Abstract
Nitrofurantoin, trimethoprim-sulfamethoxazole (TMP-SMX) and fosfomycin are first-line therapeutics for uncomplicated urinary tract infections (uUTI). While fosfomycin is the most expensive, it is also attractive due to its effectiveness against most uUTI-causing bacteria, limited risk of cross-resistance with other drugs, and single-dose delivery. In light of these competing attributes, a cost-effectiveness analysis can provide useful, standardized information about tradeoffs between fosfomycin and treatment alternatives. This paper assessed cost-effectiveness via incremental cost-effectiveness ratios (ICERs) that represented a drug’s incremental cost per additional uUTI case resolved with initial course of antibiotic therapy. The study setting was New Hampshire, USA. Total cost of treatment was lowest with TMP-SMX and highest with fosfomycin. ICERs were $84.53 and $78.59 for nitrofurantoin and $2264.29 and $2260.89 for fosfomycin under a payer and societal perspective, respectively. While no standard benchmark for our measure of cost-effectiveness exists, the high national prevalence of antibiotic stewardship efforts suggests that willingness-to-pay to increase the number of people who are successfully treated with an initial course of therapy is non-zero. Ultimately, fosfomycin may currently be considered a cost-effective option for treating uUTI in the US. As a recently off-patent drug, increased competition in the generic market may improve its cost-effectiveness in the future.
Keywords: Cost-effectiveness, fosfomycin, urinary tract infection, antibiotic resistance, antibiotic stewardship
Background
Urinary tract infections (UTIs) are the second-most commonly occurring infections in the United States.1 They are responsible for more than 35 million medical events and more than 9.3 billion dollars in medical expenditures each year.2,3 UTIs are caused by an overgrowth of pathogenic bacteria in the urinary system that results in an infection which must be treated with a prescription antibiotic.4,5 However, the answer to the question, “which antibiotic?” is not a simple one. Increasingly, the Centers for Disease Control and Prevention, the World Health Organization, and others, have pushed for providers to optimize their antibiotic choices (an activity called antibiotic stewardship), in an effort to reduce the spread of antibiotic resistance.6 Antibiotic resistance poses a significant threat to the sustained efficacy of existing antibiotic therapies.7 The high prevalence of UTIs makes them a major source of antibiotic prescriptions and therefore a source of great opportunity to reduce the risk of antimicrobial resistance through antibiotic stewardship.
Uncomplicated UTIs (uUTIs) occur when the lower urinary tract, the bladder and/or urethra, become infected. When the infection progresses to the kidneys and/or ureters, a more serious infection called pyelonephritis occurs.4 The Infectious Diseases Society of America (IDSA), which publishes evidence-based guidelines for the treatment of infectious diseases, recommends the use of 4 first-line (preferred) treatment options for uUTI: nitrofurantoin 100 mg twice daily for 5 days, trimethoprim-sulfamethoxazole (TMP-SMX) 160/800 mg twice daily for 3 days, a single 3 g dose of fosfomycin, and pivmecillinam 400 mg for 3 to 7 days, though the latter option is not yet available in America.5,8 Of the 4 treatment alternatives endorsed by the IDSA, fosfomycin is the only one that does not require multiple doses of medication over multiple days.5 The single-dose delivery of fosfomycin is advantageous from an antibiotic stewardship perspective, since adhering to doctors’ dosing and duration instructions are encouraged in the effort to fight antibiotic resistance,9,10 and patient adherence is inherently 100% when the patient is responsible for taking just one dose of medication. Further, fosfomycin has a unique chemical structure which limits its risk of cross-resistance with other drugs. Unlike with many other antibiotics, including penicillin and other beta-lactams, when bacteria do become resistant to fosfomycin their susceptibility to other, more broadly used therapies, is preseved.11
Despite its advantages, use of fosfomycin in the United States remains low. While the drug’s efficacy was a concern in the past, more recent studies have shown that the efficacy of a single 3 g dose of fosfomycin is similar to commonly prescribed targeted multi-dose alternatives and that there is no significant difference in safety.12,13 Rather, today, it is the high price of the drug that is oft-cited as prohibitive.14-16 Fosfomycin has been available in the United States as a branded drug, called Monurol, for decades. It is also newly available as a generic drug—Xiromed was the first pharmaceutical company granted approval to manufacture fosfomycin in generic form in October, 2020.17 Even still, as a generic, fosfomycin remains between 11 and 186 times more expensive than other IDSA-recommended treatment alternatives.18
It is possible that the unique advantages of fosfomycin justify its higher price. As a matter of fact, Perrault et al designed a cost minimization model and found that the treatment cost-per-case with fosfomycin was similar to that of multi-dose alternatives (TMP-SMX, nitrofurantoin, and fluoroquinolones).19 In a similar study, Sadler et al concluded that the cost per UTI resolved was lowest when fosfomycin (rather than TMP-SMX or nitrofurantoin) was used, when the likely local level of bacterial resistance to TMP-SMX was taken into account.20 However, these studies were set in Canada and England, respectively, and as such, rely on price and antibiotic resistance assumptions that are potentially very different from what would be observed in the United States. Further, neither study employed a true cost-effectiveness model, which incorporates some measure of effectiveness in order to produce useful incremental cost per incremental effectiveness ratios (ICERs) for each treatment alternative.
Therefore, the goal of this study was to adapt the models constructed by Perrault et al and Sadler et al in order to understand whether fosfomycin is a cost-effective option for treating uUTI in the United States. We measure the cost-effectiveness of TMP-SMX, nitrofurantoin, and fosfomycin as the cost per uUTI resolution with an initial course of antibiotic therapy and specify decision models that adopt both a health sector perspective and a societal perspective, given that antibiotic resistance is a societal issue. In doing so, we hope to produce information that assists healthcare providers and public health professionals in making informed decisions about optimal uUTI treatment. Additionally, given that new generic manufactures have only recently been permitted to enter the market, these data may prove helpful to payers and drug manufacturers who may be increasingly engaged modifying their formularies and negotiating value-based pricing for fosfomycin as manufacturing competition increases.
Methods
Study setting
At the highest level, this study is set within the United States (U.S.) healthcare system. The U.S. healthcare system may be aptly described as decentralized, being that no one payer (eg, the national government) renders or pays for Americans’ healthcare services. Instead, a patchwork of government (both state and national) payers, not-for-profit, and for-profit private payers pay for care on behalf of patients, while a portion of the population is uninsured.
Wherever possible, this study utilizes assumptions that are reflective of the typical costs and probabilities that would be expected in the U.S. state of New Hampshire. While the ultimate goal of this study is to understand whether fosfomycin is a cost-effective treatment alternative in the context of the U.S. healthcare system, generally, we believed it was important to limit the geographic scope of this study given that a key model parameter, the susceptibility of urine isolates to antibiotics, can vary greatly by geographic region due to differences in prescribing patterns, spread within the community and within hospitals, as well as other factors. New Hampshire is an ideal state for the purposes of this paper due to its size (small enough to minimize regional variance of bacterial resistance, yet large enough to produce robust data), fair distribution of the population by rurality (37% rural, 63% urban), and availability of data.21 To that end, the New Hampshire Department of Health and Human Services publishes detailed bacterial susceptibility data in an annual state-wide antibiogram, which is an asset to this study both because of its precision and timeliness.22 Further, the New Hampshire Insurance Department oversees one of the country’s only state-run price transparency websites, New Hampshire Healthcare Costs, and this tool is ideal for obtaining expected local costs in a cost-effectiveness analysis.23
Decision tree model
Of the 4 first-line treatment options for uUTI that are recommended by IDSA, the 3 that are currently available in the United States, TMP-SMX, nitrofurantoin, and fosfomycin, were evaluated. To compare the cost-effectiveness of these treatment alternatives, a decision tree with an arm for treatment alternative was constructed in Microsoft Excel. Both a one-way and probabilistic sensitivity analysis (PSA) were conducted in order to gauge the sensitivity of our model to parameter uncertainty. A Monte-Carlo simulation PSA, which varied probability and susceptibility assumptions within beta distributions and costs within gamma distributions, was performed using Analytic Solver Basic, an Excel add-in application.24
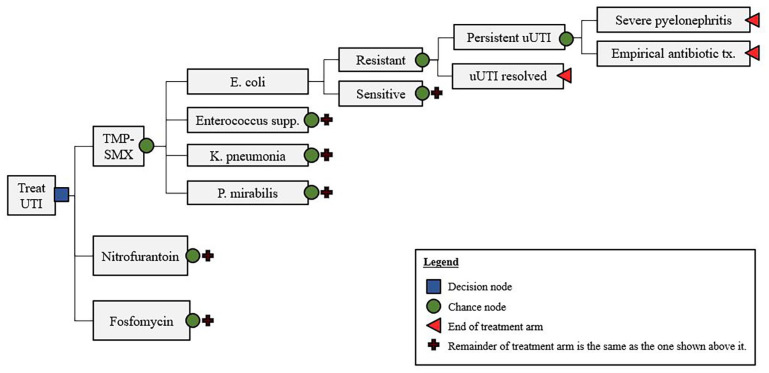
Each arm in the model, shown in Figure 1, begins with a chance node to determine which of the most common gram-negative or gram-positive organism found in New Hampshire urine isolates (i.e., E. coli, Enterococcus supp., K. pneumonia, P. mirabilis bacteria) are the cause of uUTI infection. This node is followed by a second chance node to determine whether the infection-causing bacteria are resistant or sensitive to the initial course of treatment. In either case, the uUTI infection has the possibility of either resolving or persisting, which is represented in the following chance node. If the infection persists, it may either be treated on an outpatient basis or may result in a serious case of pyelonephritis, requiring an inpatient stay. Regardless, the final chance node assumes that the infection will be resolved, given that a urine culture with an antibiotic susceptibility test will be ordered, and empirical treatment will be prescribed. Ultimately, the costs and probabilities of each branch of the model are rolled back to produce a dollar amount of the total cost for each treatment.
Figure 1.
Decision tree model.
The basic structure of our model is similar to the model conceptualized by Perrault et al19 but with the exception of a couple of key differences. First, the treatments that we evaluate reflect only those that that are recommended first-line therapeutics according to the United States’ infectious disease experts, the IDSA.5 This meant removing fluoroquinolones, which were evaluated as a treatment alternative in the Canadians’ model. In addition, the last probability node (develop severe pyelonephritis or resolve with empirical antibiotic treatment) is condensed in our model for simplicity. We make the literature-informed assumption that the costs of less severe cases or pyelonephritis which can be treated outpatient are similar to those for persistent uUTI. Thusly, we account these outcomes together as “empirical antibiotic treatment,” rather than separating them out.
The decision tree model was run twice: once assuming a payer perspective and once assuming a societal perspective. The only difference between the 2 being the exclusion of indirect costs (eg, travel and childcare costs associated with office visits, loss of output while in the hospital, etc.) in the former and the inclusion of them in the later.
Probability assumptions
The majority of the assumptions used for chance nodes (shown in Table 1) were informed by Perrault et al ’s prior study.19 However, the assumptions used for bacterial susceptibility (shown in Table 2) are an important exception. Bacterial susceptibility, which is essentially the inverse of antibiotic resistance, refers to the proportion of urine isolates that are sensitive to a given drug. The 2018 New Hampshire Antibiogram was used as the primary source for these data.22 The New Hampshire Antibiogram is a compilation of the susceptibility test results from labs throughout the state for all of the urine isolates tested in the prior year, and as such, is the best available source for state-wide susceptibility. Even still, it is not perfect. For example, data for Enterococcus spp. isolates tested against TMP-SMX and P. mirabilis isolates tested against nitrofurantoin are “. . .censored because of intrinsic resistance and/or inappropriate clinical use.”22 As a result, we assumed that in either case, the drugs would be completely ineffective (0.00% susceptibility). This assumption was verified with the antibiograms for 2 large local hospital systems, both of which censured the data or reported 0% susceptibility.25-27
Table 1.
Probability assumptions.
| Probability | Base case (%) | CI—Low (%) | CI—High (%) |
|---|---|---|---|
| uUTI caused by E. coli | 90.119 | 76.6* | 100.0* |
| uUTI caused by Enterococcus supp. | 3.019 | 7.1** | 0.0** |
| uUTI caused by K. pneumonia | 4.119 | 9.7** | 0.0** |
| uUTI caused by P. mirabilis | 2.819 | 6.6** | 0.0** |
| Sensitive isolate: UTI resolution | 94.019 | 79.9* | 100.0* |
| Resistant isolate: UTI resolution | 74.019 | 62.9* | 85.1* |
| Resistant, persistent cases that develop into severe pyelonephritis | 0.819 | 0.0* | 10.0 |
Tested base case data at ±15% of values in sensitivity analysis. High values capped at 100%, low values capped at 0%.
Re-weighted so that it is proportional to the remainder of uUTI not caused by E. coli.
Table 2.
Susceptibility assumptions.
| Base case (%) | CI—Low (%) | CI—High (%) | |
|---|---|---|---|
| TMP-SMX | |||
| E. coli | 82.622 | 70.2* | 94.9* |
| Enterococcus spp. | 0.022,25,26 | - | - |
| K. pneumoniae | 91.522 | - | - |
| P. mirabilis | 81.922 | - | - |
| Nitrofurantoin | |||
| E. coli | 97.822 | 83.1* | 100.0* |
| Enterococcus spp. | 94.822 | - | - |
| K. pneumoniae | 45.222 | - | - |
| P. mirabilis | 0.022,25,27 | - | - |
| Fosfomycin | |||
| E. coli | 100.013 | 85.0* | 100.0* |
| Enterococcus spp. | 90.613 | - | - |
| K. pneumoniae | 95.513 | - | - |
| P. mirabilis | 96.828 | - | - |
Tested base case data at ±15% of values in sensitivity analysis. High values capped at 100%, low values capped at 0%.
A second and more critical issue concerning use of the New Hampshire Antibiogram as a data source for this study is that susceptibility data for isolates tested against fosfomycin are not published at this time. Currently. “. . .hospital laboratories [in NH] do not routinely test susceptibilities for this antibiotic,”22 a practice that is likely due in part to the fact that CLSI only releases breakpoint interpretations for E. coli. As a result, base-case assumptions for fosfomycin susceptibility were supplemented with results from recent randomized control trials.13,28 Where possible, assumptions were derived from Hirsch et al’s randomized control trial, which we felt was a particularly suitable proxy for true New Hampshire values, given that it was conducted more recently (just 3 years prior to the publishing of the Antibiogram) and within close proximity to the state (Boston, MA).
In looking at Table 2, it is noteworthy that fosfomycin is the most effective treatment alternative from a susceptibility perspective for nearly every uUTI-causing bacterium (the only exception is Enterococcus spp., which are slightly more susceptible to nitrofurantoin). Further, urine isolates (particularly E. coli) have a high degree of resistance to TMP-SMX (defined by New Hampshire Antibiogram as less than 80% susceptibility).22 This is important considering that these bacteria cause 90% of uUTI infections. Finally, while effective against E. coli, nitrofurantoin has weaknesses as well, proving unfavorable activity against K. pneumoniae and P. mirabilis bacteria.
Cost assumptions
All cost assumptions (shown in Table 3) are in 2020 dollars. When cost assumptions were derived from older sources, they were inflated to 2020 dollars using the consumer price index.29
Table 3.
Cost assumptions.
| Cost (2020 USD) | Base case | CI—Low | CI—High |
|---|---|---|---|
| TMP-SMX | $0.3718 | $0.31* | $0.43* |
| Nitrofurantoin | $6.4218 | $5.46* | $7.38* |
| Fosfomycin | $68.9418 | $58.60* | $79.28* |
| Urine culture | $7.0023,31,32 | $4.0023 | $41.0023 |
| Office visit—direct costs | $169.0023 | $96.0023 | $307.0023 |
| Office visit—indirect costs | $32.2431 | $27.40* | $37.08* |
| Pyelonephritis—direct costs | $8323.1631 | $5,826.21** | $10,820.11** |
| Pyelonephritis—indirect costs | $4384.3931 | $3,069.07** | $5,699.71** |
| Empirical treatment for persistent infection*** | $2.0118 | $1.71* | $2.31* |
Tested base case data at ±15% of values in sensitivity analysis. High values capped at 100%. Low values capped at $0.00 USD.
Tested base case data at ±30% of values in sensitivity analysis.
Empirical treatment modeled is levofloxacin 750 mg.
Drug costs for the 3 treatment alternatives, as well as for the empirical treatment for persistent infections treated outpatient (levofloxacin 750 mg once daily, for 5 days), were ascertained from the Medicare National Average Drug Acquisition Cost (NADAC) database.5,18
The cost of a urine culture is reflective of the average cost paid for this test by the New Hampshire’s leading health insurer, as estimated by the state’s online price transparency tool, NH Healthcare Costs.23,30 High and low values used for the sensitivity analysis are reflective of the high and low values provided by this website as well, since they are indicative of the cash prices that a real New Hampshirite may be expected to pay. Initially, the literature was going to be used to ascertain base-case costs, but this became problematic, as many of the sources cited older sources so that the primary source dated as far back as 1998.31,32 Consequently, the price found in the literature ($30) was used to verify that the price listed on the NH Healthcare Costs was reasonable, rather than be used as the primary source. Indeed, this cost was consistent with what a New Hampshirite without insurance would pay.
Direct medical costs associated with an office visit for uUTI were also ascertained from NH Healthcare Costs website.23 Since there are a number of different levels of office visits, each with a different expected cost, a moderate-complexity, established patient visit (CPT code: 99214) was selected, following the assumption that the occurrence of high and low complexity visits offset each other in the population. Medicare reimbursement rates were used to verify the reasonableness of NH Healthcare Costs data,33 but the latter was selected as the source for base-case assumptions since it takes into account the payer mix and geographical adjustments that could be made in this state, thereby making it more contextually accurate.
Indirect costs for outpatient visits, as well as both direct and indirect and costs associated with pyelonephritis were derived from Brown et al .31 In their paper, the authors used a decision-tree model to estimate the average costs for both inpatient and outpatient cases of pyelonephritis. Indirect costs considered included travel and childcare for time at visit, and output lost due to time at visit for outpatient cases, as well as output lost due to disability and output lost to premature death for inpatient cases.31
Effectiveness
We measured the effectiveness of a treatment alternative as the probability of uUTI resolution following initial course of therapy. These values were obtained by first assigning values of 1 to individual treatment success (“uUTI resolved” endpoints, shown in Figure 1) and 0 to all others, and then rolling back each arm in order to obtain the overall probability of treatment success for each treatment alternative.
Results
Base case
Base case estimates of the total costs and effectiveness of each of the 3 treatment alternatives are shown in Table 4. The estimated total cost of treatment was similar for both of the multi-dose treatment alternatives, regardless of the perspective assumed. In either case, the difference was less than $4. The total cost of treatment with fosfomycin was $195.33 under the payer perspective and $221.81 under the societal perspective, which was $50.55 and $50.47 more expensive than the next cheapest alternative, nitrofurantoin, respectively. Estimated effectiveness, the probability of uUTI resolution following initial course of therapy, was lowest for TMP-SMX (81.78%) and highest for fosfomycin (88.17%), with nitrofurantoin falling in between the 2 (85.94%).
Table 4.
ICERs for uUTI treatment alternatives, base case.
| Drug | Total cost | Probability of resolution with first prescription (%) | Incremental cost | Incremental effectiveness (%) | ICER |
|---|---|---|---|---|---|
| Payer perspective | |||||
| TMP-SMX | $141.27 | 81.78 | - | - | - |
| Nitrofurantoin | $144.78 | 85.94 | $3.51 | 4.16 | $84.53 |
| Fosfomycin | $195.33 | 88.17 | $50.55 | 2.23 | $2264.29 |
| Societal perspective | |||||
| TMP-SMX | $168.06 | 81.78 | - | - | - |
| Nitrofurantoin | $171.33 | 85.94 | $3.27 | 4.16 | $78.59 |
| Fosfomycin | $221.81 | 88.17 | $50.47 | 2.23 | $2260.89 |
A threshold analysis was conducted to see at what cost would the total cost of fosfomycin be comparable to the multi-dose treatments. Values were tested until the rounded cost per case resolved amounted to the total cost of nitrofurantoin, the second-most expensive option ($145 in the payer perspective model and $171 in the societal perspective model). A price of $7 USD satisfied this condition.
The ICERs for each drug, which represent their incremental cost per additional uUTI case resolved with initial course of antibiotic therapy, are also shown in Table 4. When compared to the cheapest treatment alternative, TMP-SMX, the cost per additional resolution with initial course of therapy for nitrofurantoin was $84.53 under the payer perspective and $78.59 under the societal perspective. The ICER for fosfomycin was roughly $2260 in either model.
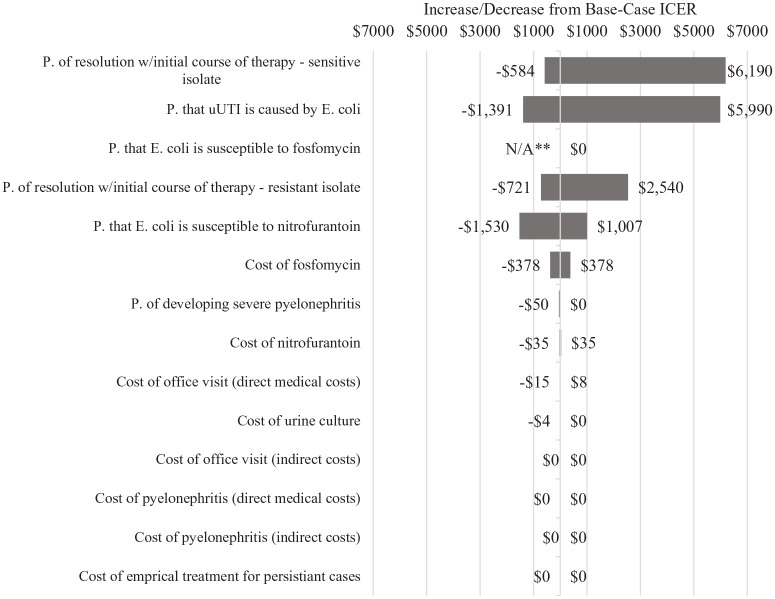
One-way sensitivity analysis
A one-way sensitivity analysis was performed in order to understand how uncertainty in any one given parameter impacted the ICER for fosfomycin when it is compared to nitrofurantoin. To accomplish this, one-by-one, each parameter was varied over its range of plausible values while the other parameters are held at their base-case level. The majority of the parameters were tested at +15% above and below their assumed base-case values. However, there were some exceptions (see Tables 1–3). Most notably, both direct and indirect costs of pyelonephritis were varied at 30% above and below their base-case values in order to reflect significant uncertainty in their assumed values. Additionally, the assumption for probability that resistant, persistent cases develop into severe pyelonephritis (0.8% in the base case), was tested up to 10%, again, due to significant uncertainty in assumed base-case values. Finally, parameters that were informed by the New Hampshire Health Costs data were varied according to the high and low values provided by the website.
The results of our sensitivity analyses, both assuming a payer perspective and a societal perspective, are shown in Figures 2 and 3, respectively.
Figure 2.
One-way sensitivity analysis, fosfomycin arm, payer perspective.
*“P.” is an abbreviation of “probability.”
*Treating uUTI with fosfomycin was a dominated strategy (ie, the ICER for fosfomycin is negative) when “p. that E. coli is susceptible to fosfomycin” was tested at the lowest point in the confidence interval.
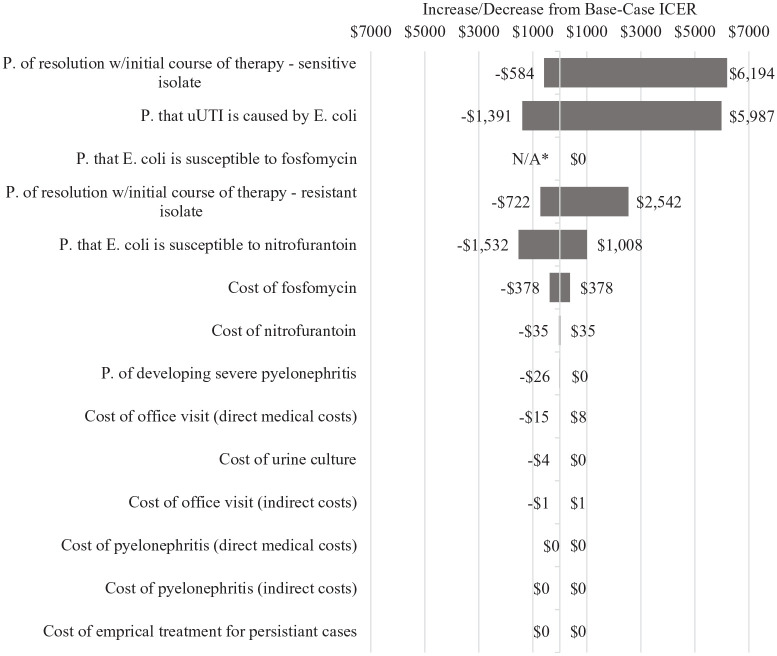
Figure 3.
One-Way sensitivity analysis, fosfomycin arm, societal perspective.
*“P.” is an abbreviation of “probability.”
**Treating uUTI with fosfomycin was a dominated strategy (ie, the ICER for fosfomycin is negative) when “p. that E. coli is susceptible to fosfomycin” was tested at the lowest point in the confidence interval.
Regardless of the perspective taken, the parameter that had the greatest impact on the ICER for fosfomycin was the probability that a uUTI infection actually resolves when the bacteria causing it are sensitive to the initial course of antibiotic treatment prescribed. The similar parameter pertaining to resistant infections also emerged as impactful. Given that these 2 parameters were those that determined effectiveness in our model (resolution of uUTI infection on initial course of therapy), this finding was not surprising.
The ICER for fosfomycin was also highly sensitive to whether uUTI infections caused by E. coli were susceptible to the first antibiotic that was prescribed and to the probability that an infection was caused by E. coli. This, too, was not surprising given that fosfomycin was assumed to be 100% effective against E. coli.
Interestingly, neither category of costs associated with pyelonephritis (direct nor indirect) had a meaningful effect on the ICER for fosfomycin, despite being tested across a range of ±30% of base case values.
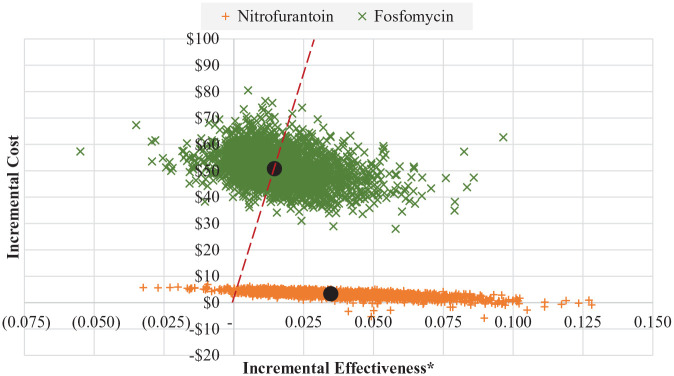
Probabilistic sensitivity analysis
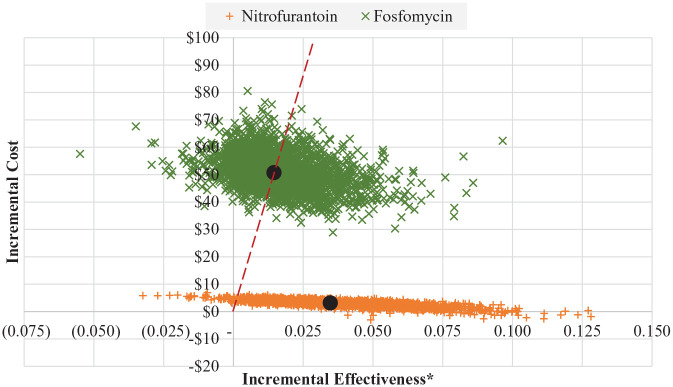
A probabilistic sensitivity analysis was conducted in order to test the sensitivity of our models when all parameters were allowed to vary simultaneously. To accomplish this, a value from each parameter’s distribution was drawn and the resulting ICER was recorded. This was done 5000 times in both models to get a range of possible ICERs. Results from this probabilistic sensitivity analysis are shown in Figures 4 and 5. The dashed line in these figures represents separates trials in which the ICER for fosfomycin were “better” (ie, lower) than the base case from those that were worse. For reference, base case ICERs are also show and are depicted as large black circles. Both under the payer and the societal perspective, 50.3% of the ICERs resulting from the PSA were better than (ie, to the right of) the base case. Additionally, just 7.8% of the replications resulted in negative incremental effectiveness and positive cost, indicating that fosfomycin was a dominated strategy.
Figure 4.
Probabilistic sensitivity analysis—payer perspective.
*Incremental effectiveness is measured as an additional uUTI case resolved with initial course of antibiotic therapy.
Figure 5.
Probabilistic sensitivity analysis—societal perspective.
*Incremental effectiveness is measured as an additional uUTI case resolved with initial course of antibiotic therapy.
The percent of all of the replications resulting from our probabilistic sensitivity analysis which had fosfomycin ICERs at or below various thresholds was also evaluated. These thresholds represented potential values of payer/societal willingness-to-pay for an additional uUTI resolved with initial antibiotic therapy. The results, shown in Table 5, were essentially the same whether the payer perspective or societal perspective was taken. When $1000, the lowest tested threshold, was applied, just 3.5% of the replications had ICERs for fosfomycin would be considered cost-effective. Increasing the willingness-to-pay threshold to $2000 and $7500 resulted in roughly one-quarter and three-quarters of the replications being classified as cost-effective, respectively.
Table 5.
Percent of probabilistic sensitivity analysis replications with fosfomycin icers at or below possible willingness-to-pay thresholds.
| Perspective taken | Willingness-to-pay threshold (%) | |||||
|---|---|---|---|---|---|---|
| $1000 | $1500 | $2000 | $5000 | $7500 | $10 000 | |
| Payer perspective | 3.5 | 13.3 | 24.7 | 63.7 | 74.6 | 79.6 |
| Societal perspective | 3.5 | 13.3 | 24.7 | 63.7 | 74.7 | 79.6 |
Discussion
The primary goal of this study was to determine whether fosfomycin, a single-dose treatment option for uUTI, could be considered a cost-effective alternative to multi-dose treatment options in the context of New Hampshire, U.S.A. The results of our study suggest that at present, where fosfomycin is only available as a costly branded drug (Monurol) and similarly-priced generic, its ICER exceeds multi-dose alternatives when effectiveness is defined as resolution of uUTI with an initial course of antibiotic therapy. However, ICERs are typically compared to benchmarks that represent willingness to pay in order to determine whether or not a treatment alternative is cost-effective. While no commonly-used benchmark exists for our measure of cost-effectiveness, it is reasonable to assume that the willingness to pay to increase the number of people who are successfully treated with an initial course of therapy for uUTI is non-zero. Most notably, avoiding additional prescriptions inherently reduces overuse of antibiotics and this is a central component of antibiotic stewardship. The CDC, alone, allocates as much as 11.25 million dollars per state every year to combat antibiotic resistance, demonstrating the societal importance of the issue.34 In addition, being successfully treated the first time is likely valuable to patients. Persistent UTI symptoms, including painful urination and urgency, can be highly unpleasant and can limit one’s daily activities. Thus, it is likely that at least some patients would be willing to pay more for an antibiotic in order to experience fewer days with symptoms. Even still, the question of whether it is worth paying more per additional case resolved with an initial course of therapy ($2260 in base-case models) is likely largely dependent on local resistance and disease patterns. This is supported by our sensitivity analyses, which showed that our models were highly sensitive to parameter uncertainty.
Our one-way sensitivity analysis revealed that pyelonephritis is too rare of an outcome for any avoidance of it downstream to have a significant effect on the cost-effectiveness of fosfomycin. Rather, the model was most sensitive to uncertainty concerning the probabilities that a uUTI case was resolved upon initial treatment, the susceptibility E. coli to treatment alternatives, and the probability that a uUTI infection was caused by E. coli. In practice, this means that stronger consideration to prescribing fosfomycin may be warranted when either, locally, the predominant cause of a uUTI are bacteria against which nitrofurantoin performs poorly or the susceptibility of E. coli to multi-dose treatment alternatives is low. Even still, PSA results suggest that situations wherein fosfomycin is dominated (ie, is less effective and more costly than nitrofurantoin) are relatively rare.
Finally, while the models employed by the authors were cost-minimization models, rather than cost-effectiveness models, it is interesting to compare our results to Perrault et al and Sadler et al’s similar studies, set in Quebec, Canada and in England, respectively.19,20 Perrault et al not only found significantly lower total cost values for all 3 therapies (TMP-SMX = 96.20CAD, nitrofurantoin = 99.09CAD,fosfomycin = 105.12 CAD), they found less variance between multi-dose and single-dose option. They noted just a $8.92 CAD difference between fosfomycin and the least costly alternative, which was likely is due in a large part to the lower price of fosfomycin (17.51 CAD, or ~13.50 USD) in Canada. Sadler et al, too, found less variance between the cost-per-case of uUTIs treated with fosfomycin versus multi-dose alternatives, even concluding that fosfomycin was the least-costly choice in areas where local resistance to TMP-SMX was greater than or equal to 30%. Again, the reported price of fosfomycin was far lower than what we observed, just 4.86€ (~5.20 2016 USD).
We conducted a threshold analysis and found that if priced at $7 (a price that is still higher than both multi-dose alternatives), the total cost of treating uUTI would be roughly the same for fosfomycin as for TMP-SMX and nitrofurantoin. This is significant given that Xiromed’s 6-month period of generic exclusivity expired very recently (April 2021), and since then, at least one additional company has received approval to manufacture single-dose fosfomycin therapy for uUTI.17 Consistent with principles of economics, studies of historical data confirm that drug prices fall significantly as the number of pharmaceutical companies manufacturing it increases, due to increased competition.35 Now that the market for fosfomycin is open to new generic manufactures, it is possible that a low-cost single-dose treatment for uUTI could come available soon. Given that the prohibitive cost of the branded drug is oft-cited as a barrier to its use, this could potentially have a significant impact on current antibiotic prescribing patterns. This is especially true in a state like New Hampshire, which places importance on combatting antimicrobial resistance.36,37
Limitations and directions for future research
When interpreting the findings of this study, it is important to consider that many of the assumptions made were specific to the state of New Hampshire and may not be reflective of all other U.S. states/regions. While limiting the scope of the study to New Hampshire was intentional, it is likely that while this decision allowed us to produce more precise estimates for the context, it also forced a compromise in generalizability. That said, this study offers an important first-look into what the cost-effectiveness of fosfomycin is in the United States and how it contrasts with other countries.
Our inability to directly include the long-term benefit of any decreased antimicrobial resistance resulting from the use of single-dose antibiotic therapy, or the potential for improved uUTI resolution for fosfomycin as a result of better medication adherence is also a significant limitation. While including these parameters would almost certainly change our results, it was not possible to account for them with available data.
Similarly, the lack of available data due to infrequent use of fosfomycin within the U.S., today, contributed to our inability to account for differences in the occurrence of adverse events across treatment alternatives. In fact, while a recent study conducted by Butler et al identified a higher adverse event rate in uUTI patients treated with TMP-SMX than in those treated with nitrofurantoin, the authors were unable to analyze the effects of fosfomycin therapy.38 Should additional data enable researchers to evaluate the comparative effect of fosfomycin therapy on the occurrence of adverse events in the future, future research should incorporate this data into cost-effectiveness models, as it could demonstrate additional value for the drug.
Aside from the beforementioned broader issues, there are a few more specific limitations pertaining to our model that are worth noting. First, the probability of uUTI resolution with initial treatment (both sensitive and resistant) was assumed to be the same for all 3 treatment alternatives. This may not be true, particularly in the case of isolates that are sensitive, where mutations may happen more or less frequently for a given therapy. Future studies may find randomized controlled trials helpful in determining whether there is variation. Second, the source we used for our base-case assumptions of direct and indirect costs of pyelonephritis, was published 15 years ago. We inflated the costs reported in Brown, Ki, and Foxman’s paper to 2020 dollars, but the true cost of treatment today may be far different, given that medical practice can evolve significantly over time and the inflation rate for medical care could be far higher than the general inflation rate. Finally, it was not ideal that our susceptibility assumptions for fosfomycin were taken from randomized control trials instead of the New Hampshire Antibiogram. Though even if this resulted in bias, it is more likely that our results are understated since New Hampshire, which is far more rural than Boston, Massachusetts (the setting of the randomized control trial), would likely have less resistance due to reduced community spread.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MK conceptualized this research study, collected data, conducted analysis, and prepared the first draft of the manuscript. MSC provided clinical and geographical expertise. RO contributed to both the study design and methodological approach and provided project leadership. All of the authors reviewed drafts for intellectual content and approved the final manuscript for publication.
ORCID iDs: Morgan Kassabian  https://orcid.org/0000-0001-8252-2280
https://orcid.org/0000-0001-8252-2280
Robert Ohsfeldt  https://orcid.org/0000-0002-6117-056X
https://orcid.org/0000-0002-6117-056X
References
- 1. Urologyhealth.org. Understanding UTIs across the lifespan. Published 2016. Accessed August 20, 2021. https://www.urologyhealth.org/healthy-living/urologyhealth-extra/magazine-archives/summer-2016/understanding-utis-across-the-lifespan
- 2. Datatools.ahrq.gov. Number of people with care in thousands by condition, United States, 1996 to 2019. Updated September 2020. Accessed August 20, 2021. https://datatools.ahrq.gov/meps-hc
- 3. Datatools.ahrq.gov. Total expenditures in millions by condition, United States, 1996 to 2019. Updated September 2020. Accessed August 20, 2021. https://datatools.ahrq.gov/meps-hc
- 4. Cdc.gov. Urinary tract infection. Updated August 27, 2019. Accessed August 20, 2021. https://www.cdc.gov/antibiotic-use/community/for-patients/common-illnesses/uti.html
- 5. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120. [DOI] [PubMed] [Google Scholar]
- 6. Cdc.gov. Core elements of antibiotic Stewardship. Updated April 7, 2021. Accessed August 20, 2021. https://www.cdc.gov/antibiotic-use/core-elements/index.html
- 7. Cdc.gov. About antibiotic resistance. Updated March 13, 2020. Accessed August 20, 2021. https://www.cdc.gov/drugresistance/about.html
- 8. Businesswire.com. UTILITY receives investigational new drug approval from US FDA. Published September 19, 2018. Accessed December 1, 2020. https://www.businesswire.com/news/home/20180918006149/en/UTILITY-Receives-Investigational-New-Drug-Approval-from-US-FDA
- 9. Cdc.gov. Antibiotic do’s & don’ts. Updated January 31, 2020. Accessed August 20, 2021. https://www.cdc.gov/antibiotic-use/do-and-dont.html
- 10. Combating antibiotic resistance. Updated October 29, 2019. Accessed August 20, 2021. https://www.fda.gov/consumers/consumer-updates/combating-antibiotic-resistance
- 11. Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev. 2016;29:321-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cai T, Tamanini I, Tascini C, et al. Fosfomycin trometamol versus comparator antibiotics for the treatment of acute uncomplicated urinary tract infections in women: A systematic review and meta-analysis. J Urol. 2020;203:570-578. [DOI] [PubMed] [Google Scholar]
- 13. Hirsch EB, Raux BR, Zucchi PC, et al. Activity of fosfomycin and comparison of several susceptibility testing methods against contemporary urine isolates. Int J Antimicrob Agents. 2015;46:642-647. [DOI] [PubMed] [Google Scholar]
- 14. Wankum M, Koutsari C, Gens K. Fosfomycin use. Pharm Times. 2017;6:6. [Google Scholar]
- 15. Shenvi C. Fosfomycin use. Emergency Physicians Monthly. 2014. Accessed August 20, 2021. https://epmonthly.com/article/fosfomycin-monurol/
- 16. Drugs.com. User reviews for monurol. Accessed August 20, 2021. https://www.drugs.com/comments/fosfomycin/monurol.html
- 17. Drugs.com. Generic monurol availability. Updated August 11, 2021. Accessed August 20, 2021. https://www.drugs.com/availability/generic-monurol.html
- 18. Data.medicaid.gov. NADAC (National Average Drug Acquisition Cost). Updated 2021. Accessed July 1, 2021. https://data.medicaid.gov/nadac
- 19. Perrault L, Dahan S, Iliza AC, LeLorier J, Zhanel GG. Cost-effectiveness analysis of fosfomycin for treatment of uncomplicated urinary tract infections in Ontario. Can J Infect Dis Med Microbiol. 2017;2017:6362804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sadler S, Holmes M, Ren S, Holden S, Jha S, Thokala P. Cost-effectiveness of antibiotic treatment of uncomplicated urinary tract infection in women: a comparison of four antibiotics. BJGP Open. 2017;1:bjgpopen17X101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruralhealthinfo.org. New Hampshire. Accessed August 20, 2021. Ruralhealthinfo.org/states/new-hampshire [Google Scholar]
- 22. New Hampshire Department of Health and Human Services, Division of Public Health Services. State of New Hampshire: 2018 State Antibiogram & Implications for Antibiotic Prescribing. New Hampshire Department of Health and Human Services, Division of Public Health Services; 2020. [Google Scholar]
- 23. NH Health Cost. NH Health Cost. Accessed July 1, 2021. Nhhealthcost.nh.gov/
- 24. FrontlineSolvers. Frontline solvers excel product overview. Accessed August 20, 2021. https://www.solver.com/products-overview
- 25. One-dh.testcatalog.org. DH Inpatient & Outpatient Urine 2021 Data. Published 2022. Accessed October 3, 2022. https://one-dh.testcatalog.org/catalogs/565/files/14151
- 26. Uvmhn.s3.amazonaws.com. Antimicrobial susceptibility results 1/1/2020-12/31/2020. Published 2021. Accessed August 20, 2021. https://uvmhn.s3.amazonaws.com/www.uvmhealth.org/assets/2020-09/gram-positive-antibiogram.pdf
- 27. Uvmhn.s3.amazonaws.com. Antimicrobial susceptibility results 1/1/2020-12/31/2020. Published 2021. Accessed August 20, 2021. https://uvmhn.s3.amazonaws.com/www.uvmhealth.org/assets/2020-09/gram-negative-antibiogram.pdf
- 28. Maraki S, Samonis G, Rafailidis PI, Vouloumanou EK, Mavromanolakis E, Falagas ME. Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob Agents Chemother. 2009;53:4508-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bls.gov. CPI inflation calculator. Accessed August 20, 2021. https://www.bls.gov/data/inflation_calculator.htm
- 30. Kff.org. Market share and enrollment of largest three insurers – individual market. Accessed August 20, 2021. kff.org/private-insurance/state-indicator/market-share-and-enrollment-of-largest-three-insurers-individual-market/?currentTimeframe=0&sortModel=%7B“colId”:“Location”,“sort”:“asc”%7D
- 31. Brown P, Ki M, Foxman B. Acute pyelonephritis among adults: cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics. 2005;23:1123-1142. [DOI] [PubMed] [Google Scholar]
- 32. Le T, Miller L. Empirical therapy for uncomplicated urinary tract infections in an era of increasing antimicrobial resistance: a decision and cost analysis. Clin Infect Dis. 2001;33:615-621. [DOI] [PubMed] [Google Scholar]
- 33. Cms.gov. Search the physician fee schedule. Updated July 01, 2021. Accessed August 20, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- 34. Arinvestments.cdc.gov. Antibiotic resistance (AR) investment map. Accessed August 20, 2021. https://arinvestments.cdc.gov/
- 35. Fda.gov. Generic competition and drug prices. Updated December 13, 2019. Accessed August 20, 2021. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/generic-competition-and-drug-prices
- 36. Dhhs.nh.gov. Antimicrobial resistance and stewardship for healthcare providers. Accessed August 20, 2021. https://www.dhhs.nh.gov/dphs/cdcs/hai/ar-providers.htm
- 37. Arinvestments.cdc.gov. AR solutions in action: CDC’s investments to combat antibiotic resistance threats. Accessed August 20, 2021. https://arinvestments.cdc.gov/PDFDocs/New-Hampshire-CDC-AR-Investments.pdf
- 38. Butler AM, Durkin MJ, Keller MR, Ma Y, Powderly WG, Olsen MA. Association of adverse events with antibiotic treatment for urinary tract infection. Clin Infect Dis. 2022;74:1408-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]