Abstract
D-dimer is an established biomarker of thromboembolism and severity in COVID-19. We and others have recently reported the dysregulation of tissue factor pathway inhibitor (TFPI), FXIII, fibrinolytic pathway, inflammatory markers, and tissue injury markers, particularly in severe COVID-19. However, association of these markers with thromboembolism in COVID-19 remains elusive. The correlation analyses between these markers in patients with moderate (non-ICU) and severe COVID-19 (ICU) were performed to delineate the potential pathomechanisms and impact of thromboembolism. We observe a negative correlation of plasma TFPI (r2 = 0.148, P = 0.035), FXIII (r2 = 0.242, P = 0.006), and plasminogen (r2 = 0.27, P = 0.003) with D-dimer, a biomarker of thromboembolism, levels in these patients. Further analysis revealed a strong positive correlation between fibrinolytic markers tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) (r2 = 0.584, P < 0.0001). Interestingly, a significant positive correlation of PAI-1, but not tPA, was observed with platelets and endothelial cells dysfunction markers P-selectin (r2 = 0.184, P = 0.01) and soluble CD40 ligand (sCD40 L) (r2 = 0.163, P = 0.02). Moreover, calprotectin (S100A8/A9) and cystatin C (CST3), previously linked with thromboembolism, exhibited positive correlations with each other (r2 = 0.339, P = 0.0007) and with the level of D-dimer independently in COVID-19. Finally, the tissue injury marker myoglobin demonstrated a strong positive correlation with D-dimer (r2 = 0.408, P = 0.0001). Taken together, inverse correlations of TFPI and FXIII with D-dimer suggest the TF pathway activation and aberrant fibrin polymerization in COVID-19 patients. The elevated level of PAI-1 is potentially contributed by activated platelets and endothelial cells. S100A8/A9 may also play roles in impaired fibrinolysis and thromboembolism, in part, through regulating the CST3. These findings strengthen the understanding of thromboembolism and tissue injury and may help in better management of thromboembolic complications in COVID-19 patients.
Keywords: TFPI, FXIII, calprotectin, Cystatin C, D-dimer, thromboembolism, tissue injury
Impact Statement
Venous thromboembolism (VTE) is one of the major clinical complications reported in severe COVID-19 and plays a driving role in tissue injury. Hence, a better understanding of the pathomechanism of VTE is required. Our correlation analysis of coagulation, inflammatory markers, and tissue injury markers with D-dimer indicates that TFPI and FXIII potentially play critical roles in the coagulation activation and thrombus embolization in COVID-19. Moreover, positive correlations of calprotectin (S100A8/A9) and cystatin C (CST3) with each other and with the level of D-dimer independently show their potential roles in VTE. Importantly, the tissue injury marker myoglobin demonstrated a positive correlation with D-dimer. These findings enhance our understanding of the pathomechanism of VTE and tissue injury frequently observed in severe COVID-19.
Introduction
SARS-CoV-2 infected patients, mostly elderly people and patients with existing comorbidity factor present severe clinical complications. 1 One of these complications includes coagulation dysfunction and impaired fibrinolysis leading to thromboembolism, multi-organ damage, and death. 2 D-dimer is an endogenous fibrin clot degradation (fibrinolysis) product and is expected in patients with venous thromboembolism (VTE). 3 The VTE including deep vein thrombosis (DVT) and pulmonary embolism (PE) is frequently reported in severe COVID-19 therefore D-dimer can effectively be used as a biomarker to diagnose VTE. Several coagulation parameters such as activated partial thromboplastin time (APTT), prothrombin time (PT), platelet count and fibrinogen, fibrin split products (FSP), and D-dimer formation are deranged in patients with severe COVID-19.4,5 It is evident that severe SARS-CoV-2 infection induces chemokine production and cytokine storm, which cause pulmonary intravascular coagulopathy and microvascular thrombosis. 6 The viral invasion induces procoagulant changes in endothelium and platelet activation through the secretion of numerous biologically active molecules. 7 SARS-CoV-2 invasion of alveolar epithelium leads to profound induction of inflammatory cytokines such as interleukin (IL)-6, IL-1, IL-8, tumor necrosis factor (TNF)-α, interferons, and chemokines such as CCL2 and CCL3. 8 This sudden increase in cytokines triggers the influx of immune cells such as macrophages, neutrophils, and T cells resulting in activation of the coagulation pathway, thrombin generation, and fibrin clot formation.9,10 Although a higher level of IL-6 is reported to induce coagulopathy through inducing tissue factor (TF) expression on immune cell population and increasing the fibrinogen level,11,12 the detailed pathomechanism of VTE in COVID-19 is still vague. This study aims to identify the potential correlations between D-dimer and coagulation, fibrinolysis, and tissue injury markers among COVID-19 patients to better understand the pathomechanisms of VTE in COVID-19.
Materials and methods
Patients and sample collection
A total of 30 hospitalized COVID-19 patients, including 15 moderate patients without pneumonia (non-intensive care unit [ICU]) and 15 severe patients with pneumonia and/or acute respiratory distress syndrome (ARDS) who required intensive care (ICU), were the subjects of the study. The patients were recruited from the Rashid Hospital, Dubai, during May–June 2020 and samples were collected after admission to either the general COVID-19 ward (moderate) or ICU (severe). The medical history and written informed consent were collected before sample collection. The patients treated with any anticoagulant were excluded from the study. The detailed exclusion and inclusion criteria and medications of these patients were reported recently.11,12 The study protocol was approved by the research ethics committees of the University of Sharjah and the Dubai Health Authority.
Assessment of coagulation, inflammation markers, and tissue injury markers
The coagulation, fibrinolysis, tissue injury markers, and inflammatory markers were assessed and reported by us recently.13,14 Briefly, the coagulation dysfunction, endothelial cell activation and fibrinolysis markers including fibrinogen, FXIII, P-selectin, soluble CD40 ligand (sCD40 L), tissue plasminogen activator (tPA), plasminogen activator inhibitor-I (PAI-I), and D-Dimer were assessed in plasma employing flow cytometry-based LegendPlex kits (BioLegend #740906, #740761) following the manual instructions. The level of tissue factor pathway inhibitor (TFPI) in plasma was assessed using an ELISA kit (Abcam #ab274392) following the manufacturer’s instruction. The level of plasma calprotectin (S100A8/A9) and cystatin C (CST3) were measured using ELISA kits (Abcam #ab267628, ThermoFisher #EHCST3) following the manual instructions.
Statistics
A linear regression analysis was performed using GraphPad Prism software to analyze the potential correlation between different coagulation, endothelial dysfunction, inflammatory markers, and tissue injury markers. A P value less than 0.05 was considered statistically significant.
Results and discussion
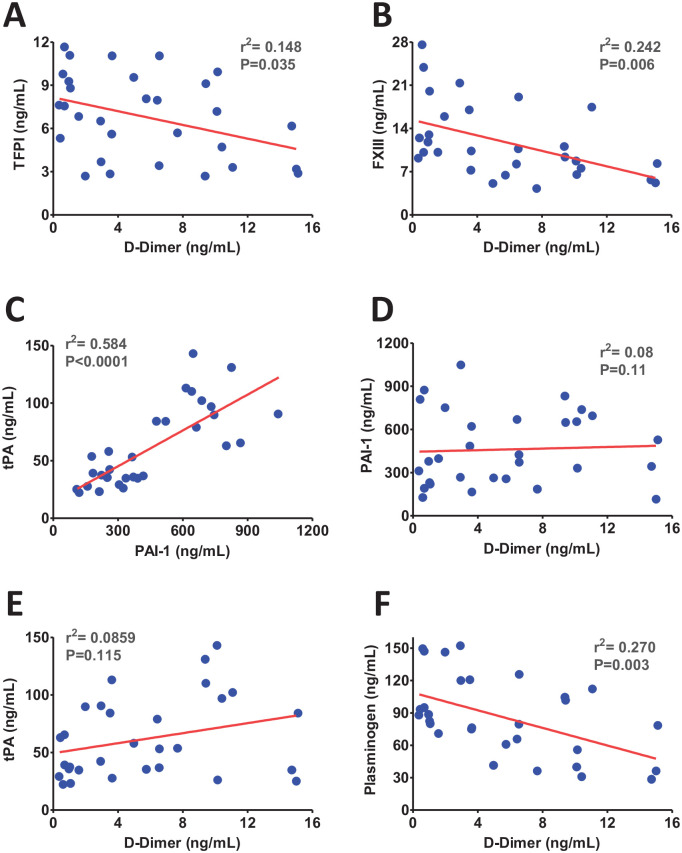
The elevated level of D-dimer is strongly correlated with COVID-19 severity, adverse clinical outcomes, and increased mortality, 15 which suggests that thromboembolism plays a pivotal role in COVID-19 pathogenesis. Our correlation analysis between dysregulated coagulation markers has shown a significant negative correlation between the levels of TFPI and D-dimer (Figure 1(A)). This is consistent with an increased TF expression on endothelial and immune cell populations in patients with coagulopathy. 16 Hence, a negative correlation between TFPI and D-dimer strongly suggests the activation of the TF-mediated coagulation pathway in COVID-19. Since endothelial cells are one of the primary sources of TFPI, particularly under inflammatory conditions, 17 next we sought to correlate the endothelial dysfunction markers P-selectin and sCD40 L with the level of TFPI in COVID-19. We found no correlation between TFPI and these endothelial activation markers (Supplemental Figure 1(A) & (B)). The results suggest that the decline in plasma TFPI levels is independent of endothelial cell dysfunction and likely occurs due to exhaustion of the immune cell population in severe COVID-19.
Figure 1.
The scatter plots from linear regression analysis show (A) inverse correlations of tissue factor pathway inhibitor (TFPI) (B) and factor XIII (FXIII) with D-dimer, (C) positive correlation between tPA and PAI-1 (D) no correlation between plasminogen activator inhibitor (PAI-1) and D-dimer (E) no correlation between tissue plasminogen activator (tPA) and D-dimer (F), and an inverse correlation between plasminogen and D-dimer. (A color version of this figure is available in the online journal.)
The negative correlation between FXIII and D-dimer in COVID-19 suggests the potential roles of FXIII in thrombus embolization (Figure 1(B)). FXIII helps stabilize thrombus by fibrin polymerization. Consequently, an acquired FXIII deficiency in COVID-19 may limit fibrin polymerization and destabilize thrombus following the activation of the coagulation pathway. Consistent with our findings, FXIII deficiency was recently reported in COVID-19 patients though no correlation with D-dimer was established in this study. 18 A negative correlation in our study suggests that FXIII deficiency may partly contribute to an increased D-dimer formation due to defective fibrin polymerization. An acquired FXIII deficiency could be one of the potential causes of bleeding manifestation previously identified in the COVID-19 patients. 19 Studies have reported that fibrinogen, along with FXIII plays a key role in the VTE; 20 however, we did not observe any correlation between fibrinogen and FXIII in COVID-19 (Supplemental Figure 1(C)). This suggests that these two markers may contribute to VTE in COVID-19 likely independent of each other.
The platelets and endothelial cells activation following severe inflammatory reaction trigger the release of tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1).21,22 Therefore, we next investigated the correlations of fibrinolytic markers tPA and PAI-1 with D-dimer. Interestingly, we observed a positive correlation between tPA and PAI-1 (Figure 1(C)). However, no correlations of tPA or PAI-1 with D-dimer were observed in COVID-19 patients (Figure 1(D) to (E)). Consistent with this finding, a recent report has also established a positive correlation between tPA and PAI-1 in COVID-19. 23 This study was performed in hospitalized critically ill patients and reported increased circulating levels of both tPA and PAI-1. In contrast, we observed a significantly elevated level of PAI-1 only in moderate cases, while an increased level of tPA was identified particularly in critically ill patients. 13 Moreover, as expected, a significant negative correlation was observed between plasminogen and D-dimer ( Figure 1(F) ). Hence, increased tPA and plasminogen may contribute to the higher level of D-dimer in severe COVID-19 patients.
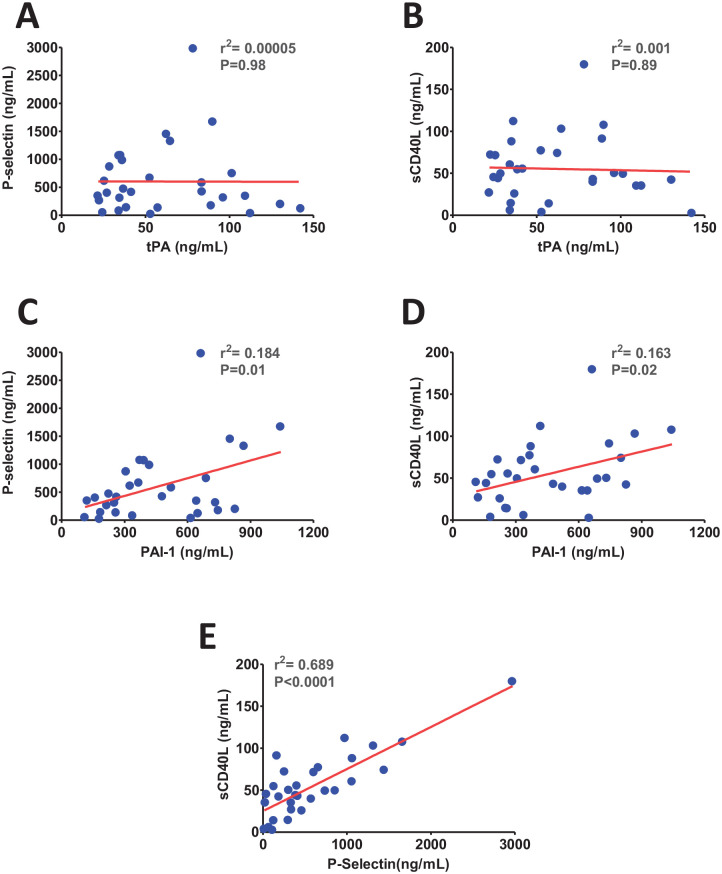
SARS-CoV-2 infection may induce the release of tPA and PAI-1 from activated platelets and endothelial cells.22,24 Therefore, next, platelet/endothelial cell activation markers P-selectin and sCD40 L were correlated with the level of tPA and PAI-1. There was no correlation between tPA and platelet/endothelial cell activation markers (Figure 2(A) to (B)); however, a significant positive correlation was observed between PAI-1 and P-selectin/sCD40 L levels in COVID-19 (Figure 2(C) to (D)). Moreover, as expected, there was a strong positive correlation between P-selectin and sCD40 L (Figure 2(E)) which indicates that the dysregulation of these two markers in COVID-19 occurs simultaneously upon platelet/endothelial cell dysfunction. Taken together, these observations suggest that most of PAI-1 at the moderate stage of SARS-CoV-2 infection is likely released from activated platelets/endothelial cells. However, elevated levels of tPA in severe patients potentially contributed due to inflammation induction (cytokine storms). The profound release of proinflammatory cytokines further activates endothelial cells and promotes immune cells infiltration. The elevated level of tPA in critically ill patients is most likely contributed by the activated immune system.
Figure 2.
The scatter plots show (A) no correlation between P-selectin and tissue plasminogen activator (tPA), (B) no correlation between soluble CD40 ligand (sCD40 L) and tPA, (C) a positive correlation between P-selectin and plasminogen activator inhibitor (PAI-1), (D), a positive correlation between sCD40 L and PAI-1, and (E) a positive correlation between P-selectin and sCD40 L. (A color version of this figure is available in the online journal.)
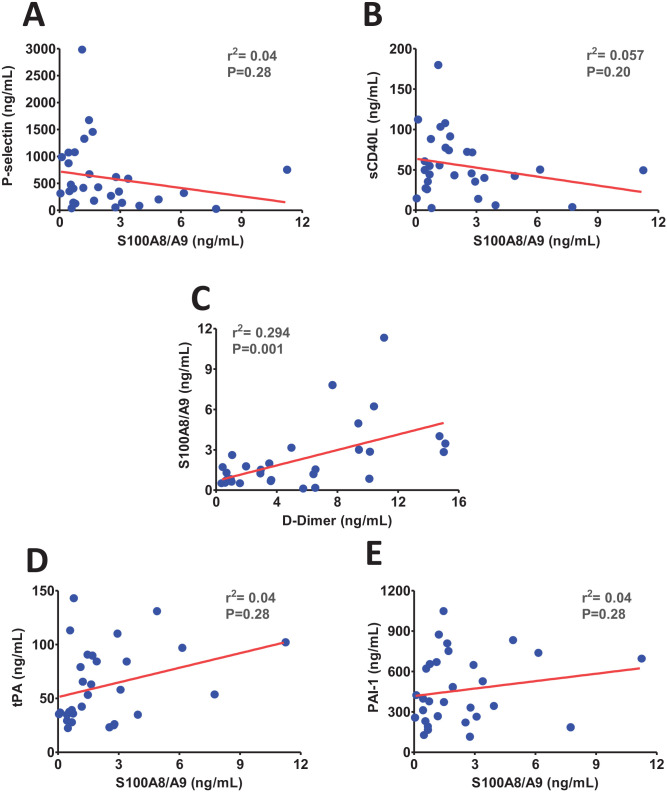
Calprotectin (S100A8/A9) plays an important role in neutrophils and platelet activation through toll-like receptor-4 (TLR4) present on these cell populations.25,26 Therefore, we next investigated the potential roles of S100A8/A9 in platelet activation and granule release post-SARS-CoV-2 infection. We found no correlations between S100A8/A9 and platelet activation markers P-selectin and sCD40 L (Figure 3(A) to (B)). Further analysis revealed a significant positive correlation between S100A8/A9 and D-dimer (Figure 3(C)). These observations suggest that S100A8/A9 may have a minimal role in SARS-CoV-2-induced platelet activation. Conversely, S100A8/A9 may play an indirect role in aberrant fibrinolysis likely by inducing the inflammation. Further assessment revealed no significant correlation of S100A8/A9 with either PAI-1 or tPA (Figure 3(D) to (E)), which further suggests the indirect roles of S100A8/A9 in impaired fibrinolysis post-SARS-CoV-2 infection.
Figure 3.
The scatter plots show (A) no correlation between P-selectin and calprotectin (S100A8/A9), (B) no correlation between soluble CD40 ligand (sCD40 L) and S100A8/A9, (C) a significant positive correlation between S100A8/A9 and D-dimer, (D) no correlation between S100A8/A9 and tissue plasminogen activator (tPA), (E), and no correlation between S100A8/A9 and plasminogen activator inhibitor (PAI-1). (A color version of this figure is available in the online journal.)
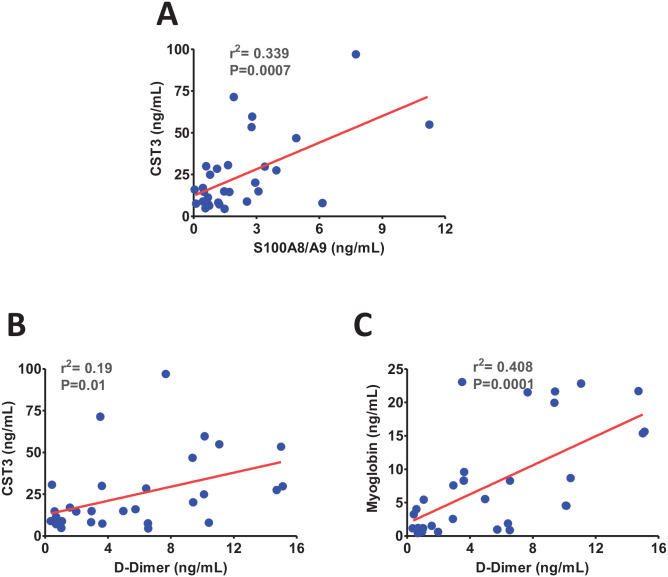
Next, we hypothesized if S100A8/A9 has any association with CST3 which is a regulator of thromboembolism and kidney injury. 27 Interestingly, S100A8/A9 has shown a strong positive correlation with the level of CST3 in COVID-19 (Figure 4(A)). Since a significant positive correlation between CST3 and D-dimer was also observed (Figure 4(B)), S100A8/A9 likely induces thromboembolism and tissue injury in COVID-19 partly through CST3 as we recently reported positive correlations of S100A8/A9 and CST3 with myoglobin. 14 Consistently, the positive correlation between myoglobin and D-dimer (Figure 4(C)) further attests to the role of thromboembolism in SARS-CoV-2 mediated tissue injury.
Figure 4.
The scatter plots show significant positive correlations between (A) calprotectin (S100A8/A9) and cystatin C (CST3), (B) CST3 and D-dimer, and (C) and between myoglobin and D-dimer. (A color version of this figure is available in the online journal.)
Taken together, the correlation analyses demonstrate the potential pathomechanisms of thromboembolism in COVID-19. A positive correlation of TFPI and FXIII with D-dimer suggests the activation of the TF-mediated coagulation pathway followed by impaired fibrin polymerization and ultimately thrombus instability. The level of plasma PAI-1, but not tPA, corresponds to the level of granule release from activated platelets and endothelial cells, which further play roles in aberrant fibrinolysis in COVID-19. Moreover, S100A8/A9 may play potential roles in aberrant fibrinolysis through, in part, regulation of CST3.
Supplemental Material
Supplemental material, sj-pptx-1-ebm-10.1177_15353702221102117 for TFPI and FXIII negatively and S100A8/A9 and Cystatin C positively correlate with D-dimer in COVID-19 by Anamika Gupta, Rizwan Qaisar, Rabih Halwani, Meganathan Kannan and Firdos Ahmad in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: AG helped with data acquisition and analysis and manuscript writing, RQ, RH, and MK helped with data interpretation and manuscript writing, and FA designed the study, acquired funding, performed data analysis and interpretation, and wrote the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study protocol was approved by the ethical review boards of the University of Sharjah and the Dubai Health Authority.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by COVID-19 (CoV-0302), Targeted (1801090144P), and Competitive (1901090162P) research grants from the University of Sharjah to Firdos Ahmad.
ORCID iDs: Rabih Halwani  https://orcid.org/0000-0002-6516-7771
https://orcid.org/0000-0002-6516-7771
Firdos Ahmad  https://orcid.org/0000-0001-6530-6068
https://orcid.org/0000-0001-6530-6068
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Gupta A, Marzook H, Ahmad F. Comorbidities and clinical complications associated with SARS-CoV-2 infection: an overview. Clin Exp Med. Epub ahead of print 1 April 2022. DOI: 10.1007/s10238-022-00821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad F, Kannan M, Ansari AW. Role of SARS-CoV-2 -induced cytokines and growth factors in coagulopathy and thromboembolism. Cytokine Growth Factor Rev 2022;63:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003;349:1227–35 [DOI] [PubMed] [Google Scholar]
- 4. Hadid T, Kafri Z, Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev 2021;47:100761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al-Tamimi AO, Yusuf AM, Jayakumar MN, Ansari AW, Elhassan M, AbdulKarim F, Kannan M, Halwani R, Ahmad F. Induction of soluble P-selectin and CD40 ligand and, FXIII deficiency promote aberrant coagulation and thromboembolism in severe COVID-19. Circ Res 2021;129:AP357 [Google Scholar]
- 6. McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020;2:e437–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kannan M, Ahmad F, Saxena R. Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood Rev 2019;37:100583. [DOI] [PubMed] [Google Scholar]
- 8. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res 2020;194:101–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation 2004;109:2698–704 [DOI] [PubMed] [Google Scholar]
- 11. Stouthard JM, Levi M, Hack CE, Veenhof CH, Romijn HA, Sauerwein HP, van der Poll T. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost 1996;76:738–42 [PubMed] [Google Scholar]
- 12. Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol 2001;115:3–12 [DOI] [PubMed] [Google Scholar]
- 13. Al-Tamimi AO, Yusuf AM, Jayakumar MN, Ansari AW, Elhassan M, AbdulKarim F, Kannan M, Halwani R, Ahmad F. SARS-CoV-2 infection induces soluble platelet activation markers and PAI-1 in the early moderate stage of COVID-19. Int J Lab Hematol. Epub ahead of print 9 March 2022. DOI: 10.1111/ijlh.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta A, Al-Tamimi AO, Halwani R, Alsaidi H, Kannan M, Ahmad F. Lipocalin-2, S100A8/A9, and cystatin C: potential predictive biomarkers of cardiovascular complications in COVID-19. Exp Biol Med (Maywood). Epub ahead of print 23 April 2022. DOI: 10.1177/15353702221091990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, Gao Y, Cai L, Wang Z, Yin P, Wang Y, Tang L, Deng J, Mei H, Hu Y. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol 2020;7:e671–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Pendurthi UR, Yi G, Rao LVM. SARS-CoV-2 infection induces the activation of tissue factor-mediated coagulation via activation of acid sphingomyelinase. Blood 2021;138:344–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ameri A, Kuppuswamy MN, Basu S, Bajaj SP. Expression of tissue factor pathway inhibitor by cultured endothelial cells in response to inflammatory mediators. Blood 1992;79:3219–26 [PubMed] [Google Scholar]
- 18. von Meijenfeldt FA, Havervall S, Adelmeijer J, Lundstrom A, Magnusson M, Mackman N, Thalin C, Lisman T. COVID-19 is Associated with an Acquired Factor XIII Deficiency. Thromb Haemost 2021;121:1668–9 [DOI] [PubMed] [Google Scholar]
- 19. Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136: 489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolberg AS. Fibrinogen and factor XIII: newly recognized roles in venous thrombus formation and composition. Curr Opin Hematol 2018;25:358–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin H, Xu L, Yu S, Hong W, Huang M, Xu P. Therapeutics targeting the fibrinolytic system. Exp Mol Med 2020;52:367–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huebner BR, Moore EE, Moore HB, Stettler GR, Nunns GR, Lawson P, Sauaia A, Kelher M, Banerjee A, Silliman CC. Thrombin provokes degranulation of platelet alpha-granules leading to the release of active plasminogen activator inhibitor-1 (PAI-1). Shock 2018;50:671–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zuo Y, Warnock M, Harbaugh A, Yalavarthi S, Gockman K, Zuo M, Madison JA, Knight JS, Kanthi Y, Lawrence DA. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci Rep 2021;11:1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan X, Zheng X, Huang Z, Lin J, Xie C, Lin Y. Involvement of S100A8/A9-TLR4-NLRP3 inflammasome pathway in contrast-induced acute kidney injury. Cell Physiol Biochem 2017;43:209–22 [DOI] [PubMed] [Google Scholar]
- 26. Xie H, Sheng L, Zhou H, Yan J. The role of TLR4 in pathophysiology of antiphospholipid syndrome-associated thrombosis and pregnancy morbidity. Br J Haematol 2014;164:165–76 [DOI] [PubMed] [Google Scholar]
- 27. Brodin EE, Braekkan SK, Vik A, Brox J, Hansen JB. Cystatin C is associated with risk of venous thromboembolism in subjects with normal kidney function—the Tromso study. Haematologica 2012;97:1008–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pptx-1-ebm-10.1177_15353702221102117 for TFPI and FXIII negatively and S100A8/A9 and Cystatin C positively correlate with D-dimer in COVID-19 by Anamika Gupta, Rizwan Qaisar, Rabih Halwani, Meganathan Kannan and Firdos Ahmad in Experimental Biology and Medicine