyeball. Bilateral stimuli of whole-screen flashes (duration, 100 ms; luminance, 180–200 cd/m2; as in our previous study [38] and other literature in rodents [42]) on a dark background (2–4 cd/m2) were generated by a pulse generator (Master-8; A.M.P.I., Jerusalem, Israel). Auditory tone stimuli (clicks; duration, 20–30 ms; intensity, 70–80 dB) on low-level background noise (25–30 dB) were delivered binaurally through a personal computer (PC) speaker located ~15 cm in front of the head. For tactile stimulation, all whiskers on each side of the head were glued to iron filings (diameter, 0.1 mm; length, ~2 mm), with the roots of whiskers maintained most closely to their resting positions and angles; the tips of some whiskers were trimmed to expose the iron filings. Two cylindrical electromagnets (diameter, 2.7 cm), positioned at a horizontal distance of ~3 cm in front of the two iron filings that had been glued to bilateral whiskers, were connected to a pulse generator (Master-8; A.M.P.I.) to deflect the whiskers (overall, ~60º; with the application of an electromagnetic field for 100 ms to attract the iron filings, after which the whiskers were allowed to return to their original positions and angles). Both eyes were covered to prevent possible visual stimulation due to whisker movement.
Sensory responses were measured for at least 100 repetitions at an inter-stimulus interval of 6 s. Data were usually collected within 12 h after initial anesthesia for preparation and surgery.
Drug Infusion, V1 Lesions, and Cutting the Optic Nerves
For ventricular infusion of muscimol, two guide cannulas (28 gauge) were bilaterally implanted in the lateral ventricles (from bregma: 0.8 mm posterior and 1.5 mm lateral; 3.5–4 mm beneath the cortical surface) and fixed on the skull with dental acrylic. Internal cannulas (32 gauge) were inserted into the guide cannulas for drug infusion (2.9 µg/1.5 µL muscimol for each injection site). Lidocaine infusion (into V1 and the lateral geniculate nucleus, LGN) and V1 lesions were as described in our previous report [41]. For V1 infusion, two glass pipettes with a tip opening of ~50 µm were placed at two ipsilateral V1 sites by micromanipulators (from bregma: 6 mm posterior and 4 mm lateral; 8 mm posterior and 3.5 mm lateral; both 0.4–0.6 mm beneath the cortical surface). At each V1 site, 0.4 μL lidocaine (2%) was infused, a treatment we have found capable of blocking neuronal firing within ~1 mm from the infusion site [41]. For LGN infusion, two guide cannulas (28 gauge), with internal cannulas (32 gauge), were bilaterally implanted in the LGN (from bregma: 4.5 mm posterior and 3.5–4 mm lateral; 4.5–5 mm beneath the cortical surface) and fixed to the skull with dental acrylic. Into each LGN site, 1 μL lidocaine (2%) was infused, capable of blocking neuronal firing within ~1.5 mm of the infusion site [41]. Bilateral V1 lesions were made mechanically by repeated removal of cortical tissue with 26-gauge needles and fine forceps, as in our previous report [41]. To cut the optical nerve, connective tissue and muscles attaching the eyeball to the orbit were first cut away, and then a cut at optic nerves was made bilaterally with fine scissors by reaching behind the eyeballs.
Anatomical Tract Tracing
Mice were anesthetized with 5% isoflurane, which was sufficient to prevent reflex responses to noxious stimuli (including the surgery and the paw pinch). To infuse the retrograde tracer cholera toxin B (CTB), the head was restrained in a stereotaxic apparatus (David Kopf Instruments) and CTB conjugated to a red fluorescent protein (mCherry) was unilaterally infused into S1 (0.4–0.5 µL of 1 mg/mL; 0.02 µL/min) using a microsyringe connected to a glass pipette (tip opening, ~50 µm; tip location, ~0.3 mm cortical depth). The injection pipette was left in place for 10 min and then slowly withdrawn. With the skin sutured, the animal was returned to the home cage. One week after tracer infusion, animals were euthanized with an extra dose of isoflurane, and perfused with 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (PBS). The brain was rapidly removed, post-fixed for 48 h in the same fixative, immerged in 20% (24 h) and 30% (24 h) sucrose in PBS, and sectioned at 40 µm on a freezing microtome (Leica CM3050 S, Leica Microsystems, Illinois, USA). The sections were incubated with 10 ng/mL 4,6-diamidino-2-phenylindole (DAPI; Beyotime, Shanghai, China) in PBS to stain nuclei. Images were captured by a fluorescence microscope (Leica DM4000 B LED, Leica Microsystems).
Data Analysis and Statistics
Liquid junction potentials (− 13 mV) were corrected. Recordings with resting potentials below − 65 mV (usually − 65 mV to − 85 mV) that had a membrane resistance of 57 ± 15 MΩ (mean ± SD) (measured from randomly-selected data) and a series resistance of 57 ± 12 MΩ (which was not compensated) were included for further analysis. To average the Vm responses evoked by sensory stimuli, spikes were removed from raw trials with a cutoff amplitude at the firing threshold (defined as the Vm value at which dV/dt >10 V/s). the baseline noise in Vm response measurements (defined as SD values of Vm noise calculated from the average trace) was 0.34 ± 0.12 mV and 0.22 ± 0.11 mV for recordings with light anesthesia and down-regulated brain activity, respectively. All Vm responses met the criterion of P <0.05 (peak amplitudes vs baseline values). Response latencies (after stimulus onset) and durations were assessed according to 2-fold-SD amplitudes in baseline noise. Unless otherwise specified, statistical significance was determined with unpaired Student’s t-test, and average values are presented as the mean ± SEM; n values refer to the number of neurons recorded.
Results
Flash-Evoked Vm Responses in Sparsely-Distributed S1 Neurons Under Light Anesthesia
We made perforated whole-cell recordings from anesthetized adult rats to investigate crossmodal responses in primary sensory cortices. Cortical neurons in layers II/III and IV and superficial layer V were recorded in the current-clamp mode to measure Vm changes (without applying holding currents). We first examined visually-evoked crossmodal responses in S1 neurons, with the presentation of a brief (100 ms) flash to both eyes (Fig. 1A). In recordings under light anesthesia with pentobarbital (n = 29 from 20 animals) or urethane (n = 8 from 4 animals), we detected Vm responses to the flash in very few S1 neurons (<5%; Fig. S2), consistent with previous findings of crossmodal spike responses in sparsely distributed sensory cortical neurons (<10%, including in the awake state) [1–3].
Flash-Evoked Excitatory Vm Responses in Widely-Distributed S1 Neurons in a Pharmacologically Down-Regulated Brain State
In the subsequent experiments, we found remarkably that a widespread population of S1 neurons exhibited excitatory crossmodal responses to the flash stimulus, as recorded in a brain state that was pharmacologically down-regulated by light anesthesia. In Fig. 1B–E, we show the responsiveness of the same cells that were first recorded under light pentobarbital anesthesia (as in Fig. S2A, C), and then in a brain state down-regulated by rapid injection of additional pentobarbital [the experimental procedures of Down p/p (Exp. I); Table S1], during which a notable reduction of spontaneous Vm oscillations was seen in our whole-cell recordings (Fig. S1A). We found that immediately after the brain state was changed to Down p/p, flash-evoked Vm depolarizations emerged in all S1 neurons recorded (n = 18 from 14 animals). However, very few of these cells (<5%) showed detectable responses during light anesthesia (Fig. 1B–E). The crossmodal Vm responses in Down p/p were all excitatory, with no inhibitory responses detected (with our perforated whole-cell recording method, inhibitory postsynaptic responses have been found to exhibit a hyperpolarizing Vm change, as shown in our previous in vivo reports in the hippocampus [37, 43]; thus, in this study, we assumed the depolarizing responses to be excitatory). In addition, these excitatory responses were subthreshold, with a small amplitude (3.7 ± 0.5 mV; Fig. 2B) and a long onset latency (137 ± 9 ms; Fig. 2C), compared with the flash-evoked excitatory Vm responses in V1 neurons in Down p/p (amplitude, 8.8 ± 1.4 mV; latency, 32 ± 2 ms; n = 11 from 8 animals; Fig. 2A).
Fig. 2.
Flash-evoked Vm responses in S1 neurons during brain activity down-regulated by various drug treatments. A For S1 recordings in Down m/p, m/u, and u/u, example Vm responses to the flash stimulus (two cells for each drug treatment), and as a comparison with those in Down p/p (as in Fig. 1B), flash-evoked Vm responses in two V1 neurons in Down p/p. B–D Summary of flash-evoked Vm responses in S1 neurons recorded in Down p/p (n = 18 from 14 animals), Down m/p (n = 13 from 6 animals), Down m/u (n = 21 from 7 animals), and Down u/u (n = 11 from 5 animals), with cumulative distributions of their amplitudes (B), onset latencies (C), and durations (D). Histograms in insets: mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; unpaired Student’s t-test. E With vehicle for muscimol infused bilaterally into the lateral ventricle under light pentobarbital anesthesia, no responses to the flash are found in S1 recordings (n = 10 from 5 animals), as shown by the Z-scores of Vm changes of all individual data (arrow, stimulus onset; gray areas, range of ±2-fold-SD in baseline noise). F With optic nerves cut and recordings performed in Down p/p [Down p/p (Exp. II) in Table S1], no responses to the flash occur in S1 recordings (n = 8 from 3 animals).
With the findings of crossmodal visual responses evoked in widely distributed S1 neurons in Down p/p, we tested other drug treatments that were also capable of down-regulating the brain states to further examine the possible induction of S1 responses to visual stimulation. It is known that pentobarbital, as used for Down p/p, mainly exerts its neuronal effects by increasing gamma-aminobutyric acid type A (GABAA) receptor activity [44], raising the possibility that GABAA receptor activity in the brain may underlie the emergence of widespread S1 responses to visual stimulation. Indeed, infusion of the selective GABAA receptor agonist muscimol into the lateral ventricle (bilaterally; 2.9 µg/1.5 µL per side) with light pentobarbital anesthesia and as in Down p/p, resulted in a significantly down-regulated brain state (i.e., Down m/p in Table S1; see Fig. S1B for the reduction of spontaneous Vm oscillations), and we again found that a large proportion (13/15 from 6 animals) of S1 neurons exhibited subthreshold excitatory Vm responses to the flash stimulus (Fig. 2A–D). With infusion of the vehicle of muscimol into the lateral ventricle, no flash-evoked Vm responses were seen in S1 cells (n = 10 from 5 animals; Fig. 2E).
In addition to the above experiments selectively increasing GABAA receptor activity, we found that in situations when brain activity was down-regulated with no direct activation or moderate activation of GABAergic transmission, crossmodal visual responses were likewise elicited in a large population of S1 neurons. In another two groups of animals, we conducted experiments with urethane anesthesia, a treatment that mainly affects intrinsic membrane excitability [44]. During light urethane anesthesia (under this condition, no flash-evoked responses were detected in the 8 S1 recordings (Fig. S2B, C), brain activity was down-regulated by muscimol injection into the lateral ventricle (Down m/u) or additional urethane injection (Down u/u) (Table S1 and Fig. S1C, D). In this situation, excitatory Vm responses were also evoked by the flash stimulus in nearly all S1 neurons (21/25 from 7 animals for Down m/u, 11/11 from 5 animals for Down u/u; Fig. 2A–D), with no inhibitory responses detected. These results demonstrated the induction of widespread S1 responses to crossmodal visual stimulation that were caused by different drug treatments, in which certain similar mechanisms (e.g., in association with the similar effects of down-regulating brain states) might be involved.
In the flash-evoked S1 responses recorded in the above experiments with brain activity down-regulated by different drugs (Figs. 1 and 2A–D), we found significant differences between the amplitudes for Down p/p versus Down m/p (P = 0.032; Fig. 2B inset), in the onset latencies for Down m/u versus Down p/p (P = 0.02) as well as Down m/p (P = 0.025) (Fig. 2C inset), and in the durations for Down p/p versus Down m/u (P <0.001) as well as Down u/u (P = 0.002) (Fig. 2D inset). Such differences were likely due to the different mechanisms of action of these drugs and/or the extent to which the brain activity was down-regulated.
Flash-Evoked S1 Responses Mainly Arise from the Visual System at Subcortical but not Cortical Levels
Next, we investigated the possible pathway in the visual system that may relay the visually-evoked S1 responses in pharmacologically down-regulated brain states. For this purpose, S1 responses to the flash stimulus were assessed in Down p/p (Down p/p (Exp. II) in Table S1). In a control experiment, when both optic nerves were cut prior to recording, no flash-evoked Vm responses were found in S1 recordings (n = 8 from 3 animals; Fig. 2F). In another two groups of animals, we made bilateral lesions of the entire V1 areas (Fig. 3A) or local lidocaine infusion in two ipsilateral sites of V1. In our recordings, these treatments did not alter the proportion of responding cells, with Vm depolarizations in response to the flash detected in all S1 recordings (n = 13 from 9 animals and 7 from 6 animals for the lesion and lidocaine groups, respectively). In addition, the amplitudes and onset latencies of these Vm responses were unchanged (Fig. 3B–F), only the durations were significantly increased by V1 lesions (Fig. 3B, C) but not by V1-lidocaine infusion (Fig. 3F).
We then infused lidocaine bilaterally into the central site of the LGN (2%, 1 μL on each side; with blocking effects on neuronal firing within ~1.5 mm from the infusion site), and found that after drug application, the flash-evoked Vm responses in S1 neurons were largely diminished (amplitudes changed from 3.1 ± 0.4 mV to 1.4 ± 0.4 mV; n = 9 from 4 animals, P < 0.001; with responses of 2 cells removed) (Fig. 4). These results suggested that the visually-evoked S1 responses were relayed by the visual thalamus but not primarily by the visual cortex, the latter appearing to have a modulatory effect on the temporal pattern (e.g., duration) of the crossmodal S1 responses.
We further found that the visual responses of S1 neurons may not arise from the somatosensory thalamus (i.e., VPM), as indicated by our extracellular unit recordings in VPM that showed a lack of flash-evoked spike responses in down-regulated brain states (Fig. 5). In these VPM recordings (n = 9 from 5 animals; Down p/p (Exp. I) in Table S1), the responses that were first measured under light pentobarbital anesthesia did not show spike responses to flash stimulation, but to tactile (whisker) stimulation (Fig. 5A, B). The following measurements in Down p/p also showed no flash-evoked spike responses in the same VPM recordings (Fig. 5C), while all simultaneously-recorded S1 neurons exhibited clear Vm responses to the flash (n = 7 from 4 animals; Fig. 5D).
Our data suggested that the flash-evoked S1 responses arose from the LGN but not the VPM. To determine whether the visual responses were transmitted directly from the LGN to S1, we performed tract-tracing experiments by infusing the retrograde tracer CTB (conjugated to mCherry) into S1 and found no retrogradely labeled (i.e., mCherry-positive) cell bodies in the LGN, but in the VPM (n = 6 mice; Fig. 6; in this experiment, mice but not rats were used, due to the smaller brain size which was more convenient for tracing). These findings indicated a lack of (or very few) LGN projections to S1, ruling out the possibility that the visually-evoked S1 responses were directly transmitted from the LGN.
Fig. 6.
No retrograde labeling in the LGN with CTB infusion in S1. A Representative coronal brain section (~0.9 mm posterior to bregma) showing CTB (conjugated to mCherry, red) infusion into S1. Nuclei are stained with DAPI (blue). B Representative coronal sections from the same mouse showing retrogradely labeled (mCherry-positive) neurons in the VPM but not in the LGN, with VPM and LGN borders (white lines in middle and right panels) outlined according to a mouse brain atlas [59] (as shown in the left for the corresponding section in the middle, ~1.8 mm posterior to bregma).
Crossmodal Responses by Stimulating Different Sensory Modalities and in Different Sensory Cortical Areas
Finally, we asked whether a widespread crossmodal response could be evoked by stimulating other sensory modalities or in other sensory cortical areas in the pharmacologically down-regulated brain state. For this purpose, crossmodal stimulus-evoked responses were assessed in Down p/p [Down p/p (Exp. I), Table S1], and S1 responses to an auditory tone (20–30 ms, 70–80 dB) were next recorded. Similar to the responsiveness to flash stimuli (Figs. 1B–E and S2A, C), although no S1 neurons in our sample (n = 14 from 8 animals) showed Vm responses to the tone stimulus during light pentobarbital anesthesia, subthreshold excitatory (but not inhibitory) Vm responses emerged in all of these cells immediately after the brain state was changed to Down p/p (Fig. 7A–C).
Then, we investigated crossmodal responses in another two primary sensory cortical areas, A1 and V1. Again, we found that when brain activity was changed to Down p/p, all cells recorded from A1 displayed subthreshold excitatory Vm responses to the flash stimulus (n = 15 from 10 animals) and the whisker stimulus (n = 13 from 9 animals) (Fig. 7); and all cells recorded from V1 displayed subthreshold excitatory Vm responses to the tone stimulus (n = 11 from 9 animals) and the whisker stimulus (n = 9 from 5 animals) (Fig. 7). Likewise, crossmodal Vm responses were detected under light anesthesia in very few (<5%) of these A1 or V1 recordings.
We further compared the amplitudes and the onset latencies of all groups of crossmodal responses recorded in Down p/p from different cortical regions or by stimulating different sensory modalities (Figs. 1 and 7). As shown in Fig. 8A, B, we found significant differences between the responses evoked by different stimuli (which perhaps had different strengths) in the same cortical region (except for a lack of differences in the onset latencies between A1 responses to flash and whisker stimuli), but no apparent differences between the responses evoked by the same stimulus in different cortical regions (except for a difference in the onset latencies between tone-evoked responses in V1 and S1). The similarity of Vm responses evoked by the same crossmodal stimulation in different sensory cortices, as reflected in the amplitudes averaged across population data and the proportion of responding cells (nearly 100%), implied a symmetrical crossmodal projection from a given sensory modality to different sensory cortical areas in terms of the overall number of crossmodal synaptic contacts.
To determine whether there were differences between the crossmodal responses evoked in different cortical layers, we further analyzed the data recorded from S1 (Fig. 8A, B) and found no significant differences in the amplitudes (P = 0.22) or onset latencies (P = 0.80) between the recordings from different cortical depth ranges that we assumed to be layers II/III (n = 10 and 4 from flash- and tone-stimulation groups, respectively) and layer IV (n = 7 and 6 from flash- and tone-stimulation groups) according to previous histological studies in S1 [45, 46] (Fig. 8C; another group of 5 S1 recordings from putative superficial layer V was not included for this comparison due to the limited data set).
Discussion
In pharmacologically down-regulated brain states, we recorded crossmodal Vm responses in a large neuronal population of the sensory cortex, including S1 responses to visual and auditory stimuli and A1 responses to visual and tactile stimuli, as well as V1 responses to auditory and tactile stimuli. These crossmodal cortical responses were excitatory and subthreshold and our results suggested that they originated from the sensory thalamus, but not the sensory cortex, of the stimulated modality. Our results indicate the sensory cortical presence of widespread excitatory crossmodal inputs.
New Insight into Crossmodal Connectivity in the Sensory System
The sensory cortical presence of crossmodal inputs has been reported by numerous studies and is thought to display a sparse distribution, as indicated by the findings of crossmodal stimulus-evoked spike responses in a small subset of neurons (<10%) [1–4]. In the present study, we made perforated whole-cell recordings in V1, A1, and S1 (in layers II/III and IV and superficial layer V) to assess subthreshold synaptic responses of sensory cortical neurons that were evoked by crossmodal stimulation. Consistent with previous reports, our recordings under light anesthesia detected crossmodal Vm responses in a small neuronal population (<5%). However, with additional drug treatment that resulted in significantly down-regulated brain activity, we found remarkably that almost all neurons (in V1, A1, and S1) exhibited subthreshold excitatory Vm responses to crossmodal stimulation, suggesting the presence of extensive crossmodal inputs in sensory cortices, which were “silent” in our recordings under light anesthesia and were active during a down-regulated brain state. Our findings provide new insight into the crossmodal connections in the sensory system by showing the electrophysiological findings that indicate a crossmodal structure with excitatory inputs to a widespread population of sensory cortical neurons.
Possible Functional Roles of the Widespread Silent Crossmodal Inputs
The crossmodal input to the widespread population of sensory cortical neurons may often exist in a “silent” status under normal physiological conditions, as we recorded during light anesthesia. Despite its silent nature, we hypothesize that this crossmodal structure is likely to have certain important functional roles, potentially involved in perception, task learning, and neural circuit remodeling following sensory loss [1, 3, 26, 36] (see Discussion below).
In perception, it has been shown that crossmodal sensory integration or neuronal responsiveness in primary sensory cortices is susceptible to modulation by brain state-associated activity, for example, with the dependence on attention, GABAergic network activity, or the intensity of salient sensory stimuli [31–35]. Given these findings, we propose that a potential role of the silent crossmodal inputs recorded in this study is the involvement in sensory perception (e.g., for binding stimulus features among different sensory modalities [26, 47]), with some of these inputs activated when certain brain state-associated activity is changed to an appropriate condition (e.g., by attention, GABAergic network transmission, or other processes). (For such a possibility, different subsets of silent inputs in a given sensory cortical area would be activated specifically by different multisensory tasks, e.g., depending on the spatial-temporal pattern of certain network activity as the potential underlying mechanism).
In addition, we hypothesized that the silent crossmodal input is recruited in task learning and converted to functional input under certain circumstances. Previous studies in the primary sensory cortex have demonstrated the induction of crossmodal plasticity after learning the association of different sensory modalities, with the emergence of crossmodal information representation or interaction recorded in an enlarged neuronal population, including in the visual cortex [10, 24–27], the auditory cortex [10, 24, 26–28], and the somatosensory cortex [12, 13]. For example, it has been shown with single-unit recording in the primate S1 region that after the animals were trained to perform a crossmodal task in which a non-tactile (visual or auditory) stimulus was associated with a tactile stimulus (the touch of an object), crossmodal spike responses to the non-tactile stimulus were induced in a few S1 neurons that were previously unresponsive [12, 13]. Functional magnetic resonance imaging in humans also showed that when an auditory-visual association had been previously learned, this association was successfully encoded in both unimodal visual cortical areas and auditory cortical areas [10, 24]. The underlying mechanism has remained unclear for the learning-induced plasticity with the emergence of crossmodal sensory cortical responses reported in previous studies. However, a possible explanation is the recruitment of some already established—but not previously functional—crossmodal inputs to sensory cortical neurons, in which the silent neurons we show in this study could be involved and undergo a plastic change (e.g., long-term potentiation, LTP) for conversion to functional neurons.
Also, we suggested that the silent crossmodal input to the sensory cortex can be potentially modified for the remodeling of representative cortical areas as found in individuals with the loss of a specific sensory input (as in blindness or deafness) [20–23] as well as with specific neonatal lesions in the brain [48, 49], via experience-dependent plasticity.
With the possible functional roles discussed above, we also propose that optogenetic technology may be a promising approach for addressing these issues experimentally. For example, the crossmodal input to the sensory cortex can be first labeled anterogradely or retrogradely for optogenetic manipulation. Then, to determine the potential role in a certain brain function, these anterogradely/retrogradely labeled crossmodal axons in the sensory cortex or labeled cell bodies in the upstream area can be selectively manipulated with optogenetic stimulation (activated or inactivated) to determine the possible influence on the brain function, the activation of the silent crossmodal inputs for generating sensory cortical activity, and the experience-induced plasticity (LTP) at silent crossmodal inputs, as discussed above for their potential roles in perception, task learning, and neural circuit remodeling following a sensory loss.
Possible Pathways for Relaying Crossmodal Cortical Responses
In the experiments conducted to examine possible pathways for relaying the crossmodal cortical responses that were elicited in pharmacologically down-regulated brain states, we recorded an evident reduction of visually-evoked Vm responses in S1 neurons following LGN-lidocaine infusion (Fig. 4), as well as a lack of visually-evoked spike responses in VPM neurons (Fig. 5A–C). These results suggest that, at the subcortical level, the crossmodal cortical responses originate from the sensory thalamus of the stimulated modality (e.g., from LGN to S1) rather than the sensory thalamus being of the modality with crossmodal cortical responses elicited (e.g., not from VPM to S1).
At the cortical level, our experiments suggest that the sensory cortex of the stimulated modality was not the main region for relaying crossmodal responses in other sensory cortical regions, as shown in Fig. 3 by the unchanged amplitudes and onset latencies of visually-evoked Vm responses in S1 neurons after V1 lesions or V1-lidocaine infusion. However, the significant increase in the duration of visually evoked S1 responses after V1 lesions (Fig. 3B, C) may reflect modulation of the sensory cortex of the stimulated modality on the temporal profiles of crossmodal responses evoked in other sensory cortices, which is likely to be accounted for by strong horizontal inhibition among different modalities of sensory cortices for crossmodal information processing, and this issue needs to be further explored.
In our tract-tracing experiments (Fig. 6), we found no retrogradely-labeled LGN neurons after the tracer CTB was infused into S1, suggesting that the visually-evoked S1 responses recorded in our electrophysiological experiments were not transmitted directly from the LGN to S1. We also found a very long onset latency in the crossmodal cortical responses (mostly >50 or 100 ms; Figs. 2C, 7C, and 8B). In the visually-evoked Vm responses in S1 neurons and V1 neurons that were recorded in Down p/p, we found that the former had an onset latency (137 ± 9 ms, SEM; Fig. 2C) much longer than the latter (32 ± 2 ms).
Given these findings, we propose that the crossmodal cortical responses were transmitted in the sensory thalamus of the stimulated modality and the following transmission by a pathway bypassing the sensory cortex of the stimulated modality. The latter transmission pathway is unclear but probably involves certain cortical regions beyond the sensory cortex due to the long onset latency recorded in the crossmodal cortical responses. Previous studies have shown a variety of cortical regions that send direct projections to primary sensory cortices, including the temporal association area, perirhinal cortex, prefrontal cortex, orbital cortex, insular cortex, retrosplenial cortex, and entorhinal cortex [50–53]. On the other hand, although there are much fewer reports, it has been found that some cortical regions (e.g., the anterior perirhinal cortex) receive direct projections from the sensory thalamus [54]. Given these previous studies, we further propose that some of the cortical regions known to have direct projections to the sensory cortex are likely to be involved in the transmission of the crossmodal responses as recorded in widespread sensory cortical neurons, particularly the higher association area that has been extensively studied and demonstrated to be an important region for crossmodal information processing [1, 55–58].
Possible Gating of Crossmodal Cortical Responses
Our data have indicated the presence of extensive crossmodal inputs to sensory cortical neurons, which were silent under light anesthesia and were active and capable of generating excitatory postsynaptic responses in a pharmacologically down-regulated brain state. These findings raise the possibility of activity-mediated gating in the brain that can determine the generation of sensory cortical responses to crossmodal stimulation. As for the possible neural mechanism of such gating, our data have suggested its dependence on GABAergic network activity (Figs. 1B–E and 7 for recordings for Down p/p and Fig. 2 for Down m/p, in which GABAergic synaptic transmission was selectively increased), but this issue remains to be further investigated.
In summary, our experiments indicate a crossmodal structure in the sensory system that consists of excitatory inputs to widespread sensory cortical neurons that were silent during recording under light anesthesia and were active when the brain activity was down-regulated with additional drug treatment. As for the possible functional role, we propose that this silent structure is likely to be involved in some brain functions that depend on crossmodal information processing or plasticities, such as sensory perception, crossmodal task learning, and neural circuit remodeling following sensory loss.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Drs. J. Tsien, L. Zhang, Y. Zhou, and J. Du for suggestions and comments on this work, and Dr. X. Yuan for the help with the tracing experiments. This work was supported by grants from the National Natural Science Foundation of China (31970957 and 31471078), the Shanghai Science and Technology Commission (19ZR1416600), and funding from 2021-JCJQ-JJ-1089.
Conflict of interest
The authors declare no competing interests concerning the subject of this study.
Footnotes
Yuan-Jie Xiao, Lidan Wang, and Yu-Zhang Liu contributed equally to this work.
Contributor Information
Zheng Zhao, Email: zzhao@brain.ecnu.edu.cn.
Zhiru Wang, Email: zrwang@brain.ecnu.edu.cn.
References
- 1.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Wallace MT, Ramachandran R, Stein BE. A revised view of sensory cortical parcellation. Proc Natl Acad Sci U S A. 2004;101:2167–2172. doi: 10.1073/pnas.0305697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimojo S, Shams L. Sensory modalities are not separate modalities: Plasticity and interactions. Curr Opin Neurobiol. 2001;11:505–509. doi: 10.1016/S0959-4388(00)00241-5. [DOI] [PubMed] [Google Scholar]
- 4.Olcese U, Iurilli G, Medini P. Cellular and synaptic architecture of multisensory integration in the mouse neocortex. Neuron. 2013;79:579–593. doi: 10.1016/j.neuron.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Sathian K, Zangaladze A. Feeling with the mind's eye: Contribution of visual cortex to tactile perception. Behav Brain Res. 2002;135:127–132. doi: 10.1016/S0166-4328(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 6.von Kriegstein K, Kleinschmidt A, Sterzer P, Giraud AL. Interaction of face and voice areas during speaker recognition. J Cogn Neurosci. 2005;17:367–376. doi: 10.1162/0898929053279577. [DOI] [PubMed] [Google Scholar]
- 7.Morrell F. Visual system's view of acoustic space. Nature. 1972;238:44–46. doi: 10.1038/238044a0. [DOI] [PubMed] [Google Scholar]
- 8.Luo H, Liu ZX, Poeppel D. Auditory cortex tracks both auditory and visual stimulus dynamics using low-frequency neuronal phase modulation. PLoS Biol. 2010;8:e1000445. doi: 10.1371/journal.pbio.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu KM, Johnston TA, Shah AS, Arnold L, Smiley J, Hackett TA, et al. Auditory cortical neurons respond to somatosensory stimulation. J Neurosci. 2003;23:7510–7515. doi: 10.1523/JNEUROSCI.23-20-07510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: A behavioral and electrophysiological study. J Cogn Neurosci. 1999;11:473–490. doi: 10.1162/089892999563544. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz JL, Berthommier F, Savariaux C. Seeing to hear better: Evidence for early audio-visual interactions in speech identification. Cognition. 2004;93:B69–B78. doi: 10.1016/j.cognition.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhou YD, Fuster JM. Visuo-tactile cross-modal associations in cortical somatosensory cells. Proc Natl Acad Sci U S A. 2000;97:9777–9782. doi: 10.1073/pnas.97.17.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou YD, Fuster JM. Somatosensory cell response to an auditory cue in a haptic memory task. Behav Brain Res. 2004;153:573–578. doi: 10.1016/j.bbr.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci. 2002;22:5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvert GA, Brammer MJ, Iversen SD. Crossmodal identification. Trends Cogn Sci. 1998;2:247–253. doi: 10.1016/S1364-6613(98)01189-9. [DOI] [PubMed] [Google Scholar]
- 16.Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci. 2005;22:2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- 17.Rockland KS, Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. Int J Psychophysiol. 2003;50:19–26. doi: 10.1016/S0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 18.Macaluso E, Frith CD, Driver J. Modulation of human visual cortex by crossmodal spatial attention. Science. 2000;289:1206–1208. doi: 10.1126/science.289.5482.1206. [DOI] [PubMed] [Google Scholar]
- 19.Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 20.Bavelier D, Neville HJ. Cross-modal plasticity: Where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- 21.Frasnelli J, Collignon O, Voss P, Lepore F. Crossmodal plasticity in sensory loss. Prog Brain Res. 2011;191:233–249. doi: 10.1016/B978-0-444-53752-2.00002-3. [DOI] [PubMed] [Google Scholar]
- 22.Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: The opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh AK, Phillips F, Merabet LB, Sinha P. Why does the cortex reorganize after sensory loss? Trends Cogn Sci. 2018;22:569–582. doi: 10.1016/j.tics.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joassin F, Pesenti M, Maurage P, Verreckt E, Bruyer R, Campanella S. Cross-modal interactions between human faces and voices involved in person recognition. Cortex. 2011;47:367–376. doi: 10.1016/j.cortex.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Goda N, Yokoi I, Tachibana A, Minamimoto T, Komatsu H. Crossmodal association of visual and haptic material properties of objects in the monkey ventral visual cortex. Curr Biol. 2016;26:928–934. doi: 10.1016/j.cub.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Bizley JK, Maddox RK, Lee AKC. Defining auditory-visual objects: Behavioral tests and physiological mechanisms. Trends Neurosci. 2016;39:74–85. doi: 10.1016/j.tins.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Kriegstein K, Giraud AL. Implicit multisensory associations influence voice recognition. PLoS Biol. 2006;4:e326. doi: 10.1371/journal.pbio.0040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atilgan H, Town SM, Wood KC, Jones GP, Maddox RK, Lee AKC, et al. Integration of visual information in auditory cortex promotes auditory scene analysis through multisensory binding. Neuron. 2018;97:640–655.e4. doi: 10.1016/j.neuron.2017.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincis R, Fontanini A. Associative learning changes cross-modal representations in the gustatory cortex. Elife. 2016;5:e16420. doi: 10.7554/eLife.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottfried JA, Dolan RJ. The nose smells what the eye sees: Crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39:375–386. doi: 10.1016/S0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 31.Desgent S, Ptito M. Cortical GABAergic interneurons in cross-modal plasticity following early blindness. Neural Plast. 2012;2012:590725. doi: 10.1155/2012/590725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim LA, Mesik L, Ji XY, Fang Q, Li HF, Li YT, et al. Cross-modality sharpening of visual cortical processing through layer-1-mediated inhibition and disinhibition. Neuron. 2016;89:1031–1045. doi: 10.1016/j.neuron.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leitão J, Thielscher A, Werner S, Pohmann R, Noppeney U. Effects of parietal TMS on visual and auditory processing at the primary cortical level—a concurrent TMS-fMRI study. Cereb Cortex. 2013;23:873–884. doi: 10.1093/cercor/bhs078. [DOI] [PubMed] [Google Scholar]
- 34.Convento S, Rahman MS, Yau JM. Selective attention gates the interactive crossmodal coupling between perceptual systems. Curr Biol. 2018;28:746–752.e5. doi: 10.1016/j.cub.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, et al. Sound-driven synaptic inhibition in primary visual cortex. Neuron. 2012;73:814–828. doi: 10.1016/j.neuron.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein BE, Stanford TR, Rowland BA. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci. 2014;15:520–535. doi: 10.1038/nrn3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu YZ, Wang Y, Tang W, Zhu JY, Wang ZR. NMDA receptor-gated visual responses in hippocampal CA1 neurons. J Physiol. 2018;596:1965–1979. doi: 10.1113/JP275094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang CF, Dan Y, Poo MM, Wang ZR. Periodic stimulation induces long-range modulation of cortical responses and visual perception. J Physiol. 2011;589:3125–3133. doi: 10.1113/jphysiol.2011.205245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: Sound frequency. J Neurophysiol. 1988;59:1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- 40.Doron NN, Ledoux JE, Semple MN. Redefining the tonotopic core of rat auditory cortex: Physiological evidence for a posterior field. J Comp Neurol. 2002;453:345–360. doi: 10.1002/cne.10412. [DOI] [PubMed] [Google Scholar]
- 41.Ma LQ, Ning L, Wang ZR, Wang YW. Visual and noxious electrical stimulus-evoked membrane-potential responses in anterior cingulate cortical neurons. Mol Brain. 2016;9:82. doi: 10.1186/s13041-016-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82:671–686. doi: 10.1016/S0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Liu YZ, Wang LD, Tang W, Wang ZR. Silent synapse unsilencing in hippocampal CA1 neurons for associative fear memory storage. Cereb Cortex. 2019;29:4067–4076. doi: 10.1093/cercor/bhy288. [DOI] [PubMed] [Google Scholar]
- 44.Iskit AB, Guc MO. Comparison of sodium pentobarbitone and urethane anesthesia in a rat model of coronary artery occlusion and reperfusion arrhythmias: Interaction with L-NAME. Pharmacol Res. 1996;33:13–18. doi: 10.1006/phrs.1996.0003. [DOI] [PubMed] [Google Scholar]
- 45.Cahusac PM. Cortical layer-specific effects of the metabotropic glutamate receptor agonist 1S, 3R-ACPD in rat primary somatosensory cortex in vivo. Eur J Neurosci. 1994;6:1505–1511. doi: 10.1111/j.1460-9568.1994.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 46.Simons DJ, Woolsey TA. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol. 1984;230:119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- 47.Senkowski D, Schneider TR, Foxe JJ, Engel AK. Crossmodal binding through neural coherence: Implications for multisensory processing. Trends Neurosci. 2008;31:401–409. doi: 10.1016/j.tins.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Sur M, Garraghty PE, Roe AW. Experimentally induced visual projections into auditory thalamus and cortex. Science. 1988;242:1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- 49.Sur M, Angelucci A, Sharma J. Rewiring cortex: The role of patterned activity in development and plasticity of neocortical circuits. J Neurobiol. 1999;41:33–43. doi: 10.1002/(SICI)1097-4695(199910)41:1<33::AID-NEU6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 50.Budinger E, Scheich H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear Res. 2009;258:16–27. doi: 10.1016/j.heares.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CCH. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci. 2010;31:2221–2233. doi: 10.1111/j.1460-9568.2010.07264.x. [DOI] [PubMed] [Google Scholar]
- 52.Froudarakis E, Fahey PG, Reimer J, Smirnakis SM, Tehovnik EJ, Tolias AS. The visual cortex in context. Annu Rev Vis Sci. 2019;5:317–339. doi: 10.1146/annurev-vision-091517-034407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang JJ, Sun P, Lv XH, Jin S, Li AN, Kuang JX, et al. Divergent projection patterns revealed by reconstruction of individual neurons in orbitofrontal cortex. Neurosci Bull. 2021;37:461–477. doi: 10.1007/s12264-020-00616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McIntyre DC, Kelly ME, Staines WA. Efferent projections of the anterior perirhinal cortex in the rat. J Comp Neurol. 1996;369:302–318. doi: 10.1002/(SICI)1096-9861(19960527)369:2<302::AID-CNE10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 55.Miller MW, Vogt BA. Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol. 1984;226:184–202. doi: 10.1002/cne.902260204. [DOI] [PubMed] [Google Scholar]
- 56.Felleman DJ, van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Nikbakht N, Tafreshiha A, Zoccolan D, Diamond ME. Supralinear and supramodal integration of visual and tactile signals in rats: Psychophysics and neuronal mechanisms. Neuron. 2018;97:626–639.e8. doi: 10.1016/j.neuron.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu CB, Yang T, Zhao H, Zhang M, Meng FC, Fu H, et al. Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull. 2016;32:191–201. doi: 10.1007/s12264-016-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konsman JP. The mouse brain in stereotaxic coordinates. Psychoneuroendocrinology. 2003;28:827–828. doi: 10.1016/S0306-4530(03)00088-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.