Abstract
Background
Monkeypox viral infection is considered as global public health and a rare disease caused by Monkeypox virus (MPXV), which is caused by smallpox-like virus and it causes pustules all over the body. MPV is an emerging zoonotic infection with sporadic occurrence globally and multiple outbreaks have been reported in African regions. The story of MPXV has been started since 1970 in Democratic republic of Cargo. The high cases of MPXV was majorly detected in Congo Rain Forest region in Africa. Animal-human (Zoonotic) transmission occurred, although the individual infected animal was not recognized. Human-human transmission occurs and is difficult until bodily fluids or respiratory droplets are exchanged. If a specific individual uses an infected person's towels or bed sheets, infection may occur.
Aim
The aim of this review is to document the methods of diagnosis, treatments (vaccines) and future role of MPXV in human population.
Output
The diagnosis is confirmed mainly through clinical diagnosis and then laboratory diagnosis such as cell-culture, serological and Polymerase Chain Reaction tests. Presently, there is no vaccine for MPXV but the smallpox vaccine will protect. The old vaccine includes antivirals approved for use against Orthopoxvirus, such as tecovirimat, which can treat up to 85 % of MPXV in humans. MPXV is now considered as transmission virus which affects from human to humans. The fatality rate was documented to be 3–10 % in children and in adults it is very low.
Conclusion
This review concludes MPXV is not as contagious as COVID-19 but proper measures should be taken as mentioned in this review to avoid MPXV. Presently, controlling MPXV presents unique challenges, and future prospective global studies in antivirals for this disease, as well as an MPXV vaccines, are recommended to eliminate this virus.
Keywords: Monkey Pox Virus (MPXV), Monkey Pox (MPX), Diagnostics, Vaccines and smallpox
Introduction
Poxviruses are members of a large and diverse family of viruses. Poxviridae is a family of double-stranded DNA viruses that replicate in the cytoplasm of infected cells. Poxviruses have been observed with electron microscopy to have brick-shaped or oval structures measuring 200–400 nm. To some extent, poxviruses' wide host range and successful evolution can be explained by their ability to regulate and modulate their hosts' immune responses. Poxviruses are also known as ancient viruses because they have been detected in insects, reptiles, birds, and mammals. According to common belief, these viruses produced a visible "pox" before invertebrates and vertebrates split. When poxvirus enters host cells via endocytosis or direct fusion with the plasma membrane, an organized sequence of events occurs that leads to viral replication [22], [4]. The details and classification of Poxviruses was shown in Table 1.
Table 1.
A classification of Poxviruses of veterinary significance.
| Family | Sub-Family | Genus | Virus |
|---|---|---|---|
| Poxviridae | Chordopoxvirinae | Orthopoxvirus | Vaccinia virus |
| Poxviridae | Chordopoxvirinae | Orthopoxvirus | Cowpox virus |
| Poxviridae | Chordopoxvirinae | Orthopoxvirus | Variola virus |
| Poxviridae | Chordopoxvirinae | Parapoxvirus | Orf virus |
| Poxviridae | Chordopoxvirinae | Parapoxvirus | Bovine popular stomatitis virus |
| Poxviridae | Chordopoxvirinae | Parapoxvirus | Pseudo cowpox virus |
| Poxviridae | Chordopoxvirinae | Parapoxvirus | Parapox virus of red deer |
| Poxviridae | Chordopoxvirinae | Unassigned | Squirrel pox virus |
| Poxviridae | Chordopoxvirinae | Capri pox virus | Goat pox virus |
| Poxviridae | Chordopoxvirinae | Capri pox virus | Sheep pox virus |
| Poxviridae | Chordopoxvirinae | Capri pox virus | Lumpy skin disease virus |
| Poxviridae | Chordopoxvirinae | Avipoxvirus | Fowl pox virus |
| Poxviridae | Chordopoxvirinae | Avipoxvirus | Pigeonpox virus |
| Poxviridae | Chordopoxvirinae | Avipoxvirus | Turkey pox virus |
| Poxviridae | Chordopoxvirinae | Avipoxvirus | Other species-specific poxviruses |
| Poxviridae | Chordopoxvirinae | Suipoxvirus | Swinepox virus |
| Poxviridae | Chordopoxvirinae | Leporipoxvirus | Myxoma virus |
| Poxviridae | Entomopoxvirinae | – | Poxviruses of insects |
The discovery of a closely related pox diseases in monkeys is called monkeypox virus (MPXV) [8]. MPXV is related to the smallpox virus and it’s a viral zoonosis [38]. The first MPXV cluster was identified in the United States in the second trimester of 2003 [15]. MPXV and other Orthopoxvirus viruses are massive double-stranded DNA viruses. Monkeypox (MPX) is a zoonotic disease that was first discovered as a human infection in the Democratic Republic of the Congo (DRC, previously Zaire) in 1970 [26]. MPXV is responsible for causing monkeypox. Animal-to-human transmission of the disease is possible because of its viral nature. It can also be transmitted from person to person [34]. The MPXV is a member of the Orthopoxvirus genus that causes human monkeypox family Poxviridae, subfamily Chordopoxvirinae. Variola virus, which causes smallpox, and vaccinia virus, which causes vaccinia, are also part of this category (the virus used in the smallpox vaccine) [41]. Because of the abundance of tropical rainforests and animals that might spread the virus, Central and Western Africa are hotspots for monkeypox. Monkeypox has undoubtedly occurred in sub-Saharan Africa for thousands of years, since humans acquired the virus through direct contact with diseased animals. The reservoir for MPXV is currently unknown. According to the research, primates serve as accidental hosts, and the reservoir is likely to be one or many species of rodents and squirrels that live in the secondary forest of central Africa [23].
In 1970, six human cases of MPV infection were recorded from the Democratic Republic of Congo, Liberia, and Sierra Leone [1]. A smallpox diagnosis was made on the basis of the clinical symptoms of each patient's sickness. They were tested extensively by numerous reference laboratories, and each one was identified as MPV. In the Democratic Republic of Congo, a 9-month-old baby was the first victim. They were between the ages of four and nine in Bouduo, Liberia. Rashes appeared on the skin of three of the children's playmates on consecutive days, indicating a possible shared exposure. This boy lived 12 kilometers away from the rest of his siblings. The patient, a 24-year-old male from Sierra Leone, had removed the stomach and intestines from a "red monkey" three to four weeks before to the commencement of disease in the sixth episode. Monkeypox had no fatal effect on any of the patients. These patients' families were also spared from the sickness. A new case has been detected since the last report, according to Henderson [8]. In 2022, a 35-year-old man acquired the second phase of the virus in the form of MPXV, presenting clinical symptoms such as low-grade fever and myalgia, and a second incidence was established in a 21-year-old male, displaying clinical symptoms such as dysuria and genital swelling. Both cases were recorded in male persons residing in the UAE in the first week of July 2022 [43].
Monkeypox is mostly found in central and west Africa, and it is more and more common in urban areas as well. Rodents and other non-human primates are examples of animal hosts [42]. MPXV has a genomic sequence of 96. 3 % is identical, cross-neutralizing immune responses between the viruses have been established [17].
Symptoms
Fever, severe headache, muscle aches, back discomfort, low energy, swollen lymph nodes, and a skin rash or lesions are typical symptoms of monkeypox. One to three days after a fever develops is when most people begin to notice a rash. Clear or yellowish fluid fills the lesions, which may or may not crust over and eventually fall off. From a few to several thousand lesions may be found on one person. The face, palms, and soles of the feet are the most commonly affected areas. The lips, genitals, and eyeballs are also common places to find them. For the most part, symptoms subside on their own within two to four weeks. Consult your doctor if you suspect that you have monkeypox-like symptoms. Inform them if you've had close contact with somebody who has been infected with monkeypox, whether it was suspected or proven [39]. Until now, the majority of MPX symptoms have disappeared within a few weeks; nevertheless, in a few cases, severe complications have resulted in death ranging from newborns to adults, including immune deficiencies [16]. The symptoms are similar to those of smallpox, with an incubation period of 8 days (4–14 days) followed by fever and a distinctive rash. In adults, the case fatality rate is often low, but in children, it can reach well over 10 % [25].
Shape of MPXV
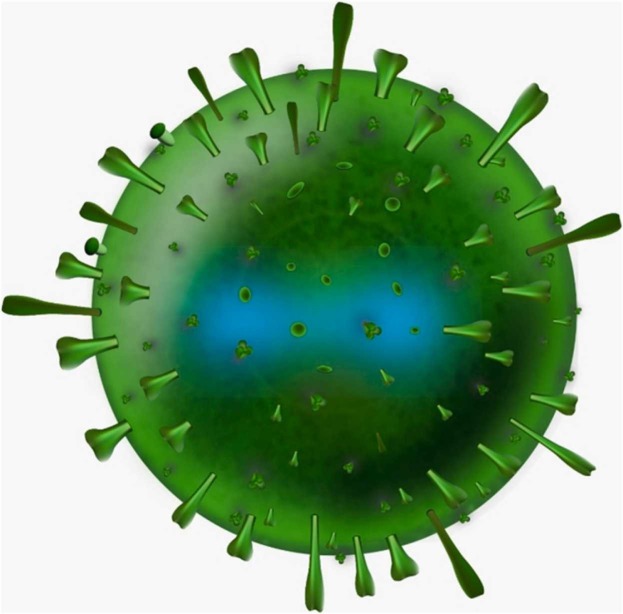
An examination of the morphology of MPXV shows that virions are brick-shaped and encased by geometrically corrugated lipoprotein outer membranes, just like other Orthopoxvirus. It is well-known that the MPXV size ranges between 200 and 250 nm. Fig. 1 describes the shape of MPXV. The linear double-stranded DNA of the MPXV genome (∼197 kb) is made up of hairpin loop, tandem, and open reading frames and is covalently coupled at the ends by palindromic hairpins. Despite the fact that MPXV is a DNA virus, its whole life cycle takes place in the cytoplasm of infected cells. All of the proteins required for viral DNA replication, transcription, virion assembly, and egress are encoded by the MPXV genome. A high number of oral polioviruses vaccines (OPVs) share the central area of their genome with genes encoding housekeeping functions, while a far lower number of OPVs share the termini region with genes encoding virus–host interactions [12], [18], [20], [24], [29], [32], [37], [6].
Fig. 1.
Monkeypox virus cell.
Pathogen
The monkeypox virus has two separate genetic clades: one from central Africa (the Congo Basin) and the other from west Africa. In the past, the Congo Basin clade was supposed to produce more severe disease and was considered to be more transmissible. Cameroon is the only country where both viral clades have been discovered. The present MPX infection in non-endemic regions should serve as a wake-up call, as it demonstrates how little or no focus has been placed on the transmission of the virus inside endemic areas. Unless the virus is contained in endemic locations, it is a warning that in present interconnected and globalized society, no region or country is immune from zoonotic viruses like MPXV. Outbreaks of MPX in endemic regions of sub-Saharan Africa must be given top priority by global health response measures. Sexually transmitted diseases, a history of syphilis, and MPXV can all be considered risk factors, but existing measures are insufficient, and only future studies will be able to confirm this hypothesis [14], [5].
Expansion of MPVX
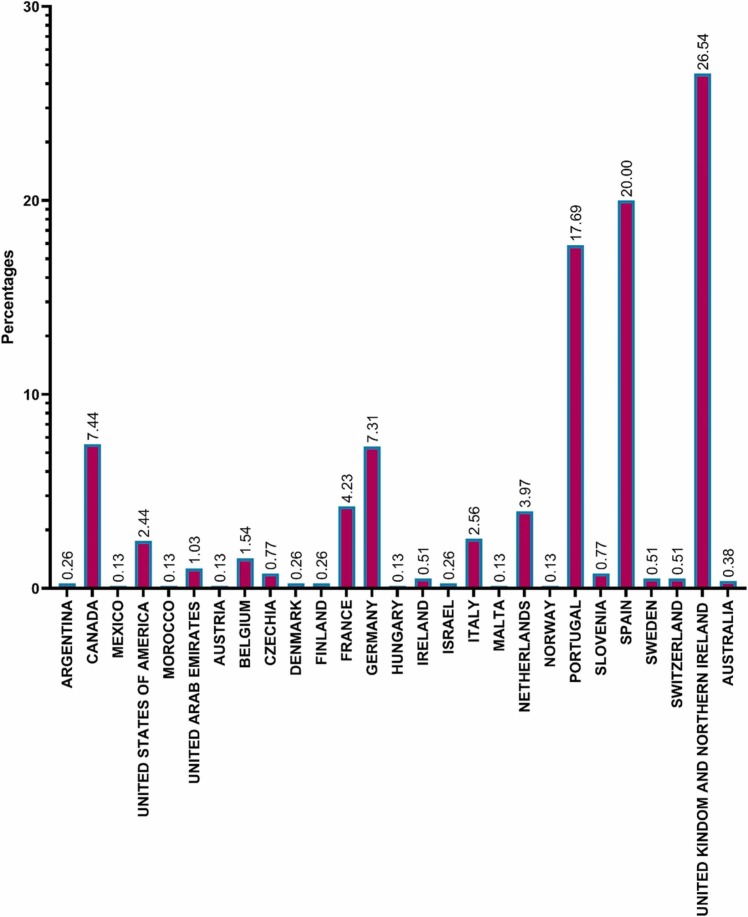
An outbreak of monkeypox occurred in United States of America (USA) in 2003 that was linked to contact with monkeypox infected pets. The rats and dormice were brought to USA from Ghana, where they shared a cage with the pets. Over 70 cases of monkeypox were reported in USA as a result of this outbreak. Nigerian tourists visiting Israel, the United Kingdom, Singapore, and USA have all been diagnosed with monkeypox, as have those from Nigeria to Israel and the United Kingdom. Several non-endemic nations reported instances of monkeypox in May 2022. Studying the epidemiology of disease, sources of infection, and transmission patterns is ongoing. Some recent cases were reported in the UAE on May 31, 2022. The source of monkeypox infection is still a mystery. It's possible, though, that non-human primates (like monkeys) from Africa and other regions could carry the virus and spread it to humans. Additionally, transmission can actually occur via placenta (which might result in congenital monkeypox), as well as through intimate contact between mother and child after birth. Although close physical contact is a well-known risk factor for transmission, it is not yet apparent if monkeypox can be transferred especially through sexual transmission channels. The second innings of MPV has been started in since 13th may and till now 780 MPVX has been identified from laboratory analysis. Fig. 2 describes the infected MPXV from different regions.
Fig. 2.
Global Prevalence of MPXV affected in2022.
Diagnosis
Pathogen detection is essential in the clinical care of patients with infectious diseases. Conventional methods of determining infectious disease etiologies such as microscopic examination; antigen detection; serology; cultures; biochemical reactions; and microscopy are still utilized. However, because of the time-consuming nature of these conventional procedures. Since the 1950 s, screening for infectious diseases has been conducted using cell-culture, serological, and molecular techniques. In comparison to serological and molecular analysis, cell culture is the most prevalent technique. One of the disadvantages of cell-culture is its time-consuming nature. However, serological tests are reliable and measure a patient's immune system, but neither directly measure the disease nor cause it. Molecular approaches have transformed infectious diseases diagnostics, primarily through the use of polymerase chain reaction (PCR). The PCR has found significant usage in science due to its high sensitivity, specificity, and ease of use in detecting known genetic sequences. Real-time PCR (RT-PCR), multiplex PCR, LAMP-PCR, and digital PCR are some of the most often used quantitative and qualitative molecular techniques. A variety of clinical diseases, such as arboviruses, COVID-19, chlamydia trachomatis, cytomegalovirus, hepatitis B Virus, hepatitis C virus, tuberculosis, human papilloma virus, enterovirus, sexually transmitted infections, and bacterial infections, could be detected using these assays to detect infectious agents and nucleic acid [28], [41], [44], [45]. Due to the pandemic of COVID-19 infectious disease, PCR, namely RT-PCR, is now well-known in the year 2020 [13].
Returning to MPXV, previous studies has reported some of the diagnostic procedures used to diagnose MPXV/Orthopoxvirus infection in clinical specimens. When accompanied with clinical and epidemiological data, such as a patient's immunization history, these tests are most effective. Viral culture or viral isolation is one of the diagnostic techniques for MPXV/Orthopoxvirus in which live virus produced from a patient's specimen is observed and characterized, which is one of the strengths of this technique. It is also useful since patient specimens from lesions are more consistent. One major drawback of this procedure is that it is time consuming; bacteria can be contaminated within the patient's sample, and viral identification must be performed for its Characterisation. Another diagnostic technique for MPXV is electron microscopy. It is possible to visually classify poxviruses other than Para poxvirus using negative staining, which provides a picture of a brick-shaped particle. Viral particles in biopsy samples, skin scabs, vesicular fluid, and viral cultures can all be detected using this method. It can spot the difference between an Orthopoxvirus and a Herpesvirus. The morphological differences between Orthopoxviruses are indistinguishable. One disadvantage of this technique is the need for an electron microscope and highly-trained workers at a major laboratory. PCR, specifically RT-PCR, will detect the existence of MPX-specific DNA markers. MPXV-2022 strains are related to the MPXV strain that was identified in 2018. Still, the MPXV-2022 strains showed 46 novel consensus mutations, including 24 nonsynonymous ones, when compared to the MPXV strain in 2018. The MPXV genome encodes 187 proteins; however, only 10 of these proteins—the D2L-like, OPG023, OPG046, OPG071, OPG105, OPG109, A27L-like, OPG153, OPG188, and OPG210 proteins—are more prone to mutation. Both OPG105 and OPG210 have three and four nucleotide changes in MPXV2022 [19], [40]. Anti-Orthopoxvirus IgG and IgM serological assays can be performed to detect Orthopoxvirus antibodies in infected patients. The advantages of IgG and IgM testing include the ability to assess prior exposure to Orthopoxvirus, which includes smallpox vaccine and a pathogen. The main disadvantages of this test are the requirements for blood collection and serum extraction. A professional technician requires to perform these tests at a prominent laboratory, one more main cons of these tests will be results can be obtained for previous chickenpox vaccination rather than MPXV. Tetracore Orthopox BioThreat Alert tests are the last tests for MPXV identification using Orthopoxvirus antigens. The major advantage of this tests is a point-of-care diagnostic technique that can also be conducted at ambient temperature with little skill and can quickly identify an active case from a patient's lesion material. In this test, MPXV is not a specified target. An endemic test is required. It is less sensitive than PCR [27].
Treatment
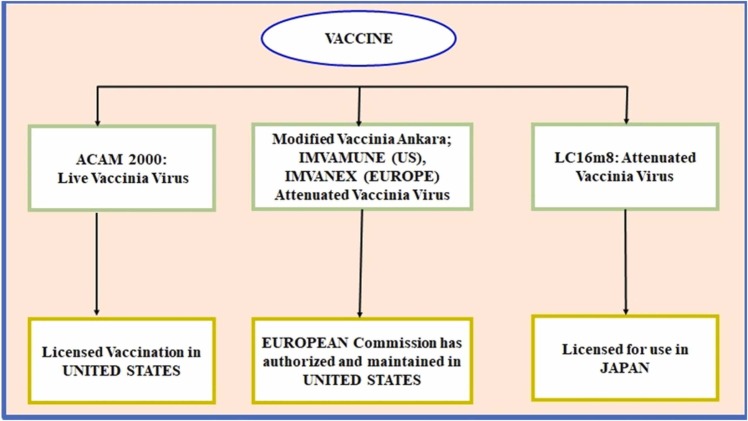
There are currently no specialized vaccines available for MPXV, although other previous vaccines for small chicken pox virus were found to be 85 % effective against MPXV [2]. The gunshot treatment for MPXV is not yet accessible, but it can be controlled by smallpox vaccination, cidofovir, ST-246, and vaccinia immune globulin (VIG) to control a monkeypox epidemic [31]. Those who had previously received a smallpox vaccination had an 85 % protection rate against MPXV [30], [7]. Compounds that show potential as antiviral treatments against Orthopoxvirus species are summarized in Fig. 3.
Fig. 3.
List of smallpox vaccines can be used for MPXV.
When an individual is afflicted or exhibits common symptoms, a diagnosis is recommended, and as shown in Fig. 3, or any sort of smallpox vaccination is recommended to control the disease, as does quarantine. Based on previous studies the fatality rate was documented in children under 10 % and there is low fatality rate in adults. In this figure, three effective vaccines that were studied for smallpox vaccines were included. Furthermore, the pros and cons of the three vaccinations, as well as the licensed countries, were thoroughly described. It is important to consider both the risks of pathogenic monkeypox disease and the potential of adverse effects from vaccines like ACAM2000 [9]. Mammalian cells are unable to completely replicate the modified Ankara virus (MVA), an attenuated vaccinia virus. Primate models exposed to fatal amounts of monkeypox virus were protected by MVA. But in primates with substantially depleted T-cell activity, this vaccine could not provide protection [10], [11], [35], [36]. The LC16m8 vaccination has been found to protect nonhuman primates from severe monkeypox disease after it was genetically engineered to limit viral replication. More than 50,000 Japanese youngsters were immunized with LC16m8 with little evidence of side effects [21], [33].
Future role of MPXV
Monkeypox is a rising global health issue that is capable of cross-border transmission and subsequent dissemination. The MPXV is not a complicated infectious disease like COVID-19, which was earlier declared a pandemic and decimated human health and global wealth. This potentially lethal pathogen's optimal infection management and treatment options have yet to be found. MPXV is often a mild virus that resolves on its own within a month, and according to the WHO, children are at greater risk than adults, with a 3–10 % fatality rate. However, Adler et al. confirmed the first use of antiviral medications in monkeypox patients, with three patients receiving brincidofovir and one receiving tecovirimat. Brincidofovir was shown to have no clinical benefit and was always associated with abnormal liver function tests. There are no adverse effects were reported before discharge in the patient treated with tecovirimat, which had a shorter duration of symptoms than other patients in the study. Extensive hospitalization was necessary for several individuals due to viral overgrowth and subsequent upper respiratory tract viral shed that occurred after crusting of all skin lesions [3]. Avoiding the handling of clothes, blanket exchange, and other clothing material that might be utilized for contact with an infected person or animal, as well as isolating the infected person, can provide protection against MPXV.
Conclusion
This review concludes with the prevalence of MPXV transmission growing globally. New vaccines, diagnostics, and antiviral treatments are being developed to assure global preparedness in the event for reappearance of smallpox. These new discoveries may potentially help with monkeypox prevention and control. WHO has declared MPXV is not as infectious as other viruses, but it can impact anyone who come into touch with afflicted humans or animals. Based on prior reports, this review concludes that MPXV is not yet pandemic, however, more epidemiological and prospective studies are required, as well as study into antivirals for this disease. This review also recommends to discover 100 % accurate vaccine for MPXV to control the global infection.
Competing interests
None declared
References
- 1.1971. A rare smallpox-like disease. WHO Chron, 25, 370–2.
- 2.Abdelaal A., Reda A., Lashin B.I., Katamesh B.E., Brakat A.M., Al-Manaseer B.M., Kaur S., Asija A., Patel N.K., Basnyat S. Preventing the next pandemic: is live vaccine efficacious against monkeypox, or is there a need for killed virus and mRNA vaccines? Vaccines. 2022;10:1419. doi: 10.3390/vaccines10091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., Osborne J.C., Rampling T., Beadsworth M.B., Duncan C.J. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alakunle E.F., Okeke M.I. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022:1–2. doi: 10.1038/s41579-022-00776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle K., Traktman P. Springer,; 2009. Viral genome replication. [Google Scholar]
- 7.Brown K., Leggat P.A. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho C.T., Wenner H.A. Monkeypox virus. Bacteriol Rev. 1973;37:1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Control C.F.D., Prevention Vaccinia (smallpox) vaccine, recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. Mortal Morb Wkly Rep. 2001;50:1–25. [PubMed] [Google Scholar]
- 10.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., Eisenberg R.J., Hartmann C.J., Jackson D.L., Kulesh D.A. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 11.Earl P.L., Americo J.L., Wyatt L.S., Espenshade O., Bassler J., Gong K., Lin S., Peters E., Rhodes Jr L., Spano Y.E. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci. 2008;105:10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito J.J., Knight J.C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985;143:230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- 13.Farasani A. Genetic analysis of the 2019 coronavirus pandemic with from real-time reverse transcriptase polymerase chain reaction. Saudi J Biol Sci. 2021;28:911–916. doi: 10.1016/j.sjbs.2020.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghazvini K., Keikha M. Human Monkeypox resurgence 2022; a new presentation as a sexual pathogen. Ann Med Surg. 2022:80. doi: 10.1016/j.amsu.2022.104267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarner J., Johnson B.J., Paddock C.D., Shieh W.-J., Goldsmith C.S., Reynolds M.G., Damon I.K., Regnery R.L., Zaki S.R., Group V.M.V.W. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis. 2004;10:426. doi: 10.3201/eid1003.030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerschlag Y., Macleod G., Papadakis G., Sanchez A.A., Druce J., Taiaroa G., Savic I., Mumford J., Roberts J., Caly L. Monkeypox infection presenting as genital rash, Australia, May 2022. Eurosurveillance. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.22.2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harapan H., Wagner A.L., Yufika A., Setiawan A.M., Anwar S., Wahyuni S., Asrizal F.W., Sufri M.R., Putra R.P., Wijayanti N.P. Acceptance and willingness to pay for a hypothetical vaccine against monkeypox viral infection among frontline physicians: a cross-sectional study in Indonesia. Vaccine. 2020;38:6800–6806. doi: 10.1016/j.vaccine.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutson C.L., Damon I.K. Monkeypox virus infections in small animal models for evaluation of anti-poxvirus agents. Viruses. 2010;2:2763–2776. doi: 10.3390/v2122763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isidro J., Borges V., Pinto M., Sobral D., Santos J.D., Nunes A., Mixão V., Ferreira R., Santos D., Duarte S. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28:1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahrling P.B., Huggins J.W., Ibrahim M.S., Lawler J.V., Martin J.W. Smallpox and related orthopoxviruses. Medical aspects of biological warfare. TMM Publ Surg Gen, Wash, DC. 2007:215–240. [Google Scholar]
- 21.Kenner J., Cameron F., Empig C., Jobes D.V., Gurwith M. LC16m8: an attenuated smallpox vaccine. Vaccine. 2006;24:7009–7022. doi: 10.1016/j.vaccine.2006.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khandelwal N., Kaur G., Kumar N., Tiwari A. Application of silver nanoparticles in viral inhibition: a new hope for antivirals. Dig J Nanomater Biostructures (DJNB) 2014:9. [Google Scholar]
- 23.Khodakevich L., Jezek Z., Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- 24.Kugelman J.R., Johnston S.C., Mulembakani P.M., Kisalu N., Lee M.S., Koroleva G., Mccarthy S.E., Gestole M.C., Wolfe N.D., Fair J.N. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20:232. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariën, J., Laudisoit, A., Patrono, L., Baelo, P., Van Vredendaal, R., Musaba, P., Gembu, G., Mande, C., Ngoy, S. & Mussaw, M. 2021. Monkeypox viruses circulate in distantly-related small mammal species in the Democratic Republic of the Congo.
- 26.Mauldin M.R., Mccollum A.M., Nakazawa Y.J., Mandra A., Whitehouse E.R., Davidson W., Zhao H., Gao J., Li Y., Doty J. Exportation of monkeypox virus from the African continent. J Infect Dis. 2022;225:1367–1376. doi: 10.1093/infdis/jiaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mccollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizusawa M. Updates on rapid diagnostic tests in infectious diseases. Mo Med. 2020;117:328. [PMC free article] [PubMed] [Google Scholar]
- 29.Moss B. The molecular basis of viral replication. Springer; 1987. The molecular biology of poxviruses. [Google Scholar]
- 30.Nasir I.A., Dangana A., Ojeamiren I., Emeribe A.U. Reminiscing the recent incidence of monkeypox in Nigeria: its ecologic-epidemiology and literature review. Port Harcourt Med J. 2018;12:1. [Google Scholar]
- 31.Rao A.K., Schulte J., Chen T.-H., Hughes C.M., Davidson W., Neff J.M., Markarian M., Delea K.C., Wada S., Liddell A. Monkeypox in a Traveler Returning from Nigeria—Dallas, Texas, July 2021. Morb Mortal Wkly Rep. 2022;71:509. doi: 10.15585/mmwr.mm7114a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remichkova M. Poxviruses: smallpox vaccine, its complications and chemotherapy. Virus Adapt Treat. 2010;2:41–46. [Google Scholar]
- 33.Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., Ogata M., Fukushi S., Mizutani T., Sata T. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80:5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sklenovská N. Springer; 2020. Monkeypox virus. Animal-origin viral zoonoses. [Google Scholar]
- 35.Smith Y.E., Bray M., Whitehouse C.A., Miller D., Mucker E., Manischewitz J., King L.R., Guroff M.R., Hryniewicz A., Venzon D. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191:372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- 36.Stittelaar K.J., Van Amerongen G., Kondova I., Kuiken T., Van Lavieren R.F., Pistoor F.H., Niesters H.G., Van Doornum G., Van Der Zeijst B.A., Mateo L. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemura M. Poxviruses and the origin of the eukaryotic nucleus. J Mol Evol. 2001;52:419–425. doi: 10.1007/s002390010171. [DOI] [PubMed] [Google Scholar]
- 38.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., Palich R., Nori A., Reeves I., Habibi M.S. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022 doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan A., Aarons E., Astbury J., Brooks T., Chand M., Flegg P., Hardman A., Harper N., Jarvis R., Mawdsley S. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26:782. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Shang J., Weng S., Aliyari S.R., Ji C., Cheng G., Wu A. Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022. J Med Virol. 2022 doi: 10.1002/jmv.28036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinstein R.A., Nalca A., Rimoin A.W., Bavari S., Whitehouse C.A. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41:1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- 42.WHO 2022. WHO situation update: Multi-country Monkeypox outbreak: situation update 〈https://www.who.int/emergencies/disease-outbreak-news/item/〉2022-DON393 -accessed June 18, 2022.
- 43.Yadav P.D., Reghukumar A., Sahay R.R., Sudeep K., Shete A.M., Raman A., Pramod V., Abraham P., Benson R., Sarin S. First two cases of Monkeypox virus infection in travellers returned from UAE to India, July 2022. J Infect. 2022 doi: 10.1016/j.jinf.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S., Rothman R.E. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004;4:337–348. doi: 10.1016/S1473-3099(04)01044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zauli, D.A.G. 2019. PCR and Infectious Diseases. Synthetic Biology-New Interdisciplinary Science. IntechOpen.