Abstract
Introduction: Neurofibromatosis type 1(NF-1) is the commonest neurocutaneous phacomatosis in children. Epilepsy is an infrequent comorbidity. Reports of seizure and Electroencephalogram (EEG) characteristics in children are sparse. Methods: A retrospective review was performed on patients with NF-1 seen between 2016–2020. Patients with co-existing epilepsy were identified. Demographic, clinical, radiological and neurophysiological data were reviewed and analyzed. Results: Out of 118 children with NF1, 16 had epilepsy. 11 patients had focal onset seizures, whereas 5 had generalized onset seizures. Most patients had easy seizure control. Focal epileptiform discharges were the most prevalent EEG abnormality. There was no significant correlation between seizure patterns and presence of intracranial tumors. Conclusion: Epilepsy is a relatively uncommon in pediatric NF-1. Seizures are often of focal semiology and likely to be easily controlled. Focal and multifocal spike epileptiform discharges are the typical interictal EEG findings. Correlation of clinical and EEG findings with intracranial lesions is poor.
Keywords: epilepsy, EEG, neurofibromatosis, children, brain tumor
Introduction
Neurofibromatosis type 1 (NF-1) is a multi-organ disorder due to the faulty production of a specific protein called Neurofibromin secondary to a germline genetic mutation in chromosome 17 at 17q11.2.1 The disease is inherited via an autosomal dominant pattern with variable clinical expression. NF-1 has distinct developmental, neurological, and dermatological features. It is the most common neurocutaneous syndrome with an average prevalence of 1/4000 and a birth incidence that is close to 1/2000 in the recent epidemiological Finish studies.2,3 Clinical manifestations include characteristic skin and central nervous system tumors, such as cutaneous and subcutaneous plexiform neurofibromas, as well as various brain and spinal cord tumors. NF-1 associated epilepsy is believed to be relatively infrequent, but other neurological comorbidities can also often occur, including intellectual disability, attention deficit hyperactivity disorder, autism spectrum disorder and chronic migraine.4
Methods
Nemours Children's Health Institutional Review Board (IRB) approval for a retrospective chart review was sought. Registry of patients with neurofibromatosis type 1 was then accessed. Using ICD-10-CM code of Q85.01 that encodes for NF-1, the medical records of patients seen at the institution over 5 years from January 2016 till December 2020 were retrieved. An approved data collection sheet was used to record desired data, including gender, presence or absence of comorbid epilepsy diagnosis, age at the time of Electroencephalogram (EEG) diagnostic study or epilepsy diagnosis, epilepsy type or classification, number and names of daily preventive seizure medications, presence of brain tumors in neuroimaging studies, other pertinent neuroimaging features, summary reports of initial EEG findings and any additional significant clinical information. After identifying patients with comorbid epilepsy, a separate data collection sheet was then utilized. Results were charted and analyzed using SPSS program version 28.
Results
During the study period from 2016 to 2020, 118 children were seen for either an initial or follow up evaluation of NF-1 at our institution. Of the total cohort, 16 patients representing 13% were diagnosed with epilepsy and subjected to further analysis (Table 1). 10 patients (63%) were females, and 6 patients (37%) were males. When gender distribution in the non-epileptic NF-1 patients was analyzed, no statistical significance of gender risk of epilepsy was found (P value of .895). Age distribution was between 1 and 18 years old, with 7, 5, and 4 patients less 5 years old, 5–12 years old, and more than 12 years old, respectively. The mean age of epilepsy onset was 8.25 ± 2.62 years old. Using the ILAE 2017 classification system and terminology manual, 11 patients (69%) were classified as having focal onset epilepsy, whereas 5 patients (31%) were classified as having generalized epilepsy. 12 patients (75%) were placed maximally on a single daily anti-seizure medication. 4 patients (25%) were completely weaned off all seizure medications during follow-up evaluations (with 2-3 years range of seizure freedom before weaning), although longer follow-up outcomes could not be traced with full accuracy. 4 patients (25%) were on two or more anti-seizure medications, consistent with the functional ILAE definition of medication resistant epilepsy. All of the16 patients (100%) had at least one EEG study. 13 patients (81%) had EEG findings of single focal, independently bi-hemispheric yet focal, or multifocal interictal epileptiform discharges. 2 (13%) patients had an EEG demonstrating diffuse and generalized epileptiform discharges whereas only one patient (6%) had a normal initial interictal EEG study (Figures 1–4 examples of EEG findings in selected 4 patients). 8 patients (50%) had intracranial tumors. Half of the tumors were optic pathway gliomas without evident brain parenchymal extension, occurring in 4 patients (25% of total cohort). By further analysis, there was no significant correlation between the location of the focal EEG epileptiform discharges and the presence or absence of the brain tumors (P value of .144).
Table 1.
Patients’ Summary of Demographic, Clinical and Testing Findings.
| No | Gender | Age | Epilepsy classification | No of ASM | Tumor in MRI | EEG findings |
|---|---|---|---|---|---|---|
| 1 | M | 16 | Focal with b/l TCS | 2 | Left Pre-septal Frontal plexiform neurofibroma, optic pathway glioma | F7/T3 spikes |
| 2 | F | 17 | Focal | 0 | Brainstem low grade glioma, optic pathway glioma | P3, O1 & P4 bilateral independent spikes |
| 3 | F | 15 | Focal | 1 | left thalamic low grade glioma, optic pathway glioma | Multifocal sharps waves |
| 4 | F | 17 | Focal/Rolandic | 1 | No tumor | Left C3/T3 spikes (sleep activated) |
| 5 | F | 10 | Focal | 1 | No tumor | Multifocal spikes |
| 6 | F | 11 | Gen tonic | 2 | Optic pathway glioma | Multifocal spikes |
| 7 | M | 3 | Focal | 0 | No tumor | independent central & temporal spikes |
| 8 | F | 5 | Focal to b/l tonic clonic | 3 | No tumor | Bursts of F4 spike and slow waves |
| 9 | M | 8 | Focal | 1 | No tumor | F3/F7 spikes during sleep |
| 10 | F | 4 | Focal | 1 | Optic pathway glioma | Right Temp focal spikes + diffuse spikes/SW |
| 11 | F | 8 | Focal | 1 | Brainstem low grade glioma | Diffuse SW, left Central & right Temporal spikes |
| 12 | M | 7 | Generalized | 1 | left brainstem low grade glioma | Bursts of High amplitude 3 Hz spike/wave discharges |
| 13 | M | 3 | Generalized | 1 | Optic pathway gliomas | Generalized bursts of poly-spikes/SW complexes |
| 14 | F | 5 | Generalized | 1 | No tumor | No epileptiform discharges initially, then diffuse |
| 15 | F | 2 | Focal | 1 | No tumor | C4, P4, Pz focal spikes |
| 16 | M | 1 | Spasms, Generalized | 3 | no tumor | Multifocal sharps with decrement |
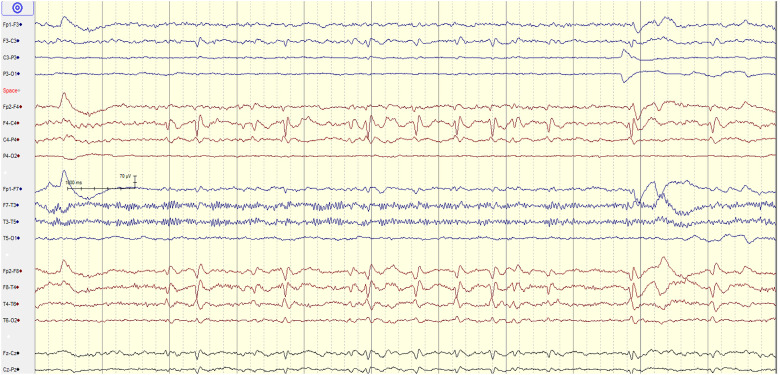
Figure 1.
10-year-old female with NF-1 & no brain tumor. EEG showing frequent, focal, right sided temporal spikes T4/T6 with a field to C4.
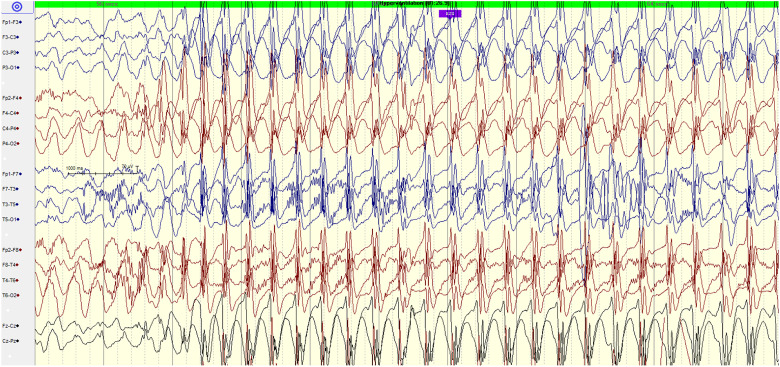
Figure 4.
7-year-old male with NF-1 and midbrain low grade glioma. EEG showing hyperventilation-provoked absence seizure with diffuse, high amplitude, 3 Hz spike/polyspike wave discharges, suggestive of generalized epilepsy.
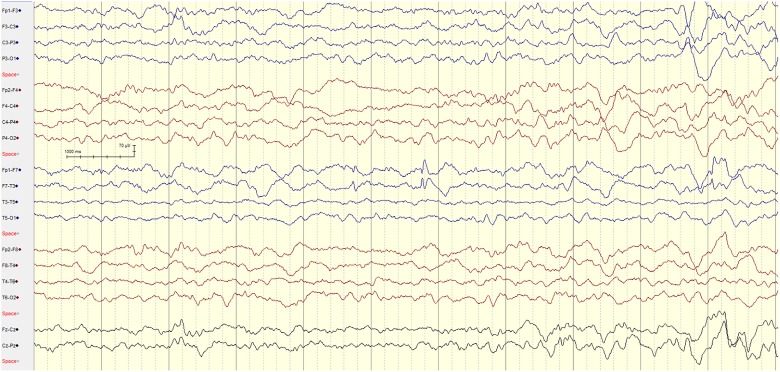
Figure 2.
8-year-old male with NF-1 with no brain tumor and focal epilepsy. EEG showing sleep-activated focal left frontal F7 spikes epileptiform discharges.
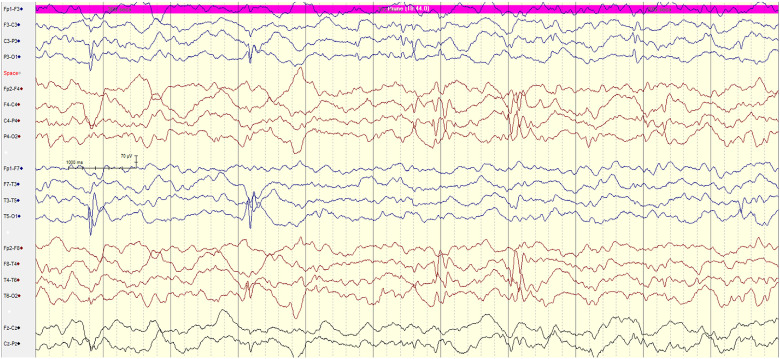
Figure 3.
8-year-old female with NF-1, brain stem tectal glioma and focal epilepsy. EEG showing multi-focal right centro—parietal C4/P4 and independent left centro-temporal C3/T3 spike epileptiform discharges.
Discussion
Epilepsy, defined as recurring unprovoked epileptic seizures, is an uncommon symptomatic manifestation of NF-1. Seizures are thought to occur at least in 6–7% of the NF-1 population.5,6 Epilepsy has been doumented in around 6.5% of 536 NF-1 patients in one of the largest cohorts by Ostendorf et al.7 Our study on the contrary revealed a significantly higher prevalence of epilepsy in NF-1 population (13%). However, a more recent study of 106 patients with NF-1 in South Korea reported a similar prevalence of 13.6%,8 suggesting that epilepsy was possibly underdiagnosed in the earlier reports. Reasons behind the variable prevalence of epilepsy in the NF-1 population can be multifactorial and related to the particular study design. In our study, we propose the prevalence might be influenced by the small sample size, possible selection bias, and potential contribution from a higher frequency of patients with more severe symptoms who were getting care in our specialized Neuro-oncology clinics.
Although the pathophysiological processes behind increased seizure risk in NF-1 patients are not completely understood, multiple etiologies appear to contribute to enhanced cortical excitability, mostly linked to associated structural brain lesions such as brain tumors and focal malformation of cortical development.9 In a systematic review by Bernardo et al including adults and children with NF-1, brain tumors, particularly low grade gliomas, were found to be the commonest etiology of epilepsy.10 However, in our cohort, there was no statistically significant correlation between presence or location of intracranial tumors and epilepsy semiology or EEG localization. This can be attributed to the unique neuroimaging characteristics of our study group, which showed brain tumors in 50% of patients but no brain tumour with invading or infiltrating features of brain parenchymal tissues in standard brain MRI studies. NF-1 can also be associated with other malformations of cortical development which are known epileptogenic foci.11 Cerebrovascular malformations, such as Moya Moya syndrome or a Sturge Weber phenotype, are rarely seen in NF-1 but can contribute to cortical excitability.12,13 Contribution of non-neoplastic spongiform neurofibromatosis unidentified bright objects (UBO) to epilepsy risk has not been confirmed as prior studies had conflicting findings.14,15
Recent reports have suggested a potential role for the Mammalian Target of Rapamycin (mTOR) pathway for neuronal hyperexcitability in NF-1 and other neurocutaneous syndromes, and hence subsequent clinical seizure predisposition.16 In observational reports of animal models, brains of mice with NF-1 showed multiple alterations and dysfunction in a number of ion channels, but a definitive linkage to epilepsy generation hypothesis has not yet been found.17 A possible explanation for those reports can be the presence of additional non-oncological, nonstructural, seizure triggers in NF-1 patients.
Epilepsy severity appears to vary across the few published pediatric studies. It is thought that epileptic patients with pre-existing NF-1 are likely to exhibit fewer seizures and a relatively less incidence of drug resistant seizures when compared to the total cohort of children with epilepsy.18 In contrast, some studies however suggested that seizures can be harder to control in the NF-1 cohort compared to the general epilepsy population with only one third of patients described as well controlled with a single anti-seizure medication.19 The mainstay of treatment for refractory epilepsy in the setting of intracranial tumors remains the gross total resection of the tumor, if possible, followed by other treatment strategies such as traditional chemotherapy or tumor targeted therapy.20,21 Success of epilepsy surgery is higher in patients with epilepsy related to temporal lobe glioma and less achieved in patients with associated hippocampal sclerosis.21,22 Radiation therapy is almost never used given the increased risk for secondary malignancies in children with NF-1.23 Our cohort showed that one fourth of the patients progressed to having frequent uncontrolled seizures consistent with drug resistant epilepsy. Similar to our observation, other reports noted that most seizures are focal onset in semiology with or without secondarily generalized features, although generalized onset seizures have been reported as well.24 Perhaps coincidentally, childhood-specific generalized epilepsy syndromes such as childhood absence epilepsy, juvenile myoclonic epilepsy, and Lennox Gastaut Syndrome were reported in few patients.25 The exact role of NF-1 gene abnormality in activating these specific epilepsy phenotypes is not yet known. Although paroxysms of convulsive status epilepticus are exceedingly rare, there have been few reports of NF-1 attributed hippocampal sclerosis, a finding that more commonly indicates a prior history of prolonged seizures.26 This neuroimaging finding was not observed in our study population. In spite of the current revolution of knowledge regarding genetic risk factors of sudden unexpected death in epilepsy (SUDEP), neither changes in NF1 gene, nor concurrent clinical epilepsy characteristics are among the known SUDEP risk factors.27
There have been only a few published studies elaborating on patterns of electroencephalogram (EEG) abnormalities in pediatric patients with NF-1. It is estimated that EEG can be abnormal in about 25% of the total NF-1 patients’ cohort.7 Interictal focal spikes and sharp waves are the most commonly reported epileptiform EEG discharges.28 Generalized spike wave discharges have an estimated prevalence of 5% in patients with NF-1 and co-existing epilepsy.24 Nonetheless, distinct generalized EEG patterns such as hypsarrhythmia and generalized poly-spike wave discharges have been occasionally reported in association with NF-1.29 Rarely, NF-1 associated epilepsy patients can exhibit an EEG pattern of continuous spike and wave in slow-wave sleep (CSWS), in the absence of overt daytime clinical seizures.30 Interestingly, it appears that EEG abnormalities can be dynamic and may evolve within a short timeframe into different patterns if serial EEG studies are done periodically.31 Although reports of causative relationship between location of brain tumors and epilepsy classification have been published, data on correlation between EEG patterns and brain tumors is sparse.32,33 In our cohort, we have not found a statistical correlation between the localization of EEG epileptiform features and the presence of intracranial tumors. However, the brain tumors in our group were mainly in the optic pathway and the infratentorial structures, with no neuroradiological evidence of cortical involvement. Similarly, a recent study of 26 patients from Turkey, which found no significant difference in the pattern of neuroimaging findings in NF-1 patients with epilepsy and those without epilepsy, as well as non-concordant epileptogenic zones with the location of brain structural pathology.34
We believe our study is one of few that have investigated the special NF-1 associated symptomatic epilepsy population, in an attempt to identify presence of specific clinical or electrophysiological biomarkers. This study is retrospective and completely descriptive, thus is subject to limitations such as recall and selection biases. The cohort size is relatively small even though it is one of the largest published reports to date. As described in the report, our cohort has not described cortical malformation, vascular anomalies, or supratentorial tumors, which are epileptogenic findings that can be seen in association with NF-1 and their absence will jeoperdize generalization of the study conclusion. The EEG studies were reviewed by different pediatric epileptologists, making it prone to inter-rater variations in interpretation, but also limiting the single-interpreter bias. It is likely that prevalence of abnormal EEG surpasses that of epilepsy in NF-1 patients, but this concept was not tested in our study, limiting the precision of EEG correlation with presence of structural brain anomalies in NF-1 patients without established diagnosis of epilepsy. It is also plausible that some patients with the diagnosis of NF-1 have a genetic predisposition to develop epilepsy that is independent from any associated neuroimaging abnormality, hence the higher prevalence compared to standard population. Thus, another limitation of our study is that none of the patients with refractory epilepsy underwent further genetic epilepsy testing to confirm the presence of additional, predisposing genetic alterations.
Conclusion
This study of electro-clinical characteristics in 16 children with coexisting NF-1 and epilepsy in our center is one of the few published series to our knowledge. Our cohort showed higher a epilepsy incidence than previously reported, predominantly focal onset of both clinical seizures and EEG discharges in most patients and lack of correlation of epilepsy characteristics with presence of intracranial tumors. These findings help to highlight the evolving knowledge of epileptogenic pathways in children with NF1, beyond the previously reported contributing effect of structural brain lesions.
Footnotes
Future Directions: We hope that a larger data set from multiple institutions can shed more light on risk factors for epilepsy for patients with NF-1 and eventually lead to clinically useful, precision-based, treatment strategies.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval to perform this retrospective study was obtained from Nemours Children's Health Institutional Review Board (Nemours—IRB) with approval number 1860968. Informed consent for patient information to be published in this article was not obtained, as the study was classified as exempt, following the Nemours IRB guidelines for retrospective, chart-review based research.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Abdulhafeez M. Khair https://orcid.org/0000-0003-1890-3972
References
- 1.Park VM, Pivnick EK. Neurofibromatosis type 1 (NF1): A protein truncation assay yielding identification of mutations in 73% of patients. J Med Genet. 1998;35(10):813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uusitalo E, Leppävirta J, Koffert Aet al. Incidence and mortality of neurofibromatosis: A total population study in Finland. J Invest Dermatol. 2015;135(3):904-906. [DOI] [PubMed] [Google Scholar]
- 3.Kallionpaa RA, Uusitalo E, Leppavirta J, Poyhonen M, Peltonen S, Peltonen J. Prevalence of neurofibromatosis type 1 in the Finnish population. Genitourin Med. 2018;20(9):1082-1086. [DOI] [PubMed] [Google Scholar]
- 4.Hirabaru K, Matsuo M. Neurological comorbidity in children with neurofibromatosis type 1. Pediatr Int. 2018;60(1):70-75. [DOI] [PubMed] [Google Scholar]
- 5.Korf BR, Carrazana E, Holmes GL. Patterns of seizures observed in association with neurofibromatosis 1. Epilepsia. 1993;34(4):616-620. [DOI] [PubMed] [Google Scholar]
- 6.Vivarelli R, Grosso S, Calabrese Fet al. et al. Epilepsy in neurofibromatosis 1. J Child Neurol. 2003;18(5):338-342. [DOI] [PubMed] [Google Scholar]
- 7.Ostendorf AP, Gutmann DH, Weisenberg JLZ. Epilepsy in individuals with neurofibromatosis type 1. Epilepsia. 2013;54(10):1810-1814. [DOI] [PubMed] [Google Scholar]
- 8.Shin A, Byun JC, Hwang SK, Kwon S, Lee YJ. Clinical characteristics of epilepsy and its risk factors in neurofibromatosis type 1: A single-center study. Ann Child Neurol. 2021;29(1):1-7. [Google Scholar]
- 9.Gutmann DH, Parada LF, Silva AJ, Ratner N. Neurofibromatosis type 1: Modeling CNS dysfunction. J Neurosci. 2012;32(41):14087-14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernardo P, Cinalli G, Santoro C. Epilepsy in NF1: A systematic review of the literature. Childs Nerv Syst. 2020;36(10):2333-2350. [DOI] [PubMed] [Google Scholar]
- 11.Guerrini R, Dobyns WB. Malformations of cortical development: Clinical features and genetic causes. Lancet Neurol. 2014;13(7):710-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budişteanu M, Burloiu CM, Papuc SMet al. et al. Neurofibromatosis type 1 associated with moyamoya syndrome. Case report and review of the literature. Rom J Morphol Embryol. 2019;60(2):713-716. [PubMed] [Google Scholar]
- 13.Gowda VK, Srinivasan VM, Srinivas SM, Chadaga H. A rare association of Sturge Weber syndrome with neurofibromatosis type-1. Indian J Pediatr. 2018;85(8):703-704. [DOI] [PubMed] [Google Scholar]
- 14.Mimouni-Bloch A, Kornreich L, Kaadan W, Steinberg T, Shuper A. Lesions of the corpus callosum in children with neurofibromatosis 1. Pediatr Neurol. 2008;38(6):406-410. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh HY, Fung HC, Wang CJ, Chin SC, Wu T. Epileptic seizures in neurofibromatosis type 1 are related to intracranial tumors but not to neurofibromatosis bright objects. Seizure. 2011;20(8):606-611. [DOI] [PubMed] [Google Scholar]
- 16.Citraro R, Leo A, Constanti A, Russo E, De Sarro G. mTOR pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis. Pharmacol Res. 2016;107:333-343. [DOI] [PubMed] [Google Scholar]
- 17.Stafstrom CE, Staedtke V, Comi AM. Epilepsy mechanisms in neurocutaneous disorders: Tuberous sclerosis complex, neurofibromatosis type 1, and Sturge-weber syndrome. Front Neurol. 2017;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorrentino U, Bellonzi S, Mozzato Cet al. Epilepsy in NF1: Epidemiologic, genetic, and clinical features. A monocentric retrospective study in a cohort of 784 patients. Cancers (Basel). 2021;13(24):6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santoro C, Bernardo P, Coppola A, et al. Seizures in children with neurofibromatosis type 1: Is neurofibromatosis type 1 enough? Ital J Pediatr. 2018;44(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pecoraro A, Arehart E, Gallentine Wet al. Epilepsy in neurofibromatosis type 1. Epilepsy Behav. 2017;73:137-141. [DOI] [PubMed] [Google Scholar]
- 21.Barba C, Jacques T, Kahane Pet al. Epilepsy surgery in neurofibromatosis type 1. Epilepsy Res. 2013;105(3):384-395. [DOI] [PubMed] [Google Scholar]
- 22.Gales J, Prayson RA. Hippocampal sclerosis and associated focal cortical dysplasia-related epilepsy in neurofibromatosis type I. J Clin Neurosci. 2017;37:15-19. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia S, Chen Y, Wong FLet al. Subsequent neoplasms after a primary tumor in individuals with neurofibromatosis type 1. J Clin Oncol. 2019;37(32):3050-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkantrakorn K, Geller TJ. Seizures in neurofibromatosis 1. Pediatr Neurol. 1998;19(5):347-350. [DOI] [PubMed] [Google Scholar]
- 25.Runke M, Salanova V. Epilepsy due to a cortical malformation in a neurofibromatosis type 1 patient. Seizure. 2013;22(6):476-479. [DOI] [PubMed] [Google Scholar]
- 26.Algın Dİ, Tezer FI, Oguz KK, Bilginer B, Soylemezoglu F, Saygi S. Pharmacoresistant seizures in neurofibromatosis type 1 related to hippocampal sclerosis: Three case presentation and review. J Clin Neurosci. 2019;64:14-17. [DOI] [PubMed] [Google Scholar]
- 27.Sahly AN, Shevell M, Sadleir LG, Myers KA. SUDEP risk and autonomic dysfunction in genetic epilepsies. Auton Neurosci. 2021;237:102907. [DOI] [PubMed] [Google Scholar]
- 28.Yerdelen D, Koc F, Durdu M, Karakas M. Electrophysiological findings in neurofibromatosis type 1. J Neurol Sci. 2011;306(1-2):42-48. et al. [DOI] [PubMed]
- 29.Caraballo RH, Portuondo E, Fortini PS. Neurofibromatosis and epilepsy. J Pediatr Epilepsy. 2016;5:59-63. [Google Scholar]
- 30.Bhat S, Ming X, Dekermenjian R, Chokroverty S. Continuous spike and wave in slow-wave sleep in a patient with Rett syndrome and in a patient with Lhermitte-Duclos syndrome and neurofibromatosis 1. J Child Neurol. 2014;29(12):NP176–80. [DOI] [PubMed] [Google Scholar]
- 31.Patel SH, Carson RP, Jordan LC, Pagano LM. Rapid ictal transition of focal epilepsy to infantile spasms in neurofibromatosis type 1 captured with EEG. Epilepsy Behav Rep. 2020;14:100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong TS, Grant R, Gilbert MR, Lee JW, Norden AD. Epilepsy in glioma patients: Mechanisms, management, and impact of anticonvulsant therapy. Neuro Oncol. 2016;18(6):779-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinonez J, Ruxmohan S, Paesani S, Patel A, Edaki O. Glioma-induced seizure in a neurofibromatosis type 1 patient: A case report. Cureus. 2021;13(11):e19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serdaroglu E, Konuskan B, Karli Oguz K, Gurler G, Yalnizoglu D, Anlar B. Epilepsy in neurofibromatosis type 1: Diffuse cerebral dysfunction? Epilepsy Behav. 2019;98(Pt A):6-9. [DOI] [PubMed] [Google Scholar]