One of the most devastating symptoms of autism spectrum disorder (ASD), which affects ~0.70% of children in Mainland China [1], is compromised social skills [2]. As it is critical for individuals' survival and adaptive behavioral developments, those diagnosed with ASD may constantly battle the difficulties of mundane social interactions. Although noted and assessed by physicians, psychiatrists, and pediatricians with tremendous efforts to find treatments capable of improving social skills in ASD patients, none have yet been satisfactory. One of the major obstacles that hampers the development of new treatment strategies is the genetic heterogeneity attributed to ASD. While genomic evidence has suggested a list of possible culprits, none have been effective targets for ASD treatment for multiple reasons, from unclarified molecular mechanisms to ineffective drug discovery [3]. Nevertheless, with novel techniques such as opto-/chemo-genetics and viral vector-mediated neuronal tracing, researchers have unexpectedly found that various risk factors, either genetic or environmental, tend to converge on certain common social circuits. Thus, compared to investigating ASD at a traditional genetic-molecular level, a much more promising strategy is looking into the common social circuits that underlie ASD.
5-Hydroxytryptamine (5-HT), also known as serotonin, is a monoamine neurotransmitter mainly synthesized in the raphe nuclei and then delivered throughout other brain regions, where they play significant yet versatile roles in anxiety/depression formation, neuroendocrine system functioning, and reward-related behavior, all of which are closely correlated with social behaviors. Thus, the hypothesis concerning the involvement of 5-HT in the neurodevelopment of ASD is one of the longest standing [4]. Clinical evidence dating back to 1961 has reported that an abnormal bloodstream 5-HT level occurs in autistic children [5]. Recently, opposing changes of serotonergic innervation in the amygdala were reported in patients with either ASD or Williams syndrome who exhibit dichotomous social behaviors [6], emphasizing a possible contribution of 5-HT to social behaviors. In addition, the latest study showed that the availability of the 5-HT transporter (5-HTT), which is the main player responsible for extracellular 5-HT clearance, is lower in the brain of adult individuals with ASD [4]. Interestingly, 5-HTT expression and distribution is altered in either neuroligin 2- or neurexin-null mutant mice [7, 8]; these are classic synaptic partners tightly associated with ASD pathology [9, 10]. Furthermore, 5-HT neurons lacking neuroligin 2 or neurexins exhibited anomalous excitability and impaired 5-HT release in the dorsal raphe (DR) nuclei [7, 8], giving the probability that social behaviors mediated by neurexins/neuroligin 2 are modulated by serotonergic circuits. Nonetheless, the existence and detailed picture of the specific serotonergic circuits underlying social behavior remain mysterious.
It has been reported that mice with genetically-depleted brain serotonergic projections display social impairments and communication deficits [11], implying that changes or manipulations of 5-HT signaling influence social behaviors [6], yet the detailed circuits for 5-HT-modulated social behaviors were undetermined. A leading study was conducted by Malenka and his colleges [12] when they investigated the role of oxytocin in the nucleus accumbens (NAc), a key component of the mesocorticolimbic reward circuits [13]. They demonstrated that oxytocin acts as a social reinforcement signal within the NAc and selective ablation of presynaptic NAc oxytocin receptors (OTRs) completely abolishes social reward behavior in mice. Although the NAc receives OTR-containing inputs from multiple brain regions, genetic deletion of OTRs specifically from the DR , which provides 5-HT innervation to the NAc, blocks the reinforcing properties of social interactions. Furthermore, oxytocin-induced synaptic plasticity requires the activation of NAc 5-HT1b receptors, and their blockade prevents social reward. Together, these results suggest that the rewarding properties of social interaction in mice require coordinated activity involving both oxytocin and 5-HT in the NAc, pointing to a critical role of 5-HT related circuits in social behaviors.
Malenka’s group further confirmed that 5-HT release in the NAc rescues the sociability deficits in a mouse model of autism [14]. Specifically, stimulation of 5-HT release in the NAc enhances sociability while inhibiting DR 5-HT neurons, through soma or terminal manipulation, compromises sociability [14]. In mice, decreases of 5-HTergic neuronal activity in the DR region (Nestin-Cre with region-specific targeting) or 5-HT neurons (Sert-Cre with neuron subtype specificity) induce deficits in social behavior. To determine whether manipulation of the DR–NAc 5-HTergic neural circuit is a target for treating social defects in ASD patients, a mouse model was used with conditional deletion of corresponding genetic susceptibility that mimics ASD patients with copy number variation on chromosome 16p11.2. The authors showed that the behavior of mice with 16p11.2 deletion has sociability deficits that are rescued by optogenetic activation of DR 5-HT neuronal somata or with DR–NAc terminal activation. In addition, these outcomes required and were mimicked by activating 5-HT1b receptors in the NAc. Overall, this study provides the first hint of an unexpected role of 5-HT activity in the NAc pertaining to sociability functioning, and suggests that targeting this mechanism may prove therapeutically beneficial.
Interestingly, a latest study from the same group further emphasized the importance of 5-HT released from the medial raphe (MR) nucleus in the modulation of social memory stability [15], a critical step for a social animal to recognize and remember familiar conspecifics. It is well-known that the hippocampal dorsal CA2 (dCA2) and ventral CA1 (vCA1) subregions are crucial for social memory formation, yet the upstream input regions projecting to the dCA2 and vCA1 remain uncharacterized. Strikingly, with genetically-modified TRAP2;Ai14 (in vivo c-Fos labeling) mice as subjects for social interaction assay, they found that after interaction with novel mice the c-Fos-positive neurons were significantly increased in the medial septum (MS) and dCA2 compared to novel object or control condition (empty cage). More importantly, chemogenetic manipulation of 5-HT activity in the MS bi-directionally regulates social memory formation and optogenetic inhibition of the MS–dCA2 circuit disrupts social memory, while the initial sociability is unaffected. Since neuromodulators play roles in social behaviors while MS neurons express receptors for both oxytocin and 5-HT [12], the authors then explored the potential roles of oxytocin and 5-HT in MS-mediated social memory. Interestingly, social memory was not influenced by infusion of an OTR antagonist but was prevented by a 5-HT receptor 1B (5-HT1b) antagonist, suggesting that 5-HT released in the MS is necessary for social memory. By TRIO (tracing the relationship between input and output) monosynaptic rabies virus tracing, the authors identified a monosynaptic input from the MR onto MS cells projecting to the dCA2. Optogenetic inhibition of MR 5-HT inputs in the MS during the initial social interaction did not affect baseline sociability and inanimate object memory but impaired social memory. Consistent with this, activation of 5-HT inputs in the MS during the initial novel social interaction generated long-lasting social memory. Lastly, the authors generated a mouse model with haploinsufficiency for the ASD-susceptibility genes neuroligins 1, 2, and 3 [16] (Nlgn123het mice) which exhibited normal baseline sociability but impaired social memory [15]. Infusion of a 5-HT1b receptor agonist into the MS rescued the social memory deficit but had no effect on inanimate object memory or baseline sociability.
Based on the above mechanistic studies, they further explored possible preclinical treatments in ASD mouse models [17]. First tested was the effects of the recreational drug MDMA, a chemical that boosts self-confidence, enhances sociability, and facilitates communication in humans. The three-chamber sociability test and juvenile interaction test both yielded reversed sociability deficits in all ASD mouse models after MDMA administration. Furthermore, MDMA-treated normal mice exhibited enhanced sociability. However, MDMA binds to the 5-HTT with high affinity, causing excessive release of 5-HT via a reverse transport mechanism that makes it addictive, posing a substantial risk in clinical application. Thus, the researchers turned to other alternatives capable of enhancing the 5-HTergic circuit. One serotonin receptor sub-type named 5-HT1b attracted attention in previous studies. Further experiments showed that replacing MDMA with CP-94253, a type of 5-HT1b receptor agonist, reverses the social behavior deficit in multiple ASD mouse models, while having no effects on control mice. Overall, these findings suggested 5-HT and its related circuits as plausible targets for treating the social deficits in ASD.
However, several concerns and potential drawbacks require attention in 5-HT-related drug or treatment development and future clinical applications. First, 5-HT appears to mediate different kinds of behaviors in disparate brain regions: for example, forebrain expression of 5-HT1b receptors is essential for adult aggression and impulsivity [18], and dorsal periaqueductal gray 5-HT receptors are involved in modulating defensive behaviors in mice [19]. Thus, global application of pharmacological interventions altering 5-HT activity may lead to activation in a series of brain regions that receive DR projections, causing other psychological issues. Second, 5-HT heteroreceptors perform distinct functions at different developmental stages. An early postnatal period of 5-HT1b receptor expression contributes to the development of adult aggression, while certain heteroreceptors acting during adulthood mediate impulsivity [18], indicating that 5-HT mediates distinct functions at different developmental stages, and requires tailored treatment schemes for different age groups. Third, 5-HT neurons in the raphe nuclei are heterogeneous and possess a variety of idiosyncratic molecular hallmarks, which may orchestrate different functions and give rise to various behaviors. Therefore, to identify specific subtypes responsible for distinct functions using techniques like single-cell sequencing or the spatial transcriptome may contribute to clinical applications. Last, that neuromodulators such as 5-HT tend to act in networks [20] must also be taken into consideration. Thus, a comprehensive understanding of how social interaction is initiated, maintained, and remembered will likely require simultaneous examination of the actions of multiple neuromodulators in a range of brain areas (Fig. 1).
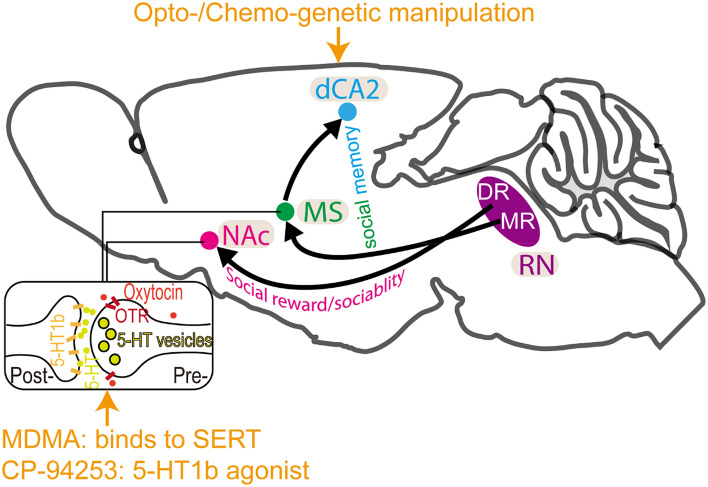
Fig. 1.
5-HT modulates social behaviors via distinct neural circuits. The raphe nuclei (RN) are the main source of 5-HT in the CNS. Dorsal RN (DR) 5-HT release into the NAc is required for social reward by coordinating the functions of oxytocin and is also essential for sociability with specifically activated 5-HT1b receptors. In addition, medial RN (MR) 5-HT release in the MS is critical for social memory stability by modulating the MS–dCA2 circuit that also requires 5-HT1b receptors. Manipulation of these circuits with opto-/chemo-genetics and enhancement of 5-HT signaling rescues the social behavioral deficits in mouse models of ASD.
Despite a probable contribution of 5-HT to social behavior and ASD has been proposed for decades, the direct neurobiological evidence is just emerging. Here, we recapitulated a series of studies basically from the same group that all clearly showed that 5-HT modulates different aspects of social behaviors with distinct neuronal circuits. Typically, DR 5-HT release into the NAc is required for social reward by coordinating the functions of oxytocin [12] and specifically activated 5-HT1b receptors are also essential for sociability [14]. In addition, MR 5-HT release in the MS is critical for social memory stability by modulating the MS–dCA2 circuit [15]. Importantly, manipulation of these neural circuits with opto-/chemo-genetics and enhancement of 5-HT signaling rescues the social behavioral deficits in mouse models of ASD.
Acknowledgements
This Insight article was supported by grants from the Key-Area Research and Development Program of Guangdong Province (2019B030335001), the National Natural Science Foundation of China (32000690), and the National Social Science Foundation of China (20&ZD296).
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Guangyi Yang and Hongyan Geng have contributed equally to this work.
References
- 1.Zhou H, Xu X, Yan WL, Zou XB, Wu LJ, Luo XR, et al. Prevalence of autism spectrum disorder in China: A nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull. 2020;36:961–971. doi: 10.1007/s12264-020-00530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodges H, Fealko C, Soares N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9:S55–S65. doi: 10.21037/tp.2019.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vorstman JAS, Parr JR, SMoreno-De-Lucaoares D, Anney RJL, Nurnberger JI, Jr, Hallmayer JF. Autism genetics: Opportunities and challenges for clinical translation. Nat Rev Genet. 2017;18:362–376. doi: 10.1038/nrg.2017.4. [DOI] [PubMed] [Google Scholar]
- 4.Andersson M, Tangen Ä, Farde L, Bölte S, Halldin C, Borg J, et al. Serotonin transporter availability in adults with autism-a positron emission tomography study. Mol Psychiatry. 2021;26:1647–1658. doi: 10.1038/s41380-020-00868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/S0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 6.Lew CH, Groeniger KM, Hanson KL, Cuevas D, Greiner DMZ, Hrvoj-Mihic B, et al. Serotonergic innervation of the amygdala is increased in autism spectrum disorder and decreased in Williams syndrome. Mol Autism. 2020;11:12. doi: 10.1186/s13229-019-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A, Matsui A, Abe M, Sakimura K, Sasaoka T, Uemura T, et al. Neurexins in serotonergic neurons regulate serotonin transmission and complex mouse behaviors. bioRxiv 2021, 10.1101/2021.12.09.471904. [DOI] [PMC free article] [PubMed]
- 8.Ye R, Quinlan MA, Iwamoto H, Wu HH, Green NH, Jetter CS, et al. Physical interactions and functional relationships of neuroligin 2 and midbrain serotonin transporters. Front Synaptic Neurosci. 2016;7:20. doi: 10.3389/fnsyn.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trobiani L, Meringolo M, Diamanti T, Bourne Y, Marchot P, Martella G, et al. The neuroligins and the synaptic pathway in autism spectrum disorder. Neurosci Biobehav Rev. 2020;119:37–51. doi: 10.1016/j.neubiorev.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Kane MJ, Angoa-Peréz M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, et al. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: Possible relevance to autism. PLoS One. 2012;7:e48975. doi: 10.1371/journal.pone.0048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Y, Gao G, Liu K, Shi X, Cheng MX, Xiong Y, et al. Projections from D2 neurons in different subregions of nucleus accumbens shell to ventral pallidum play distinct roles in reward and aversion. Neurosci Bull. 2021;37:623–640. doi: 10.1007/s12264-021-00632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature. 2018;560:589–594. doi: 10.1038/s41586-018-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu XT, Morishita W, Beier KT, Heifets BD, Malenka RC. 5-HT modulation of a medial septal circuit tunes social memory stability. Nature. 2021;599:96–101. doi: 10.1038/s41586-021-03956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia QQ, Xu J, Liao TL, Yu J, Shi L, Xia J, et al. Neuroligins differentially mediate subtype-specific synapse formation in pyramidal neurons and interneurons. Neurosci Bull. 2019;35:497–506. doi: 10.1007/s12264-019-00347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh JJ, Llorach P, Cardozo Pinto DF, Wenderski W, Christoffel DJ, Salgado JS, et al. Systemic enhancement of serotonin signaling reverses social deficits in multiple mouse models for ASD. Neuropsychopharmacology. 2021;46:2000–2010. doi: 10.1038/s41386-021-01091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nautiyal KM, Tanaka KF, Barr MM, Tritschler L, le Dantec Y, David DJ, et al. Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron. 2015;86:813–826. doi: 10.1016/j.neuron.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pobbe RLH, Zangrossi H, Jr, Blanchard DC, Blanchard RJ. Involvement of dorsal raphe nucleus and dorsal periaqueductal gray 5-HT receptors in the modulation of mouse defensive behaviors. Eur Neuropsychopharmacol. 2011;21:306–315. doi: 10.1016/j.euroneuro.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avery MC, Krichmar JL. Neuromodulatory systems and their interactions: A review of models, theories, and experiments. Front Neural Circuits. 2017;11:108. doi: 10.3389/fncir.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]