Abstract
Introduction:
Debate on the association between the use of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) and the risk of developing cancer has been ongoing for decades. This study aimed to generate reliable results by analysing observational studies published in the decade after our last meta-analysis was conducted.
Methods:
We searched Embase and Medline databases on 21 January 2021 for cohort and case-control studies. Two researchers independently reviewed the literature and assessed the title and abstract of each publication. The I2 statistic used to evaluate the heterogeneity of the effect measures. Risk of bias was qualitatively assessed using the Newcastle–Ottawa scale.
Results and discussion:
We included an additional 16 cohort, 6 nested case-control, and 9 conventional case-control studies in the updated analysis. Overall HRs decreased, while overall relative risks increased.
Conclusion:
Our results show some protective effects through the hazard ratio and some detrimental effects through the relative risk. Large-scale investigations of cohorts followed up for decades are needed to clarify association.
Plain Language Summary
Introduction: Two types of drug, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), have been linked to the risk of developing cancer. We performed a meta-analysis by aggregating individual studies looking into the cancer risk of ACEIs and ARBs.
Methods: We searched for articles on Embase and Medline databases until 21 January, 2021. Two researchers independently reviewed the literature and assessed the title and abstract of each publication.
Results: Overall, the hazard ratio showed less than 1, while the relative risks showed higher than 1.
Conclusion: Our results show some protective effects through the hazard ratio and some detrimental effects through the relative risk. Evidence supporting the risk of developing cancer is insufficient to prevent prescribing ACEIs or ARBs for patients with high blood pressure.
Keywords: angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, cancer, hypertension, meta-analysis
Introduction
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are used to treat hypertension.1,2 They are considered first-line treatments for patients with diabetes, chronic kidney disease, and heart disease2 as first-line agents for reducing the risk of heart disease, proteinuria, and the expression of plasminogen activator inhibitor-13 in patients with cancer. These drugs also reportedly have a beneficial effect in patients with heart failure with preserved ejection fraction.4
Renin-angiotensin system (RAS) maintains overall blood pressure and electrolyte balance, with angiotensin playing an important role in regulating the system.5 Angiotensin II promotes cell proliferation and neovascularisation,6 as well as cancer growth, through VEGF-mediated angiogenesis.7 ACEIs and ARBs can reduce the risk of cancer by inhibiting the RAS system through these biological mechanisms, and this has been partially demonstrated in experimental animal and human studies.6,8
The first meta-analysis focusing on the relationship between hypertensive drugs and cancer was conducted in 2001.9 It included both randomised control trials and observational studies but found no significant association between cancer and ACEI or ARB.9 However, two subsequent meta-analyses focusing on randomised controlled trials (RCTs) found that ARBs or a combination of ACEI and ARB modestly increased the risk of cancer.1,10 However, these findings were criticised because patients were followed up for only 2–5 years, making it difficult to demonstrate causality or to determine the possibility that untreated hypertension could increase occurrence of certain types of cancer).11 A meta-analysis of observational studies also found no significant association between ACEI or ARB and cancer. Analyses of studies that followed patients for more than 5 years, to reduce the limitations of RCT meta-analyses, revealed potential benefits of these substances against cancer.12
The most recent RCT meta-analysis reported no evidence of consistent antihypertensive use affecting cancer risk.13 Considering that findings from numerous observational studies published since 2011 may be novel, we performed a meta-analysis by adding papers published after 2011. A previous meta-analysis published in 2011 assessed overall risk by aggregating odds ratios (ORs) and hazard ratios (HRs) into relative risk (RR).12 In this study, we subdivided effect measures into RRs and HRs because the data in most long-term follow-up cohort studies included herein were analysed using these measures.
Methods
Protocol registration
This meta-analysis was conducted in accordance with PRISMA guidelines.14 The study protocol is registered with PROSPERO (registration no: CRD42021231789).
Selection criteria
We selected only the papers in which primary cancer was diagnosed by using national register ICD code or in hospital. There was no limit to the type of cancer or the duration of follow-up. Skin cancer was limited to only malignant melanoma similar to the methodology of the previous meta-analysis. Any cancer was the opposite of specific cancer, and all cancers referred to both any cancer and specific cancer. We analysed studies of ACEIs or ARBs that reported effect measures, including risk ratios, ORs, and HRs. Cohort and case-control studies were included in the analysis. Publications using data from the same database were included if they analysed different diseases.
Search strategy
We searched Embase and Medline databases in 21 January 2021 using the following terms: ACE inhibitor OR ACEI OR angiotensin converting enzyme inhibitor OR Angiotensin I converting enzyme inhibitor OR kinase II inhibitor OR dipeptidyl carboxypeptidase inhibitor OR ARB OR angiotensin receptor blocker OR angiotensin receptor antagonist OR antihypertensive OR antihypertension OR blood pressure lowering AND cancer OR malignancy OR malignancies OR malignant neoplasm OR tumour OR carcinoma OR carcinogenesis OR pre-cancer AND ratio OR risk OR hazard OR outcome OR prognosis OR mortality OR morbidity OR prevalence OR incidence OR odds. The search was limited to study titles and abstracts and was not restricted to a specific language.
Selection process
Two authors (KS, JY) independently searched the databases and assessed the title and abstract of each publication retrieved using the search strategy. Full-text articles were reviewed to determine their suitability for inclusion in the analysis. Disagreements were resolved through discussion.
Data extraction
The following data were extracted during the screening phase: title, abstract, journal name, author name(s), publication year, and publication type. Information on study design, study population, effect measures, follow-up period, age, sex, cancer type, drug class, region, and adjusted variables was also extracted.
Summary measures
We aggregated the ORs and RRs into RRs based on a previously described methodology. Furthermore, we computed the HR because it is considered the rate of event or outcome in one group relative to another group over a specific period and is similar but distinct from other effect measures.
Risk of bias in individual studies
The risk of bias in the selected cohort and case-control studies was qualitatively assessed using the Newcastle-Ottawa scale. Assessment tools are presented in the supplementary materials. The authors (KS, JY) independently assessed the risk of bias in the studies and verified the quality of the evidence. Discrepancies were resolved through discussion. Cohort and case-control study scores were classified as good, fair, or poor based on the Agency for Healthcare Research and Quality standard. The sensitivity analysis was performed on studies evaluated as ‘good’ quality as a result of risk of bias assessment.
Publication bias across studies
We used Egger’s test to evaluate publication bias using STATA 13 software. We used funnel plots to visually evaluate publication bias.
Statistical analyses
Data are shown as crude and adjusted ORs, RRs, and HRs, with 95% confidence intervals. The classification of I2 statistics, as presented by Higgins et al.,15 was used to evaluate the heterogeneity of the effect measures. Heterogeneity was considered low, moderate, or high if I2 values were 25%, 50%, or 75%, respectively. The random effect method was used when heterogeneity exceeded 50%; otherwise, the fixed-effect method was used. If an integrated value was required, the calculation was performed using the Higgins method.15 Results were presented using the Review Manager 5.4 software.
Results
Study selection and characteristics
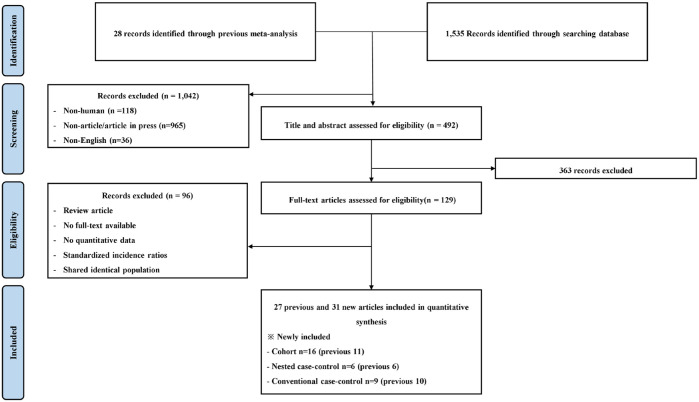
The titles and abstracts of 492 records were screened. A full-text review was conducted for 129 publications, and 61 articles (28 previous and 31 new) were selected for inclusion in the study (Figure 1). The previously analysed studies consisted of 12 cohort, 6 nested case-control, and 10 conventional case-control studies. The new studies consisted of 16 cohort, 6 nested case-control, and 9 conventional case-control studies. The characteristics of the studies included in the analysis were presented in Supplementary Tables 1 and 2.
Figure 1.
PRISMA flowchart.
Overall results
Hazard ratio
The adjusted HRs for all, any, breast, and smoking-related cancers were less than 1 (Table 1). The crude HRs for any, lung, and smoking-related cancers were also less than 1.
Table 1.
Subgroup analyses of association between ACEi or ARB and risk of cancer.
| Type of cancer | All studies | |||
|---|---|---|---|---|
| Adjusted | Crude | |||
| HR (95% CI) | I² (%) /(cohort studies) |
HR (95% CI) | I² (%) /(cohort) |
|
| All cancer risk | 0.83 (0.75–0.93)* | 94/(17) | 0.87 (0.74–1.01) | 96/(7) |
| Any | 0.65 (0.57–0.74)* | 86/(5) | 0.70 (0.59-0.83)* | 95/(3) |
| Breast | 0.73 (0.69–0.77)* | 39/(4) | 0.84 (0.60-1.18) | 87/(3) |
| Lung | 0.68 (0.58–0.80)* | 65/(4) | 0.68 (0.60-0.76)* | 37/(3) |
| Colon/rectal | 0.98 (0.84–1.13) | 54/(3) | 0.91 (0.76-1.10) | 85/(4) |
| Prostate | 0.99 (0.88–1.12) | 73/(4) | 0.71 (0.59-0.86)* | 0/(2) |
| Hematologic | 0.57 (0.43-0.76)* | –/(1) | – | –/– |
| Genitourinary | 0.89 (0.67-1.19) | 93/(2) | – | –/– |
| Female reproductive | 1.01 (0.75-1.36) | –/(1) | – | –/– |
| Pancreatic | 0.86 (0.71-1.05) | 0/(1) | – | –/– |
| Smoking-related cancer** | 0.68 (0.58–0.80)* | 65/(4) | 0.68 (0.60-0.76)* | 37/(3) |
Statistically significant (p < 0.05), **Includes oesophageal, lung, and kidney cancers
CI, confidence interval; HR, hazard ratio; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Relative risk
Crude and adjusted combined RRs were analysed using a previously described method (Table 2). Adjusted RRs were higher than 1 in kidney cancer and melanoma. Crude RRs were higher than 1 in all, kidney, and hematologic cancers. Subgroup analyses were conducted for cohort and nested case-control studies. Adjusted and crude RR values for cohort and nested case-control studies were less than 1 in colon/rectal cancer, while higher than 1 in kidney cancer and melanoma.
Table 2.
Subgroup analyses of the association between ACEi or ARB and the risk of cancer based on study design.
| Type of cancer | All studies | Cohort and nested–case control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Previous | Adjusted | Crude | Previous | Adjusted | Crude | |||||
| RR (95% CI) | I² (%) /(cohort/ case–control) |
RR (95% CI) | I² (%) /(cohort/ case–control) |
RR (95% CI) | I² (%) /(cohort/ case–control) |
RR (95% CI) | I² (%) /(cohort/ case–control) |
|||
| All cancers | 0.96 (0.90–1.03) | 1.02 (0.99–1.05) | 72/(10/31) | 1.11 (1.05–1.17)* | 93/(2/32) | 0.90 (0.83–0.97)* | 0.99 (0.96–1.03) | 66/(10/12) | 1.12 (1.03–1.21)* | 96/(2/14) |
| Any | 0.85 (0.73–0.98)* | 1.01 (0.94–1.08) | 87/(3/6) | 1.00 (0.94–1.07) | 93/(1/6) | 0.80 (0.68–0.95)* | 0.99 (0.97–1.02) | 29/(3/3) | 0.98 (0.94–1.03) | 52/(2/3) |
| Breast | 0.99 (0.90–1.08) | 1.00 (0.98–1.02) | 48/(3/9) | 1.01 (0.95–1.08) | 79/(0/8) | 0.98 (0.89–1.09) | 0.97 (0.94–1.00) | 26/(3/4) | 0.96 (0.89–1.03) | 68/(0/4) |
| Lung | 0.95 (0.69–1.31) | 1.04 (1.00–1.08) | 58/(0/5) | 1.05 (1.00–1.10)* | 77/(0/4) | 1.01 (0.64–1.58) | 1.05 (1.03–1.08) | 39(0/3) | 1.05 (1.00–1.11) | 83/(0/3) |
| Oesophagus | 0.73 (0.57–0.94) | 0.79 (0.60–1.04) | –/(0/1) | 0.81 (0.62–1.05) | –/(0/1) | 0.73 (0.57–0.94) | 0.79 (0.60–1.04) | –/(0/1) | 0.81 (0.62–1.05) | –/(0/1) |
| Stomach | 0.84 (0.52–1.37) | 1.11 (0.88–1.40) | –/(0/1) | 1.21 (0.97–1.50) | –/(0/1) | 0.84 (0.52–1.37) | 1.11 (0.88–1.40) | –/(0/1) | 1.21 (0.97–1.50) | –/(0/1) |
| Colon/rectal | 0.98 (0.82–1.16) | 0.97 (0.85–1.12) | 91/(0/6) | 1.03 (0.92–1.15) | 90/(0/5) | 0.97 (0.77–1.22) | 0.87 (0.83–0.91)* | 0/(0/3) | 0.94 (0.90–0.98)* | 0/(0/3) |
| Kidney | 1.50 (1.01–2.23)* | 1.22 (1.15–1.30)* | 28/(1/7) | 1.58 (1.37–1.81)* | 60/(0/6) | 0.75 (0.34–1.66) | 1.20 (1.13–1.28)* | 43/(1/2) | 1.77 (1.49–2.11)* | 82/(0/2) |
| Prostate | 1.00 (0.87–1.16) | 1.09 (0.98–1.21) | 88/(1/7) | 1.11 (1.01–1.21)* | 86/(0/6) | 0.88 (0.80–0.97) | 0.95 (0.92–0.99)* | 18/(1/3) | 0.97 (0.94–1.00) | 0/(0/3) |
| Female reproductive | 1.04 (0.76–1.41) | 1.01 (0.63–1.62) | 77/(0/2) | 0.93 (0.74–1.17) | 0/(0/1) | 1.0 (0.7–1.4) | – | – | – | – |
| Melanoma | 1.09 (1.00–1.19)* | 1.08 (1.01–1.15)* | 0/(2/3) | 1.36 (0.97–1.91) | 94/(1/2) | 1.1 (1.0–1.2) | 1.09 (1.00–1,19) | 37/(2/0) | 2.36 (2.02–2.75)* | 0/(1/0) |
| Hematologic | 0.85 (0.60–1.20) | 1.03 (0.94–1.13) | 0/(0/2) | 1.10 (1.01–1.20)* | 31/(0/1) | 0.88 (0.61–1.29) | 1.10 (1.01–1.20)* | 31/(0/1) | 1.10 (1.01–1.20)* | 31/(0/1) |
| Pancreas | – | 1.10 (0.60–2.02) | –/(0/1) | – | – | – | – | – | – | – |
| Liver | – | 1.20 (0.93–1.56) | 0/(0/2) | 1.25 (0.98–1.58) | 0/(0/2) | – | 1.13 (0.79-1.62) | –/(0/1) | 1.30 (0.95–1.78) | –/(0/1) |
| Smoking–related cancer** |
1.04 (0.77–1.40) | 1.07 (1.02–1.13)* | 63(1/11) | 1.19 (1.07–1.32)* | 95/(0/10) | 0.86 (0.64–1.16) | 1.07 (1.02–1.13)* | 66/(1/5) | 1.15 (0.99–1.33) | 97/(0/5) |
Statistically significant (p < 0.05), **Includes oesophageal, lung, and kidney cancers.
CI, confidence interval; RR, relative risk; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Risk of bias in individual studies
Supplementary Tables 3 and 4 show detailed assessments of risk of bias in individual studies. Of the 17 cohort studies, 5 were rated ‘good’, 1 was rated ‘fair’, and 11 were rated ‘poor’. Of the 16 case–control studies, 6 were rated ‘good’, 7 were ‘fair’, and 3 were rated ‘poor’ (Supplementary table 5).
Publication bias across studies
Funnel plots were used to represent the results of cancer risk (Supplementary Figures 1–3). No significant publication bias was observed based on Egger’s regression test (p > 0.05).
Sensitivity analysis
The overall sensitivity analysis results for high quality studies were presented in Table 3. There was a considerable change in the confidence interval as the number of included studies decreased.
Table 3.
Sensitivity analyses for high-quality studies.
| Sensitivity analyses | HR (95% CI) | I² (%) /(cohort/case–control) |
RR (All) | I² (%) /(cohort/case–control) |
RR (cohort and nested case-control) |
I² (%) /(cohort/case–control) |
|---|---|---|---|---|---|---|
| Adjusted (all) | 0.83 (0.75–0.93)* | 94/(17/0) | 1.02 (0.99–1.05) | 72/(10/31) | 0.99 (0.96–1.03) | 66/(10/12) |
| Adjusted (high-quality studies) |
0.86 (0.66-1.11) | 97/(5/0) | 1.05 (0.99-1.12) | 74/(2/14) | 0.98 (0.88-1.10) | 68/(2/7) |
| Crude (all) | 0.87 (0.74–1.01) | 96/(7/0) | 1.11 (1.05–1.17)* | 93/(2/32) | 1.12 (1.03-1.21)* | 96/(2/14) |
| Crude (high-quality studies) |
0.78 (0.68-0.88)* | 85/(4/0) | 1.12 (1.02-1.23)* | 93/(1/14) | 1.12 (0.90-1.30) | 94/(1/7) |
Statistically significant (p < 0.05).
CI, confidence interval; HR, Hazard ratio; RR, relative risk.
Discussion
The literature review and updated meta-analysis of observational studies showed an inverse correlation between HR and RR. Similar to previous meta-analyses, RRs associated with kidney cancer and melanoma were significantly higher than 1. HRs indicated that ACEIs/ARBs are associated with lower risk of lung and breast cancers.
We computed HRs and RRs separately. Effect measures, including ORs, RRs, and HRs, are often applied interchangeably in meta-analyses and are statistically integrated for diseases that are relatively rare in the general population.16 RR represents the fold increase in risk that an individual in one category is at compared with an individual in another category.17 HR represents the rate at which a disease occurs in one group relative to another group over a specific period and is also referred to as a form of RR independent of the study period.16 However, HR and RR differ slightly and which is computed depends on the study population and design. We postulated that analysing HRs and RR separately could lead to different interpretations.
Previous RCT meta-analyses found that ARBs or one ARB combined with an ACEI increased the risk of cancer.1,10 This may be because ACEIs can inhibit the RAS and reduce the risk of cancer, whereas ARBs can stimulate angiogenesis by inducing excessive angiotensin II type 2 receptor activity by inhibiting angiotensin II type 1 receptor.1,18 However, there is no clinical evidence in support of this hypothesis.18 In addition, following up the subjects for less than 3 years did not clearly illustrate the causal relationship between these drugs and cancer.12 A previous meta-analysis of observational studies of cohorts followed up for more than 5 years found a decreased risk of cancer, contradicting the RCT results.12 In this case, the level of evidence may be higher due to the relatively longer follow-up period and the suitability of biological mechanisms.
The RR results obtained here were similar to those obtained in a previous meta-analysis. However, the overall values in this study converged to 1 compared with the values in the previous meta-analysis, indicating a decreased association. Kidney cancer and melanoma were also significantly increased. Untreated hypertension may have a greater effect on kidney cancer than ACEIs or ARBs, while melanoma may be affected by the photosensitising effect of ACEIs and ARBs.19,20
HRs computed for cohort studies indicated an overall reduction in the risk of lung and breast cancer. ARB has been reported to be associated with inhibition of AT1R, which activates cell migration, cell proliferation, inflammation, and angiogenesis in lung cancer.21 On the other hand, ACEIs are known to induce lung accumulation of bradykinin and substance P, which promote tumour proliferation and angiogenesis).22 Breast cancer is necessary to clarify cancer-specific biological plausibility. The reported minimum induction period for most solid cancers is 10 years.23 Therefore, large-scale studies of cohorts followed up for decades are needed to clarify association
Our study has several limitations. First, there is a possibility of confounding by indication bias as a specific and prominent source of the disparate results between cohort and case-control studies. Second, interpreting adjusted values only is not sufficient because these tend to vary between studies. Third, some studies analysed patients from the same registries, potentially resulting in selection bias in the study population. Finally, publication bias could not be evaluated precisely because the number of samples used to compute the adjusted values was unknown.24 Despite these limitations, our study has several strengths. First, the data sources are reliable and included large numbers of samples because many studies used patients in national registries. Second, we included more studies than previously analysed, thereby improving the reliability of our findings. Third, we separated effect measures to provide diverse interpretations. Finally, we included a long-term follow-up study to provide additional evidence on the strong correlation between these drugs and cancer.
Conclusion
Our results show some protective effects through the hazard ratio and some detrimental effects through the relative risk. Evidence supporting the risk of developing cancer is insufficient to prevent prescribing ACEIs or ARBs for patients with hypertension. However, the causal relationship between cancer and ACEIs or ARBs needs to be verified by following a defined cohort for 10 or more years.
Supplemental Material
Supplemental material, sj-docx-1-taw-10.1177_20420986221129335 for Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and cancer risk: an updated meta-analysis of observational studies by Kayeong Shin, Jiwoo Yang, Yeuni Yu, Eunjeong Son, Kihun Kim and Yun Hak Kim in Therapeutic Advances in Drug Safety
Acknowledgments
None.
Footnotes
ORCID iD: Yun Hak Kim  https://orcid.org/0000-0002-9796-8266
https://orcid.org/0000-0002-9796-8266
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kayeong Shin, Department of Medicine, School of Medicine, Pusan National University, Busan, Republic of Korea.
Jiwoo Yang, Department of Medicine, School of Medicine, Pusan National University, Busan, Republic of Korea.
Yeuni Yu, Biomedical Research Institute, Pusan National University Hospital, Busan, Republic of Korea.
Eunjeong Son, Division of Critical Care Medicine, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea.
Kihun Kim, Department of Occupational and Environmental Medicine, Kosin University Gospel Hospital, Busan 49267, Republic of Korea.
Yun Hak Kim, Department of Anatomy and Department of Biomedical Informatics, School of Medicine, Pusan National University, Yangsan 50612, Republic of Korea.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Kayeong Shin: Data curation; Formal analysis; Investigation; Visualisation; Writing – original draft.
Jiwoo Yang: Data curation; Formal analysis; Investigation; Visualisation; Writing – original draft.
Yeuni Yu: Methodology; Software; Validation.
Eunjeong Son: Data curation; Formal analysis; Investigation; Validation; Visualisation.
Kihun Kim: Data curation; Formal analysis; Methodology; Software; Validation; Visualisation; Writing – review & editing.
Yun Hak Kim: Conceptualisation; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Research Centre program [grant number NRF-2018R1A5A2023879], the Basic Science Research Programme [grant number NRF-2020R1C1C1003741] through a National Research Foundation of Korea grant funded by the Korean government.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Data sharing is not applicable to this article as no datasets were generated or analysed in the current study.
References
- 1. Sipahi I, Debanne SM, Rowland DY, et al. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol 2010; 11: 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herman LL, Padala SA, Annamaraju P, et al. Angiotensin Converting Enzyme Inhibitors (Acei). Treasure Island, FL: StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 3. Souza VBD, Silva EN, Ribeiro ML, et al. Hypertension in patients with cancer. Arquivos Brasileiros de Cardiologia 2015; 104: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallo G, Tocci G, Fogacci F, et al. Blockade of the neurohormonal systems in heart failure with preserved ejection fraction: a contemporary meta-analysis. Int J Cardiol 2020; 316: 172–179. [DOI] [PubMed] [Google Scholar]
- 5. Fountain JH, Lappin SL. Physiology, renin angiotensin system. Treasure Island, FL: StatPearls Publishing, 2017. [PubMed] [Google Scholar]
- 6. Rosenthal T, Gavras I. Renin–angiotensin inhibition in combating malignancy: a review. Anticancer Res 2019; 39: 4597–4602. [DOI] [PubMed] [Google Scholar]
- 7. Wegman-Ostrosky T, Soto-Reyes E, Vidal-Millán S, et al. The renin-angiotensin system meets the hallmarks of cancer. J Renin Angiotensin Aldosterone Syst 2015; 16: 227–233. [DOI] [PubMed] [Google Scholar]
- 8. Yoshiji H, Kuriyama S, Noguchi R, et al. Angiotensin-I converting enzyme inhibitors as potential anti-angiogenic agents for cancer therapy. Curr Cancer Drug Targets 2004; 4: 555–567. [DOI] [PubMed] [Google Scholar]
- 9. Grossman E, Messerli FH, Goldbourt U. Antihypertensive therapy and the risk of malignancies. Eur Heart J 2001; 22: 1343–1352. [DOI] [PubMed] [Google Scholar]
- 10. Bangalore S, Kumar S, Kjeldsen SE, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324 168 participants from randomised trials. Lancet Oncol 2011; 12: 65–82. [DOI] [PubMed] [Google Scholar]
- 11. Lindholm LH, Carlberg B. Blood-pressure drugs and cancer: much ado about nothing? Lancet Oncol 2011; 1212: 66–88. [DOI] [PubMed] [Google Scholar]
- 12. Yoon C, Yang H-S, Jeon I, et al. Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: a meta-analysis of observational studies. Cmaj 2011; 183: E1073–E1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Copland E, Canoy D, Nazarzadeh M, et al. Antihypertensive treatment and risk of cancer: an individual participant data meta-analysis. Lancet Oncol 2021; 22: 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MJ, Mckenzie JE, Bossuyt PM, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. A’Court C, Stevens R, Heneghan C. Against all odds? Improving the understanding of risk reporting. Br J Gen Pract 2012; 62: e220–e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George A, Stead TS, Ganti L. What’s the risk: differentiating risk ratios, odds ratios, and hazard ratios? Cureus 2020; 12: e10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen J, Huang YM, Wang M, et al. Renin–angiotensin system blockade for the risk of cancer and death. J Renin Angiotensin Aldosterone Syst 2016; 17: 1470320316656679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nardone B, Majewski S, Kim AS, et al. Melanoma and non-melanoma skin cancer associated with angiotensin-converting-enzyme inhibitors, angiotensin-receptor blockers and thiazides: a matched cohort study. Drug Safe 2016; 40: 249–255. [DOI] [PubMed] [Google Scholar]
- 20. Seretis A, Cividini S, Markozannes G, et al. Association between blood pressure and risk of cancer development: a systematic review and meta-analysis of observational studies. Sci Rep 2019; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rachow T, Schiffl H, Lang SM. Risk of lung cancer and renin–angiotensin blockade: a concise review. J Cancer Res Clin Oncol 2021; 147: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hicks BM, Filion KB, Yin H, et al. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018; 363; k4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fraser DK. Latency period of radiation-induced cancer. CMAJ 2011; 183: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin ZC, Zhou XH, He J. Statistical methods for dealing with publication bias in meta-analysis. Stat Med 2015; 34: 343–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taw-10.1177_20420986221129335 for Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and cancer risk: an updated meta-analysis of observational studies by Kayeong Shin, Jiwoo Yang, Yeuni Yu, Eunjeong Son, Kihun Kim and Yun Hak Kim in Therapeutic Advances in Drug Safety