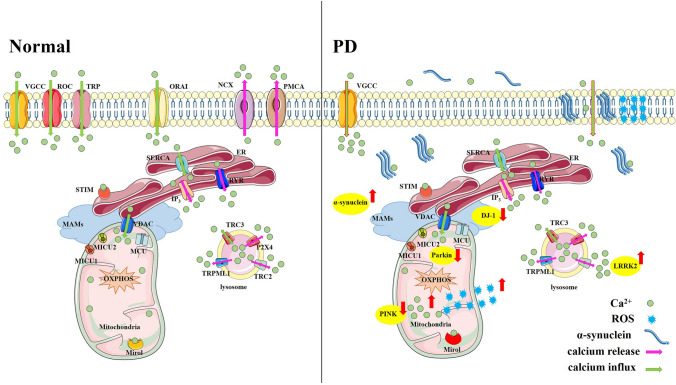
Disturbances of Ca2+ homeostasis have been reported to be associated with the development of Parkinson’s disease (PD) (Fig. 1), which is characterized by the selective vulnerability of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Different from the dopaminergic neurons in the ventral tegmental area (VTA), which mainly rely on hyperpolarization-activated cyclic nucleotide-gated/voltage-gated channels for pacing and have a strong Ca2+-buffering capacity, SNpc neurons mostly depend on L-type Ca2+ channels (LTCCs) for their basal activity. Also, the density of LTCCs is higher in the SNpc than in the VTA, which leads to a higher Ca2+ load and an increase the sensitivity of neurons to neurotoxins associated with PD [1]. Therefore, a difference in Ca2+ homeostasis has been proposed to be one of the most likely reasons for the selective degeneration of dopaminergic neurons in the SNpc rather than in the VTA region in PD [1]. Since α-synuclein aggregation, mitochondrial oxidative stress, and lysosome dysfunction are major risk factors of PD, potential interactions between Ca2+ and the above factors, and potential treatments for PD that target Ca2+ are discussed.
Fig. 1.
Calcium homeostasis in the physiological state and Parkinson’s disease Under physiological conditions, Ca2+ shuttles between the extracellular space, cytoplasm, endoplasmic reticulum (ER), mitochondria, lysosomes, and Golgi bodies, and maintains a stable amplitude and frequency of Ca2+ changes in different cellular compartments, which are strictly regulated by many types of Ca2+ channels or Ca2+ pumps. In the Parkinson’s disease state, aggregated α-synuclein, mutant PD-related genes (such as LRRK2, DJ-1, Parkin, and PINK), alter the levels of Ca2+ channels or damaged the cell membrane, causing imbalanced Ca2+ signaling between different cellular compartments, and thereby contribute to nigral dopaminergic neuron death. VGCC, voltage-gated Ca2+ channel; ROC, receptor-operated channel; TRP, transient receptor potential channel; ORAI, Ca2+ release-activated Ca2+ channel modulator; NCX, Na+/Ca2+ exchanger; PMCA, Ca2+ ATPase; ER, endoplasmic reticulum; SERCA, sarco-endoplasmic reticulum Ca2+-ATPase; RYR, ryanodine receptor; STIM, stromal interaction molecule; IP3, inositol-1, 4, 5-triphosphate; VDAC, voltage-dependent anion channel; MAM, mitochondria-associated endoplasmic reticulum membrane; MCU, mitochondrial Ca2+ uniporter; MICU1/2, mitochondrial Ca2+ uptake 1/2; OXPHOS, oxidative phosphorylation; TRC2/3, two-pore channels 2/3; P2X4, ATP-gated multi-ion channel; TRPML1, mucolipin-1; LRRK2, leucine-rich repetitive kinase 2; ROS, reactive oxygen species.
Calcium Homeostasis and α-Synuclein
Abnormal aggregation of α-synuclein is thought to be one of core pathogenic factors in PD. Ca2+ can bind to the C-terminus of α-synuclein, regulate the secretion of α-synuclein, and promote the formation of α-synuclein aggregates. Alpha-synuclein is mainly located at presynaptic terminals, where high Ca2+ fluctuations occur, and participates in synaptic vesicle endocytosis. Both the N-terminus and the C-terminus of α-synuclein interact with isolated synaptic vesicles; however, the binding with the C-terminus is regulated by Ca2+, which increases the lipid-binding capacity of α-synuclein [2]. Also, the C-terminus binding of Ca2+ to α-synuclein causes changes in the non-amyloid component domain and promotes the formation of β-folded structures, thus resulting in the formation of α-synuclein aggregates. Ca2+ and α-synuclein have been suggested to affect vesicle pool homeostasis through two mechanisms, one by promoting inter-vesicle interactions, and the other by the binding of synaptic vesicles to the plasma membrane, which may affect their access to voltage-gated Ca2+ channels. Also, α-synuclein has been reported to be secreted in the mouse striatum and in cultured neurons through a Ca2+-dependent mechanism, which is tightly regulated by gamma-aminobutyric acid transmission [3]. In PD patient-derived dopaminergic neurons with lost ATP13A2/PARK9 function, disrupted lysosomal Ca2+ homeostasis (decreased storage of lysosomal Ca2+ and increased cytosolic Ca2+) impairs lysosomal exocytosis, which impairs axonal and somatic α-synuclein secretion and promotes the accumulation of α-synuclein. When the lysosomal Ca2+ channel mucolipin-1 (TRPML1) is activated, lysosomal exocytosis is upregulated, which promotes α-synuclein secretion and prevents the cellular accumulation of α-synuclein [4]. Recently, inositol-1, 4, 5-triphosphate kinase B (ITPKB) has been shown to be associated with sporadic PD, and inhibition or knockdown of ITPKB reduces α-synuclein aggregation by affecting endoplasmic reticulum (ER)-to-mitochondria Ca2+ transport, which causes an accumulation of Ca2+ in mitochondria [5]. As a Ca2+-binding protein, calbindin also plays important roles in maintaining Ca2+ homeostasis. Both dementia with Lewy bodies and a unilateral rotenone mouse model have revealed that α-synuclein aggregates are not present in cells expressing calbinin-D28k, suggesting that calbinin-D28k is protective against α-synuclein aggregation and cell loss [6]. Similar evidence has been found in macaques expressing calbindin in the striatum by adenoviral vector injection: dopaminergic neurons express more α-synuclein on the non-calbindin-recruited control side after 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) administration; on the other hand, recruitment of calbindin into nigral dopaminergic neurons protects against MPTP-induced neurodegeneration [7].
Meanwhile, α-synuclein also affects Ca2+ signaling. Both the unfolded monomeric and oligomeric states of α-synuclein can induce an increase in intracellular Ca2+; however, only oligomeric α-synuclein induces Ca2+-dependent cell death. In neurons with α-synuclein triplication derived from human-induced pluripotent stem cells, excessive α-synuclein oligomers are incorporated into the membrane, which causes abnormal Ca2+ influx and derived ferroptosis [8].
Calcium Homeostasis and Mitochondria
In PD, mitochondrial Ca2+ signaling contributes to the death of nigral dopaminergic neurons, mainly by regulating the production of adenosine triphosphate (ATP) and mitochondrial oxidant stress. An α-synuclein-dependent increase of cytosolic Ca2+ in SNpc neurons has been found to be followed by an elevation of mitochondrial Ca2+ and mitochondrial oxidation [9]. In cultured hippocampal neurons, a cytosolic Ca2+ increase triggered by LTCC-mediated Ca2+ influx has reported to promote mitochondrial ATP synthesis [10]. As shown in primary cultured neurons and in mouse slices, dopaminergic neurons in the SNpc exhibit higher levels of mitochondrial oxidant stress under resting and pathology-free conditions. However, mitochondrial oxidative stress in VTA dopaminergic neurons is much lower [9, 11]. Mitochondrial oxidative stress has been reported to be enhanced by increased Ca2+ through Cav1, followed by the oxidation of dopamine, misfolding of α-synuclein, and impaired lysosomal function [12]. Systemic application of Cav1 channel inhibitors in mice, or inhibition of mitochondrial Ca2+ influx, could protect nigral dopaminergic neurons by reducing mitochondrial oxidative stress, slowing down mitophagy, and normalizing mitochondrial quality [11], suggesting that regulation of mitochondrial oxidative stress through Ca2+ signaling is beneficial for PD.
Mitophagy is a form of selective autophagy that degrades damaged mitochondria through autophagy. It is regulated by the PD-related gene PINK1 and the Parkin protein, and is important for mitochondrial quality control that is also affected by Ca2+. Loss of PINK1 is reported to be associated with reduced mitochondrial Ca2+ uptake or impaired mitochondrial Ca2+ outflow (promoting mitochondrial Ca2+ overload) due to depolarization [13]. Parkin can adjust the level and turnover of the mitochondrial Ca2+ uniporter (MCUC) regulator—MICU1/2 [14]. Also, PD-linked mutations in leucine-rich repetitive kinase 2 (LRRK2) increase the expression of MCU1 and MICU1 and reduce mitochondrial Ca2+ efflux [15].
Calcium Homeostasis and Lysosomes
Recently, abnormal lysosomal Ca2+ in PD has attracted increasing attention. Decreased content of the lysosomal Ca2+ store, followed by altered lysosomal morphology, has been reported in the fibroblasts of PD patient carrying the N370S mutation in β-glucocerebrosidase [16]. LRRK2 also functions as a regulator of lysosomal Ca2+; it is localized to the membranes of late endosomes and lysosomes, and regulates endocytic membrane trafficking in an Rab7-dependent manner. In fibroblasts from PD patients carrying the G2019S mutation in LRRK2, NAADP (nicotinic acid adenine dinucleotide phosphate) stimulates lysosomal Ca2+ release with the involvement of the two-pore channel and Lsm12 [17]. Meanwhile, an enlarged lysosomal system and increased perinuclear aggregation have also been reported, and these defects are corrected by the inhibition of NAADP. TRPML1 is another Ca2+-release channel on the lysosome that plays an essential role in lysosomal exocytosis and α-synuclein secretion, and the lysosomal Ca2+ release mediated by activated TRPML1 can rescue α-synuclein secretion and prevent the cellular α-synuclein accumulation in PD [4].
Calcium Homeostasis and Mitochondria-Associated ER Membranes (MAMs)
The transfer of Ca2+ between the ER and mitochondria depends on the Ca2+ transfer system located on the MAMs. Most of the Ca2+ in mitochondria originates from MAMs, which are closely connected to mitochondria and also associated with PD. Alpha-synuclein is also located on the MAMs, and the A30P mutation in α-synuclein shows reduced MAM localization. Both α-synuclein and its disease-causing mutants (A30P and A53T) have been shown to bind to the mitochondrial binding protein VAPB, thereby impeding its association with ER-specific PTPIP51 and disrupting the ER-mitochondrion contact site [18]. ITPKB (inositol-trisphosphate 3-kinase B), a new kinase that regulates MAMs, has recently been reported to negatively regulate α-synuclein aggregation by affecting ER-mitochondrion Ca2+ transport [5]. Recently, mutations in the Ca2+-binding EF and GTPase domains of Miro1, which is an outer mitochondrial membrane protein, have been identified in patients with sporadic PD, and these mutations cause reduced ER-mitochondrion contact sites, resulting in a reduced cytoplasmic Ca2+ buffering capacity of the mitochondria of fibroblasts [19]. Meanwhile, these mutations also increase mitophagy. Furthermore, the ER-mitochondrion connectivity is also regulated by familial PD-related proteins, such as DJ-1, which has been identified as an important component of the IP3R-GRP 75-VDAC 1 complex that mediates ER-mitochondrion Ca2+ transfer [20]. The loss of DJ-1 function leads to destruction of the IP3R-GRP 75-VDAC 1 complex and reduction of mitochondrial Ca2+ levels after IP3R stimulation. In addition, the loss of DJ-1 also results in increased levels of the ER-mitochondrion contact site IP3R3, suggesting that IP3R3 forms aggregates when it loses its association with the IP3R 3-GRP 75-VDAC 1 complex.
Treatment and Prospects
Accumulating evidence indicates the important role of imbalanced Ca2+ homeostasis in the development of PD, and Ca2+ channel blockers (such as nimodipine, isradipine, dihydropyridines, nifedipine, felodipine, and benidipine), and Ca2+-binding protein (such as S100B) have shown potential therapeutic effects in animal models of PD or clinical trials. However, treatment effects on PD that target lysosomal Ca2+ channels and MAMs are still limited. Also, the dynamic monitoring of Ca2+ is particularly important, especially for the storage and release of Ca2+ from organelles, such as the ER, mitochondria, and lysosomes. And specific drugs that target these processes may be beneficial for PD prevention or treatment. In addition, the interactions between Ca2+ and other metal ions, such as iron [21], which contribute to the development of PD, are also worthy of further investigation.
Acknowledgements
This insight was supported by the National Foundation of Natural Science of China (32170984), and the Natural Science Foundation of Shandong Province (ZR2020YQ23 and ZR2019BC008).
Contributor Information
Leilei Chen, Email: leileichen2019@qdu.edu.cn.
Junxia Xie, Email: jxiaxie@public.qd.sd.cn.
References:
- 1.Verma A, Ravindranath V. Ca V1.3 L-type calcium channels increase the vulnerability of substantia nigra dopaminergic neurons in MPTP mouse model of Parkinson's disease. Front Aging Neurosci. 2020;11:382. doi: 10.3389/fnagi.2019.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lautenschläger J, Stephens AD, Fusco G, Ströhl F, Curry N, Zacharopoulou M, et al. C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat Commun. 2018;9:712. doi: 10.1038/s41467-018-03111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emmanouilidou E, Minakaki G, Keramioti MV, Xylaki M, Balafas E, Chrysanthou-Piterou M, et al. GABA transmission via ATP-dependent K+ channels regulates α-synuclein secretion in mouse striatum. Brain. 2016;139:871–890. doi: 10.1093/brain/awv403. [DOI] [PubMed] [Google Scholar]
- 4.Tsunemi T, Perez-Rosello T, Ishiguro Y, Yoroisaka A, Jeon S, Hamada K, et al. Increased lysosomal exocytosis induced by lysosomal Ca2+ channel agonists protects human dopaminergic neurons from α-synuclein toxicity. J Neurosci. 2019;39:5760–5772. doi: 10.1523/JNEUROSCI.3085-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apicco DJ, Shlevkov E, Nezich CL, Tran DT, Guilmette E, Nicholatos JW, et al. The Parkinson's disease-associated gene ITPKB protects against α-synuclein aggregation by regulating ER-to-mitochondria calcium release. Proc Natl Acad Sci U S A. 2021;118:e2006476118. doi: 10.1073/pnas.2006476118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rcom-H'cheo-Gauthier AN, Davis A, Meedeniya ACB, Pountney DL. Alpha-synuclein aggregates are excluded from calbindin-D28k-positive neurons in dementia with Lewy bodies and a unilateral rotenone mouse model. Mol Cell Neurosci. 2016;77:65–75. doi: 10.1016/j.mcn.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Inoue KI, Miyachi S, Nishi K, Okado H, Nagai YJ, Minamimoto T, et al. Recruitment of calbindin into nigral dopamine neurons protects against MPTP-Induced Parkinsonism. Mov Disord. 2019;34:200–209. doi: 10.1002/mds.107. [DOI] [PubMed] [Google Scholar]
- 8.Angelova PR, Choi ML, Berezhnov AV, Horrocks MH, Hughes CD, De SM, et al. Alpha synuclein aggregation drives ferroptosis: An interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020;27:2781–2796. doi: 10.1038/s41418-020-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberman OJ, Choi SJ, Kanter E, Saverchenko A, Frier MD, Fiore GM, et al. Α-synuclein-dependent calcium entry underlies differential sensitivity of cultured SN and VTA dopaminergic neurons to a parkinsonian neurotoxin. eNeuro 2017, 4: ENEURO.0167–17.2017. [DOI] [PMC free article] [PubMed]
- 10.Hotka M, Cagalinec M, Hilber K, Hool L, Boehm S, Kubista H. L-type Ca2+ channel-mediated Ca2+ influx adjusts neuronal mitochondrial function to physiological and pathophysiological conditions. Sci Signal. 2020;13:eaaw6923. doi: 10.1126/scisignal.aaw6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman JN, Ilijic E, Yang B, Sanchez-Padilla J, Wokosin D, Galtieri D, et al. Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J Clin Invest. 2018;128:2266–2280. doi: 10.1172/JCI95898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbulla LF, Song PP, Mazzulli JR, Zampese E, Wong YC, Jeon S, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson's disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang E, Qu DB, Huang TW, Rizzi N, Boonying W, Krolak D, et al. PINK1-mediated phosphorylation of LETM1 regulates mitochondrial calcium transport and protects neurons against mitochondrial stress. Nat Commun. 2017;8:1399. doi: 10.1038/s41467-017-01435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matteucci A, Patron M, Vecellio Reane D, Gastaldello S, Amoroso S, Rizzuto R, et al. Parkin-dependent regulation of the MCU complex component MICU1. Sci Rep. 2018;8:14199. doi: 10.1038/s41598-018-32551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludtmann MHR, Kostic M, Horne A, Gandhi S, Sekler I, Abramov AY. LRRK2 deficiency induced mitochondrial Ca2+ efflux inhibition can be rescued by Na+/ Ca2+/Li + exchanger upregulation. Cell Death Dis. 2019;10:265. doi: 10.1038/s41419-019-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilpatrick BS, Magalhaes J, Beavan MS, McNeill A, Gegg ME, Cleeter MWJ, et al. Endoplasmic Reticulum and lysosomal Ca2+ stores are remodelled in GBA1-linked Parkinson disease patient fibroblasts. Cell Calcium. 2016;59:12–20. doi: 10.1016/j.ceca.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JY, Guan X, Shah K, Yan JS. Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles. Nat Commun. 2021;12:4739. doi: 10.1038/s41467-021-24735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paillusson S, Gomez-Suaga P, Stoica R, Little D, Gissen P, Devine MJ, et al. Α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017;134:129–149. doi: 10.1007/s00401-017-1704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossmann D, Berenguer-Escuder C, Bellet ME, Scheibner D, Bohler J, Massart F, et al. Mutations in RHOT1 disrupt endoplasmic Reticulum-mitochondria contact sites interfering with calcium homeostasis and mitochondrial dynamics in Parkinson's disease. Antioxid Redox Signal. 2019;31:1213–1234. doi: 10.1089/ars.2018.7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Ma XP, Fujioka H, Liu J, Chen SD, Zhu XW. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc Natl Acad Sci U S A. 2019;116:25322–25328. doi: 10.1073/pnas.1906565116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen LL, Li C, Xie JX. Axonal iron transport might contribute to iron deposition in Parkinson's disease. Neurosci Bull. 2021;37:275–277. doi: 10.1007/s12264-020-00585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]