Abstract
Introduction:
Experiencing psychosocial stress is associated with poor health outcomes such as hypertension and obesity, which are risk factors for developing cardiovascular disease. African American women experience disproportionate risk for cardiovascular disease including exposure to high levels of psychosocial stress. We hypothesized that psychosocial stress, such as perceived stress overload, may influence epigenetic marks, specifically DNA methylation (DNAm), that contribute to increased risk for cardiovascular disease in African American women.
Methods:
We conducted an epigenome-wide study evaluating the relationship of psychosocial stress and DNAm among African American mothers from the Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure (InterGEN) cohort. Linear mixed effects models were used to explore the epigenome-wide associations with the Stress Overload Scale (SOS), which examines self-reported past-week stress, event load and personal vulnerability.
Results:
In total, n = 228 participants were included in our analysis. After adjusting for known epigenetic confounders, we did not identify any DNAm sites associated with maternal report of stress measured by SOS after controlling for multiple comparisons. Several of the top differentially methylated CpG sites related to SOS score (P < 1 × 10−5), mapped to genes of unknown significance for hypertension or heart disease, namely, PXDNL and C22orf42.
Conclusions:
This study provides foundational knowledge for future studies examining epigenetic associations with stress and other psychosocial measures in African Americans, a key area for growth in epigenetics. Future studies including larger sample sizes and replication data are warranted.
Keywords: DNA methylation, stress, cardiovascular disease, African American, EWAS, women’s health
Introduction
Evidence indicates that stress is an important risk factor in regards to cardiovascular disease.1,2 For example, stress has been significantly associated with hypertension, a key risk factor for cardiovascular disease.3-5 Black women have the unique intersectional reality of experiencing stressors such as discrimination on the basis of gender and race also known as “gendered racism,”6 that has been shown to negatively affect health outcomes among African American women.7,8 Other stressors such as parenting stress9 and financial stress disproportionately affect Black women10 and have been associated with adverse health outcomes.11
Women report higher overall levels and different types of stress compared with men 12; and sex differences in pathophysiological and psychosocial mechanisms related to stress have been reported.13-15 It is likely that genomic and social factors, including pyschosocial stress, are driving hypertension disparities.16 Changes in DNA methylation can be influenced by environmental stressors, and has been identified as a potential mechanism by which stress may predispose individuals to chronic health conditions such as hypertension, obesity, and diabetes.17-19 Previous research reported that parenting stress was associated with loss of methylation at over 90 cytosine-phosphate-guanine (CpG) sites, notably in genes associated with the stress signaling pathway including poly (ADP-ribose) polymerase-1 (PARP-1).9 These findings indicated that higher levels of parenting stress were significantly associated with DNA methylation (DNAm) changes among young African American mothers.
To explore whether psychosocial stress is associated with DNA methylation, we conducted an epigenome-wide association study (EWAS) among young African American mothers who participated in the Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure (InterGEN) Study. We hypothesized that there would be associations between perceived stress and DNA methylation, given prior literature that have reported the linkages between stress and DNA methylation.20-22
Materials and Methods
For the present secondary analysis, we examined data from the InterGEN study, which was a prospective cohort study that examined the effects of genetic, epigenetic, and psychosocial factors on blood pressure in 250 mother/child dyads. Eligibility criteria included the following characteristics: mothers (⩾21 years old) who self-identified as African American or Black, spoke English and had no diagnosis of mental illnesses that could prevent collection of psychosocial measures. The mother-child dyads were recruited from childcare centers in Connecticut over a 5-year period between 2014 and 2019, and participated in 4 interviews over a period of 2 years. Sociodemographic measures as well as other self-reported psychosocial measures (eg, parenting stress, perceived racism and discrimination, and depression) were collected through using Audio Computer-Assisted Self-Interview (ACASI) software. Additional recruiting measures and practices have been previously described.23,24 Institutional Review Board (IRB) approval was obtained from Yale University, New York University and Columbia University.
Survey measures
The Stress Overload Scale (SOS) is a validated instrument that was developed to measure psychosocial stress.25 The SOS is a practical, multiculturally sensitive, and brief questionnaire that captures perception of overload, which is a state of feeling overwhelmed or overextended. It was derived using community-based samples and has been used in diverse populations. The SOS is comprised of 2 subscales that total 24 items (min: 24; max: 119). The 2 subscales include a 12-item personal vulnerability scale (min: 12; max 59) and a 12-item event load scale (min: 12; max: 60). Both measures capture levels of self-reported vulnerability (ie, feelings of powerlessness and frailty) in the past week as well as self-reported past-week event-load (ie, external demands and pressures).25 Items are scored on a 5-point Likert scale from 1 (not at all) to 5 (a lot). Subscales were calculated by adding totals in each subscale, and the total SOS score was calculated by summing the total subscale scores.
Potential confounding variables
Mothers self-reported their age, if they were currently smokers (yes/no) and other demographic data at the initial interview. We adjusted for age and smoking, which are known confounders in epigenetic studies.26 We also adjusted for batch effects and potential heterogeneity in cell proportions from saliva using the reference-free EWAS method by Houseman et al.27 This is also a method frequently utilized in previous research and literature.28
DNA collection, processing and DNA methylation profiling
Saliva was collected from participants for DNA analysis using Oragene (OG)−500 format tubes.29 This procedure requires that participants fill a tube with 2 mL of saliva. DNA extraction and processing was performed based on protocol and standard DNA processing for ReliaPrep, which has been described previously.23 To maintain confidentiality and ensure accuracy of analyses, tubes were barcoded and stored in a laboratory freezer. A barcode scanning system was used for DNA pipetting with robotic workstations to maintain the accuracy and integrity during the transfer process from tubes to plates. This process ensured that accurately identified participants’ DNA for correct merging to genotype calls.
To quantify DNA methylation, the Illumina Infinium Methylation EPIC (850K) BeadChip was used, and quantile-normalization of beta values was completed for autosomal CpG sites. Laboratory-based quality control procedures (missing rate <10% and no sex mismatch) were implemented for each saliva sample. CpG sites were excluded if they had a missing rate greater than 10% (n = 3343), overlapped with single nucleotide polymorphisms (n = 87 074), or were listed in the recent Illumina quality notice (n = 977). Ultimately, a total of 756 448 autosomal sites were included in the association analyses.
Statistical analyses
An epigenome-wide association study (EWAS) analysis of total stress overload scores was conducted among InterGEN mothers (N = 228). We modeled DNAm beta-values as the dependent variable and linear mixed effects models with 2 random effects (batch and chip) were applied to ascertain epigenetic associations with stress overload scores adjusted for age, smoking status and cell-type heterogeneity. We used the reference-free EWAS method to account for cell proportions for saliva samples.27 Principal components (PCs) were also derived using DNAm data30 and we included the top 10 PCs as fixed-effect covariates to account for potential population stratification. False discovery rate (FDR) was used to correct for multiple comparisons and FDR-corrected P < .05 was defined as EWAS significance threshold. We utilized this FDR given its use in other studies in InterGEN that has been previously discussed.31 We conducted analyses using the R statistical package environment32 and a selection of packages from Bioconductor.
Results
In our study sample of 250 mothers, 16 were missing data for the SOS total score. Of the 234 participants with complete SOS data, 6 participants’ DNAm samples did not pass quality assessment and were excluded from analysis. A total of N = 228 mothers were included in the EWAS analysis of stress overload, and characteristics of the study sample are summarized in Table 1. The mean maternal age was 31.3 years. Fifty-eight (25.4%) mothers self-reported that they were smokers. Over half of the sample had completed at least some college (59.2%). With regards to income, nearly half of the sample (46.1%) earned less than or equal to $15 000. More than two-thirds (66.2%) of the sample designated single as their marital status. Mothers reported a mean score of 59.9 (s.d. = 24.2) for stress overload. The mean scores for personal vulnerability and event load were 26.2 (s.d. = 12.4) and 33.7 (s.d. = 13.3) respectively.
Table 1.
Descriptive statistics of study sample (N = 228).
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Age (years) | 31.3 (5.7) |
| Stress Overload Scale total score | 59.9 (24.2) |
| Personal Vulnerability subscale | 26.2 (12.4) |
| Event Load subscale | 33.7 (13.3) |
| Current smokers | 58 (25.4) |
| Education level | |
| Less than high school | 12 (5.3) |
| High school | 81 (35.5) |
| Some college or higher | 135 (59.2) |
| Annual household income | |
| Less than $5000 | 51 (22.4) |
| $5000 to $9999 | 29 (12.7) |
| $10 000 to $14 999 | 25 (11.0) |
| $15 000 to $19 999 | 18 (7.9) |
| $20 000 or above | 101 (44.3) |
| Marital Status | |
| Married | 52 (22.8) |
| Single | 151 (66.2) |
| Divorced | 11 (4.8) |
| Separated | 3 (1.3) |
| Living with significant other | 11 (4.8) |
We did not find significant associations between SOS score and DNA methylation among InterGEN mothers. (Figures 1 and 2) However, several of the top (P < 1 × 10−5) differentially methylated CpGs were positionally mapped to PXDNL, C22orf42, and SND1 genes in mothers (Table 2). Two genes, PXDNL and C22orf42, were identified as top signals for the total stress overload scores in mothers and were also top hits for 2 of the stress overload subscales: personal vulnerability and event load (Supplemental Tables 1 and 2). Hypomethylation of cg20043066 (mapped to intron 4 of PXDNL) was associated with increased total stress overload score (P = 7 × 10−7) as well as increased event load (P = 9.5 × 10−6). Additionally, hypomethylation of cg24060571 (mapped to 927 bp upstream of C22orf42) was associated with increased total stress score (P = 4.6 × 10−6) and increased personal vulnerability (P = 1 × 10−5).
Figure 1.

Quantile-quantile plot of epigenome-wide associations with self-reported stress for mothers. Associations of DNAm level at each CpG site with total stress score was tested using linear mixed model adjusted for age, smoking, cell-type proportions and top 10 principal components. The total sample size is n = 228, and the overall inflation factor is 0.983 showing well-controlled type I error rate. Observed -log10(p) (y-axis) was plotted against expected -log10(p) derived from a uniform distribution (x-axis). Red straight line is y = x and the red curved lines indicate the 95% confidence interval for the expected −log10(p).
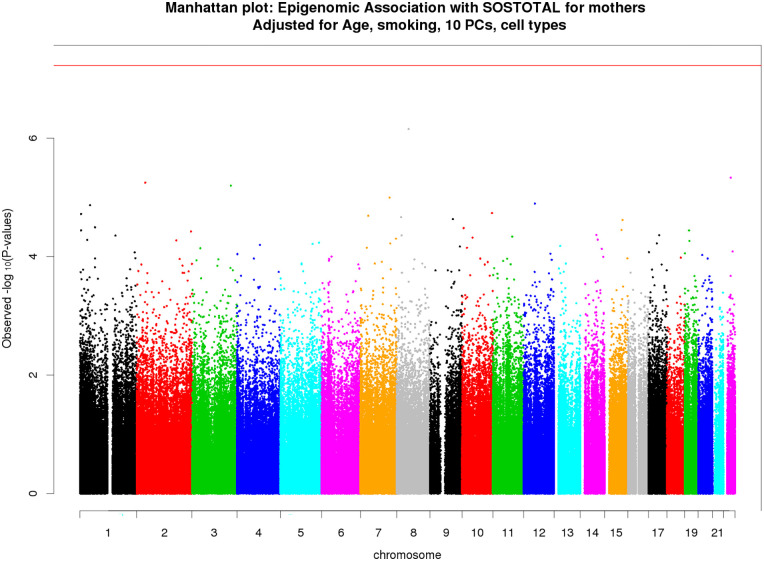
Figure 2.
Manhattan plot of epigenome-wide associations with self-reported stress for mothers. Associations of DNAm level at each CpG site with total stress score was tested using linear mixed model adjusted for age, smoking, cell-type proportions and top 10 principal components. The total sample size is n = 228. −log10(p) of each CpG site is plotted against its genomic position (hg19). Red line indicates the P-value cutoff for epigenome-wide significance.
Table 2.
Top epigenetic associations with self-reported stress for mothers adjusted for age, smoking, cell-type proportions and 10 principal components.
| CpG | CHR | BP* | Gene | Beta | SE | P-value |
|---|---|---|---|---|---|---|
| cg20043066 | 8 | 52467264 | PXDNL | −4.9 × 10−4 | 9.4 × 10−5 | 7.0 × 10−7 |
| cg24060571 | 22 | 32556170 | C22orf42 | −1.5 × 10−4 | 3.1 × 10−5 | 4.6 × 10−6 |
| cg17866650 | 2 | 37798344 | −6.4 × 10−4 | 1.3 × 10−4 | 5.6 × 10−6 | |
| cg08575835 | 3 | 170448125 | 1.4 × 10−4 | 2.9 × 10−5 | 6.3 × 10−6 | |
| cg10057227 | 7 | 127607004 | SND1 | −1.4 × 10−4 | 3.0 × 10−5 | 1.0 × 10−5 |
Base-pair position using human genome build hg19.
Discussion
This study explored associations between perceived stress overload and epigenome-wide DNA methylation in African American mothers. In this cohort of African American women, perceived stress overload was not associated with differences in epigenome-wide DNA methylation. However, we did identify differentially methylated sites based on stress overload scores that warrant follow-up testing in larger cohorts with improved power to detect significant differences across the epigenome.
PXDNL, the gene that encodes the peroxidasin-like protein, has been found to be exclusively expressed in the heart33 and previous research suggests that mutations in the PXDNL gene may lead to ventricular arrhythmias.34 Although the exact function of peroxidasin-like protein remains unknown, it has been implicated in cardiovascular health.35-37 Other peroxidases in the heart are responsible for breaking down hydrogen peroxide (H2O2), a common reactive oxygen species generated during aerobic respiration with known roles in the etiology of hypertension.38-40 It is possible that if hypomethylation of this gene is associated with increased expression, it may be an adaptive marker associated the cellular responsiveness to experiencing increased stress. Further research is required to understand the exact consequences of differential methylation in the expression of PXDNL, and how that could contribute to the etiology of hypertension.
Another gene that exhibited a large difference in methylation associated with stress was C22orf42, a validated open reading frame that has been found to be expressed profusely in testis, prostate, some brain cells and adrenal gland.41,42 There is growing evidence to suggest that there is a relationship between stress in early life and the HPA axis. Some studies have linked the stress and HPA activation with mental health outcomes43 and alcohol dependence.44 However, there is still a critical research gap regarding the role of the protein produced by C22orf42 and its potential role in cardiovascular health.
The construct of stress overload among African American women is complex and may have been underreported as given cultural nuances and norms that normalize portraying strength and suppressing stress.45 Others have explained how maladaptive coping mechanisms such as John Henryism, which involves “high effort coping” may lead to adverse health outcomes; particularly as it pertains to cardiovascular disease risk.46,47 Other constructs such as the “Strong Black Woman Schema” or “Superwoman Syndrome” posit that despite exposure to stress and discrimination, African Americans may feel compelled to relay strength whilst not acknowledging or suppressing their perceptions of these stressors.48,49 In turn, this may have deleterious health effects and may elevate risk for hypertension and cardiovascular risk.50 Steinhardt et al.51 found that psychosocial intervention that explored stress, resilience, and diabetes self-management among diabetic African Americans found improvements in physical risk factors such as BMI and systolic and diastolic blood pressure that were statistically significant as well as in psychosocial factors such as empowerment and diabetes self-management. However, in regards to stress, there were no statistically significant changes. These findings suggest that that while individuals’ perception of their stress may not change, the underlying effects of stress could be influencing epigenetic changes to occur.
A prior study examining stress, coping and methylation of blood pressure-related genes in InterGEN, showed that stress overload and coping strategies were associated with epigenetic changes, though these relationships did not hold after accounting for multiple testing adjustment.52 Additionally, stress and coping were not found to be significantly associated with blood pressure candidate genes.52 This present analysis expanded on the previous study by employing an epigenome-wide approach to assess potential associations of stress overload with DNAm. Other InterGEN analyses have also demonstrated the importance of chronic stressors such as neighborhood stress and experiencing racial discrimination and how exposure to such stressors negatively impact mental and physical health.53-55
It is imperative that future research explore culturally-sensitive ways to capture stress and examine possible changes to stress levels over time. While there are several validated measures to capture stress such as the Perceived Stress Scale (PSS) (past 30 days stress),56 Weekly Stress Inventory (WSI) (past week stress),57 and others, there is no consensus for measuring stress/ideal timeframes for measurement. Similarly, this is also apparent with measures of stress in childhood,58,59 Longitudinal data regarding the examination and dynamic nature of stress is needed.
This study addressed a gap in the literature on epigenomic associations with stress among a cohort of African American women, a group that is often underrepresented in epigenomic studies.60 However, there are some limitations to consider. First, we do not have a replication analysis for our results. Future studies can expand on our sample size using multiple cohorts with meta analyses to increase power for gene discovery. Given our sample size, it is possible that we were underpowered to detect these associations. Also, our measurement of stress may not have captured the full spectrum of stressors faced by African American women. Studies have shown that African American women face a variety of stressors, including stress related to parenting,9 as well as racism and gender discrimination.61 Additionally, the SOS assesses past week levels of stress and may not be representative of cumulative stress, which may have a considerable influence on DNAm. Lastly, it is also important to consider that there are many other social determinants outside the scope of this analysis that could have a considerable effect on stress and/or DNAm such as neighborhood stress/safety62 and social support,63 for example.
Conclusion
There has been a significant interest in expanding knowledge on how social epigenomics may be influencing disparities; particularly among African American women who are disproportionately burdened by health disparities. Despite increased interest, there remains a dearth of epigenetic studies that include African Americans participants. This analysis provides a foundation for future studies examining epigenetic associations with stress and other psychosocial measures among African Americans.
Supplemental Material
Supplemental material, sj-docx-1-gae-10.1177_25168657221126314 for Stress Overload and DNA Methylation in African American Women in the Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure Study by Jolaade Kalinowski, Yunfeng Huang, Martin A Rivas, Veronica Barcelona, Michelle L Wright, Cindy Crusto, Tanya Spruill, Yan V Sun and Jacquelyn Y Taylor in Epigenetics Insights
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institutes of Health, National Institute of Nursing Research [R01NR013520].
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: J.K., M.R., V.B., M.W., C.C., T.S., Y.S., contributed to the writing of the manuscript and provided critical review, Y.H. contributed to data to the analysis and provided critical review of the manuscript.
ORCID iDs: Michelle L Wright  https://orcid.org/0000-0002-9348-8740
https://orcid.org/0000-0002-9348-8740
Jacquelyn Y Taylor  https://orcid.org/0000-0002-0858-7358
https://orcid.org/0000-0002-0858-7358
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Caceres BA, Barcelona V, Crusto C, Taylor JY. Exploring psychosocial mediators of the associations of lifetime trauma and Body Mass Index in African American Women. Health Equity. 2020;4:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brondolo E, Byer K, Gianaros P, et al. Stress and Health Disparities: Contexts, Mechanisms and Interventions Among Racial/Ethnic Minority and Low Socioeconomic Status Populations. American Psychological Association; 2017. [Google Scholar]
- 3. Spruill TM. Chronic psychosocial stress and hypertension. Curr Hypertens Rep. 2010;12:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spruill TM, Butler MJ, Thomas SJ, et al. Association between high perceived stress over time and incident hypertension in black adults: findings from the Jackson Heart Study. J Am Heart Assoc. 2019;8:e012139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalinowski J, Kaur K, Newsome-Garcia V, et al. Stress interventions and hypertension in black women. Womens Health. 2021;17:17455065211009751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis JA, Williams MG, Peppers EJ, Gadson CA. Applying intersectionality to explore the relations between gendered racism and health among Black women. J Couns Psychol. 2017;64:475-486. [DOI] [PubMed] [Google Scholar]
- 7. Lewis TT, Kravitz HM, Janssen I, Powell LH. Self-reported experiences of discrimination and visceral fat in middle-aged African-American and Caucasian women. Am J Epidemiol. 2011;173:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Everson-Rose SA, Lutsey PL, Roetker NS, et al. Perceived discrimination and incident cardiovascular events: the multi-ethnic study of Atherosclerosis. Am J Epidemiol. 2015;182:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright ML, Huang Y, Hui Q, et al. Parenting stress and DNA methylation among African Americans in the InterGEN study. J Clin Transl Sci. 2017;1:328-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hughes M, Kiecolt KJ, Keith VM. How racial identity moderates the impact of financial stress on mental health among African Americans. Ment Health Soc. 2014;4:38-54. [Google Scholar]
- 11. Moran KE, Ommerborn MJ, Blackshear CT, Sims M, Clark CR. Financial Stress and risk of coronary heart disease in the Jackson Heart Study. Am J Prev Med. 2019;56:224-231. [DOI] [PubMed] [Google Scholar]
- 12. Xu X, Bao H, Strait K, et al. Sex differences in perceived stress and early recovery in young and middle-aged patients with acute myocardial infarction. Circulation. 2015;131:614-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113-132. [DOI] [PubMed] [Google Scholar]
- 14. Bangasser DA, Curtis A, Reyes BA, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:896-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52:318-327. [DOI] [PubMed] [Google Scholar]
- 16. Musemwa N, Gadegbeku CA. Hypertension in African Americans. Curr Cardiol Rep. 2017;19:129. [DOI] [PubMed] [Google Scholar]
- 17. Millis RM. Epigenetics and hypertension. Curr Hypertens Rep. 2011;13:21-28. [DOI] [PubMed] [Google Scholar]
- 18. Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopomo A, Burgio E, Migliore L. Epigenetics of obesity. Prog Mol Biol Transl Sci. 2016;140:151-184. [DOI] [PubMed] [Google Scholar]
- 20. Klengel T, Pape J, Binder EB, Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacol. 2014;80:115-132. [DOI] [PubMed] [Google Scholar]
- 21. Unternaehrer E, Luers P, Mill J, et al. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Transl Psychiatry. 2012;2:e150-e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murgatroyd C, Patchev AV, Wu Y, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559-1566. [DOI] [PubMed] [Google Scholar]
- 23. Taylor JY, Wright ML, Crusto CA, Sun YV. The intergenerational impact of genetic and psychological factors on blood pressure (InterGEN) study: Design and methods for complex DNA analysis. Biol Res Nurs. 2016;18:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crusto CA, Barcelona de Mendoza V, Connell CM, Sun YV, Taylor JY. The intergenerational impact of genetic and psychological factors on blood pressure study (InterGEN): Design and methods for recruitment and psychological measures. Nurs Res. 2016;65:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amirkhan JH. Stress overload: A new approach to the assessment of stress. Am J Community Psychol. 2012;49:55-71. [DOI] [PubMed] [Google Scholar]
- 26. Rakyan VK, Down TA, Balding DJ, Beck S. Epigenome-wide association studies for common human diseases. Nat Rev Genet. 2011;12:529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Houseman EA, Kile ML, Christiani DC, Ince TA, Kelsey KT, Marsit CJ. Reference-free deconvolution of DNA methylation data and mediation by cell composition effects. BMC Bioinformatics. 2016;17:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barcelona de Mendoza V, Wright ML, Agaba C, et al. A systematic review of DNA methylation and preterm birth in African American women. Biol Res Nurs. 2017;19:308-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bahlo M, Stankovich J, Danoy P, et al. Saliva-derived DNA performs well in large-scale, high-density single-nucleotide polymorphism microarray studies. Cancer Epidemiol Biomarkers Prev. 2010;19:794-798. [DOI] [PubMed] [Google Scholar]
- 30. Barfield RT, Almli LM, Kilaru V, et al. Accounting for population stratification in DNA methylation studies. Genet Epidemiol. 2014;38:231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barcelona V, Wang Z, Crusto C, Hui Q, Sun YV, Taylor JY. High blood pressure in pregnancy, DNA methylation, and later blood pressure in African American women enrolled in the InterGEN study. Birth. 2020;47:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2013. [Google Scholar]
- 33. Péterfi Z, Tóth ZE, Kovács HA, et al. Peroxidasin-like protein: a novel peroxidase homologue in the human heart. Cardiovasc Res. 2014;101:393-399. [DOI] [PubMed] [Google Scholar]
- 34. Barajas-Martinez H, Smith M, Hu D, et al. Susceptibility to ventricular arrhythmias resulting from mutations in FKBP1B, PXDNL, and SCN9A evaluated in hiPSC cardiomyocytes. Stem Cells Int. 2020;2020:8842398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bathish B, Paumann-Page M, Paton LN, Kettle AJ, Winterbourn CC. Peroxidasin mediates bromination of tyrosine residues in the extracellular matrix. J Biol Chem. 2020;295:12697-12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma QL, Zhang G-G, Peng J. Vascular peroxidase 1: a novel enzyme in promoting oxidative stress in cardiovascular system. Trends Cardiovasc Med. 2013;23:179-183. [DOI] [PubMed] [Google Scholar]
- 37. Bhave G, Cummings CF, Vanacore RM, et al. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol. 2012;8:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Investig. 1980;65:128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blankenberg S, Rupprecht HJ, Bickel C, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605-1613. [DOI] [PubMed] [Google Scholar]
- 40. Ardanaz N, Yang XP, Cifuentes ME, et al. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension. 2010;55:116-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dunham I, Shimizu N, Roe BA, et al. The DNA sequence of human chromosome 22. Nature. 1999;402:489-495. [DOI] [PubMed] [Google Scholar]
- 42. Takamitsu E, Otsuka M, Haebara T, et al. Identification of human N-myristoylated proteins from human complementary DNA resources by cell-free and cellular metabolic labeling analyses. PLoS One. 2015;10:e0136360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Juruena MF, Eror F, Cleare AJ, Young AH. The role of early life stress in HPA Axis and Anxiety. Adv Exp Med Biol. 2020;1191:141-153. [DOI] [PubMed] [Google Scholar]
- 44. Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34:468-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beauboeuf-Lafontant T. You have to show strength: an exploration of gender, race, and depression. Gender Soc. 2007;21:28-51. [Google Scholar]
- 46. James SA, Hartnett SA, Kalsbeek WD. John Henryism and blood pressure differences among black men. J Behav Med. 1983;6:259-278. [DOI] [PubMed] [Google Scholar]
- 47. Felix AS, Shisler R, Nolan TS, et al. High-effort coping and cardiovascular disease among women: A systematic review of the John Henryism hypothesis. J Urban Health. 2019;96:12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woods-Giscombé CL. Superwoman schema: African American women’s views on stress, strength, and health. Qual Health Res. 2010;20:668-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Belgrave FZ, Abrams JA. Reducing disparities and achieving equity in African American women’s health. Am Psychol. 2016;71:723-733. [DOI] [PubMed] [Google Scholar]
- 50. Abrams JA, Maxwell M, Pope M, Belgrave FZ. Carrying the world with the grace of a lady and the grit of a warrior: deepening our understanding of the “Strong Black Woman” schema. Psychol Women Q. 2014;38:503-518. [Google Scholar]
- 51. Steinhardt MA, Mamerow MM, Brown SA, Jolly CA. A resilience intervention in African American adults with type 2 diabetes. Diabetes Educ. 2009; 35:274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brown KM, Hui Q, Huang Y, et al. Association between stress and coping with DNA methylation of blood pressure-related genes among African American women. Chronic Stress. 2019;3:2470547019879088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Condon EM, Barcelona V, Ibrahim BB, Crusto CA, Taylor JY. Racial discrimination, mental health, and parenting among African American Mothers of preschool-aged children. J Am Acad Child Adolesc Psychiatry. 2022;61:402-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Basile Ibrahim B, Barcelona V, Condon EM, Crusto CA, Taylor JY. The association between neighborhood social vulnerability and cardiovascular health risk among Black/African American women in the InterGEN Study. Nurs Res. 2021;70:S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barcelona de Mendoza V, Huang Y, Crusto CA, Sun YV, Taylor JY. Perceived racial discrimination and DNA methylation among African American women in the InterGEN Study. Biol Res Nurs. 2018;20:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-396. [PubMed] [Google Scholar]
- 57. Brantley P, Bodenlos JS, Cowles M, Whitehead D, Ancona M, Jones GN. Development and validation of the Weekly Stress Inventory-Short Form. J Psychopathol Behav Assess. 2007;29:54-59. [Google Scholar]
- 58. Kassam-Adams N. The Acute Stress Checklist for Children (ASC-Kids): development of a child self-report measure. J Trauma Stress. 2006;19:129-139. [DOI] [PubMed] [Google Scholar]
- 59. Vanaelst B, De Vriendt T, Huybrechts I, Rinaldi S, De Henauw S. Epidemiological approaches to measure childhood stress. Paediatr Perinat Epidemiol. 2012;26:280-297. [DOI] [PubMed] [Google Scholar]
- 60. Mudd-Martin G, Cirino AL, Barcelona V, et al. Considerations for cardiovascular genetic and genomic research with marginalized racial and ethnic groups and Indigenous Peoples: A Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2021;14:e000084. [DOI] [PubMed] [Google Scholar]
- 61. Perry BL, Harp KL, Oser CB. Racial and gender discrimination in the stress process: implications for African American Women’s Health and well-being. Sociol Perspect. 2013;56:25-48. [PMC free article] [PubMed] [Google Scholar]
- 62. Giurgescu C, Nowak AL, Gillespie S, et al. Neighborhood environment and DNA methylation: implications for cardiovascular disease risk. J Urban Health. 2019;96:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Surkan PJ, Hong X, Zhang B, et al. Can social support during pregnancy affect maternal DNA methylation? Findings from a cohort of African-Americans. Pediatr Res. 2020;88:131-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-gae-10.1177_25168657221126314 for Stress Overload and DNA Methylation in African American Women in the Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure Study by Jolaade Kalinowski, Yunfeng Huang, Martin A Rivas, Veronica Barcelona, Michelle L Wright, Cindy Crusto, Tanya Spruill, Yan V Sun and Jacquelyn Y Taylor in Epigenetics Insights