Abstract
Background:
The question of which parameters may be informative on venetoclax outcome in chronic lymphocytic leukemia (CLL) is still unclear. Furthermore, the choice to treat with venetoclax can be challenging in patients with baseline characteristics or comorbidities that may potentially favor some specific adverse events.
Objectives:
This study was aimed to evaluate whether age, fitness status, patients’/disease characteristics, or concomitant medications may predict outcomes in CLL patients receiving venetoclax.
Design:
Retrospective observational study.
Methods:
Impact of age, presence of Cumulative Illness Rating Scale (CIRS) >6 or severe organ impairment (CIRS3+), Eastern Cooperative Oncology Group–Performance Status (ECOG-PS), renal function, and concomitant medications were retrospectively analyzed on treatment management (definitive discontinuation due to toxicity, discontinuation due to toxicity, Tox-DTD; permanent dose reduction, PDR) and survival [progression free survival (PFS), event free survival (EFS), overall survival (OS)] in unselected patients receiving venetoclax monotherapy in common practice.
Results:
A total of 221 relapsed/refractory patients were included. Tox-DTD and PDR were reported in 5.9% and 21.7%, respectively, and were not influenced by any fitness parameter, age, number or type of concomitant medication, baseline neutropenia, or impaired renal function. None of these factors were associated with tumor lysis syndrome (TLS) development. Age and coexisting conditions had no influence on PFS and EFS. At univariate analysis, OS was significantly shorter only in patients with ECOG-PS >1 (p < 0.0001) and elderly (⩾65 years) with CIRS >6 (p = 0.014) or CIRS3+ (p = 0.031). ECOG-PS >1 retained an independent role only for EFS and OS. While Tox-DTD affected all survival outcomes, no differences in PFS were reported among patients permanently reducing dose or interrupting venetoclax for > 7 days.
Conclusion:
Clinical outcome with venetoclax is not influenced by comorbidities, patients’ clinical characteristics, or concomitant medications. Differently from other targeted therapies, this demonstrates that, except ECOG-PS, none of the parameters generally considered for treatment choice, including baseline neutropenia or impaired renal function, should rule the decision process with this agent. Anyway, if clinically needed, a correct drug management does not compromise treatment efficacy and may avoid toxicity-driven discontinuations.
Plain Language Summary
Chapter 1: Why was this study done?
Chapter 2: Which are the main findings of the study?
Chapter 3: How these findings may impact on clinical practice?
Coexisting conditions and concomitant medications do not affect venetoclax management and survival in chronic lymphocytic leukemia
• The question of which parameters may be informative on venetoclax outcome in chronic lymphocytic leukemia is still unclear. Furthermore, the choice to treat with venetoclax can be challenging in patients with baseline characteristics or comorbidities that may potentially favor some specific adverse events (e.g. compromised renal function or baseline neutropenia).
• In our large series of patients treated outside of clinical trials, we demonstrated that neither age, fitness, comorbidities nor concomitant medications impact on venetoclax management and survival. Importantly, patients presenting with baseline neutropenia or impaired renal function did not have a higher rate of dose reductions or toxicity-driven discontinuations, thus further underlining that venetoclax may be safely administered even in those categories with no preclusions.
• Differently from other targeted agents, our data demonstrate that none of the baseline factors commonly considered in treatment decision process retains a role with venetoclax. Finally, permanent dose reductions and temporary interruptions did not adversely impact PFS suggesting that, if clinically needed, a correct drug management should be adopted with no risk of compromising venetoclax efficacy.
Keywords: CIRS, CLL, comorbidities, discontinuations, ECOG, fitness, reduction, targeted therapies, venetoclax
Introduction
The introduction of novel agents, B-cell receptor (BCR) inhibitors and B-cell lymphoma 2 (Bcl-2) antagonists, has radically changed chronic lymphocytic leukemia (CLL) treatment scenario.1 These drugs appear to be generally well tolerated, offering an attractive treatment approach even in the elderly population.2–5 The role of age, Eastern Cooperative Oncology Group–Performance Status (ECOG-PS), and comorbidities has been well established with chemotherapy or chemoimmunotherapy;6–8 nevertheless, it is not clear which parameters may have a predictive value with those target agents in the common clinical practice. Few studies have addressed the role of age and fitness status on BCR inhibitors management and outcome.9–11 In two retrospective studies of ibrutinib-treated patients outside clinical trials, Cumulative Illness Rating Scale (CIRS) and ECOG-PS emerged as reliable tools in predicting treatment tolerance and survival.9,11 Among other parameters considered at baseline, in the study published by Tedeschi et al.,11 only neutropenia independently influenced all the survival outcomes.
As regards venetoclax, most of the venetoclax analyses so far have focused on the role of age.12–14 Similar quality and duration of responses in elderly compared with younger patients were observed both in trials and in common practice with venetoclax.13,14 Furthermore, age was not detrimental to treatment management and tolerability.12,14 Considering that every target agent has a unique and specific toxicity profile, renal function has a crucial role in patients treated with the Bcl-2 antagonist. A retrospective analysis by Roeker et al.15 showed that a creatinine clearance (CrCl) <80 ml/min was associated with a higher risk of tumor lysis syndrome (TLS).
In most of venetoclax clinical trials, CrCl <50 ml/min was considered as an exclusion criterion12,16,17 while in the CLL14 study, that led to venetoclax obinutuzumab approval in unfit untreated CLL patients, only patients with a CrCl <30 ml/min were excluded. Furthermore, in the same trial, CIRS was considered as a key inclusion criterion.5
To the best of our knowledge, no studies so far have been specifically addressed to determine the role of CIRS or other fitness parameters on venetoclax management and outcomes. Moreover, the impact of concomitant medications, including CYP3A4 inhibitors, that may alter venetoclax plasma levels, has never been analyzed.
In the present retrospective study, we evaluated the impact of age, ECOG-PS, comorbidities, patients’ and disease characteristics at baseline as well as concomitant medications, in a large population receiving venetoclax monotherapy outside of clinical trials.
Patients and methods
The population of this study includes unselected consecutive relapsed/refractory (R/R) patients treated with venetoclax monotherapy outside of clinical trials in 16 Italian centers from December 2016 to August 2021. Venetoclax was given on label, and irrespective of this analysis, all patients signed an informed consent for treatment administration and data collection. All patients’ details have been deidentified and considering the retrospective observational nature of the study, approval exemption was given. All patients receiving at least one dose of venetoclax treatment were considered. To assess whether any clinical baseline condition may predict venetoclax tolerance and influence drug management, thus avoiding both the bias of a co-administered anticancer drug and fixed duration schedule, we chose to analyze patients treated with continuous monotherapy. Medical records were reviewed to determine patients’ characteristics at the time of venetoclax initiation including age, ECOG-PS, CrCl calculated according to the Cockcroft–Gault equation, concomitant nephropathy (intended as disease or damage of the kidney, which can eventually decline in kidney failure),18 presence of grade 3–4 neutropenia, number of concomitant medications, previous lines of therapy, previous ibrutinib administration, Rai stage, ImmunoGlobuline Heavy Chain Variable region (IGHV) mutational status, del(11q) and del(17p) by fluorescent in situ high hybridization, and TP53mut by Sanger Sequencing. Biological tests were locally performed. As a univocal definition of polypharmacy does not exist, polypharmacy was numerically defined as the concomitant use of >3 prescribed drugs assumed on regular basis.19 Short-term course reliever drugs were excluded. Furthermore, comorbidities were evaluated at the time of venetoclax initiation, and the Charlson Comorbidity Index (CCI) and CIRS score were calculated.20,21 As performed for other studies, medical conditions that were deemed to be complications of CLL (e.g. anemia, thrombocytopenia, and splenomegaly) were not included as part of the total CIRS score.8,9,11 According to previous CLL studies, patients were considered as having a high comorbidity burden if the total CIRS score was greater than 6 (CIRS >6). Patients were also assessed for the presence of CIRS3+ defined as a severe impairment (score 3 or 4) in any single organ system.22
Patients were stratified for age (<65 years versus ⩾65 years), ECOG-PS (0–1 versus >1), CCI (0–2 versus >2), CrCl (<30 versus 30–49 versus ⩾50), prior lines of therapy (1 versus >1), CIRS (⩽6 versus >6), and CIRS3+ (present versus absent). We analyzed the impact of age and patients’ fitness (that includes ECOG-PS, CIRS, CIRS3+, CCI) on treatment management [defined as definitive treatment discontinuation due to toxicity (Tox-DTD) or permanent dose reduction (PDR)], on progression free survival (PFS, defined as time from treatment initiation to disease progression or death), event free survival (EFS, defined as time from treatment initiation to treatment discontinuation, disease progression, or death), and overall survival (OS, defined as time from treatment initiation to death). Survival functions for the time-to-event variables were estimated by the Kaplan–Meier method and the related strata compared using the log-rank test. Venetoclax was administered with weekly ramp-up schedule over 5 weeks to the recommended daily dose of 400 mg according to prescribing information for CLL.23
To investigate the impact of selected patients’ characteristics (age, patients’ fitness, concomitant medications >3, CYP3A4 inhibitors, nephropathy CrCl, baseline neutropenia) before starting venetoclax therapy and of disease characteristics [del(17p) and TP53mut, del(11q), IGHV unmutated status, previous lines of therapy, previous ibrutinib treatment] on the time to Tox-DTD, PDR, PFS, EFS, and OS, univariate and multivariate Cox regression models were fitted. The multivariate models were obtained through a model building strategy that consisted of a number of steps. First, covariates that were significant in the univariate models at α = 0.25 were selected. After this step, models were re-assessed in terms of standard error of covariates to identify potential correlations and influence of collinearity on such covariates and on the dependent variable. If correlation was high, only the most clinically meaningful covariate was retained. In addition, at this stage, if covariates were not significant at α = 0.05, the fit of the model was assessed with and without nonsignificant covariates through model performance parameters such as R2 and Akaike’s information criterion (AIC). If the model fits the data better without them and there was no clinical justification to keep such covariates, they were removed from the model. Clinically relevant interaction terms were created and kept in the model if significant at α = 0.10. Following these steps, final models were then used to obtain hazard ratios and their 95% confidence intervals for each outcome. Statistical analysis was performed using the SAS (Statistical Analysis System) version 9.4 (SAS Institute, Cary, NC, USA).
The reporting of this study conforms to the STROBE statement;24 STROBE statement checklist is available at Supplemental Appendix 1. The data generated in this study are available upon request from the corresponding author.
Results
A total of 221 R/R patients treated with venetoclax monotherapy outside of clinical trials were analyzed. Median prior lines of therapy were 2 (range 1–9). In Table 1, patients’ and disease characteristics are summarized. Median age was 70 years (range 27–81 years), 63.8% of patients being ⩾65 years. Overall, 16.7% presented an ECOG-PS >1. Median CIRS score for the whole population was 5 (range 0–19) with a CIRS >6 recorded in 37.6% of patients. In 25.8% of patients, a severe impairment of a single organ system was observed (CIRS3+). 19.9% of cases concomitantly presented CIRS >6 and CIRS3+. When patients were stratified according to CrCl, 3.2% had a value <30 ml/min, 19.5% between 30 and 49 ml/min, and 77.4% ⩾50 ml/min. A concomitant diagnosis of nephropathy was present in 7.2% of cases. 16.7% started treatment with grade 3–4 baseline neutropenia. About half of patients (53.4%) were in treatment with 3 or more concomitant medications with a median number of 4 (range 0–13). In 22.6%, concomitant treatments were represented by CYP3A4 inhibitors. As regards disease characteristics, most patients (67%) had an IGHV unmutated status while 43% presented with del(17p) and TP53 mutation. All patients performed venetoclax ramp-up according to venetoclax prescribing information for CLL.23 After a median follow-up of 17.3 months (range 0.8–58.3), 136 (61.5%) are continuing treatment: 88/221 (39.8%) at the dose of 400 mg daily and 48/221 (21.7%) at a permanent lower dose (300 mg/daily in 21 patients; 200 mg/daily in 25, and 100 mg/daily in 2 patients). The main reason for PDR was recurrent drug-induced neutropenia (60.4%) followed by drug-to-drug interference (10.4%) and infections (8.3%). Venetoclax-induced neutropenia leading to PDR was recorded in 15 among the 37 patients with baseline neutropenia.
Table 1.
Patients’ characteristics.
| Patients’ characteristics total (N = 21) | Value N (%) |
|---|---|
| Median age, years (range) <65 years/⩾65 years |
70.0 (27–81) 80 (36.1)/141 (63.8) |
| Sex: male/female | 145 (65.6)/76 (34.4) |
| ECOG-PS 0–1/>1 |
184 (83.3)/37 (16.7) |
| CIRSa median (range) CIRSa ⩽6/CIRSa >6 CIRSa 3+ CIRS >6 and CIRS3+ |
5 (0–19) 138 (62.4)/83 (37.6) 57 (25.8) 44 (19.9) |
| CCI median (range) CCI <2/CCI ⩾2 |
5 (0–24) 47 (21.3)/174 (78.7) |
| CrCl (ml/min) ⩾50/30–49/<30 |
169 (77.4)/42 (19.5)/7 (3.2) |
| Nephropathy | 27 (7.2) |
| Grade 3–4 neutropenia | 37 (16.7) |
| Median N concomitant medications (range) Polypharmacyb CYP3A4 inhibitors |
4 (0–13) 118 (53.4) 50 (22.6) |
| Rai stage 0–2 3–4 |
106 (48) 115 (52) |
| Prior Tx median (range) 1–2 ⩾3 |
2 (1–9) 126 (57) 95 (43) |
| IGHV unmutated del(17p) and TP53mut del(11q) High riskc |
148 (67.0) 95 (43.0) 56 (25.3) 184 (83.3) |
CCI, Charlson Comorbidity Index; CIRS, Cumulative Illness Rating Scale; CrCl, creatinine clearance; ECOG-PS, Eastern Cooperative Oncology Group–Performance Status; Tx, therapy.
Medical conditions that deemed to be complications of CLL not included as part of the total CIRS score.
Polypharmacy defined as >3 concomitant medications.
High risk defined as: del(17p) or TP53mut or del(11q) or unmutated IGHV.
Venetoclax was definitively discontinued in 85 (38.5%) due to: CLL progression and Richter’s transformation (17.2% and 9%, respectively), transplant procedure (3.6%), toxicity (5.9%), secondary malignancies (1.4%), and other reasons (i.e. patient or physician decision, 1.4%). Median time to Tox-DTD was 2.3 months (range 0.1–12.2 months), infections (53.8%), and cytopenia (30.8%) being the major causes. Pneumonia was the most common infection reported.
In the whole population, a transient dose reduction (for at least one transient episode1 day) of dose reduction was necessary in 48 cases (21.7%); in 34 of them, venetoclax dose reduction was permanently maintained, while venetoclax was maintained permanently at an inferior dose in 34 of them.
Treatment was temporarily interrupted for at least 1 day in 69 patients (31.2%), being ⩾7 days in 46 cases (20.8%). Infections, neutropenia, and TLS development were the most frequent events leading to temporary discontinuation (42%, 17.4%, and 11.6% of 69 patients temporary interrupting the drug). Venetoclax dosing and discontinuations are shown in Table 2.
Table 2.
Venetoclax dosing and discontinuations.
| Rate, % (proportion) | |
|---|---|
| Achieved 400 mg daily | 100% (221/221) |
| Maintained 400 mg daily | 39.8% (88/221) |
| Permanently reduced venetoclax dose due to toxicity | 21.7% (48/221) |
| Interrupted venetoclax for ⩾7 days | 20.8% (46/221) |
| Definitively discontinued venetoclax due to toxicity | 5.9% (13/221) |
TLS, was observed overall in 27 patients (12.2%), being only laboratory in the majority of them (88.9%) and did not lead to any permanent discontinuation.
None of the parameters considered among age, ECOG-PS, CIRS >6, CIRS3+, CCI >2, polypharmacy, CrCl <50, and nephropathy showed to be associated with a higher risk of TLS. Neither age nor patients’ fitness had an influence on PDR and Tox-DTD, even when patients were stratified according to age (<65 versus ⩾65 years). None of the variables considered at baseline, including polypharmacy, had an impact on drug management. Only trend toward increased PDR was observed with baseline a neutropenia (p = 0.0531).
Patients temporarily interrupting venetoclax for ⩾7 days did not show a higher risk of PDR and Tox-DTD. TLS development did not translate in a permanent venetoclax discontinuation or dose reduction.
Number of previous lines (1 versus >1) and prior ibrutinib treatment were not associated with PDR while both significantly correlated with Tox-DTD (p = 0.0427 and p = 0.0235, respectively).
The median of PFS, EFS, and OS for the whole population was 38.6, 32.5, and not reached, respectively. Presence of del(17p) and TP53 mutations significantly influenced PFS, EFS, and OS, while no differences were observed when patients were stratified according to IGHV mutational status and 11q deletion. Patients who had received more than one line prior to venetoclax and those who received previous ibrutinib showed significantly inferior survival outcomes.
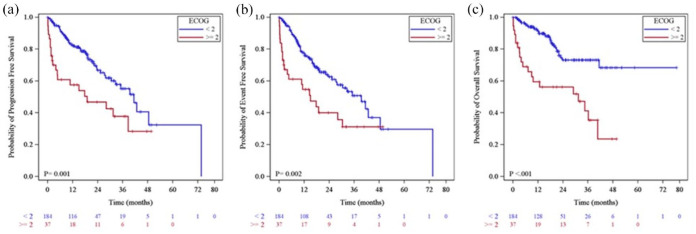
ECOG-PS >1 was the only fitness parameter influencing all three survival outcomes (p = 0.0017, p = 0.0021 and p < 0.0001 for PFS, EFS, and OS, respectively; Figure 1).
Figure 1.
(a) Progression free survival, (b) event free survival, and (c) overall survival by ECOG-PS.
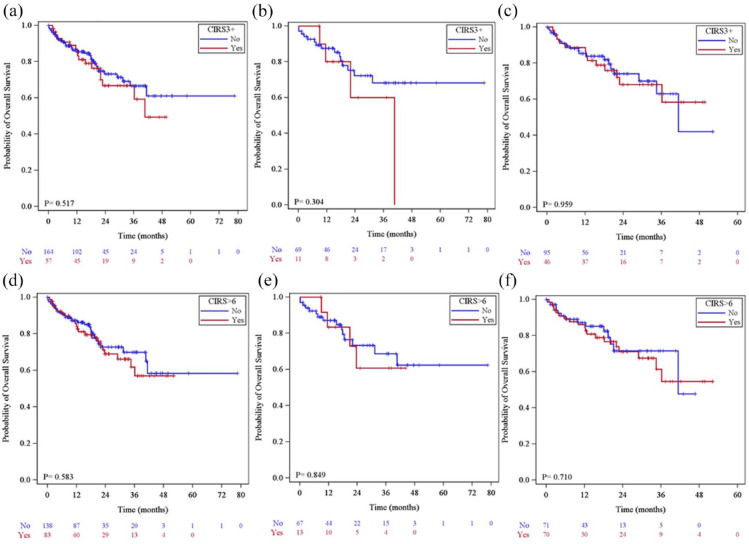
Although CIRS > 6 and CIRS3+ in the whole population and in younger patients (<65 years) had no impact on PFS, EFS, and OS, only in the elderly both were detrimental on OS (Figure 2).
Figure 2.
(a) CIRS > 6 and CIRS3+ overall and by age: overall survival by CIRS3+ in the whole population, (b) in patients <65 years, (c) in patients ⩾65 years, (d) overall survival by CIRS >6 in the whole population, (e) in patients <65 years, (f) in patients ⩾65 years.
Age, baseline neutropenia, and concomitant medications were not associated with inferior prognosis. While PDR did not affect any survival parameters, Tox-DTD led to significantly worse PFS, EFS, and OS (p < 0.0001). Furthermore, a cut-off of >7 days of interruption affected only OS.
The results from univariate analysis are shown in Table 3.
Table 3.
Univariate analysis for venetoclax.
| PFS | EFS | OS | PDR | Tox-DTD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | 0.96 (0.60–1.53) | 0.8534 | 0.79 (0.51–1.21) | 0.2769 | 1.12 (0.64–1.98) | 0.6942 | 1.03 (0.78–1.37) | 0.8263 | 1.12 (0.65–1.47) | 0.4340 |
| CIRS >6 | 1.02 (0.64–1.63) | 0.9272 | 0.83 (0.54–1.30) | 0.4193 | 1.17 (0.67–2.02) | 0.5837 | 0.89 (0.67–1.20) | 0.4459 | 1.01 (0.76–1.33) | 0.9554 |
| CIRS3+ | 1.22 (0.75–1.98) | 0.4312 | 1.28 (0.81–2.02) | 0.2860 | 1.21 (0.68–2.16) | 0.5178 | 0.83 (0.60–1.15) | 0.2607 | 0.90 (0.66–1.22) | 0.5016 |
| ECOG–PS | 2.26 (1.36–3.75) | 0.0017 | 2.15 (1.32–3.50) | 0.0021 | 3.53 (2.01–6.20) | <0.0001 | 0.88 (0.59–1.31) | 0.5221 | 0.82 (0.58–1.17) | 0.2751 |
| CCI | 1.34 (0.73–2.44) | 0.3408 | 1.15 (0.66–1.98) | 0.6246 | 2.90 (0.89–9.50) | 0.0784 | 0.76 (0.54–1.07) | 0.1140 | 0.97 (0.70–1.33) | 0.8307 |
| CrCl | 1.22 (0.72–2.06) | 0.4576 | 1.08 (0.65–1.80) | 0.7552 | 1.09 (0.58–2.05) | 0.7809 | 0.80 (0.57–1.12) | 0.1907 | 0.97 (0.71–1.34) | 0.8732 |
| Neutrop | 1.34 (0.75–2.41) | 0.3209 | 1.47 (0.85–2.55) | 0.1645 | 1.46 (0.75–2.85) | 0.2635 | 0.69 (0.47–1.01) | 0.0531 | 0.96 (0.67–1.36) | 0.8025 |
| Polyph | 1.09 (0.68–1.76) | 0.7290 | 0.99 (0.64–1.55) | 0.9771 | 1.34 (0.74–2.41) | 0.3329 | 0.91 (0.68–1.20) | 0.4954 | 1.06 (0.80–1.40) | 0.7042 |
| CYP3A4 | – | – | – | 0.83 (0.58–1.18) | 0.3119 | 0.90 (0.66–1.24) | 0.5267 | |||
| TLS | 1.07 (0.56–2.04) | 0.8365 | 0.95 (0.50–1.80) | 0.8839 | 1.37 (0.67–2.81) | 0.3952 | 0.94 (0.63–1.41) | 0.7584 | 0.96 (0.64–1.43) | 0.8271 |
| >1 prior Tx | 3.20 (1.53–6.68) | 0.0020 | 2.79 (1.48–5.26) | 0.0016 | 2.80 (1.19–6.59) | 0.0018 | 0.85 (0.63–1.15) | 0.3011 | 0.73 (0.47–0.99) | 0.0427 |
| Prior ibrutinib | 3.27 (1.76–6.07) | 0.0002 | 3.07 (0.76–5.37) | <0.0001 | 2.71 (1.32–5.57) | 0.0066 | 0.91 (0.69–1.21) | 0.5171 | 0.72 (0.55–0.96) | 0.0235 |
| 17p-/TP53 | 2.04 (1.28–3.25) | 0.0029 | 1.64 (1.07–2.53) | 0.0245 | 1.98 (1.13–3.47) | 0.0165 | – | – | ||
| 11q-/TP53 | 1.05 (0.63–1.75) | 0.8527 | 0.99 (0.61–1.61) | 0.9801 | 1.98 (0.66–2.16) | 0.5486 | – | – | ||
| UNM IGHV | 1.43 (0.77–2.66) | 0.2642 | 1.47 (0.81–2.68) | 0.2043 | 1.23 (0.60–2.55) | 0.5707 | – | – | ||
| PDR | 0.99 (0.57–1.73) | 0.9903 | 1.06 (0.64–1.78) | 0.8226 | 0.78 (0.39–1.54) | 0.4763 | – | – | ||
| Tox–DTD | 25.9 (11.2–59.8) | <0.0001 | 36.1 (15.7–83.2) | <0.0001 | 20.2 (7.28–56.1) | <0.0001 | – | – | ||
All p values are reported in Italics; statistically significant p values are reported in bold.
CCI, Charlson Comorbidity Index; CI, confidence interval; CIRS, Cumulative Illness Rating Scale; CrCl, creatinine clearance; ECOG-PS, Eastern Cooperative Oncology Group–Performance Status; Neutrop, baseline neutropenia; PDR, permanent dose reduction; Polyph, polypharmacy; TLS, tumor lysis syndrome; Tox-DTD, toxicity-related discontinuation; Tx, therapy; UNM, unmutated.
At multivariate analysis, neither fitness status nor all the other baseline variables considered retained an independent role on Tox-DTD and PDR (Table 4). Again, ECOG >1 confirmed to be the only parameter independently affecting EFS and OS (p = 0.0112 and p < 0.0001, respectively).
Table 4.
Cox proportional regression hazards model on PFS, EFS, OS, Tox-DTD, and PDR.
| PFS | EFS | OS | Tox-DTD | PDR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | 0.99 (0.55–1.81) | 0.984 | 0.79 (0.46–1.37) | 0.402 | 0.91 (0.45–1.83) | 0.783 | 1.08 (0.79–1.46) | 0.636 | 1.07 (0.78–1.48) | 0.664 |
| ECOG-PS | 1.67 (0.91–3.07) | 0.096 | 2.00 (1.17–3.42) | 0.011 | 3.41 (1.84–6.32) | <0.0001 | 0.85 (0.58–1.25) | 0.421 | 0.97 (0.64–1.48) | 0.897 |
| CIRS3+ | 1.07 (0.60–1.91) | 0.815 | 1.36 (0.78–2.37) | 0.282 | 1.12 (0.58–2.18) | 0.741 | 0.89 (0.62–1.29) | 0.550 | 0.95 (0.64–1.40) | 0.777 |
| CIRS >6 | 0.90 (0.49–1.68) | 0.742 | 0.67 (0.37–1.21) | 0.179 | 0.89 (0.43–1.84) | 0.751 | 1.03 (0.72–1.47) | 0.887 | 0.98 (0.67–1.44) | 0.925 |
| Polypharmacy | 1.01 (0.49–2.07) | 0.978 | 1.03 (0.56–1.93) | 0.915 | 1.18 (0.53–2.64) | 0.685 | 1.09 (0.78–1.54) | 0.615 | 1.08 (0.76–1.52) | 0.678 |
| Neutropenia | 0.93 (0.47–1.83) | 0.838 | 1.09 (0.59–2.03) | 0.779 | 0.92 (0.43–1.97) | 0.824 | 1.14 (0.77–1.69) | 0.508 | 0.74 (0.49–1.11) | 0.143 |
| CrCl | 1.16 (0.63–2.14) | 0.639 | 1.11 (0.63–1.98) | 0.717 | 1.14 (0.56–2.29) | 0.721 | 1.02 (0.71–1.46) | 0.933 | 0.88 (0.59–1.31) | 0.529 |
CI, confidence interval; CIRS, Cumulative Illness Rating Scale; CrCl, creatinine clearance; ECOG-PS, Eastern Cooperative Oncology Group–Performance Status; PDR, permanent dose reduction; Tox-DTD, toxicity-related discontinuation.
Discussion
Novel inhibitors in all phase III randomized trials demonstrated improved PFS compared with chemoimmunotherapy.3–5,16,25–27 In some of these studies, age and coexisting conditions were applied as treatment selection criteria to better define the advantage of the novel drugs in this setting of difficult-to-treat population. Considering that all these agents have a specific spectrum of adverse events, there is still an unmet need to identify patient-related characteristics that may predict treatment tolerance and survival.
Venetoclax has a relevant role in the treatment of CLL both as single agent and in combination with anti-CD20 monoclonal antibodies. Many data in the literature clearly demonstrate the activity, durable responses and good safety profile of the Bcl-2 antagonist.5,12,16 Based on its favorable tolerability, the CLL14 trial was specifically addressed to patients with a compromised renal function or comorbidities (CIRS >6). Despite the high median CIRS score of the population analyzed, this study confirmed a low rate of high-grade toxic effects.5
It is well known from ibrutinib experience that there are inconsistencies from clinical trials compared with common practice on data regarding discontinuations rate and toxicity.28–31 This is mainly due to differences in patients’ characteristics, control of adverse events, and physicians’ experience on drug management.
There are not many studies exploring venetoclax tolerability outside of clinical trials, with the majority focused mostly on the role of age and renal function in respect to TLS and dose adjustments.14,15,32–34 Furthermore, outcomes have been analyzed only in relation to age without considering the possible interference of other patient-related factors.
Although venetoclax in first line has recently been approved, cumulated clinical experience with the Bcl-2 antagonist mostly regards heavily pre-treated diseases.32–36 This is consistent with a population of patients of advanced age, often presenting with multiple comorbidities, assuming concomitant medications and deep immunosuppression. To assess whether any clinical baseline condition may predict venetoclax tolerance and influence drug management, thus avoiding both the bias of a co-administered anticancer drug and fixed duration schedule, we chose to analyze patients treated with continuous monotherapy.
This large series is characterized by elderly patients with a relevant proportion of high comorbidity burden with 25% presenting a severe organ impairment. Furthermore, about 20% would have been excluded from most clinical trials due to compromised renal function (CrCl <50 ml/min). Importantly, in contrast with that what previously reported with BCR inhibitors,10,28–30 the low rate of toxicity-driven discontinuations seen with venetoclax in our series is consistent with that observed in common practice (7.7–21%)14,15,33 and confirms the reproducibility of data from clinical trials (10–14.9%).12,37
Our analysis confirms14 that Tox-DTD, mostly due to cytopenia, is an early event with venetoclax (median time 2.3 months), not related to age. Considering that the upper range limit of time to Tox-DTD in our series is about 1 year, this latter data appears informative also in the setting of fixed venetoclax schedule. Moreover, similar results were reported by Mato et al.34
Furthermore, in our series, a higher risk of definitive discontinuation was observed in patients previously treated with ibrutinib. This observation may be related to a reduced marrow reserve due to a higher number of previous treatments, including multiple lines of chemoimmunotherapy.
Importantly, this is the first study showing that none of fitness parameters nor the other baseline characteristics we considered had a significant influence on toxicity-related definitive treatment discontinuation. In contrast, the higher rate of PDR observed in our series compared with clinical trials (13–17%)12,37 is possibly related to more stringent criteria on dose adjustments required by protocols and to the attitude of physicians in common practice to reduce treatment dose, rather than interrupting therapeutic continuity.
Importantly, age, ECOG-PS, and comorbidities did not result in a suboptimal venetoclax dosing, thus further confirming that the Bcl-2 antagonist may be safely administered even in frail patients. Permanent dose reduction did not increase the risk of progression or death at the Cox multivariate analysis. This highlights the importance of appropriate treatment managing to ensure optimal outcomes for patients receiving venetoclax.
As expected, Tox-DTD independently influenced survival outcomes and notably we found that prolonged dose interruption >7 days did not adversely impact PFS, as observed in both trials and common practice.15,37 Neutropenia and characteristics that may be associated with higher risk of TLS are generally considered as tools of special interest with venetoclax that may potentially preclude the eligibility to this agent.
Presence of baseline neutropenia emerged as an unexpected important factor influencing survival in our previous large experience of CLL patients treated with ibrutinib.11 Considering also that Bcl-2 is crucial for neutrophil precursor survival, we analyzed the role of neutropenia at baseline that is often seen in elderly heavily pre-treated patients. Notably, baseline neutropenia in this series did not translate into higher discontinuation or dose reduction rates, and in contrast with that previously observed with ibrutinib, it has no impact on patients’ survival.
It is noteworthy that, differently from data reported by Mato et al.37 in which more than 40% of patients temporary interrupted venetoclax at least once due to neutropenia, in our experience this adverse event led to transient interruption only in a minority, 12/221 patients (5.4%). This may be possibly explained by physicians’ attitude toward clinical management of hematological toxicities rather than treatment interruption.
As regards TLS development, it is difficult to compare our series with others. The insufficiency of data on TLS risk prior to venetoclax initiation is due to the lack of uniformity in physicians’ attitude to perform computed tomography (CT) scan. Nevertheless, while a higher rate of laboratory TLS was recorded,12,14,15,34,37 only few patients (1.8%) developed clinical TLS compared with the literature, thus probably mirroring a maturate experience on the adherence to TLS-prophylaxis safety measures.
Moreover, none of patients experiencing TLS definitively discontinued venetoclax, suggesting a greater physicians’ confidence with the possible management of the drug even in case of this Adverse event (AE).
Even with a lower cut-off of CrCl of 50 ml/min,15 in our population, we cannot confirm the role of impaired renal function as an additional risk factor in predicting TLS. Noteworthy, presence of coexisting conditions, and in particular nephropathy, did not show to increase TLS in our series.
Finally, survivals were comparable in patients presenting with concomitant nephropathy or impaired renal function, thus underlining that venetoclax treatment in these categories should not be a priori avoided.
It is well known that, as for Summary of Product Characteristics, concomitant use of a strong CYP3A inhibitor with venetoclax is contraindicated at therapy start and during ramp up phase, while at least 50% dose reduction is required on other treatment phases and with moderate inhibitors.23 In clinical practice, it is not easy to evaluate clinicians’ awareness on the correct pharmacovigilance, and there are not data reporting the role of these drugs in retrospective studies.
The role of the number of concomitant medications, in particular CYP3A inhibitors, on venetoclax treatment has never been analyzed. Importantly, these medications, and in general polypharmacy, did not influence rate of persistent dose reductions and Tox-DTD; furthermore, they did not affect any survival outcome.
Total CIRS score is generally considered an effective tool in categorizing patients’ fitness in CLL.2,5,8,38,39 Nevertheless, its value in predicting treatments outcome has not been consistently replicated across clinical trials.10,40 On the contrary, a clear interaction has recently been observed in retrospective analysis in unselected patients receiving ibrutinib.9,11
To our knowledge, this is the first study exploring the role of ECOG-PS and comorbidities, not only on treatment management but also on patients’ survival. Notably, both CIRS >6 and CIRS3+ were not detrimental to PFS and EFS.
Although not leading to treatment adjustments, both comorbidity burden and severity were predictors of inferior OS in our elderly population. We may speculate that in this setting, patient-related factors rather than disease control by venetoclax may project their adverse effects on survival.
As observed with ibrutinib,11 ECOG-PS, resulted to be the only parameter predicting inferior outcomes at univariate analysis. Compared with other fitness evaluating scales, ECOG-PS is immediate and easy-to-use and may represent an accurate tool to assess treatment feasibility and outcomes for patients with CLL receiving targeted agents.
The results of this study have clearly demonstrated that none of the patient-related factors analyzed should be considered informative for PFS with venetoclax. Further studies on common practice are needed to confirm this observation. As ECOG-PS in this series was the only variable independently influencing EFS and OS, this may imply that patients’ level of functioning and ability to care for daily activities, may be highly relevant and informative on survival.
This study is limited by its retrospective design and may include biases associated with possible missing data. As venetoclax represented the real last salvage therapy for many of these patients, however, we tend to exclude the possibility of selection bias and consider that all patients were treated with the Bcl-2 antagonist whatever the baseline clinical features. Furthermore, it is possible to speculate that the physicians’ attitude could have been more prone to manage adverse events maintaining treatment dose intensity.
Finally, as a growing number of treatment options can be offered earlier to CLL relapsing after immunochemotherapy, the optimal selection of individual treatment for each patient is challenging. Therefore, aside from biologic characteristics, it is crucial to identify patient-specific risk factors allowing a diversification and personalization of therapies. As a consequence, from our results, none of patients’ fitness features nor any specific comorbidity may preclude the choice to start a treatment with venetoclax.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207221127550 for Coexisting conditions and concomitant medications do not affect venetoclax management and survival in chronic lymphocytic leukemia by Anna Maria Frustaci, Giovanni Del Poeta, Andrea Visentin, Paolo Sportoletti, Alberto Fresa, Candida Vitale, Roberta Murru, Annalisa Chiarenza, Alessandro Sanna, Francesca Romana Mauro, Gianluigi Reda, Massimo Gentile, Marzia Varettoni, Claudia Baratè, Chiara Borella, Antonino Greco, Marina Deodato, Giulia Zamprogna, Roberta Laureana, Alessandra Cipiciani, Andrea Galitzia, Angelo Curto Pelle, Francesca Morelli, Lucio Malvisi, Marta Coscia, Luca Laurenti, Livio Trentin, Marco Montillo, Roberto Cairoli and Alessandra Tedeschi in Therapeutic Advances in Hematology
Acknowledgments
The authors thank Antonio Vita, Fondazione Malattie del Sangue, Rete Ematologica Lombarda for their support. The study is in memory of Giovanni Del Poeta who died during the writing of the manuscript.
Footnotes
ORCID iDs: Anna Maria Frustaci  https://orcid.org/0000-0003-2587-7901
https://orcid.org/0000-0003-2587-7901
Paolo Sportoletti  https://orcid.org/0000-0002-5630-9862
https://orcid.org/0000-0002-5630-9862
Alberto Fresa  https://orcid.org/0000-0002-8084-9009
https://orcid.org/0000-0002-8084-9009
Andrea Galitzia  https://orcid.org/0000-0002-9122-4258
https://orcid.org/0000-0002-9122-4258
Marco Montillo  https://orcid.org/0000-0003-0010-032X
https://orcid.org/0000-0003-0010-032X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Anna Maria Frustaci, Department of Hematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Piazza Ospedale Maggiore 3, Milano 20162, Italy.
Giovanni Del Poeta, Hematology, Department of Biomedicine and Prevention, University Tor Vergata, Roma, Italy.
Andrea Visentin, Hematology and Clinical Immunology Unit, Department of Medicine, University of Padova, Padova, Italy.
Paolo Sportoletti, Centro di Ricerca Emato-Oncologica (CREO), Department of Medicine and Surgery, Institute of Hematology, University of Perugia, Perugia, Italy.
Alberto Fresa, Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy.
Candida Vitale, Division of Hematology, A.O.U. Città della Salute e della Scienza di Torino and Department of Molecular Biotechnology and Health Sciences, Università di Torino, Torino, Italy.
Roberta Murru, Hematology and Stem Cell Transplantation Unit, Ospedale Oncologico A.Businco, ARNAS ‘G. Brotzu’, Cagliari, Italy.
Annalisa Chiarenza, Hematology Division, A.O.U. Policlinico ‘G. Rodolico-S.Marco’, Catania, Italy.
Alessandro Sanna, Hematology, Department of Oncology, AOU Careggi, Firenze, Italy.
Francesca Romana Mauro, Hematology, Department of Translational and Precision Medicine, ‘Sapienza’ University, Roma, Italy.
Gianluigi Reda, U.O.C. Ematologia, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico di Milano, Milano, Italy.
Massimo Gentile, Hematology Section, Cosenza Hospital, Cosenza, Italy.
Marzia Varettoni, Division of Hematology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Claudia Baratè, Department of Clinical and Experimental Medicine, Section of Hematology, University of Pisa, Pisa, Italy.
Chiara Borella, Department of Hematology, Ospedale San Gerardo, Monza, Italy.
Antonino Greco, Department of Hematology, Azienda Ospedaliera Giovanni Panìco, Tricase, Italy.
Marina Deodato, Department of Hematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy.
Giulia Zamprogna, Department of Hematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy.
Roberta Laureana, Hematology, Department of Biomedicine and Prevention, University Tor Vergata, Roma, Italy.
Alessandra Cipiciani, Centro di Ricerca Emato-Oncologica (CREO), Department of Medicine and Surgery, Institute of Hematology, University of Perugia, Perugia, Italy.
Andrea Galitzia, Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy.
Angelo Curto Pelle, Hematology Division, A.O.U. Policlinico ‘G. Rodolico-S.Marco’, University of Catania, Catania, Italy.
Francesca Morelli, Hematology, University of Florence, Firenze, Italy.
Lucio Malvisi, AliraHealth, Verona, Italy.
Marta Coscia, Division of Hematology, A.O.U. Città della Salute e della Scienza di Torino and Department of Molecular Biotechnology and Health Sciences, Università di Torino, Torino, Italy.
Luca Laurenti, Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy.
Livio Trentin, Hematology and Clinical Immunology Unit, Department of Medicine, University of Padova, Padova, Italy.
Marco Montillo, Department of Hematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy.
Roberto Cairoli, Department of Hematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy.
Alessandra Tedeschi, Department of Hematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy.
Declarations
Ethics approval and consent to participate: All patients signed an informed consent for treatment administration and data collection. All patients’ details have been deidentified and considering the retrospective observational nature of the study, approval exemption was given.
Consent for publication: Not applicable.
Author contributions: Anna Maria Frustaci: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Writing – review & editing.
Giovanni Del Poeta: Data curation; Validation.
Andrea Visentin: Data curation; Validation.
Paolo Sportoletti: Data curation; Validation.
Alberto Fresa: Data curation; Validation.
Candida Vitale: Data curation; Validation.
Roberta Murru: Data curation; Validation.
Annalisa Chiarenza: Data curation; Validation.
Alessandro Sanna: Data curation; Validation.
Francesca Romana Mauro: Data curation; Validation.
Gianluigi Reda: Data curation; Validation.
Massimo Gentile: Data curation; Validation.
Marzia Varettoni: Data curation; Validation.
Claudia Baratè: Data curation; Validation.
Chiara Borella: Data curation; Validation.
Antonino Greco: Data curation; Validation.
Marina Deodato: Data curation; Formal analysis; Validation; Writing – original draft.
Giulia Zamprogna: Data curation; Formal analysis; Validation; Writing – original draft.
Roberta Laureana: Data curation; Validation.
Alessandra Cipiciani: Data curation; Validation.
Andrea Galitzia: Data curation; Validation.
Angelo Curto Pelle: Data curation; Validation.
Francesca Morelli: Data curation; Validation.
Lucio Malvisi: Formal analysis; Methodology; Validation; Writing – review & editing.
Marta Coscia: Data curation; Validation.
Luca Laurenti: Data curation; Validation.
Livio Trentin: Data curation; Validation.
Marco Montillo: Data curation; Formal analysis; Supervision; Validation; Writing – review & editing.
Roberto Cairoli: Data curation; Validation.
Alessandra Tedeschi: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Supervision; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.M.F.: Janssen, AbbVie, BeiGene, AstraZeneca Honoraria and Advisory Board; M.C.: – Janssen: Membership on an entity’s Board of Directors or advisory committees, Research Funding and Speakers Bureau – AbbVie: Honoraria, Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau – Shire and Gilead: Honoraria, Membership on an entity’s Board of Directors or advisory committees – Karyopharm Therapeutics: Research Funding; P.S.: AbbVie and Janssen Honoraria; C.V.: Janssen Honoraria; R.M.: AbbVie, Janssen, and AstraZeneca Honoraria; G.R.: Janssen, AbbVie, and Gilead Membership on an entity’s Board of Directors or advisory committees; M.V.: Janssen, AbbVie, AstraZeneca, BeiGene Advisory Board; L.L.: AbbVie, Roche, Gilead, Janssen Honoraria; M.M.: – Roche Honoraria and Research Funding – AbbVie, Janssen, and Gilead Honoraria and Speakers Bureau – AstraZeneca and Verastem Honoraria; A.T.: Janssen, AbbVie, BeiGene, and Sunesis Honoraria and Speakers Bureau. Giovanni del Poeta, Andrea Visentin, Alberto Fresa, Annalisa Chiarenza, Alessandro Sanna, Francesca Romana Mauro, Massimo Gentile, Claudia Baratè, Chiara Borella, Antonino Greco, Marina Deodato, Giulia Zamprogna, Roberta Laureana, Alessandra Cipiciani, Andrea Galitzia, Angelo Curto Pelle, Francesca Morelli, Lucio Malvisi and Livio Trentin have no conflict of interest to declare.
Availability of data and materials: All data produced in this study are available upon reasonable request to the authors.
References
- 1. Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol 2021; 96: 1679–1705. [DOI] [PubMed] [Google Scholar]
- 2. Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373: 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020; 395: 1278–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 43–56. [DOI] [PubMed] [Google Scholar]
- 5. Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 2019; 380: 2225–2236. [DOI] [PubMed] [Google Scholar]
- 6. Martell RE, Peterson BL, Cohen HJ, et al. Analysis of age, estimated creatinine clearance and pretreatment hematologic parameters as predictors of fludarabine toxicity in patients treated for chronic lymphocytic leukemia: a CALGB (9011) coordinated intergroup study. Cancer Chemother Pharmacol 2002; 50: 37–45. [DOI] [PubMed] [Google Scholar]
- 7. Vojdeman FJ, Van’t Veer MB, Tjønnfjord GE, et al. The HOVON68 CLL trial revisited: performance status and comorbidity affect survival in elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma 2017; 58: 594–600. [DOI] [PubMed] [Google Scholar]
- 8. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370: 1101–1110. [DOI] [PubMed] [Google Scholar]
- 9. Gordon MJ, Churnetski M, Alqahtani H, et al. Comorbidities predict inferior outcomes in chronic lymphocytic leukemia treated with ibrutinib. Cancer 2018; 124: 3192–3200. [DOI] [PubMed] [Google Scholar]
- 10. Gordon MJ, Huang J, Chan RJ, et al. Medical comorbidities in patients with chronic lymphocytic leukaemia treated with idelalisib: analysis of two large randomised clinical trials. Br J Haematol 2021; 192: 720–728. [DOI] [PubMed] [Google Scholar]
- 11. Tedeschi A, Frustaci AM, Mauro FR, et al. Do age, fitness, and concomitant medications influence management and outcomes of patients with CLL treated with ibrutinib? Blood Adv 2021; 5: 5490–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davids MS, Hallek M, Wierda W, et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res 2018; 24: 4371–4379. [DOI] [PubMed] [Google Scholar]
- 13. Roberts AW, Ma S, Kipps TJ, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood 2019; 134: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eyre TA, Kirkwood AA, Gohill S, et al. Efficacy of venetoclax monotherapy in patients with relapsed chronic lymphocytic leukaemia in the post-BCR inhibitor setting: a UK wide analysis. Br J Haematol 2019; 185: 656–669. [DOI] [PubMed] [Google Scholar]
- 15. Roeker LE, Fox CP, Eyre TA, et al. Tumor lysis, adverse events, and dose adjustments in 297 venetoclax-treated CLL patients in routine clinical practice. Clin Cancer Res 2019; 25: 4264–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2018; 378: 1107–1120. [DOI] [PubMed] [Google Scholar]
- 17. Kater AP, Arslan Ö, Demikran F, et al. Efficacy of venetoclax in patients with relapsed/refractory chronic lymphocytic leukemia: primary endpoint analysis of the international phase 3b phase trial (Venice I). In: EHA virtual conference, 11–21 June 2020; 294976; S156. [Google Scholar]
- 18. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2, Suppl. 1): S1–266. [PubMed] [Google Scholar]
- 19. Mortazavi SS, Shati M, Keshtkar A, et al. Defining polypharmacy in the elderly: a systematic review protocol. BMJ Open 2016; 6: e010989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 21. Salvi F, Miller MD, Grilli A, et al. A manual of guidelines to score the modified Cumulative Illness Rating Scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc 2008; 56: 1926–1931. [DOI] [PubMed] [Google Scholar]
- 22. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968; 16: 622–626. [DOI] [PubMed] [Google Scholar]
- 23. European Medicine Agency. European public assessment report: Venclyxto, 11 January 2022, https://www.ema.europa.eu/en/medicines/human/EPAR/venclyxto
- 24. Von Elm E, Altman DG, Egger M, et al. STROBE initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 25. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib–rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 2019; 381: 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 2018; 379: 2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 2020; 38: 2849–2861. [DOI] [PubMed] [Google Scholar]
- 28. Winqvist M, Asklid A, Andersson PO, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A Study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica 2016; 101: 1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. UKCLL and UK CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica 2016; 101: 1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol 2015; 1: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with ibrutinib. J AM Coll Cardiol 2019; 74: 1667–1678. [DOI] [PubMed] [Google Scholar]
- 32. Bouclet F, Calleja A, Dilhuydy MS, et al. Real-world outcomes following venetoclax therapy in patients with chronic lymphocytic leukemia or Richter syndrome: a FILO study of the French compassionate use cohort. Ann Hematol 2021; 100: 987–993. [DOI] [PubMed] [Google Scholar]
- 33. Mato AR, Thompson M, Allan JN, et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica 2018; 103: 1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mato AR, Roeker LE, Eyre TA, et al. A retrospective comparison of venetoclax alone or in combination with an anti-CD20 monoclonal antibody in R/R CLL. Blood Adv 2019; 3: 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mato AR, Hill BT, Lamanna N, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol 2017; 28: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 36. Innocenti I, Morelli F, Autore F, et al. Venetoclax in CLL patients who progress after B-cell receptor inhibitor treatment: a retrospective multi-centre Italian experience. Br J Haematol 2019; 187: e8–e11. [DOI] [PubMed] [Google Scholar]
- 37. Mato AR, Sharman JP, Biondo JML, et al. The impact of early discontinuation/dose modification of venetoclax on outcomes in patients with relapsed/refractory chronic lymphocytic leukemia: post-hoc analyses from the phase III MURANO study. Haematologica 2022; 107: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016; 17: 928–942. [DOI] [PubMed] [Google Scholar]
- 39. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goede V, Bahlo J, Chataline V, et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: results of the CLL9 trial of the German CLL study group. Leuk Lymphoma 2016; 57: 789–796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207221127550 for Coexisting conditions and concomitant medications do not affect venetoclax management and survival in chronic lymphocytic leukemia by Anna Maria Frustaci, Giovanni Del Poeta, Andrea Visentin, Paolo Sportoletti, Alberto Fresa, Candida Vitale, Roberta Murru, Annalisa Chiarenza, Alessandro Sanna, Francesca Romana Mauro, Gianluigi Reda, Massimo Gentile, Marzia Varettoni, Claudia Baratè, Chiara Borella, Antonino Greco, Marina Deodato, Giulia Zamprogna, Roberta Laureana, Alessandra Cipiciani, Andrea Galitzia, Angelo Curto Pelle, Francesca Morelli, Lucio Malvisi, Marta Coscia, Luca Laurenti, Livio Trentin, Marco Montillo, Roberto Cairoli and Alessandra Tedeschi in Therapeutic Advances in Hematology