Abstract
Introduction: Sturge-Weber syndrome (SWS) is often associated with drug resistant epilepsy. The literature is unclear as to how often these patients can be weaned off of antiepileptic drugs (AEDs) to become seizure-free. Case Description: We describe two patients with SWS. After initial treatment with various AEDs, breakthrough seizures still occurred. However, after periods with no seizure activity, they were weaned off of their medications. They have been off for 4 and 3 years and seizure-free for 13 and 12 years, respectively. No surgical procedure was necessary. Conclusion: We hypothesize that spontaneous involution or pathological disconnection of the vascular malformations might underly the patients’ recovery. The initial aggressive therapy, close follow-up, choice of AEDs, or natural evolution of the disease may have played a role in their recovery. Therefore, in patients with SWS and lesional structural epilepsy, medication freedom is possible and invasive management options including surgery should be discussed carefully.
Keywords: resolved epilepsy, genetic epilepsy, structural epilepsy, leptomeningeal angiomatosis
Introduction
Sturge-Weber syndrome (SWS) is a neurocutaneous syndrome characterized by the presence of some or all the following: facial trigeminal capillary angiomas, choroidal angiomas and leptomeningeal pial angiomas leading to port-wine stains (PWS), glaucoma, and neurological complications such as epilepsy, hemiplegia, and developmental delay.1–3 Epilepsy is the most common comorbidity and usually the initial neurological manifestation of SWS.1,3 As such, it contributes significantly to the prognosis of SWS.1 Epilepsy occurs in about 70–100% of patients, and they may have several daily or monthly seizures, causing severe disability.1 Most patients develop drug resistant epilepsy (DRE).1–4
“Resolved epilepsy” is defined by Fisher et al5 in the official practical guide of the International League Against Epilepsy as “individuals who had an age-dependent epilepsy syndrome but are now past the applicable age or those who have remained seizure-free for the last 10 years, with no seizure medicines for the last 5 year”. We describe two patients with SWS and DRE in whom we were able to discontinue their antiepileptic drugs (AEDs) and are on their way to resolved epilepsy. We hypothesize that patients with DRE secondary to SWS may have spontaneous remission due to spontaneous involution or pathological disconnection of the vascular malformations might be the plausible cause.
Cases
This study is a retrospective case series of two patients with SWS and DRE at the Montreal Children's Hospital. The patients were followed-up for 16 years. At present, the patients have been off medication respectively for four and three years.
Patient 1
Patient 1 is a 17-year-old male with SWS diagnosed at age 3 weeks. He was born with a PWS in the right trigeminal nerve cutaneous distribution V1. He started to have focal impaired awareness seizures at age 4 months. He had left head and eye deviation and clonic movements of the left extremity of up to 25 minutes duration. Midazolam, diazepam, phenobarbital, and phenytoin were required to resolve the seizures over 1 week. He again had a cluster of seizures at 9 months provoked by fever. Carbamazepine and clobazam were started as maintenance therapy and nitrazepam was added as needed with fever. The maximum doses reached were 35 mg/kg/day and 1 mg/kg/day, respectively. Nevertheless, at 14–15 months and 3 years of age he had frequent seizures (unprovoked by fever, upper respiratory tract infections, or trauma).
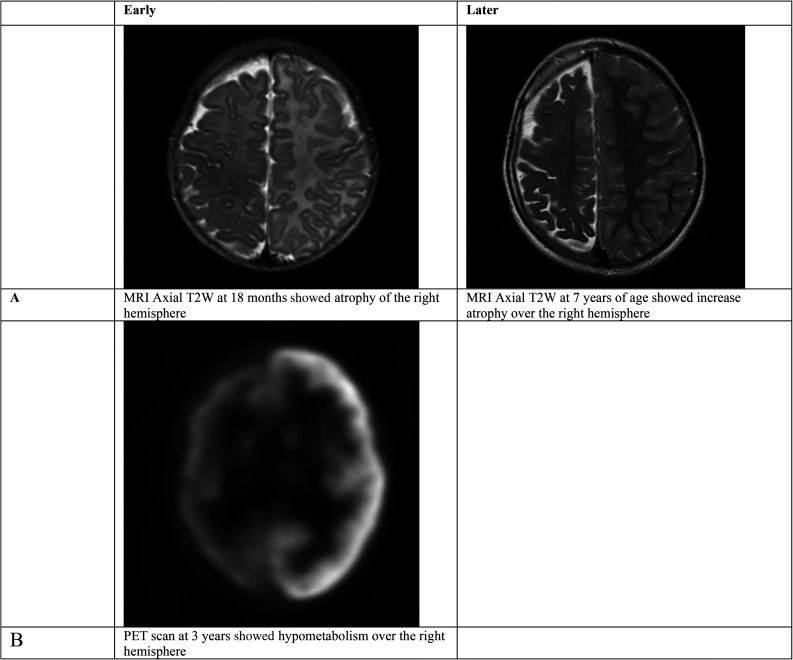
Magnetic resonance imaging (MRI) of the brain at 1 and 4 months of age showed the characteristic features of SWS, including enlargement of the right choroid plexus in the trigonal and occipital horn, moderate atrophy of the right cerebral hemisphere, and extensive enhancement of the right cerebral hemisphere with demonstration of the sulcations at all sites. This indicates leptomeningeal enhancement and confirmed involvement of the entire right hemisphere (Figure 1A). Fluorodeoxyglucose-positron emission tomography at 3 years of age showed right hemisphere hypometabolism (Figure 1B). Over the years, no further atrophy was observ after the age 7.
Figure 1.
Brain MRI (A) and PET scan (B) for patient 1 early and later in his life..
His first electroencephalogram (EEG) (at age 5 months) showed a moderate to severe disturbance of cerebral activity and at times a burst suppression and continuous disturbance over the right hemisphere. However, his EEGs never showed definite interictal epileptic abnormalities.
His last seizure occurred at 3 years of age. He was evaluated for epilepsy surgery. As no further seizures were observed, he was eventually taken off clobazam at 12 years of age and carbamazepine at 13 years of age. After 4 years post taper of AEDs (Table 1), seizures have not recurred. He is currently taking aspirin. His development is stable, but he has an attention deficit syndrome, mild intellectual disability, and behavioral issues. He has right choroidal hemangioma without glaucoma. An EEG following the taper showed no interictal epileptic activity.
Table 1.
Summary of the Patients’ Clinical Information.
| Patient | First seizure | Last seizure | Type of seizures | Status | Hemip. | Glauc. | AEDs needed for maintainance | Years without seizures | Years off medication | Last EEG | Cognitive deficits |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Male) | 4 m | 3 y | Focal | Yes | Yes | No | Clobazam Carbamazepine |
13 y | 4 y | No IE | ADHD ID Behavioral |
| 2 (Female) | 3 y | 4 y | Focal | Yes | No | No | Clobazam Topiramate |
12 y | 3 y | No IE | Normal |
Abbreviations: AEDs, antiepileptic drugs; ADHD, attention deficit hyperactivity syndrome; Hemip, hemiplegia; Glauc, glaucoma; ID, intellectual disability; IE, interictal; m, months; y, years.
Patient 2
Patient 2 is a 16-year-old female with SWS. She was born with faint PWS over the right trigeminal nerve cutaneous distribution V1. She started to have seizures at 3 years of age with a 40 minutes episode of status epilepticus (focal to bilateral tonic-clonic seizures with head/eye deviation to the left). She required lorazepam and phenytoin to stop the seizure, and she was discharged on clobazam and topiramate as maintenance therapy within 2 weeks.
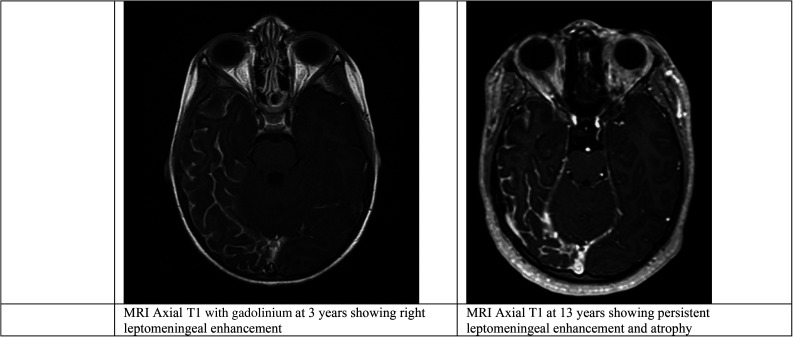
Her MRI showed the features of SWS involving extensive enhancement of the right cerebral hemisphere, particularly in the right temporal and occipital lobes. Enhancement of a large right choroid plexus extending throughout the right lateral ventricle and pial enhancement over the surface of the brain extending upwards to the superior temporal region was also observed. There was obvious atrophy, which is known to be age-related in SWS. Magnetic resonance venography showed the cortical veins were distributed symmetrically with normal dural sinuses. Her MRI at 6 years of age showed reduced leptomeningeal enhancement and no further atrophy. At 13 years of age, avid enhancement was seen with no further atrophy (Figure 2).
Figure 2.
Brain MRI for patient 2, early and later in her life..
Her initial EEGs showed right posterior disturbance of cerebral activity over the right posterior quadrant and frequent right posterior electrographic and electroclinical seizures with onset over the right fronto-central and posterior temporal regions that lasted up to 20 minutes. Follow-up EEGs showed rare interictal epileptic activity over the right frontal region at 10 years of age and a follow-up EEG was normal by 12 years of age. At age 13, a prolonged EEG showed rare generalized epileptiform spike and wave discharges during sleep, but a 3H-EEG was normal again at 15 years of age.
She had her last seizure at age 4 years. She was weaned off clobazam at 13 years of age and topiramate at 14 years of age. Maximum doses reached were 1 mg/kg/day and 10 mg/kg/day, respectively. On a follow-up visit at age 16 years, there was no recurrence of seizures. She remained on aspirin. Her development is within normal limits for her age. She attends regular school but she experiences some school learning difficulties in mathematics. She has no ocular abnormalities.
Discussion and Conclusions
The course of epilepsy varies in SWS. A study by Arzimanoglou and Aicardi1 involving 23 patients with SWS showed that about 65% of patients had DRE. However, the other 35% had relatively controlled epilepsy. Only two were completely weaned off medications.
A study by Sujansky and Conradi4 including 171 patients with SWS reported that seizures were seen in at least 80% of patients (a possible underestimation). Full seizure control was achieved in 50% and partial seizure control was achieved in 39% of the patients. The study did not mention the number of patients who had resolved epilepsy.
As seen in our patients, Kossoff et al2 mentioned that seizure-free-periods could be seen in 39% of patients, with a median seizure-free period of 1 year (range 0.5–10 years). Patients who were able to discontinue medication were not mentioned in this article, but our patients had longer periods of seizure freedom. However, it is unclear if surgical intervention could have prevented thepsychosocial problems in patient one.
Our hypothesis was based on the imaging findings in cases of SWS, which described reduced perfusion or cerebral blood flow and impaired vasomotor reactivity suggested by SPECT (single photon emission computed tomography),6 reduced glucose metabolism suggested by positron emission tomography (PET), or vessel occlusion or malformations on cerebral angiography.7 This hypoperfusion/ischemia might be the pathophysiology underlying the disconnection-like phenomenon (auto-disconnection) leading to potential resolved epilepsy in patients with SWS.
On the other hand, the recurrence or clinical deterioration in patients might be related as well to vasomotor changes or thrombosis in the angiomas.6,7 Following seizures or hemiplegia, cortical enhancement or perfusion metabolic mismatch could be seen in the angioma or extend beyond the angioma, possibly explaining the progressive deterioration and expansion of damage.3,8,9
In conclusion, our small case series demonstrates that even with DRE in patients with SWS, resolution of epilepsy is possible, and patients can ultimately be medication-free. In this context, patients can have either a seizure-free periods or periods of high seizure burden but can eventually be weaned off medication. Absence of seizures and mild residual interictal epileptic abnormalities may assist medication weaning and avoid invasive epilepsy surgery. Finally, these two cases show that even with structural lesional epilepsy, medication freedom is possible without surgery.
Footnotes
Abdulla Alawadhi, Dubai Medical College & Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, United Arab Emirates.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Both patients and their families gave consent for their cases to be documented in this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Abdulla Alawadhi https://orcid.org/0000-0001-7014-3056
References
- 1.Arzimanoglou A, Aicardi J. The epilepsy of Sturge-Weber syndrome: clinical features and treatment in 23 patients. Acta Neurol Scand Suppl. 1992;86(S140):18–22. [DOI] [PubMed] [Google Scholar]
- 2.Kossoff EH, Ferenc L, Comi AM. An infantile-onset, severe, yet sporadic seizure pattern is common in Sturge-Weber syndrome. Epilepsia. 2009;50(9):2154–2157. [DOI] [PubMed] [Google Scholar]
- 3.Sudarsanam A, Ardern-Holmes SL. Sturge-Weber syndrome: from the past to the present. Eur J Paediatr Neurol. 2014;18(3):257–266. [DOI] [PubMed] [Google Scholar]
- 4.Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10(1):49–58. [DOI] [PubMed] [Google Scholar]
- 5.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. [DOI] [PubMed] [Google Scholar]
- 6.Roach E, Riela A, McLean W, Stump D. (eds). Aspirin therapy for Sturge-Weber syndrome. ANNALS OF NEUROLOGY; 1985: LIPPINCOTT-RAVEN PUBL 227 EAST WASHINGTON SQ, PHILADELPHIA, PA 19106.
- 7.Chugani HT, Mazziotta JC, Phelps ME. Sturge-Weber syndrome: a study of cerebral glucose utilization with positron emission tomography. J Pediatr. 1989;114(2):244–253. [DOI] [PubMed] [Google Scholar]
- 8.Terdjman P, Aicardi J, Sainte-Rose C, Brunelle F. Neuroradiological findings in Sturge-Weber syndrome (SWS) and isolated pial angiomatosis. Neuropediatrics. 1991;22(3):115–120. [DOI] [PubMed] [Google Scholar]
- 9.Alkonyi B, Miao Y, Wu J, et al. A perfusion-metabolic mismatch in Sturge-Weber syndrome: a multimodality imaging study. Brain Dev. 2012;34(7):553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]